Abstract
Basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (cSCC) are the most frequent cancers in humans, with cumulative ultraviolet radiation exposure, aging, and immunodepression as the main risk factors. In most cases, these malignancies arise in the head and neck area, and they can be treated with locoregional therapies. A minority of cases require systemic therapy. Currently, Sonic Hedgehog inhibitors (i.e., vismodegib and sonidegib) have been approved for advanced BCC, while the PD-1 checkpoint inhibitor cemiplimab has been approved as a first-line treatment for cSCC and as a second-line treatment for BCC. Nevertheless, there is a clinical need for an effective and safe systemic therapies for advanced synchronous (syn) BCC/cSCC not amenable to local treatments. International guidelines do not provide specific recommendations for patients affected by this condition, and no case reports on the full-dose association of these medications have been previously reported. Here, we present the cases of two elderly patients affected by synBCC/cSCC of the head and neck, who received combined therapy with cemiplimab and sonidegib at full dose and standard schedule, achieving remarkable clinical benefit and long-term responses, without major adverse events. The instance of a feasible treatment for patients with advanced synBCC/cSCC will become increasingly frequent with the advancement of life expectancy in the global population, and the synergistic activity of targeted therapies and immunotherapy—administered either in association or sequentially—deserves to be further explored.
Keywords: cutaneous squamous cell carcinoma, basal cell carcinoma, synchronous carcinomas, immunotherapy, checkpoint inhibitors, hedgehog inhibitors, case report
Introduction
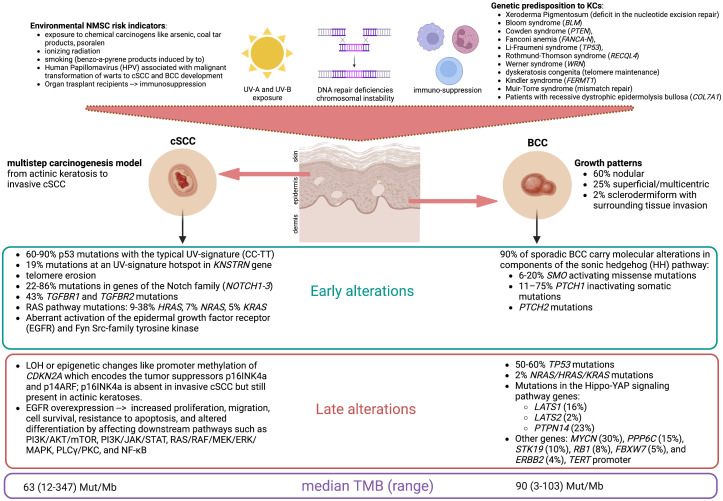
Non-melanoma skin cancers (NMSCs) are the most common malignancies in humans. Keratinocyte cancers (KCs) as basal squamous cell carcinoma (BCC) and cutaneous squamous cell carcinoma (cSCC) account for 99% of NMSCs, with a BCC-to-SCC prevalence ratio between 1:1 and 10:1, depending on the population, ethnic group, and gender (1). In the general population, BCC has higher incidence rates than cSCC, while the reverse is seen in the population of solid organ transplant recipients (2). Advanced cSCC has higher lethality than BCC, and the overall incidence of cSCC increased by more than 200% between 1990 and 2019 (3). The DNA damage from ultraviolet (UV-A and UV-B) radiation exposure is the major risk factor for both histotypes; BCC is caused by intensive UV exposure in childhood/adolescence, while cSCC is related to cumulative UV exposure over decades ( Figure 1 ) (4). Synchronous BCC/cSCC (synBCC/cSCC) is not infrequent: the 3-year cumulative risk of developing a BCC in patients with a prior SCC is 44%, while the risk of developing a cSCC in patients with a prior BCC is approximately 6% (5). In a recent retrospective study on the Australian population, the 5-year cumulative risk of subsequent keratinocyte carcinoma after a first diagnosis of synBCC/cSCC was 51%, with an annualized 5-year incidence rate of 16,634/100,000 person-years at risk (6). Another retrospective study on 969 patients diagnosed in Portugal observed that cases with a history of skin cancer had a risk of 17.0% of developing new skin neoplasms. Moreover, the main risk factors for the development of metachronous lesions were advanced age and detection of synchronous neoplasms at first diagnosis (7). The last GLOBOCAN report showed that, in 2020, more than 63,700 patients succumbed to NMSC-specific death on a global scale. Therefore, the appropriate management of the advanced forms of NMSC is a relevant global health issue (8).
Figure 1.
Keratinocytes are the prevalent resident cell types within the epidermis. Common risk factors of keratinocyte cancers include sun exposure, DNA repair deficiencies leading to chromosomal instability, and immunosuppression. Frequently, these factors are intermingled, as DNA photoproducts are almost exclusively repaired by the nucleotide excision repair pathway, and cancerous cells escape from detection in the presence of a defective immune system. This figure reports the main risk factors for the development of cutaneous squamous cell carcinoma (cSCC) and basal cell carcinoma (BCC) with the most frequent molecular alterations found in the early and late developments of these lesions and their relative prevalence (%). The median tumor mutational burden (TMB), expressed in the number of mutations per megabase (Mut/Mb), is correlated with genomic instability and is considered high if >10 Mut/Mb. BCCs harbor a low level of immune cell infiltration, but HHi therapy increases CD8+ and CD4+ T-cell infiltration and sensitivity to immune checkpoint inhibitors. (Figure created by the authors with BioRender).
The majority of KCs arise in the head and neck area and can be cured with surgery, cryo-/photodynamic therapy, or radiotherapy (9). Locally advanced BCC (laBCC) and cSCC not amenable to local treatments are aggressive malignancies with low chemosensitivity. Nevertheless, in the last decade, the rise of targeted therapy and immune checkpoint inhibitors has favorably changed the prognosis of patients affected by advanced KCs. Nearly 90% of sporadic BCCs harbor mutations in the genes of the Sonic Hedgehog (HH) pathway, and up to 70% harbor mutations in the PTCH1 gene on chromosome 9q coding for the patched-1 receptor (10, 11). Hedgehog inhibitors (HHi) are approved by the Food and Drug Administration (FDA) and the European Medical Agency (EMA) for the treatment of unresectable BCCs on the basis of two pivotal studies: the single-arm phase II ERIVANCE BCC with vismodegib given 150 mg orally daily (12) and the phase II BOLT with sonidegib given 200 mg orally daily (dose approved for use) (13). These trials showed an objective response rate (ORR) by central review of 43% and 56%, respectively, although with some differences in the criteria of response evaluation (14).
As both BCC and cSCC harbor a high tumor mutational burden (TMB) due to radiation-induced DNA damage (15, 16), immunotherapy with anti-PD-1 agents has been explored as a potential therapeutic strategy (17–19). The single-arm phase II study EMPOWER-CSCC-1 in a population of patients with cSCC treated with the PD-1 checkpoint inhibitor cemiplimab (350 mg flat dose intravenously every 3 weeks) showed an ORR of 44% (95% CI 32–55), leading to the FDA and EMA approval of first-line treatment for locally advanced/metastatic cSCC (20). In February 2021, the FDA approved cemiplimab also for the treatment of laBCC in patients who progressed on HHi or were intolerant to prior HHi therapy, on the basis of a non-randomized phase II trial reporting an ORR of 31% (95% CI 21–42), with 6% of complete responses and 25% of partial responses; serious treatment-related adverse events (AEs) occurred in 35% of the study population (21). Recently, a phase II basket study (NCT03012581) included 32 advanced BCC patients to evaluate the efficacy and safety of the PD-1 inhibitor nivolumab after the failure of HHi. Disease control was achieved in terms of complete responses (12.5%), partial responses (18.8%), and stable diseases (43.8%) (22). Despite the high TMB, BCC has a low immune infiltration, but HHi therapy increases CD8+ and CD4+ T-cell infiltration, suggesting a synergistic effect of the combination/sequence of treatment with HHi and checkpoint inhibitors on the management of laBCC (23).
Currently, the international guidelines do not provide specific recommendations for the first-line therapy of patients with advanced synBCC/cSCC. Therefore, clinicians face complex decisions for the treatment of these cases in real-world practice. A previous proof-of-principle non-randomized trial on 16 patients with laBCCs tested anti-PD-1 pembrolizumab with (n = 7) or without (n = 9) vismodegib, reporting an overall response rate (ORR) of 29% for the anti-PD-1/HHi doublet and 44% for anti-PD-1 monotherapy (24). Investigators concluded that pembrolizumab is active against BCCs and that the response of the anti-PD-1/HHi group was not superior to the anti-PD-1 monotherapy group; however, no specific information about the safety of anti-PD-1/HHi combination was provided. Here, we report two cases of synchronous laBCC and cSCC of the head and neck area treated with cemiplimab and sonidegib in two different tertiary referral centers. Both patients gave their written permission to use information—including photographs—about their clinical history for the purposes of this paper. All the reported AEs are graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 (25). The staging was given according to the clinical TNM Staging System of the cSCC and BCC of the head and neck (AJCC/UICC 8th Edition).
Case 1: Cemiplimab plus sonidegib
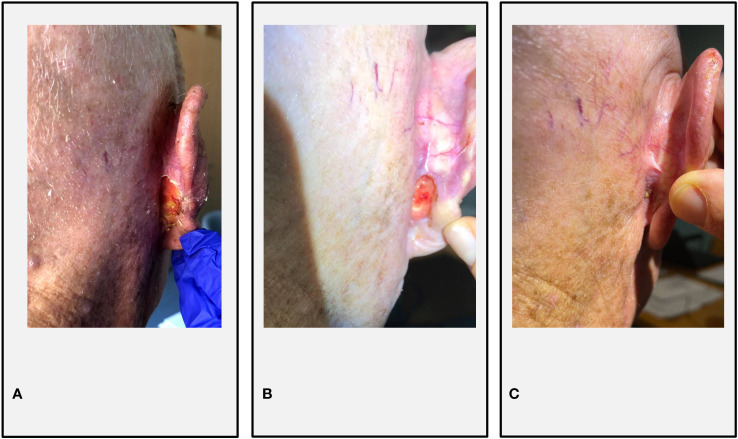
A 76-year-old Caucasian man with a history of multiple excisions of localized KCs was referred for a bulky recurrence of cSCC with sarcomatoid features of the vertex. The lesion had been treated 20 months before with R1 surgery (positive lateral margins) followed by three courses of palliative hemostatic radiotherapy, the last delivered 1 month before the first visit to our institution. The patient presented with a large and painful vegetating mass of the vertex characterized by a fibrinous central ulcerated–hemorrhagic area and peripheral necrosis ( Figure 2A ). The baseline skin examination revealed multiple BCCs, mostly localized in the head and neck region. The patient had a good performance status (ECOG PS 1) and did not present significant comorbidities; he did not report a family history of malignant neoplasms of the skin. A whole-body computer tomography scan excluded distant metastases (cTNM cT3N0M0). On October 2019, the patient started cemiplimab 350 mg intravenously every 3 weeks, achieving a dimensional reduction of the cSCC on the vertex ( Figures 2B, C ) and numerical reduction of the multiple diffuse BCCs, without reporting immune-related adverse events (irAEs). After 6 months from the start of immunotherapy, the patient reported rapid onset of a painful, hemorrhagic lesion in the right auricular area. At clinical examination, the ulcerated lesion was infiltrating the external auditory meatus, and a biopsy confirmed the clinical suspect of BCC (cT3) ( Figure 2D ). A multidisciplinary evaluation deemed the lesion not amenable to radical surgery or radiotherapy. In order to maximize the symptomatic control and maintain the clinical benefit of the cSCC, sonidegib at standard dose and schedule was started in November 2020, while continuing cemiplimab administration at the usual dose. A prompt clinical response on the laBCC was observed ( Figures 2E, F ), while the cSCC at the vertex maintained clinical and radiological partial response. On the last follow-up visit, the patient had completed 31 cycles of cemiplimab and 10 cycles of sonidegib. The combined treatment was well tolerated, with mild HHi-related AEs (myalgia G1, alopecia G1) and no evidence of irAEs. In August 2021, the patient succumbed to the consequences of sepsis, deemed unrelated to the oncological treatment.
Figure 2.
Case 1. (A) Bulky recurrence on the vertex of cSCC at cycle 1 (C1) of cemiplimab; (B) partial response at C23 of cemiplimab and C4 of sonidegib; (C) stable disease at C30 of cemiplimab and C10 of sonidegib. (D) Infiltrative BCC extended to the right external auditory meatus at C18 of cemiplimab and C1 of sonidegib; (E) partial response at C23 of cemiplimab and C4 of sonidegib; (F) complete response of BCC at C30 of cemiplimab and C10 of sonidegib.
Case 2: Sonidegib followed by cemiplimab
An 83-year-old Caucasian man with a history of multiple excisions of head and neck cSCCs and BCCs was evaluated for the onset of 3-cm right fronto-temporal recurrence of cSCC (cT2) and a painful right retroauricular BCC infiltrating the auricular cartilage (cT3) ( Figure 3A ). The patient had a good performance status (ECOG PS 1) and did not present significant comorbidities; he did not report a family history of malignant neoplasms of the skin. After a multidisciplinary evaluation, in April 2020, the patient underwent a multimodal therapeutic approach to the lesions. Surgical removal of the right fronto-temporal cSCC was performed (R0, with perineural invasion), followed by adjuvant radiotherapy (total dose 66 Gy in 33 fractions); meanwhile, he started systemic treatment with sonidegib. HHi therapy was well tolerated, except for mild dysgeusia and asthenia (G1). After 2 months of sonidegib therapy, a significant clinical reduction of the retroauricular BCC was achieved ( Figure 3B ). In December 2020, a right retroangulomandibular adenopathy occurred, histologically diagnosed as cSCC metastasis. Since neither surgery nor radiotherapy was deemed feasible, cemiplimab at standard dose was started. Sonidegib was discontinued after 10 cycles, considering the good radiological response obtained at the retroauricular level and the HHi-related toxicities reported (muscle spasms G2, dysgeusia G2, and asthenia G2). The patient experienced a mild infusion reaction at the first cycle (lower back pain and flushing). At the last follow-up on 22 November 2022, the patient completed 27 cycles of cemiplimab, obtaining a complete response with clinical benefit ( Figure 3C ) without significant AEs from the sequential HHi/anti-PD-1 therapy.
Figure 3.
Case 2. (A) Retroauricular BCC infiltrating the right auricular cartilage at C1 of sonidegib; (B) partial response at C10 of sonidegib; (C) complete response at C10 of cemiplimab. A computer tomography scan of the neck reported a complete response of the cSCC nodal relapse at C7 of cemiplimab.
Discussion
To the best of our knowledge, these are the first clinical reports on the concomitant and sequential full-dose administration of sonidegib and cemiplimab in patients with inoperable synchronous laBCC/cSCC. In our cases, the HHi/anti-PD-1 association proved to be an effective and well-tolerated strategy. In Case 1, the preauricular laBCC was resistant to immunotherapy, and the association of sonidegib with cemiplimab enabled achieveing a complete response of the laBCC, while maintaining the benefit from anti-PD-1 on the cSCC. In Case 2, sonidegib was started to treat a retroauricular laBCC, achieving a partial response; then, cemiplimab was started for a nodal relapse of cSCC, obtaining a complete response of both lesions. These two different therapeutic approaches to synchronous laBCC/SCC, imbricating the PD-1 inhibitor cemiplimab with HHi in concurrent and sequential ways, were both well tolerated despite the age of the patients, who managed to continue the systemic treatments for almost 22 months in Case 1 and at least 30 months in Case 2 ( Figure 4 ).
Figure 4.
Timeline showing the multimodal treatments received and the responses achieved by each patient since surgery to systemic therapy. The clinical staging of cutaneous squamous cell carcinoma (cSCC) and basal cell carcinoma (BCC) is based on the TNM AJCC/UICC 8th Edition criteria. For resected lesions, surgical margin status (R1, microscopically positive; R0, negative) and positive perineural invasion (PNI+) are reported. Adjuvant radiotherapy treatment is quantified in Gray (Gy) per fraction (fx). Blue blocks correspond to cemiplimab immunotherapy given intravenously (IV) at the flat dose of 350 mg once every 21 days (q21); yellow blocks correspond to sonidegib targeted therapy given orally every day (QD) in cycles of 28 days (q28). Responses are estimated according to the RECIST v.1.1 criteria (PR, partial response; CR, complete response). (Figure created with BioRender).
Over the last 5 years, the landscape of treatments for advanced KCs has profoundly changed, and HHi/anti-PD-1 combination therapy is currently under evaluation. The Sonic Hedgehog pathway promotes tumor-associated macrophage polarization (26) and induction of PD-L1 expression (27). Therefore, HHi may promote an adaptive immune response in BCC, fostering a synergistic effect with anti-PD-1 checkpoint inhibitors (28).
Ongoing clinical trials are evaluating the combination of anti-PD-1 plus HHi in laBCC. The design of a phase II open-label non-randomized clinical trial with cemiplimab and sonidegib administered in a pulsed schedule was presented at the ESMO Congress 2021 (NCT04679480): the study planned to include 20 patients with advanced BCC receiving sonidegib in a run-in phase, followed by combination therapy of cemiplimab at standard dose plus sonidegib in a 2-week cycle every 4 weeks (pulsed therapy: 2 weeks on, 2 weeks off) to evaluate primarily the best response at any time between treatment start and 26 weeks after the initiation of the treatment, and secondarily the tumor response at 26 weeks, changes in histology/immunogenicity of the tumor, and treatment safety (29). In addition, a phase I study with pembrolizumab and sonidegib for solid malignancies is currently ongoing, including patients with stage IV cSCC of the head and neck as well (NCT04007744).
Even though laBCC and unresectable cSCC have comparable risk factors and immunologic characteristics, they are two entities with different pathogenesis, molecular alterations, clinical presentation, and evolution (30, 31). Despite a common sensitivity to PD-1 inhibitors, the spectrum of responses is heterogeneous and reflects the microenvironment composition. Therefore, instead of a one-size-fits-all approach with immunotherapy, our report highlights the opportunity of considering the two components of advanced synchronous BCC/cSCC as distinct malignancies that may require—at least in certain cases—a systemic treatment with both HHi and PD-1 inhibitors in order to achieve symptomatic control and durable objective responses.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
EC, CG, AO, FC, CB, LDL, CM, AA, LL, LFL, PB, and CR participated in the concept design of the manuscript. EC, CG, PB, and CR drafted the introduction and discussion sections. EC designed Figures 1 and 4 . AO, FC, and CB retrieved the clinical information and pictures of Case 1. CG, AA, and LL retrieved the clinical information and pictures of Case 2. LDL and LFL provided critical feedback on the manuscript content and figures. All authors contributed to the revision of the final manuscript and approved the submitted version.
Funding Statement
The open access publication of this work was supported by a grant of SunPharma, which had no role in the manuscript concept, writing and revision.
Conflict of interest
Author LDL received conference honoraria/advisory board fees from EISAI, MSD, Merck Serono, McCann Healthcare, Eli Lilly, Sanofi, Sunpharma, IPSEN, Bayer, and Roche. Author LL received occasional conference honoraria/advisory board fees from Astrazeneca, Bayer, MSD, Merck-Serono, AccMed, and Neutron Therapeutics, Inc. Author PB received advisory board or conference honoraria from Merck, Sanofi-Regeneron, Merck Sharp & Dohme, Sun Pharma, Angelini, Nestlè, and Elevar. Author CR received honoraria from SunPharma. None of these relationships interfere with the intellectual content in the current manuscript.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Ciążyńska M, Kamińska-Winciorek G, Lange D, Lewandowski B, Reich A, Sławińska M, et al. The incidence and clinical analysis of non-melanoma skin cancer. Sci Rep (2021) 11(1):4337. doi: 10.1038/s41598-021-83502-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Madeleine MM, Patel NS, Plasmeijer EI, Engels EA, Bouwes Bavinck JN, Toland AE, et al. Epidemiology of keratinocyte carcinomas after organ transplantation. Br J Dermatol (2017) 177(5):1208–16. doi: 10.1111/bjd.15931 [DOI] [PubMed] [Google Scholar]
- 3. Institute for Health Metrics and Evaluation . Global burden of disease 2019 results. Available at: http://ghdx.healthdata.org/gbd-results-tool.
- 4. Leiter U, Keim U, Garbe C. Epidemiology of skin cancer. In: Reichrath J, editor. Sunlight, vitamin d and skin cancer. Advances in experimental medicine and biology, vol. 1268 . Cham: Springer; (2020). doi: 10.1007/978-3-030-46227-7_6 [DOI] [PubMed] [Google Scholar]
- 5. Marcil I, Stern RS. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer: A critical review of the literature and meta-analysis. Arch Dermatol (2000) 136(12):1524–30. doi: 10.1001/archderm.136.12.1524 [DOI] [PubMed] [Google Scholar]
- 6. Ragaini BS, Blizzard L, Venn A. Risk of subsequent keratinocyte carcinomas after a first diagnosis in Tasmania, Australia. Australas J Dermatol (2022) 64 (1):108–117. doi: 10.1111/ajd.13938 [DOI] [PubMed] [Google Scholar]
- 7. Duarte AF, Sousa-Pinto B, Haneke E, Correia O. Risk factors for development of new skin neoplasms in patients with past history of skin cancer: A survival analysis. Sci Rep (2018) 8(1):15744. doi: 10.1038/s41598-018-33763-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 9. Kansara S, Bell D, Weber R. Surgical management of non melanoma skin cancer of the head and neck. Oral Oncol (2020) 100:104485. doi: 10.1016/j.oraloncology.2019.104485 [DOI] [PubMed] [Google Scholar]
- 10. Pellegrini C, Maturo MG, Di Nardo L, Ciciarelli V, Gutiérrez García-Rodrigo C, Fargnoli MC. Understanding the molecular genetics of basal cell carcinoma. Int J Mol Sci (2017) 18(11):2485. doi: 10.3390/ijms18112485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Di Nardo L, Pellegrini C, Di Stefani A, Ricci F, Fossati B, Del Regno L, et al. Molecular alterations in basal cell carcinoma subtypes. Sci Rep (2021) 11(1):13206. doi: 10.1038/s41598-021-92592-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sekulic A, Migden MR, Basset-Seguin N, Garbe C, Gesierich A, Lao CD, et al. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer (2017) 17(1):332. doi: 10.1186/s12885-017-3286-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dummer R, Guminksi A, Gutzmer R, Lear JT, Lewis KD, Chang ALS, et al. Long-term efficacy and safety of sonidegib in patients with advanced basal cell carcinoma: 42-month analysis of the phase II randomized, double-blind BOLT study. Br J Dermatol (2020) 182(6):1369–78. doi: 10.1111/bjd.18552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dummer R, Ascierto PA, Basset-Seguin N, Dréno B, Garbe C, Gutzmer R, et al. Sonidegib and vismodegib in the treatment of patients with locally advanced basal cell carcinoma: a joint expert opinion. J Eur Acad Dermatol Venereology (2020) 34(9):1944–56. doi: 10.1111/jdv.16230 [DOI] [PubMed] [Google Scholar]
- 15. Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med (2017) 9(1):34. doi: 10.1186/s13073-017-0424-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chang D, Shain AH. The landscape of driver mutations in cutaneous squamous cell carcinoma. NPJ Genom Med (2021) 6(1):61. doi: 10.1038/s41525-021-00226-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McNamara MG, Jacobs T, Lamarca A, Hubner RA, Valle JW, Amir E. Impact of high tumor mutational burden in solid tumors and challenges for biomarker application. Cancer Treat Rev (2020) 89:102084. doi: 10.1016/j.ctrv.2020.102084 [DOI] [PubMed] [Google Scholar]
- 18. Goodman AM, Kato S, Cohen PR, Boichard A, Frampton G, Miller V, et al. Genomic landscape of advanced basal cell carcinoma: Implications for precision treatment with targeted and immune therapies. Oncoimmunology (2017) 7(3):e1404217. doi: 10.1080/2162402X.2017.1404217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goodman AM, Kato S, Chattopadhyay R, Okamura R, Saunders IM, Montesion M, et al. Phenotypic and genomic determinants of immunotherapy response associated with squamousness. Cancer Immunol Res (2019) 7(6):866–73. doi: 10.1158/2326-6066.CIR-18-0716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Migden MR, Khushalani NI, Chang ALS, Lewis KD, Schmults CD, Hernandez-Aya L, et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: Results from an open-label, phase 2, single-arm trial. Lancet Oncol (2020) 21(2):294–305. doi: 10.1016/S1470-2045(19)30728-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stratigos AJ, Sekulic A, Peris K, Bechter O, Prey S, Kaatz M, et al. Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: An open-label, multi-centre, single-arm, phase 2 trial. Lancet Oncol (2021) 22(6):848–57. doi: 10.1016/S1470-2045(21)00126-1 [DOI] [PubMed] [Google Scholar]
- 22. Véron M, Chevret S, Grob JJ, Beylot-Barry M, Saiag P, Fléchon A, et al. Safety and efficacy of nivolumab, an anti-PD1 immunotherapy, in patients with advanced basal cell carcinoma, after failure or intolerance to sonic hedgehog inhibitors: UNICANCER AcSé NIVOLUMAB trial. Eur J Cancer (2022) 177:103–11. doi: 10.1016/j.ejca.2022.09.013 [DOI] [PubMed] [Google Scholar]
- 23. Heppt MV, Gebhardt C, Hassel JC, Alter M, Gutzmer R, Leiter U, et al. Long-term management of advanced basal cell carcinoma: Current challenges and future perspectives. Cancers (Basel) (2022) 14(19):4547–7. doi: 10.3390/cancers14194547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chang ALS, Tran DC, Cannon JGD, Li S, Jeng M, Patel R, et al. Pembrolizumab for advanced basal cell carcinoma: An investigator-initiated, proof-of-concept study. J Am Acad Dermatol (2019) 80(2):564–6. doi: 10.1016/j.jaad.2018.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.. Common terminology criteria for adverse events (CTCAE), Vol. 155 (2017) U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf [Google Scholar]
- 26. Petty AJ, Li A, Wang X, Dai R, Heyman B, Hsu D, et al. Hedgehog signaling promotes tumor-associated macrophage polarization to suppress intratumoral CD8+ T cell recruitment. J Clin Invest (2019) 129(12):5151–62. doi: 10.1172/JCI128644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chakrabarti J, Holokai L, Syu L, Steele NG, Chang J, Wang J, et al. Hedgehog signaling induces PD-L1 expression and tumor cell proliferation in gastric cancer. Oncotarget (2018) 9(100):37439–57. doi: 10.18632/oncotarget.26473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Otsuka A, Dreier J, Cheng PF, Nägeli M, Lehmann H, Felderer L, et al. Hedgehog pathway inhibitors promote adaptive immune responses in basal cell carcinoma. Clin Cancer Res (2015) 21(6):1289–97. doi: 10.1158/1078-0432.CCR-14-2110 [DOI] [PubMed] [Google Scholar]
- 29. Boerlin A, Ramelyte E, Maul J, Nägeli MC, Dummer. R. 1089TiP - efficacy and tolerability of anti-PD1 antibody in combination with pulsed hedgehog inhibitor in advanced basal cell carcinoma. Ann Oncol (2021) 32(suppl_5):S867–905. doi: 10.1016/annonc/annonc706 [DOI] [Google Scholar]
- 30. Cies M, Mannavola F, Lospalluti L, Sergi MC, Cazzato G, Filoni E, et al. Non-melanoma skin cancers: Biological and clinical features. Int J Mol Sci (2020) 21(15):5394. doi: 10.3390/ijms21155394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hall ET, Fernandez-Lopez E, Silk AW, Dummer R, Bhatia S. Immunologic characteristics of nonmelanoma skin cancers: Implications for immunotherapy. Am Soc Clin Oncol Educ Book (2020) 40:1–10. doi: 10.1200/EDBK_278953 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.