Abstract
Introduction
Tumor recurrence in choroidal melanoma has been associated with decreased overall survival due to metastatic spreading. To detect risk factors of local recurrence and side effects, we analyzed tumor planning and treatment parameters in patients with recurrence of choroidal melanoma after treatment with robotic-assisted radiosurgery (CyberKnife).
Methods
Six hundred ninety-four patients treated with CyberKnife between 2005 and 2019 were retrospectively reviewed. Age, gender, best-corrected visual acuity, tumor height, and diameter were recorded. Treatment planning and radiation doses were reviewed. Salvage therapy, overall survival, metastasis, and complications were recorded.
Results
Seventy-four patients showed local recurrence. Local recurrence occurred after 42.1 months post CyberKnife treatment (mean; range: 5–100 months). Fourteen out of 74 patients (18.9%) died during follow-up. Recurrence treatment included enucleation in 51 patients (68.9%) and radiosurgery in 19 patients (25.7%). Treatment planning without contrast medium MRI, radiation dose of less than 21 Gy, and insufficient margin delineation were identified as risk factors incrementing local control.
Discussion
Robotic-assisted radiosurgery (CyberKnife) is a suitable treatment option for large choroidal melanoma up to 12 mm. Patients with significantly better visual acuity received repeat CyberKnife treatment as salvage therapy and showed an eye retention rate of 81%.
Keywords: Choroidal melanoma, Radiation treatment, Recurrence treatment, Survival, Metastasis, CyberKnife
Introduction
The treatment of primary choroidal melanoma without evidence of metastasis includes globe-conserving therapy or enucleation. Since the Collaborative Ocular Melanoma Study's (COMS) randomized trial did not demonstrate superiority of enucleation in terms of mortality during the 12 years of follow-up period [1], increasing interest has been placed on eye preservative treatment options for choroidal melanoma [2].
In the management of patients with choroidal melanoma, local tumor control is important, as patients have a decreased overall survival due to the development of organ metastases [3, 4]. Treatment failure because of local recurrence implicates secondary treatment involving either radiation therapy or enucleation of the eye.
Ophthalmic and radiation oncologists define local control as either cessation of growth or tumor shrinkage [5, 6]. The literature analysis suggests that local control rates of radiation-treated uveal melanomas depend on radiation dose, tumor location, and largest basal diameter [7].
While different radiation treatments have been used so far, each of them endangers local control due to specific risk factors. Plaque brachytherapy is the most common treatment modality for choroidal melanoma; beginning in the 1960s, multiple case series have been published describing the use of 106Ru or 125I eye plaques in the treatment of melanomas [9]. The average local recurrence rate for the available isotopes is 9.6% [2]. The widely quoted multicentric COMS reported a local treatment failure rate of 10.3%. Studies with superior local control used intraoperative ultrasound for the detection and confirmation of correct plaque placement [10].
Photon-based external beam radiation therapy (GammaKnife radiosurgery) showed similar rates of local treatment failure (2–16%) [11]. However, reports on local control following stereotactic radiosurgery are scarce since the treatment has been developed more recently [12, 13, 14, 15]. To date, the largest comparable study in the literature for treatment of choroidal melanoma with GammaKnife radiosurgery determined advanced tumor stages (T3-T4), the only risk factor threatening local control [12]. There are different hypothesis for local control failure. Namely, early recurrence suggests that the tumor may not have been within the targeted zone (“geographic miss”), while late failure suggest that the tumor may have been radiation-resistant ab initio [9].
It is challenging to find an appropriate radiation dose that balances proper treatment and tumor shrinkage but does not provoke radiation-associated complications. The COMS, for example, uses a higher minimum-tumor dose than other non-study centers commonly use (100 Gy vs. 70 Gy) − with comparable local control rates in plaque brachytherapy [16]. The same issue occurs referring to stereotactic radiosurgery: the risk of radiation-related, ocular side effects in the anterior segment of the eye is higher since radiation beam travels through this segment in order to reach the tumor [9], and therefore, decline or loss of visual acuity may occur.
In order to detect risk factors of local recurrence and ocular side effects, we analyzed all patients with recurrence of choroidal melanomas primarily treated with robotic-assisted radiosurgery (CyberKnife) at the Department of Ophthalmology at the Ludwig Maximilians University in Munich between 2005 and 2019. Patients with local recurrence were evaluated regarding visual outcome and tumor regression after salvage therapy and possible indicators and risk factors in treatment planning and procedure.
Methods
A retrospective review of all patients who were diagnosed with choroidal melanoma and treated with robotic-assisted radiosurgery (CyberKnife) at the Department of Ophthalmology at the Ludwig Maximilians University in Munich, Germany, was performed. For the present analysis, we evaluated all patients with recorded local recurrence following robotic-assisted radiosurgery.
Local recurrence was defined based on tumor growth in ultrasound examination, widefield fundus photography and documentation, or new extraocular growth. In case of suspect but uncertain findings in the ultrasound examination, it was repeated after four to 6 weeks. Additionally and for treatment planning, a MRI scan was performed in most of the cases. In order to ensure sufficient data quality, only patients with a minimum follow-up time of 6 months after recurrence treatment were included.
We recorded age, gender, best-corrected visual acuity (BCVA), tumor height, and diameter following standardized A-/B-scan ultrasound and photo documentation at first presentation, before recurrence therapy, and at the last follow-up; furthermore, salvage therapy management and complication occurrence and management were included. We were specifically interested in possible reasons and risk factors for local recurrence and thus, investigated radiation planning protocol, treatment parameters (radiation dose), and tumor localization (p = posterior, pe = posteroequatorial, e = equatorial, a-e = anterior-equatorial, a = anterior) according to standardized ultrasound. A complete oncologic workup was commenced at the time of first presentation to our department and systemic therapy was started by an oncologist.
Written informed consent was obtained before treatment and risks, and chances as well as treatment options were discussed with the patient. Recurrence treatment strategy was decided individually with each patient and depended on tumor prominence and visual acuity. This study protocol was reviewed and approved by Ethikkommission der Universität München, approval number 22-0618. Informed consent was not necessary for study participation as data were analyzed anonymously.
CyberKnife radiotherapy was performed as a standardized outpatient procedure and has been described in detail, before [17]. In brief, target volume was defined by an interdisciplinary team composed of ophthalmologists, medical physicists, and radiation oncologists using gadolinium-contrast-enhanced MRI and computer tomography (CT, 1.0 and 1.2 mm slices) as well as all previously obtained clinical data including widefield imaging, clinical examination, and ultrasonography results. A 1-mm margin in all directions and 2 mm posteriorly was added to the visible tumor to create a planning target volume.
A nonisocentric inverse algorithm was used for treatment planning (Multiplan®, Accuray Incorporated, Sunnyvale, CA, USA). Radiation was delivered in a single fraction with a CyberKnife system (Accuray Inc.) during a net radiation time of approximately 25 min. During the radiation, as today's state of the art, retrobulbar anesthesia was performed to achieve akinesia of the globe within the orbit.
Statistical Analysis
The results of qualitative variables are articulated using frequencies (%) and results of continuous variables are expressed by medians and interquartile range. Time to death was estimated using the Kaplan-Meier product-limit method. Survival curves were calculated according to strata of the baseline factors and compared using the logrank test. Strata of the continuous variables were determined based on median values. Results were considered significant if the p value was <0.05.
Results
We reviewed all 694 patients with choroidal melanoma treated with robotic-assisted radiosurgery following CyberKnife radiosurgery between January 2005 and December 2019 and − for the present analysis − considered all patients with local control failure during follow-up time. Patients with incomplete data and follow-up of less than 6 months after recurrence treatment were excluded from the study. Overall, 74 of 594 patients (12.4%) showed local recurrence after 42.1 months post CyberKnife treatment (mean; range: 5–100 months) and met the inclusion criteria. Forty-two (56.8%) of them were female.
Their median age was 64 years (range: 30–82 years). Mean follow-up time was 61.0 months (range: 13.3–153.6 months).
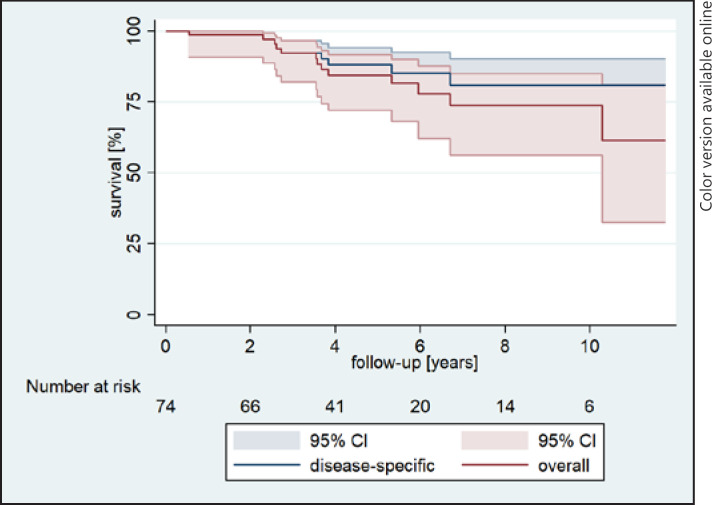
Overall and disease-specific survival is shown in Figure 1. In brief, the eye preservation rate was 27.0%, while a total of 54 eyes were enucleated. A total of 14 patients developed metastasis with liver metastases in 9 patients and multiple metastases in 5 patients. Ultimately, 10 of these patients died due to metastasis development.
Fig. 1.
Kaplan-Meier curve shows overall survival in percent (%) following years after the first CyberKnife treatment.
Comparison of Recurrence Group and Overall Cohort
Of the overall cohort, 278 (n = 46.8%) were female, their mean age was 63.2 years (standard deviation, SD, 13.9). The mean follow-up time for this group was 43 months. Eye retention was achieved in 486 (81.8%). Furthermore, during the follow-up, 77 patients died (13%, 31 patients died due to nondisease-related causes). Sixty-eight of 594 patients in the overall cohort developed metastasis. The tumor size did not diverge between the groups. Eye retention was significantly better in the overall cohort than in the recurrence group (p < 0.0001, online suppl. Fig. 1; for all online suppl. material, see www.karger.com/doi/10.1159/000527915). Disease-specific survival rates are not different in both groups (online suppl. Fig. 2).
Ultrasound Parameters and TNM Classification (8th AJCC)
Median tumor height, largest basal diameter, and internal reflectivity at first diagnosis were 5.65 mm (median; range: 1.6–12.1 mm; SD: 2.60), 11.83 mm (median; range: 4.1–21.5 mm; SD: 3.70), and 50.0% (median; range: 23–78%, SD: 13.4%), respectively. At first presentation, 16 patients were in T1, 31 patients in T2, 21 patients in T3, and 6 patients in T4 tumor stages according to the 8th edition of AJCC cancer staging. Six months post radiosurgery tumor height, largest basal diameter, and internal reflectivity decreased to 3.90 mm (median; range: 0.8–10.3 mm, SD: 2.2), 10.0 (median; range: 4.0–18.0 mm, SD: 10.9), and 66.0% (median; range: 30–93%, SD: 14.2), respectively, which was statistically significant (p < 0.001 for all).
In patients with suspected recurrence, an increase of 1.95 mm (median; range: 0.10–9.5 mm) in tumor height was observed. Sixty-four patients had a decrease in internal reflectivity to a median of 12.0% (range: 0–42%), whereas 10 patients showed an increase in internal reflectivity. Patients, who received salvage enucleation had significantly more prominent tumors before the first CyberKnife treatment (i.e., at the time of first tumor detection; p = 0.004) than patients with salvage CyberKnife treatment (online suppl. Fig. 1).
20 patients (27.0%) had posterior tumor location, 23 patients (31.1%) posteroequatorial, 11 patients (14.9%) equatorial, 9 patients (12.2%) anteroequatorial, and 11 patients (14.9%) anterior located tumors. Local recurrence with extraocular growth was observed in 14 patients (18.9%). 50% of these tumors were initially located anteriorly. Twelve of the patients with extraocular growth were enucleated (85.7%) as they had poor BCVA. For further details about patient characteristics, see Table 1.
Table 1.
Characteristics of patients with local recurrence treated with CyberKnife radiosurgery
| Total patients | 74 |
| Gender (male/female), n (%) | M/F = 32/42 (M = 43.2%) |
| Treatment site (right/left), n (%) | RE/LE = 36/38 (R = 48.6%) |
| Age, median (Q1–Q3), years | 64 (30–82) |
| Tumor height, median ± SD (Q1–Q3), mm | 5.65±2.61 (1.6–12.1) |
| Largest basal diameter, median ± SD (Q1–Q3), mm | 11.83±3.65 (4.1–21.5) |
| Local recurrence, median ± SD (Q1–Q3), months | 38.50±26.2 (5–100) |
| Total follow-up, mean ± SD (Q1–Q3), months | 54.70±34.20 (13.3–153.6) |
| Tumor location as defined by standardized ultrasound | |
| Posterior | 20 |
| Posteroequatorial | 23 |
| Equatorial | 11 |
| Anterioequatorial | 9 |
| Anterior | 11 |
| TNM stage as defined by AJCC classification scheme (8th edition) | |
| T1 | 16 |
| T2 | 31 |
| T3 | 21 |
| T4 | 6 |
RE, right eye; LE, left eye; SD, standard deviation.
Treatment Parameters, Treatment Planning, and Recurrence Therapy
Patients were treated with a median prescription dose of 20 Gy (range: 18–22 Gy) and a median isodose of 70%. Forty out of 74 patients (54.1%) were treated with less than 21 Gy of radiation dose and seven patients had 65% of isodose (Table 2). The retrospective analysis of radiation planning revealed that 37 patients (50%) did not have contrast MRI (meaning they instead had a CT scan in advance) in advance and 48 patients (64.9%) showed insufficient margin delineation in the radiation planning process.
Table 2.
Treatment parameters, treatment planning, and recurrence therapy
| Treatment parameters | N |
|---|---|
| Prescription dose | |
| 18 Gy | 5 |
| 19 Gy | 4 |
| 20 Gy | 30 |
| 21 Gy | 34 |
| 22 Gy | 1 |
| Treatment planning | |
| Without contrast medium MRI | 37 |
| Insufficient margin delineation | 48 |
| Recurrence therapy | |
| Salvage radiotherapy (CyberKnife) | 19 |
| Enucleation | 51 |
| Radiosurgery followed by enucleation | 2 |
| Systemic medication | 1 |
| Pars-plana-vitrectomy and tumor resection | 1 |
SD, standard deviation; MRI, magnet resonance tomography. Median prescription dose was 20±0.87 Gy (Q1–Q3: 18–22); this equaled an isodose of 70±1.94% (Q1–Q3 65–70%).
Recurrence treatment included primary enucleation in 51 patients (68.9%) versus repeated radiosurgery (CyberKnife) in 19 patients (25.7%). The main reason for primary enucleation was worse BCVA (for details see below). Two patients were treated with CyberKnife but had an enucleation, hereafter (5.4%). One patient received a pars-plana-vitrectomy with tumor endoresection, and one patient had systemic immunotherapy with Nivolumab (Table 2). Except for the two patients with enucleation after salvage CyberKnife treatment, the patients showed a reduction of tumor height in follow-up ultrasound examinations. No further recurrence was detected in these patients.
Development of Visual Acuity
Visual acuity was 0.40 logMAR (median; range: 3.00–0.00 logMAR) at first diagnosis, 2.20 logMAR (median; range: 3.00–0.10 logMAR) at time of recurrence, and 3.00 logMAR at the last follow-up visit (median; range: 3.00–2.300). Sixty-three patients (85.1%) had visual acuity of finger counting or less at the time of the last follow-up. Eleven patients (14.9%) had visual acuity better than finger counting with 0.90 logMAR (mean; range: 1.50–0.30 logMAR).
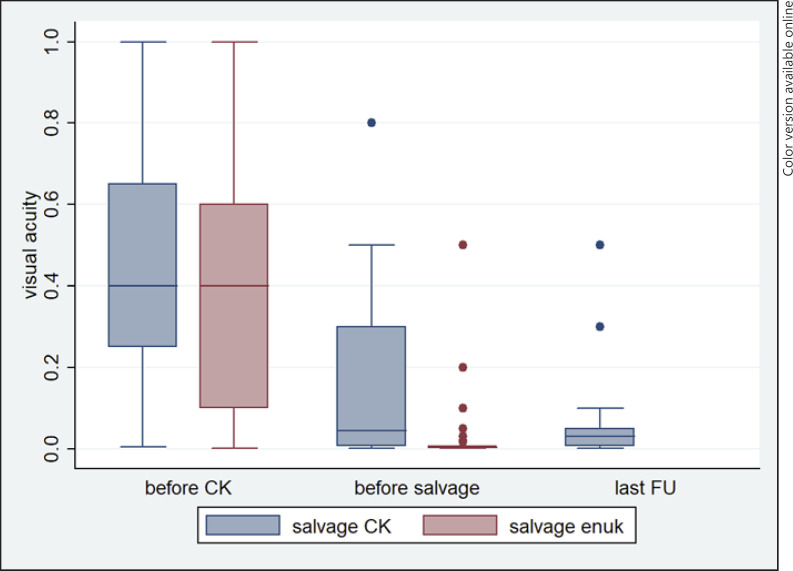
When comparing patients with salvage CyberKnife and patients with salvage enucleation therapy, patients that received CyberKnife had significantly better visual acuity before salvage treatment (p < 0.001). Patients with extraocular growth when recurrence was detected were more likely enucleated (85.7%) since the BCVA was ranging between no light perception (3.0 logMAR) and light perception (2.70 logMAR). However, eye retention is less important if the visual acuity was worse, beforehand. Furthermore, 12 patients had to be excluded (6 patients with missing data on visual acuity, 2 patients with different salvage therapy, 4 patients with enucleation following salvage CyberKnife, respectively). The progression of visual acuity in the mentioned two groups is shown in Figure 2.
Fig. 2.
Visual acuity (logMAR) development of patients treated with salvage CyberKnife versus patients treated with salvage enucleation before the first treatment, before salvage therapy, and at the last follow-up, respectively.
Treatment-Associated Complications
Regarding all treated patients (N = 568), the most frequent complication was serous retinal detachment in 209 patients (36.8%). The incidence of secondary neovascular glaucoma was observed in 18.1% of the eyes (n = 103). Of them, 40 patients (38.8%) had to be enucleated. Radiation retinopathy was observed in 72 patients (12.7%).
In the recurrence group, the most frequently observed complication was the occurrence of neovascular glaucoma in 35 patients (47.3%) after a median of 53 months post CyberKnife treatment (range: 5–92 months). Eleven of 35 patients with occurrence of neovascular glaucoma had choroidal melanoma with ciliary body involvement (31.4%). Eight patients received local antiglaucoma drops in combination with antiglaucoma operations (22.9%). Twenty-seven out of 54 eyes (50%) were enucleated due to uncontrolled neovascular glaucoma.
Furthermore, common complications included serous retinal detachment in 30 of 74 patients (40.5%), vitreous bleeding in 21 patients (28.4%), and the occurrence of subsequent central radiation retinopathy with macula edema in 10 patients (Finger radiation retinopathy stage 3, 13.5%) [18]. Twelve patients received pars-plana-vitrectomies due to vitreous bleeding or retinal detachment (16.2%).
Discussion
We report on 74 patients with recurrence of choroidal melanoma after treatment with robotic-assisted radiosurgery (CyberKnife) between 2005 and 2019. Recurrence treatment included enucleation in 51 patients (68.9%) and repeat radiosurgery in 19 patients (25.7%). Possible risk factors for local control failure were treatment planning without contrast medium MRI, radiation dose of less than 21 Gy, and insufficient margin delineation. Complication rates were higher than in the group without local recurrence; the most common complication was neovascular glaucoma (47.3%) followed by retinal detachment (40.5%). Ten of 74 patients died due to the development of metastasis.
Local control failure after globe-preserving therapy is a common complication that might increase morbidity to the vision and eye [19]. Therefore, minimizing the risk of local treatment failure and recurrence is incumbent.
To date, plaque brachytherapy, which is available since the 1960s, is the most common treatment of choroidal melanoma. Yet, local failure rates for this long-standing treatment are reported to range from 0% [20] to 27% [21] with a weighted average of 9.6% [2]. Tabandeh and colleagues significantly reduced local failure rates of plaque brachytherapy by the use of intraoperative ultrasonography to modify or verify plaque position and guarantee sufficient tumor coverage [10]. Chang reported that in about 36% of the treated eyes, the 125I plaque needed centration to the tumor margin [22]. Yet, neither the tumor size nor tumor location was associated with the need to reposition the plaque [22]. Likewise in other surgical procedures, the precise placement of the plaque follows a significant learning curve [23].
Reports on local control following stereotactic radiosurgery are scarce since the treatment has been developed more recently [12, 13, 14, 15]. Dunavoelgyi et al. [13] described 10 years of follow-up and outcome of 212 patients treated with stereotactic radiosurgery. The tumor median height at first diagnosis was 4.8 mm and decreased to 2.6 mm after treatment. Similarly, Krema and his research group [14] treated 64 patients with juxtapapillary choroidal melanoma with a median tumor height of 4.2 mm. They report a local control of 94% at the end of follow-up (median: 37 months). Modorati et al. [15] described 12 years of outcome of 78 patients with a median tumor height of 6.1 mm. Remarkably, Wackernagel et al. [12] described outcome and local control in a similar population but additionally took different factors influencing local control into account. The Austrian study group treated 189 patients with Gamma-Knife radiosurgery for choroidal melanomas. They found advanced tumor stages (T3-T4), the only risk factor threatening local control. Treatment dose was not significantly associated with tumor control failure [12].
In comparison to the studies mentioned above, we observe a higher local recurrence rate of 12.5% in a median follow-up of 54.7 months in our study population. However, with a median tumor height of 5.65 mm at first diagnosis and median basal diameters of 12.2 mm, we treated more prominent tumors with larger basal diameter than groups reporting a lower local recurrence rate. Besides, we used a similar but different treatment modality (GammaKnife vs. CyberKnife; 30–70 Gy vs. 18–22 Gy radiation, respectively). Most importantly, our treatment regimen is not comparable to the studies mentioned above [12, 13, 14, 15]: while they performed fractionated radiation therapy with higher total prescription doses ranging from 30 to 70 Gy and a treatment time of several days, we treated patients with prescription doses of 18–22 Gy during a single outpatient session.
Our survival rate of 82% at 4.5 years of follow-up compares well to Modorati et al. (81.9%) [15]. When comparing both groups, disease-specific survival rates, interestingly, are not different in both groups. As online supplementary Figure 2 shows, dropout rates during the follow-up are higher in the overall cohort. This might be as patients with an uneventful follow-up are more likely to skip regular appointments, while patients with problematic disease (and metastasis) show up, regularly.
Other studies [14] showed superior survival rates of 90% and metastasis rate of only 15%. This might primarily be caused by a shorter follow-up time of only 36 months [14]. Despite the importance of maximizing local control, survival rates for patients with choroidal melanoma have not changed over the past four decades. Other factors, such as genetics, are suggested to play an important role with respect to survival [24, 25]. Regarding the visual acuity development presented by Krema et al. [14], patients showed visual acuity of 0.50 (median; logMAR) at first diagnosis, which decreased to counting fingers or less in 80% of all patients. Similarly, the visual acuity of 0.40 at first diagnosis (median; logMAR) decreased to a median of 3.00 logMAR at the last follow-up in our cohort. Sixty-three of our patients (85.1%) had visual acuity of counting fingers or less at the last follow-up. Nevertheless, decision-making on salvage therapy management took tumor prominence and visual acuity into account. Patients, who received salvage CyberKnife in our cohort, had significantly flatter tumors (p = 0.004) and better visual acuity (p = 0.0008) compared to patients with salvage enucleation therapy. As anticipated, eye retention was significantly higher in the overall cohort than in the recurrence group (p < 0.0001, online suppl. Fig. 3).
While neovascular glaucoma was more common in the recurrence than in the overall population (47.3% vs. 17.3%), other complications like the incidence of retinal detachment were comparable (40.5% vs. 35.2%, respectively). In the recurrence group, neovascular glaucoma appeared after a median time of 53 months after CyberKnife treatment. Of the patients that developed serous retinal detachment, 28.4% had vitreous bleeding. Twelve patients required pars-plana-vitrectomy. However, most of the complications were distributed equally compared to all patients treated with CyberKnife for ocular melanoma (data not shown). Nevertheless, the comparison to other studies is difficult since they describe complication rates in all patients, while we only describe patients with local recurrence [15]. Modorati et al. [15], for example, reported a neovascular glaucoma rate of 18.7% involving all treated patients.
To date the literature on treatment-associated factors that increase local tumor control in robotic-assisted radiosurgery is scarce. Hence, we specifically analyzed the treatment planning procedures and treatment regimen as influence factors for local recurrence in our study population. We previously found local control rates for ciliary body melanomas treated with 18–22 Gy at a 70% isodose to be 85% after 3 years [26]. Interestingly, there was a significant difference between patients treated with a prescription dose of 21 Gy (local control rate 90.5%) and patients treated with a prescription dose of 20 Gy or less (local control rate 71%) [26]. Thus, we declare a prescription dose of less than 21 Gy as insufficient from today's point of view. Of the present cohort, 40 patients (54%) were treated with less than 21 Gy prescription dose.
The retrospective analysis of treatment planning unveiled that 37 patients (50%) did not have contrast medium MRI before radiation planning. Today, contrast medium MRI is an essential tool of the treatment planning protocol for every patient with choroidal melanoma. The value of contrast medium MRI has been established in previous studies [27, 28, 29]. Due to its excellent soft-tissue contrast and spatial resolution, MRI facilitates a distinction between benign and malignant lesions [30].
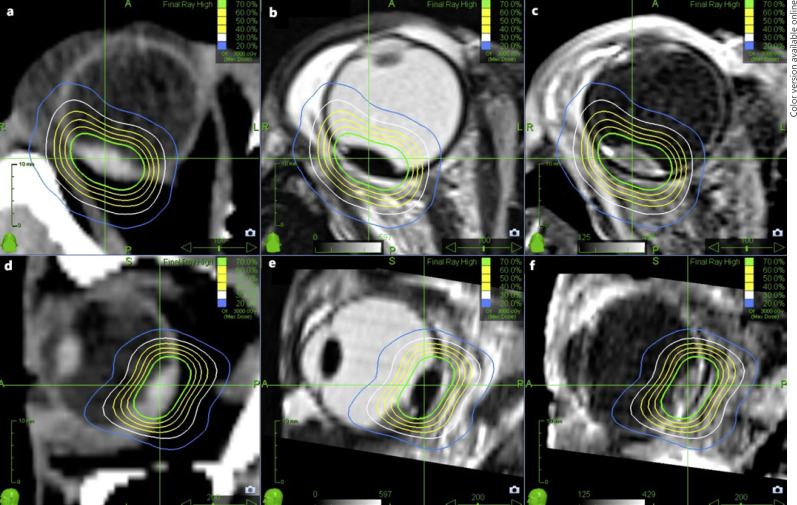
Still, we did find patients, with contrast medium MRI-based treatment planning, a prescription dose of 21 Gy and local control failure. The analysis of treatment planning showed that predominantly in these cases the flat tumor margin delineation was narrow or insufficient. It is visible more distinctly in MRI than in CT scans, likewise is the differentiation between subretinal fluids and tumor facilitated (Fig. 3). Due to limited availability at the beginning of the study period, of 48 patients with retrospectively insufficient tumor delineation, only 21 patients had contrast medium MRI in advance (43.8%).
Fig. 3.
Axial (a) and sagittal contrast medium enhanced CT (d) of choroidal melanoma with respective radiation isodoses with indistinct tumor margin delineation due to image modality. T2-weighted axial MRI (b), T2-weighted sagittal MRI (e), as well as contrast medium enhanced T1-weighted axial T1 (c) and sagittal (f) MRI with radiation isodoses of patient treated with CyberKnife for choroidal melanoma. The tumor margin can be recognized superiorly and distinction between tumor and subretinal fluids is possible.
Notwithstanding, a small number of 11 patients developed local recurrence despite optimized treatment (prescription dose of 21 Gy contrast medium MRI and sufficient tumor margin delineation). As described by Finger and colleagues [9], there is no way to predict the radiosensitivity of a particular choroidal melanoma prior to treatment. Intrinsic, tumor-specific factors (e.g., cell type, melanin content, oxygenation) as well as gene status (e.g., monosomy 3) are common aspects that affect tumor radiosensitivity [31]. Whether local control failure is related to the development of metastatic disease or is more likely to occur in more aggressive tumors remains unclear [2].
Without any doubt, there are some limitations that need to be considered. First, the comparability of our results with other studies is limited due to different radiation modality, prescription doses, and treatment periods; we report experiences of 15 years in the treatment of choroidal melanomas using robotic-assisted radiosurgery. Moreover, we treated tumors with tumor heights of up to 12 mm that are usually primarily enucleated. Our treatment regimen involves a single outpatient procedure, which is comfortable for elderly patients with multiple medical conditions. Including all cases with local recurrence, we observed 74 recurrences in 594 patients treated over 15 years. Patients, who received salvage CyberKnife therapy showed eye retention up to 81% until the last follow-up. We are not aware of a further recurrence in the patients receiving salvage radiosurgery. Besides poor visual acuity, insufficient treatment planning was a risk factor for enucleation.
Summing up, robotic-assisted radiosurgery (CyberKnife) is a suitable treatment option for large choroidal melanoma up to 12 mm. Patients with significantly better visual acuity received repeat CyberKnife treatment as salvage therapy and showed an eye retention rate of 81% despite local recurrence. Over the past years, we went through a constant learning progress that led to the use of radiation doses of minimum 21 Gy [26], proper planning of treatment including contrast-based MRIs, ultra-widefield photo documentation of tumor delineation, and sufficient safety distance around the tumor edges to increase local control. In this manner, we were able to increase local control as described in details, elsewhere [26].
Statement of Ethics
Written informed consent was obtained before treatment and risks and prognosis as well as treatment options were discussed with the patient. This study protocol was reviewed and approved by Ethikkommission der Universität München, approval number 22-0618. Informed consent was not necessary for study participation as data were analyzed anonymously.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
Each author declares that there are no sources of funding received for the research submitted to the journal.
Author Contributions
Valerie Schmelter drafted the manuscript and collected the data. Frederick Schneider helped with data collection, writing the paper, and statistical analysis. Alexander Muacevic and Paul Foerster conducted the study. Christoph Fuerweger analyzed the data. Siegfried G. Priglinger, Stefanie R. Guenther, Raffael Liegl, Paul Foerster, and Alexander Muacevic revised the manuscript for important intellectual content. All authors read the final version of the manuscript and agreed with its submission.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Funding Statement
Each author declares that there are no sources of funding received for the research submitted to the journal.
References
- 1.Collaborative Ocular Melanoma Study Group The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report No. 28. Arch Ophthalmol. 2006;124((12)):1684–1693. doi: 10.1001/archopht.124.12.1684. [DOI] [PubMed] [Google Scholar]
- 2.Chang MY, McCannel TA. Local treatment failure after globe-conserving therapy for choroidal melanoma. Br J Ophthalmol. 2013;97((7)):804–811. doi: 10.1136/bjophthalmol-2012-302490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Char DH, Kroll S, Phillips TL. Uveal melanoma. Growth rate and prognosis. Arch Ophthalmol. 1997;115((8)):1014–1018. doi: 10.1001/archopht.1997.01100160184007. [DOI] [PubMed] [Google Scholar]
- 4.Vrabec TR, Augsburger JJ, Gamel JW, Brady LW, Hernandez C, Woodleigh R. Impact of local tumor relapse on patient survival after cobalt 60 plaque radiotherapy. Ophthalmology. 1991;98((6)):984–988. doi: 10.1016/s0161-6420(91)32193-6. [DOI] [PubMed] [Google Scholar]
- 5.Char DH, Castro JR, Kroll SM, Irvine AR, Quivey JM, Stone RD. Five-year follow-up of helium ion therapy for uveal melanoma. Arch Ophthalmol. 1990;108((2)):209–214. doi: 10.1001/archopht.1990.01070040061031. [DOI] [PubMed] [Google Scholar]
- 6.Fontanesi J, Meyer D, Xu S, Tai D. Treatment of choroidal melanoma with I-125 plaque. Int J Radiat Oncolo Biol Phys. 1993;26((4)):619–623. doi: 10.1016/0360-3016(93)90278-4. [DOI] [PubMed] [Google Scholar]
- 7.Fontanesi J, Hetzler D, Ross J. Effect of dose rate on local control and complications in the reirradiation of head and neck tumors with interstitial iridium-192. Int J Radiat Oncol Biol Phys. 1989;17((2)):365–369. doi: 10.1016/0360-3016(89)90452-5. [DOI] [PubMed] [Google Scholar]
- 8.Egan KM, Gragoudas ES, Seddon JM, Glynn RJ, Munzenreider JE, Goitein M, et al. The risk of enucieatlon after proton beam irradiation of uveal melanoma. Ophthalmology. 1989;96((9)):1377–1383. doi: 10.1016/s0161-6420(89)32738-2. discussion 82–3. [DOI] [PubMed] [Google Scholar]
- 9.Finger PT. Radiation therapy for choroidal melanoma. Surv Ophthalmol. 1997;42((3)):215–232. doi: 10.1016/s0039-6257(97)00088-x. [DOI] [PubMed] [Google Scholar]
- 10.Tabandeh H, Chaudhry NA, Murray TG, Ehlies F, Hughes R, Scott IU, et al. Intraoperative echographic localization of iodine-125 episcleral plaque for brachytherapy of choroidal melanoma. Am J Ophthalmol. 2000;129((2)):199–204. doi: 10.1016/s0002-9394(99)00315-3. [DOI] [PubMed] [Google Scholar]
- 11.Simonová G, Novotný J, Jr, Liscák R, Pilbauer J. Leksell gamma knife treatment of uveal melanoma. J Neurosurg. 2002;97((5 Suppl)):635–639. doi: 10.3171/jns.2002.97.supplement. [DOI] [PubMed] [Google Scholar]
- 12.Wackernagel W, Holl E, Tarmann L, Mayer C, Avian A, Schneider M, et al. Local tumour control and eye preservation after gamma-knife radiosurgery of choroidal melanomas. Br J Ophthalmol. 2014;98((2)):218–223. doi: 10.1136/bjophthalmol-2013-304031. [DOI] [PubMed] [Google Scholar]
- 13.Dunavoelgyi R, Dieckmann K, Gleiss A, Sacu S, Kircher K, Georgopoulos M, et al. Local tumor control, visual acuity, and survival after hypofractionated stereotactic photon radiotherapy of choroidal melanoma in 212 patients treated between 1997 and 2007. Int J Radiat Oncol Biol Phys. 2011;81((1)):199–205. doi: 10.1016/j.ijrobp.2010.04.035. [DOI] [PubMed] [Google Scholar]
- 14.Krema H, Somani S, Sahgal A, Xu W, Heydarian M, Payne D, et al. Stereotactic radiotherapy for treatment of juxtapapillary choroidal melanoma: 3-year follow-up. Br J Ophthalmol. 2009;93((9)):1172–1176. doi: 10.1136/bjo.2008.153429. [DOI] [PubMed] [Google Scholar]
- 15.Modorati G, Miserocchi E, Galli L, Picozzi P, Rama P. Gamma knife radiosurgery for uveal melanoma: 12 years of experience. Br J Ophthalmol. 2009;93((1)):40–44. doi: 10.1136/bjo.2008.142208. [DOI] [PubMed] [Google Scholar]
- 16.Accuracy of diagnosis of choroidal melanomas in the collaborative ocular melanoma study. COMS report no. 1. Arch Ophthalmol. 1990;108((9)):1268–1273. doi: 10.1001/archopht.1990.01070110084030. [DOI] [PubMed] [Google Scholar]
- 17.Muacevic A, Nentwich M, Wowra B, Staerk S, Kampik A, Schaller U. Development of a streamlined, non-invasive robotic radiosurgery method for treatment of uveal melanoma. Technol Cancer Res Treat. 2008;7((5)):369–373. doi: 10.1177/153303460800700503. [DOI] [PubMed] [Google Scholar]
- 18.Finger PT, Kurli M. Laser photocoagulation for radiation retinopathy after ophthalmic plaque radiation therapy. Br J Ophthalmol. 2005;89((6)):730–738. doi: 10.1136/bjo.2004.052159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bechrakis NE, Petousis V, Willerding G, Krause L, Wachtlin J, Stroux A, et al. Ten-year results of transscleral resection of large uveal melanomas: local tumour control and metastatic rate. Br J Ophthalmol. 2010;94((4)):460–466. doi: 10.1136/bjo.2009.162487. [DOI] [PubMed] [Google Scholar]
- 20.McCannel TA, Chang MY, Burgess BL. Multi-year follow-up of fine-needle aspiration biopsy in choroidal melanoma. Ophthalmology. 2012;119((3)):606–610. doi: 10.1016/j.ophtha.2011.08.046. [DOI] [PubMed] [Google Scholar]
- 21.Leonard KL, Gagne NL, Mignano JE, Duker JS, Bannon EA, Rivard MJ. A 17-year retrospective study of institutional results for eye plaque brachytherapy of uveal melanoma using (125)I, (103)Pd, and (131)Cs and historical perspective. Brachytherapy. 2011;10((4)):331–339. doi: 10.1016/j.brachy.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Chang MY, Kamrava M, Demanes DJ, Leu M, Agazaryan N, Lamb J, et al. Intraoperative ultrasonography-guided positioning of iodine 125 plaque brachytherapy in the treatment of choroidal melanoma. Ophthalmology. 2012;119((5)):1073–1077. doi: 10.1016/j.ophtha.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Shah NV, Houston SK, Murray TG, Markoe AM. Evaluation of the surgical learning curve for I-125 episcleral plaque placement for the treatment of posterior uveal melanoma: a two decade review. Clin Ophthalmol. 2012;6:447–452. doi: 10.2147/OPTH.S30307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh AD, Turell ME, Topham AK. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 2011;118((9)):1881–1885. doi: 10.1016/j.ophtha.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 25.Bensoussan E, Thariat J, Maschi C, Delas J, Schouver ED, Hérault J, et al. Outcomes after proton beam therapy for large choroidal melanomas in 492 patients. Am J Ophthalmol. 2016;165:78–87. doi: 10.1016/j.ajo.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 26.Liegl R, Schmelter V, Fuerweger C, Foerster MH, Muacevic A, Priglinger SG, et al. Robotic cyberknife radiosurgery for ciliary body melanoma. Ophthalmol Retina. 2020;4((9)):954–956. doi: 10.1016/j.oret.2020.03.031. [DOI] [PubMed] [Google Scholar]
- 27.Jaarsma-Coes MG, Goncalves Ferreira TA, van Haren GR, Marinkovic M, Beenakker JWM. MRI enables accurate diagnosis and follow-up in uveal melanoma patients after vitrectomy. Melanoma Res. 2019;29((6)):655–659. doi: 10.1097/CMR.0000000000000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kivelä T, Eskelin S, Mäkitie T, Summanen P. Exudative retinal detachment from malignant uveal melanoma: predictors and prognostic significance. Invest Ophthalmol Vis Sci. 2001;42((9)):2085–2093. [PubMed] [Google Scholar]
- 29.Beenakker JWM, Ferreira TA, Soemarwoto KP, Genders SW, Teeuwisse WM, Webb AG. Clinical evaluation of ultra-high-field MRI for three-dimensional visualisation of tumour size in uveal melanoma patients, with direct relevance to treatment planning. Magn Reson Mater Phy. 2016;29((3)):571–577. doi: 10.1007/s10334-016-0529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foti PV, Longo A, Reibaldi M, Russo A, Privitera G, Spatola C, et al. Uveal melanoma: quantitative evaluation of diffusion-weighted MR imaging in the response assessment after proton-beam therapy, long-term follow-up. Radiol Med. 2017;122((2)):131–139. doi: 10.1007/s11547-016-0697-3. [DOI] [PubMed] [Google Scholar]
- 31.Hall EJ, Brenner DJ. The radiobiology of radiosurgery: rationale for different treatment regimes for AVMs and malignancies. Int J Radiat Oncol Biol Phys. 1993;25((2)):381–385. doi: 10.1016/0360-3016(93)90367-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.