Abstract
Malignant tumor, the leading cause of death worldwide, poses a serious threat to human health. For decades, natural product has been proven to be an essential source for novel anticancer drug discovery. Shikonin (SHK), a natural molecule separated from the root of Lithospermum erythrorhizon, shows great potential in anticancer therapy. However, its further clinical application is significantly restricted by poor bioavailability, adverse effects, and non-selective toxicity. With the development of nanotechnology, nano drug delivery systems have emerged as promising strategies to improve bioavailability and enhance the therapeutic efficacy of drugs. To overcome the shortcoming of SHK, various nano drug delivery systems such as liposomes, polymeric micelles, nanoparticles, nanogels, and nanoemulsions, were developed to achieve efficient delivery for enhanced antitumor effects. Herein, this review summarizes the anticancer pharmacological activities and pharmacokinetics of SHK. Additionally, the latest progress of SHK nanomedicines in cancer therapy is outlined, focusing on long circulation, tumor targeting ability, tumor microenvironment responsive drug release, and nanosystem-mediated combination therapy. Finally, the challenges and prospects of SHK nanomedicines in the future clinical application are spotlighted.
Keywords: shikonin, cancer therapy, pharmacokinetics, nanomedicine, drug delivery
Graphical Abstract
Introduction
Malignant tumor is the leading cause of mortality worldwide.1 Chemotherapy remains the primary therapy option for cancer patients to improve survival rate. Although encouraging progress has been made in clinical chemotherapy, disadvantages such as drug resistance and severe side effects are still witnessed, failing to exert a satisfactory effect. Thus, it is necessary to explore novel antitumor agents with excellent efficacy and reduced toxicity to overcome the above problem in cancer treatment.
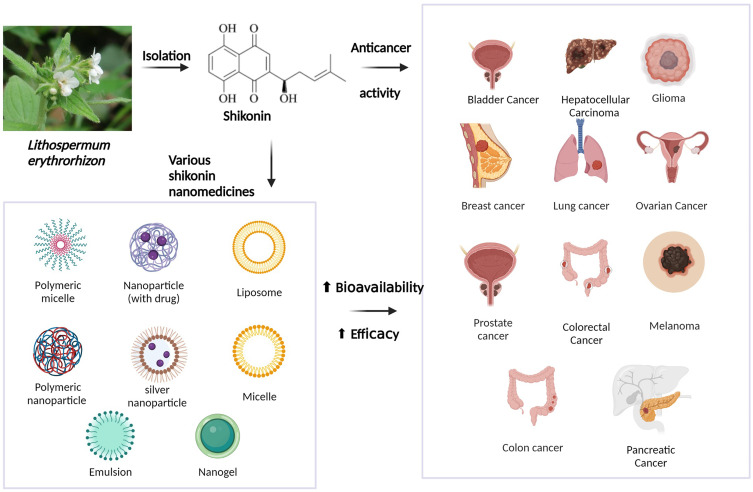
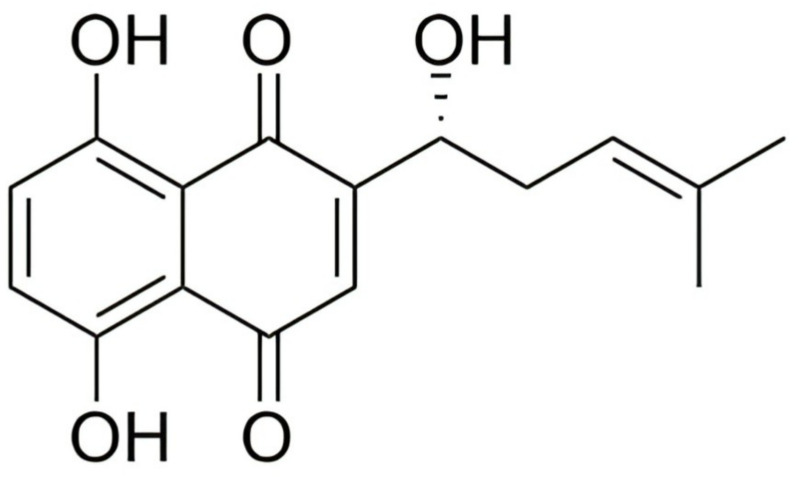
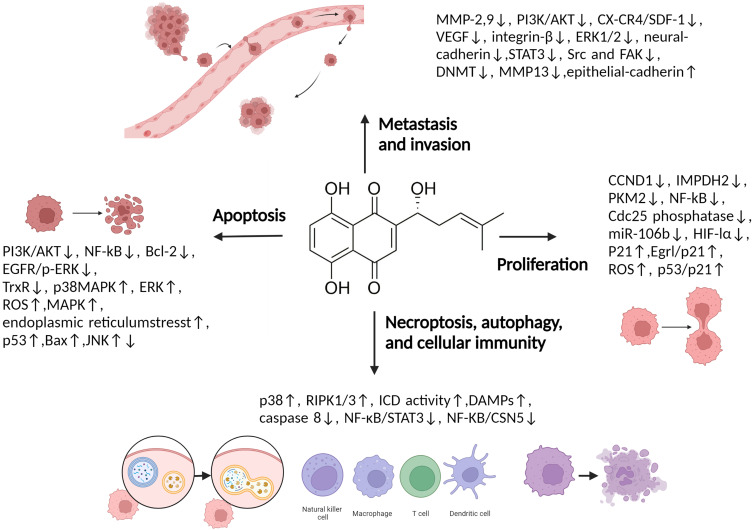
Natural products extracted or optimized from nature have made great progress in anticancer therapy.2–5 SHK ((±)-5,8-dihydroxy-2-(1-hydroxy-4-methyl-3-pentenyl)-1,4-naphthoquinone) (Figure 1), a natural naphthoquinone separated from the Lithospermum erythrorhizon is a traditional Chinese Medicine used to treat several diseases, such as anaphylactoid purpura, burns, eczema, measles.6 Apart from botanical sources, the source of SHK involves chemical synthesis and biosynthesis, which may impel the clinical application of SHK.7,8 Recently, studies have shown that SHK possesses a favorable therapeutic effect on cancer growth, development, and metastasis by regulating cancer cell proliferation, apoptosis, autophagy, necroptosis, and inducing immunogenic cell death (ICD) (Figure 2). In addition, SHK could regulate immunosuppressive tumor microenvironment through inhibiting glycolysis of tumor cells and repolarizing tumor-associated macrophages (TAMs). However, SHK is a hydrophobic natural molecule with unsatisfactory solubility, rapid intestinal absorption, obvious “first pass” effect, and rapid clearance, leading to low oral bioavailability.9,10 For instance, SHK displays a half-life of 15.15 ± 1.41 h and Cmax of 0.94 ± 0.11 μg/ml in rats when administered intravenously.11 In addition, SHK could induce serious skin sensitization, suicidal erythrocyte death, and potential toxicity in combination with other drugs through related metabolic enzymes.12–16 Notably, due to these disadvantages, its further application is significantly restricted.
Figure 1.
Chemical structure of shikonin.
Figure 2.
Multiple molecular targets modulated by SHK in cancer therapy.
Abbreviations: CCND1, Cyclin D1 gene; NF-κB, Nuclear factor kappa-B; Cdc25, Cell division cycle 25; HIF-1α, Hypoxia-inducible factor 1α; PKM2, Pyruvate kinase muscle isozyme M2; IMPDH2, Inosine-5’-monophosphate dehydrogenase 2; EGR1, Early growth response 1; ROS, Reactive oxygen species; PI3K, Phosphoinositide 3-kinase; AKT, Protein kinase B; Bcl-2, B-cell lymphoma-2; EGFR, Epithelial growth factor receptor; p-ERK, Phosphorylated extracellular regulated protein kinases; TrxR, Thioredoxin reductase; MAPK, Mitogen-activated protein kinase; ERK, Extracellular regulated protein kinases; ASK1, Apoptosis signal-regulating kinase 1; MKK3, Mitogen-activated Protein Kinase Kinase 3; Bax, Bcl-2-associated X protein; JNK, C-Jun-N-terminal kinase; MMP, Matrix metalloproteinase; CXCR4, Type IV C-X-C-motif chemokine receptor; SDF-1, Stromal cell–derived factor-1; VEGF, Vascular endothelial growth factor; STAT3, Signal transducer and activator of transcription 3; SRC, Proto-oncogene tyrosine-protein kinase Src; FAK, Focal adhesion kinase 1; DNMT1, Human DNA methyltransferase 1; RIP3, Receptor-interacting protein kinase 3; DAMPs, Damage associated molecular patterns; CSN5, COP9 signalosome 5.
Nanomedicine has attracted considerable attention over the past few decades, possessing advantages, such as prolonged circulation time, improved drug bioavailability, controllable drug release, enhanced therapeutic efficacy, and alleviated side effects. Additionally, increasing literature has demonstrated that developing nano drug delivery systems can improve bioavailability and strengthen the therapeutic efficacy of drugs without chemical modification. Encouragingly, Onivyde®, an irinotecan liposome injection approved by FDA, is harnessed to treat metastatic pancreatic cancer patients after gemcitabine therapy.17 Recent studies have demonstrated its encouraging anticancer activity in various malignancies such as pancreatic, esophago-gastric, and colorectal cancer.18 Various nanomedicines including Myocet®, Mepact®, Marqibo®, Doxil®, Abraxane®, Apealea®, and Vyxeos® were approved by FDA or EMA.19–21 Therefore, nano drug delivery systems are effective approaches to improving the therapeutic efficiency of drugs. Given the shortcoming of SHK, enormous efforts have been devoted to developing various nano drug delivery systems such as liposomes, polymeric micelles, nanoparticles, nanogels, nanoemulsions, and chimeric advanced drug delivery nano systems (Chi-aDDnSs), for efficient delivery of SHK.
This review summarizes the anticancer activities and pharmacokinetics of SHK. Moreover, the latest updates of SHK nanomedicines in anticancer therapy are overviewed, with emphasis on long circulation, tumor targeting ability, tumor microenvironment responsive drug release, and nanosystem-mediated combination therapy. Such SHK nanomedicine exhibits improved bioavailability and outstanding therapeutic efficacy, which show great potential for ameliorating the limitations of SHK and facilitating clinical application.
Anti-Cancer Activities of SHK
SHK exerts a favorable therapeutic effect on breast, colon, lung, and other cancers via regulating cancer cell proliferation, apoptosis, metastasis, invasion, autophagy, necroptosis, and cellular immunity. The detailed roles of SHK in cancer treatment are shown in Table 1.
Table 1.
The Anti-Cancer Activity of SHK in vitro
| Cancer Types | Cell Lines | Role | Potential Mechanism | Ref. |
|---|---|---|---|---|
| Breast Cancer | MCF-7 | Inhibit cell proliferation | Inhibit Cdc25 phosphatase | [25] |
| MDA-MB-231 | Inhibit cell proliferation Induce apoptosis |
Up-regulate P21 Inhibit the ERK pathway |
[26] | |
| MDA-MB-231, 4T1 | Inhibit cell proliferation | Inhibit IMPDH2 | [24] | |
| MCF-7, SHK-BR-3 | Inhibit cell proliferation Induce apoptosis |
Downregulate EGFR/p-ERK through ERα and GPER suppression | [23] | |
| MDA-MB-231 | Induce necrosis and apoptosis | Self-ubiquitination and degradation of cIAP1 and cIAP2 leading to the decreased ubiquitination of RIP1 | [48] | |
| MDA-MB-231, 4T1 | Inhibit cells metastasis and invasion | Reverse the epithelial-to-mesenchymal transition | [44] | |
| Cervical Carcinoma | Hela | Inhibit cell proliferation | Inhibit Cdc25 phosphatase | [25] |
| Glioma | SHG-44, U87, U251 | Induce necroptosis | Promote ROS overgeneration and RIP1/RIP3 necrosome formation | [47] |
| U87MG, HS683, M059K | Induce apoptosis | Induce ROS production, GSH depletion, mitochondrial transmembrane potential destruction, p53 up-regulation, and PARP [poly(ADP-ribose) polymerase] cleavage | [136] | |
| Lung Cancer | A549 | Inhibit cell proliferation | Up-regulate P21 Inhibit the ERK pathway |
[26] |
| A549 | Inhibit cells metastasis and invasion | Inhibit integrin β1 expression Suppress the ERK1/2 signaling pathway |
[36] | |
| H1975, H1650 | Induce apoptosis | Inhibit TrxR and activate the EGFR proteasomal degradation pathway | [49] | |
| Myeloma | RPMI8226, IM9 | Induce apoptosis | Inhibit the phosphorylation of IGF-1 receptors | [32] |
| B16F10, female C57BL/6JNarl mice | Induce cellular immunity | Induce ICD | [48] | |
| A375 | Induce autophagy and apoptosis | Activate ROS-mediated endoplasmic reticulum stress and P38 pathway | [31] | |
| Osteosarcoma | U2OS | Inhibit cells metastasis and invasion | Suppress MMP13 | [40] |
| U2OS | Inhibit cell proliferation | Up-regulate P21 Inhibit the ERK pathway |
[26] | |
| Pancreatic Cancer | PANC-1 | Inhibit cell proliferation | Up-regulate P21 Inhibit the ERK pathway |
[26] |
| Prostate Cancer | PC3, DU145, LNCaP, and 22Rv1 | Induce necroptosis | Decrease caspase8 and increase pRIP1 and pRIP3 | [46] |
| PC-3, DU145 | Inhibit cells metastasis and invasion | Reduce MMP-2/-9 expression via AKT/mTOR and ROS/ERK1/2 pathways | [41] | |
| DU-145, PC-3 | Induce apoptosis | Trigger ROS production, ER stress, and calpain activity Enhance pro-apoptotic Bax expression and Bcl-2 inhibition Disrupt MMP |
[30] | |
| Ovarian Cancer | A2780 | Induce apoptosis | Decrease Bcl-2 expression Increase Bax expression, and caspase-9 and caspase-3 cleavage |
[153] |
| Hepatocellular Carcinoma | HepJ5 and Mahlavu | Inhibit cells metastasis and invasion | Downregulate the expression of vimentin and MMP-2 and −9 | [154] |
| Metastatic Colorectal Cancer | NCM460, HT29, HCT116 and SW480 | Inhibit cells metastasis and invasion | Upregulate SIRT2 via phospho-ERK inhibition | [37] |
| Colon Cancer | Caco-2 cells | Enhance NK cell proliferation | Improve p-ERK1/2, GranB, perforin, and p-Akt expression | [56] |
| HCT116, SW620 | Inhibit cell proliferation | Inhibit hypoxia-inducible factor- 1α signaling | [27] | |
| Bladder Cancer | T24, SW870 | Induce necroptosis | Inhibit PKM2 activity | [155] |
Abbreviations: cIAP1, Cellular inhibitor of apoptosis 1; cIAP2, Cellular inhibitor of apoptosis 2; ERα, Estrogen receptor α; GSH, Glutathione; GPER, G-protein-coupled estrogen receptor; IGF-1, Insulin-like growth factor-1; mTOR, Mammalian target of rapamycin; P-Akt, Phosphorylated Akt; p-ERK1/2, Phosphorylated extracellular signal-regulated kinases 1/2; RIP1, Receptor-interacting protein kinase 1; SIRT2, Sirtuin 2.
Inhibit Cell Proliferation
SHK exhibits a general anti-cancer effect on various cancer cells including lung adenocarcinoma (A549 cell), triple-negative breast cancer (MDA-MB-231 cell), pancreatic cancer (PANC-1 cell), and osteosarcoma (U2OS cell). Its cellular mechanisms might be arresting the cell cycle at the G2/M phase due to its P21 up-regulation.22 In addition, anti-cancer effects on MCF-7 and SK-BR-3 cells appear to be concerned about EGFR/p-ERK downregulation via Erα and GPER inhibition.23 Moreover, SHK induces triple-negative breast cancer (TNBC) cell growth suppression is probably due to inhibiting IMPDH2 which is always involved in TNBC formation and progression.24 One research indicates that SHK’s anti-proliferation activity in cancer cells (MCF-7, HeLa, K562, and tsFT210) was perhaps through inhibition of the Cdc25s.25 Furthermore, SHK led to cell proliferation inhibition and cell-cycle arrest in hypoxia-induced human colon cancer cell lines by inhibiting the hypoxia-inducible factor-1α signaling pathway.27
Induce Apoptosis
Apoptosis is a form of programmed cell death, but cancer cells avoid apoptosis through various mechanisms, including overexpressing or stabilizing antiapoptotic BCL-2 family proteins and inactivating apoptosis transcription factors.28 Among them, the BCL-2 family can regulate apoptosis by controlling the permeability of the mitochondrial outer membrane.29 A study has shown SHK induced-apoptosis occurs in human glioma cells through multiple pathways including inducing ROS production, GSH depletion, mitochondrial transmembrane potential disruption, p53 up-regulation, and PARP cleavage.29 In addition, SHK can induce apoptosis of prostate cancer cells by induction of endoplasmic reticulum stress, and mitochondrial dysfunction.30 Similarly, activation of endoplasmic reticulum stress also plays a role in inducing apoptosis of melanoma A375 cells.31 For gefitinib-resistant non-small cell lung cancer, SHK promoted apoptosis through EGFR degradation induced by ROS generation.49 Furthermore, Kimura et al discovered SHK induced apoptosis via inhibiting the phosphorylation of IGF-1 receptors in myeloma cells.32
Inhibit Cells Metastasis and Invasion
Persistent growth and metastasis are two basic characteristics of malignant tumors. Metastasis has been one of the challenges of cancer treatment and diagnosis.33,34 Cancer metastasis involves a complex set of processes, such as cell adhesion to the extracellular matrix (ECM), cell invasion, angiogenesis, and lymphangiogenesis.35 In one study, SHK inhibited invasion and metastasis of lung cancer A549 cells by inhibiting integrin β1 expression and ERK1/2 signaling pathway.36 In addition, SHK suppressed colorectal cancer cell invasion through the ERK1/2/SIRT2 pathway.37 The metastasis process needs the degradation of ECM components by proteolytic enzymes. MMPs, including MMP-2, 9, and 13, are the primary extracellular matrix-degrading enzymes.38,39 SHK suppressed hepatocellular carcinoma cell, osteosarcoma, and prostate cancer cell migration by restraining the expression or activity of MMP.40,41,154 Epithelial-to-mesenchymal transformation (EMT) is crucial to the establishment of metastasis in tumor progression.42,43 Research suggests SHK could suppress triple-negative breast cancer cell metastasis through reversing EMT.44
Induce Necroptosis, Autophagy, and Cellular Immunity
Necroptosis, a lytic, programmed cell death pathway involves deubiquitination of TNF signaling and RIP1, phosphorylation of RIP1 and RIP3, inactivation of Caspase 8, and phosphorylation of MLKL.45 In docetaxel-resistant prostate cancer cells, SHK induced necroptosis by decreasing caspase 8 and increasing pRIP1 and pRIP3.46 Similarly, SHK induced glioma cell necroptosis by ROS overproduction and promoting RIP1/RIP3 necrosome formation.47 Wang et al identified that self-ubiquitination and degradation of cIAP1 and cIAP2 induced by SHK further led to a significantly decreased ubiquitination of RIP1, thus inhibiting MDA-MB-231 cell survival and accelerating necroptosis.48 In addition, SHK induced a dominant necroptosis to avoid cancer resistance in MCF-7, HEK293 and their drug-resistant lines with overexpression of P-glycoprotein, Bcl-2, or Bcl-x.50
Autophagy is a type II programmed cell death, and the regulation of autophagy can be an effective intervention strategy for cancer treatment.51 Enhanced autophagy is accompanied by SHK-stimulated RIPK1 and RIPK3-dependent necroptosis.51 Furthermore, SHK-induced autophagy directly contributes to DAMP upregulation which is the characteristic of ICD.52–54 In a study, increased DAMPs expression and a highly mature state of dendritic cells indicated SHK induced a significant ICD effect.55 Natural killer cells, a type of innate lymphocytes, play a role in controlling cancer progression. A study confirmed SHK enhanced NK cell proliferation and cytotoxicity of Caco-2 cells by regulating the expression of P-ERK1/2 and P-AKt.56 PD-L1 is the primary ligand of programmed death 1 (PD-1). PD-L1 expression is an immune evasion mechanism exploited by various malignancies.57–59 SHK promoted PD-L1 degradation and inhibited pancreatic cancer cells immune evasion by inhibiting the NF-κB/STAT3 and NF-κB /CSN5 signaling pathways.60 TAM, a major part of the tumor microenvironment, is concerned about the progression of tumor. M2-TAMs exert anti-inflammatory and tumorigenic characteristics.61 SHK can reduce the production of lactate which is an important driving factor of TAM2 polarization, thus repolarizing M2-TAMs.62 Furthermore, SHK inhibits tumor growth in mice by suppressing PKM2-mediated aerobic glycolysis.63,64
Pharmacokinetics of SHK
For many years, the in vivo process of SHK has been described mainly from rat animal models. The pharmacokinetic parameters in rat plasma were t1/2β at 630.7 ± 124.9 min; Cmax at 83.6 ± 8.8 ng/ml; Tmax at 1.0 ± 0.0 min; Vd at 136.6 ± 10.5 l/kg after intravenous administration of a 5 mg/kg SHK.156 The blood concentration of SHK was 0.48 μg/mL at 0.5 h after oral administration of a 25 g/kg SHK.157 Interestingly, SHK was not detected at 10 h in the plasma When the intravenous dose was 1.5 mg/kg.10 Animal experiments showed that SHK had an obvious “first pass” effect in rats, with drug concentration highest in bile and liver.
Drug metabolism enzymes (DMEs) participate in the metabolism, and/or detoxification of exogenous substance. Most organs are provided with numerous DMEs, including phase I and phase II metabolic enzymes and phase III transporters.65 SHK effectively upregulated protein expression of phase I enzymes, phase II enzymes, and phase III drug transporters.9 The metabolism of SHK was studied using rat liver microsomes cultured in vitro.66 The results revealed that NADH increased the metabolic rate of SHK. And microsome concentration exhibits an obvious effect on metabolic rate. The hydroxylation of the naphthoquinone nucleus was the principal metabolic pathway of SHK, followed by generating glucuronide conjugates and excreting in the bile and urine. The bacterial transformation of SHK has been investigated using human intestinal bacteria. Bacteroides fragilis subsp. thetaotus could transform SHK extensively into ten metabolites.67
When accessing the efficacy of drugs, safety issues must pay more attention to. SHK resulted in obvious animal weight loss when inhibited renal interstitial fibrosis as a glycolysis inhibitor.13 In addition, SHK could trigger serious skin sensitization at a low concentration.14 Moreover, SHK can promote Ca2+ entry and induce eryptosis, the suicidal erythrocyte death when treating human erythrocytes.15 A study reveals SHK might cause toxicity, especially drug-drug interactions based on an atypical inhibitory effect on CYP.16
In conclusion, SHK is a hydrophobic natural molecule with unsatisfactory solubility, rapid intestinal absorption, obvious “first pass” effect, and low oral bioavailability. In addition, existing literatures have revealed its adverse effects and non-selective toxicity. Thus, much more attention should be paid for effective application of SHK.
SHK Nanomedicines for Cancer Therapy
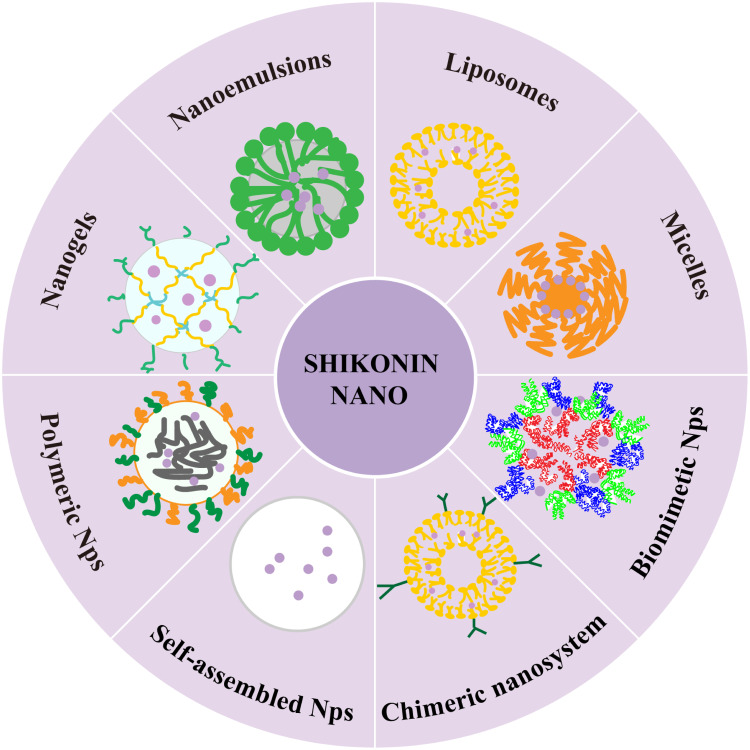
The existing literatures show that SHK has poor solubility and chemical stability in aqueous medium, which seriously limits its bioactivity in vivo. In addition, low oral bioavailability and the non-selective toxicity have restricted its application. Considering the promising advantages of nanomedicines to improve bioavailability, reduce adverse reaction and enhance the therapeutic efficacy of drugs, various SHK nanomedicines including liposomes, polymeric micelles, nanoparticles, nanoemulsions, nanogels, and chimeric advanced drug delivery nano systems have been developed to overcome the pharmacokinetic limitations of SHK (Figure 3). Briefly, the preparation method and physicochemical property of SHK nanomedicines are shown in Table 2.
Figure 3.
Various types of SHK-based nano-delivery systems for cancer therapy.
Table 2.
The Preparation and Physicochemical Property of SHK Nanomedicines
| Nanomedicines | Materials or Modified | Preparation | Size (nm) | Zeta Potential (mV) | Encapsulation Efficiency (%) | Drug Loading Capacities (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Liposomes | Soya bean phospholipid and cholesterol | Ethanol injection method | 145.4 ± 10.6 | −38.72 ± 2.43 | 80.37 ± 3.89 | – | [70] |
| PEG-modified | Thin-film hydration method | 62.3–121.3 | −11.54− −27.93 | 75.64–89.40 | – | [74] | |
| RGD-modified | Thin-film hydration method | 117.53 ± 3.05 | −15.37 ± 0.91 | 94.89 ± 1.83 | – | [78] | |
| HA-coated | Thin-film hydration method | 173 ± 5 | −36.5 ± 2.1 | 93.2 | 3.3 | [80] | |
| Biotinylated | Thin-film hydration method | 150–220 | 22–35 | – | – | [87] | |
| pH and GSH sensitive | Thin-film hydration method | ∼124 | –14 | >95 | 9.1 | [158] | |
| ATP as the internal water phase | Thin-film hydration method | ∼110 | ∼–15 | – | 16.7 | [96] | |
| Micelles | PID118-PLA39 | Dialysis methods | 98 | – | – | – | [99] |
| PEI-PCL, PEG-PCL | Evaporation technique | 91.5 ± 11.3 | 2.3 ± 0.3 | 82.4 ± 4.2 | 9.4 ± 0.6 | [160] | |
| FA-PEG-FA-mediated red cell membrane camouflaged, triphenylphosphine (TPP) decorated | Solvent-diffusion technique | ∼60 | ∼–10 | ∼60 | ∼3 | [105] | |
| Nanoparticles | AgNPs | Chemical reduction method | 106 | –23.8 | – | – | [114] |
| Ab-armed, pegylated | Emulsion-solvent evaporation technique | 120–250 | −35 | 42 | 8.6 | [117] | |
| Lf-modified PEG-PLGA NPs | Emulsion and solvent evaporation method | 174 | −9.7 | 92.45 | 15.57 | [11] | |
| DSPE-PEG2K-FA and PEI−PCL | Nano-precipitation method | 70–140 | ∼5 | 79.5 ± 3.2 | 5.1 ± 0.7 | [159] | |
| DSPE-PEG2K-FA and PEI−PCL | Evaporation technique | 106 ± 6 | 1.8 ± 1.0 | 80.3 ± 2.7 | 5.2 ± 0.9 | [123] | |
| DOPC, cholesterol and DSPE-PEG2k | Solvothermal method and evaporation technique | 174 | – | – | 6.53 | [126] | |
| Mannosylated lactoferrin nano-system | Heat-driven protein self-assembly process | 150 | 30 | 48.58 ± 4.21 | 1.43 ± 0.26 | [62] | |
| Albumin/lactoferrin hybrid biomimetic nanoparticles | High-speed dispersion emulsification-homogenization method | 153 | ∼−20 | – | 3.2 | [129] | |
| RBC-4T1 hybrid membrane camouflaged, ICG/PEI@HPB NPs | Two-step controlled etching method | 124.3 ± 3.5 | −12.87 | – | – | [130] | |
| Fe(III)-SHK supramolecular nanomedicine | Self-assembly method | 56.15 | 21.5 | – | – | [131] | |
| Nanoemulsion | T7/AS1411 modified | Nano-precipitation method | ∼35 | ∼−25 | ∼80 | ∼4 | [137] |
| Nanogel | STP-decorated, disulfide bond-crosslinked | Sequential dispersion and dialysis approach | 75 | −2.21 | – | 7.49 | [141] |
| Chi-aDDnSs | Hyperbranched polymers (PFH-16-OH, PFH-32-OH, PFH-64-OH), liposome | Evaporation technique | 170.3 ± 29.0 169.4 ± 23.7 177.9 ± 21.5 185.0 ± 10.9 |
−0.75 ± 3.32 −1.00 ± 3.11 0.50 ± 2.69 1.97 ± 0.75 |
5.41 ± 0.70 12.96 ± 0.60 45.61 ± 3.03 47.60 ± 1.12 |
– | [143] |
| PEGylated, hyperbranched polymers, liposome | Ultrasonic dispersion technology | 96.3–238.2 | −7.84− −21.87 | – | – | [144] |
Notes: The encapsulation efficiency (%) is defined by the concentration of the incorporated drug detected in the formulation over the initial concentration used to make the formulation. Drug loading capacity (%) is the amount of drug loaded per unit weight of the nanoparticle.
Abbreviations: DOPC, 1,2-dioleoyl-sn-glycero-3-phosphocholine; ATP, Adenosine triphosphate; RGD, Arginyl-glycyl-aspartate; CD44, Cluster of differentiation 44; FA, Folic acid; DSPE-PEG2K-FA, FA-conjugated 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[(polyethylene glycol)-2000]; ICG, Indocyanine green; NADH, Nicotinamide adenine dinucleotide; PID, Poly(N-isopropylacrylamide)-c-N,N-dimethylacrylamide random copolymer; PEG, Polyethylene glycol; PEG-PCL, Polyethylene glycol-polycaprolactone; PEI−PCL, Polyethyleneimine−poly (epsilon-caprolactone); PEI-PCL, Polyethyleneimine-polycaprolactone; PLA, Polylactic acid; PD-L1, Programmed death ligand 1; STP, Sarcoma-targeting peptide; AgNPs, Silver nanoparticles; TEM1, Tumor endothelial marker 1; TAMs, Tumor-associated macrophages.
Liposomes
Liposomes are spherical vesicles consisted of lipid bilayers. Due to their unique structure, hydrophobic agents can be embedded in lipid bilayers and hydrophilic agents encapsulated in the central aqueous chamber.68 Various liposomes such as conventional liposomes, long-circulating liposomes, actively targeted liposomes, and multifunctional liposomes have been reported to load SHK to improve the anticancer effect.
Conventional Liposomes
To meet urgent requirements for nutrient and oxygen supplies in tumors, the tumor blood vessels endothelial cell gap is large, the vascular wall smooth muscle layer is lacking, and the angiotensin receptor function is deficient. In addition, the tumor tissue lacks lymphatic vessels, prohibiting the return of lymph fluid. Thus macromolecular substances could easily pass through the vascular wall and accumulate in tumor tissues, and can not be carried away by lymphatic reflux, which is called enhanced permeability and retention (EPR) effect.69 EPR effect has become the basis of many nanomedicines to achieve tumor targeting. Xia et al prepared SHK-loaded liposome (sh-L) via the thin-film hydration method.70 The results showed that sh-L could reduce toxicity to endothelial cells, inhibit migration and trigger angiogenesis reduction. Tumor angiogenesis,71 one of the “hallmarks of cancer”, is critical to tumor growth, progression, and metastasis. The study provides implications for further research on the development of SHK nanomedicine based on inhibiting angiogenesis.
Long Circulating Liposomes
Despite the advantages, liposomes binding with serum proteins are easily caught by the reticuloendothelial system cells, failing to deliver drugs to target sites.72 To prolong circulation time and increase stability, long-circulating liposomes have been developed.72 One of the most significant ways to develop long-circulating liposomes is decorating them with a hydrophilic polymer, such as PEG.73 Conventional and PEGylated SHK-loaded liposomes were prepared,74 confirming that decorating PEG to the SHK-loaded liposomal surface decreased particle size (reduction varied between 17–45%), improved entrapment efficiency (increments varied from 13 to 18%), increased stability and resulted in more release (increase approximately 20–30%).
Actively Targeted Liposomes
Ligand-directed nanomedicines are expected to decrease side effects to normal cells, increase drug uptake at target sites and enhance anticancer efficacy, which is mainly accomplished by attaching ligands on the surface of drug carriers and then aggregating drugs around solid tumors by specific recognition and binding of ligand receptors on the tumor cell membrane.75
RGD is an integrin recognition sequence which can bind to the ανβ3 receptor overexpressed on the angiogenic endothelium in various tumor.76,77 RGD functionalized SHK-loaded liposome (RGD-SSLs-SHK) was developed.78 RGD-SSLs-SHK showed superior cellular uptake via receptor-mediated endocytosis. Compared with SSLs-SHK, higher cytotoxicity to MDA-MB-231 cells was seen, without significant difference in that of MCF-7 cells, which might be due to the specific binding of RGD to ανβ3 receptors which are expressed on MDA-MB-231 cells rather than MCF-7 cells. CD44, a glycoprotein, is overexpressed in various tumor cell surfaces, including breast cancer.79 HA, a principal CD44 binding molecule, exerts a preferable prospect in decorating nanocarriers. HA-modified SHK liposomes (HA-SHK-Lip) were developed for TNBC therapy.80 Cellular uptake of HA-SHK-Lip in MDA-MB-231 cell was higher than SHK-Lip and free SHK, which is attributed to the CD44 receptor-mediated endocytosis. HA-SHK-Lip displayed more significant anticancer efficacy than SHK and SHK-Lip in vitro and vivo.
Multifunctional Liposomes
The complex tumor microenvironment prevents the drug from efficient delivery and brings great obstacles to tumor treatment.81 However, Based on tumor-specific pathological stimuli such as acidic microenvironments, redox milieu environments, and overexpressed enzymes, stimuli-responsive nanomedicines have shown excellent performance in realizing controlled drug release and tumor targeting.82,83 Currently, the co-administration of two or more drugs can enhance anticancer efficacy by targeting different molecular pathways and reduce toxicity via decreasing individual drug doses.84,85 Especially, co-administration of chemical drugs and immunomodulators is attracting researchers’ attention.86 Due to the attractive characteristics mentioned above, the combination of stimulus-sensitive design, active targeting, co-administration, and cancer immunotherapy strategy has been reported to develop multifunctional SHK liposomes.
SHK and N-end rule inhibitor RFC11 were loaded into biotinylated liposomes.87 SHK could induce necroptosis in various cancers via upregulating RIPK1/RIPK3/MLKL expression.88,89 Interestingly, inhibiting the N-end rule pathway is conducive to increase the stability of RIPK1, sensitizing cells to necroptosis.90–92 The cellular uptake was strikingly higher than the non-targeting counterparts in the CT26 cell line. In addition, the biotinylated liposome codelivery of RFC11 and SHK showed a synergistic antitumor effect in vitro and vivo via RIP1 and RIP3-dependent necroptosis. ICD could enhance the anticancer efficiency of certain drugs by integrating direct tumor cell killing with stimulated anticancer immunity.93 But the dosage of SHK to trigger effective ICD may cause hepatoxicity. SHK at a low dose was combined with Anthracyclines to develop LipMS,158 exhibiting a pH and GSH dual-sensitive release pattern mediated by copper. LipMS significantly inhibited melanoma growth and increased necrosis areas and apoptotic cells in comparison with single-encapsulated liposomes and the mixture. In addition, AST levels in all groups were higher than those in a normal saline group except LipMS1:1, indicating LipMS1:1 did not cause hepatotoxicity. Furthermore, LipMS1:1 reduced cardiotoxicity which is a well-known side effect of Anthracyclines.6 Further study showed LipMS triggered a significant ICD effect, immune memory, and did not form new tumors in the process. ICD is often accompanied with autophagy.94 But autophagy could suppress immune response by robbing immune-related antigens.95 Hydroxychloroquine (HCQ), an autophagy inhibitor, can increase SHK triggered immune-related antigens exposure in colon cancer. But the reduction of ATP results from autophagy suppression, weakening anticancer immune response. To solve the problem, LipHCQa was prepared by pH gradient with ATP wrapped in the aqueous phase.96 The result showed the ATP in the aqueous phase of LipHCQa compensated the ATP reduction caused by autophagy suppression. Further, coadministration of LipSHK and LipHCQa at appropriate dosage achieved superior anticancer efficacy without suppressing the immune response.
Liposome is an efficient drug delivery system for SHK, with increased stability, long circulation, controlled release, and enhanced anticancer efficacy. Compared with passively targeted liposomes, actively targeted liposomes effectively enhance the cellular uptake of SHK and further improve the overall antitumor effect. Moreover, the co-administration of SHK and other drugs in liposomes amplified the anticancer effect and reduced the unfavorable effect.
Polymeric Micelles
Polymeric micelles are formed by amphiphilic polymers that self-assemble into nanostructures with hydrophilic shells and hydrophobic cores.97,98 The shell protects the micelle from aggregation and precipitation and the core can hold hydrophobic drugs.
Su et al formulated SHK-loaded thermosensitive micelle (STN) for breast cancer therapy.99 STN exhibited rapid SHK release (90%) at tumor sites with temperatures 3°C–5°C higher than normal tissue. The entrapment of SHK into the thermosensitive biodegradable micelles improved its accumulation and cytotoxicity in breast cancer in vitro and vivo. Apart from single-loaded micelles, mixed micelles (SHK/siIDO1-HMs) loading SHK and IDO-1 knockdown siRNA (siIDO1) were prepared to enhance immunotherapy for colon cancer160 Cytotoxic T lymphocyte (CTL) activation triggered by ICD could induce tumor regression by IFN-γ secretion. But IFN-γ secretion can promote the generation of indoleamine 2, 3-dioxygenase-1 (IDO-1), which may be a chief culprit of the immunosuppressive tumor microenvironment (ITM).100–102 Calreticulin (CRT) exposure, ATP secretion, extracellular significant release of HMGB1, and maturing rate of DCs all revealed SHK/siIDO1-HMs induced ICD notably. IDO-1, as an immunosuppressive enzyme, catalyzes the degradation of tryptophan to kynurenine.103,104 Decreased IDO-1, and reduced kynurenine to tryptophan ratio in SHK/siIDO1-HMs group suggested SHK/siIDO1-HMs resolved the contradiction between ICD-triggered anticancer immunity and IDO-1 induced immunosuppression. In addition, the lowest tumor volume and weight, and prolonged survival in SHK/siIDO1-HMs group indicated SHK/siIDO1-HMs exerted outstanding anticancer effect in the CT26 tumor mouse model.
Mitochondria-targeted drug treatment is a potential strategy to overcome TNBC. SHK can not only induce apoptosis by inhibiting mitochondrial energy production, but also exhibit significant inhibitory effects on TNBC.44,106,107 Triphenylphosphine (TPP) has been generally used as a specific ligand to mediate the mitochondrial targeting. SHK-loaded micelles prepared with TPP-PEG-Hyd-PCL/TPP-PEG-PCL/mPEG-PCL (ThTM/SHK) was incubated with FA-PEG-FA modified-red blood cells membranes to develop red blood cells membranes camouflaged micelle (ThTM/SHK@FP-RBCm) for mitochondria-targeted TNBC therapy.105 Both FP-RBCm coating and TPP on micelles contribute to tumor lesion distribution, receptor-mediated cellular uptake, and electrostatic attraction-dependent mitochondrial targeting, thereby maximizing inhibitory effects on mitochondrial biosynthesis in TNBC cells. In vivo, ThTM/SHK@FP-RBCm suppressed TNBC tumor growth, lung metastasis, and mitochondrial biogenesis.
SHK-loaded polymeric micelles exhibited a controlled drug release pattern at target sites. Compared with single-loaded-polymeric micelles, dual-loaded-polymeric micelles resolved the contradiction between ICD-triggered anticancer immunity and IDO-1-induced immunosuppression. Moreover, “right-side-out” membrane-camouflaged SHK loaded-micelle significantly strengthened the antitumor efficacy.
Nanoparticles
Nanoparticles (NPs) have been developed with suitable size and surface characteristics to improve biodistribution and prolong circulation time.108 Due to the attractive properties of NPs, a wide range of NPs including metallic nanoparticles, polymeric nanoparticles, lipid-polymer hybrid nanoparticles, biomimetic nanoparticles, and self-assembled nanoparticles have been reported to load SHK.
Metallic Nanoparticles
Silver nanoparticles (AgNPs), as a type of metallic nanoparticles,109 have been broadly investigated due to their excellent properties such as easy to prepare and versatile functionalization sites.84 In addition, AgNPs are considered to be an excellent synergistic agent.110–113 SHK-loaded-silver nanoparticles (SHK-AgNPs) were prepared to achieve a synergistic effect for lung cancer.114 SHK-AgNPs were stable under physiological conditions and showed preferred distribution in lung. Furthermore, IC50 of SHK-AgNPs on A549 cells was lower than that of free SHK, silver nitrate, and phytomediated AgNPs, suggesting SHK and silver nanoparticles had a synergistic effect on inhibiting A549 cells.
Polymeric Nanoparticles
Polymeric nanoparticles have attracted numerous attention due to its fascinating properties such as drugs protection, controlled release, specific targeting.115 Poly (lactic-co-glycolic) acid (PLGA), a biodegradable copolymer of polylactic and polyglycolic acid approved by the FDA, shows great potential for drug delivery due to its advantages such as biodegradability, biocompatibility, and versatility.116
PEG and TEM1/endosialin-targeting antibody (Ab) modified PLGA NPs were synthesized to load SHK for ovarian cancer therapy.117 TEM1 is a protein mostly expressed on the surface of endothelial cells in newly formed blood vessels and cancer cells,118 making TEM1-specific targeting a promising approach for cancer therapy. Through active and specific targeting mediated by anti-TEM1 Ab/scFv, these NPs accumulated within the TME in which 80% SHK was released within 24–48 hours, indicating higher drug accumulation and continuous release of SHK in TME. Moreover, the NPs induced superior cytotoxic effects on ovarian cancer. Lf-modified PEG-PLGA NPs (Lf-NPs) were engineered to deliver SHK for glioma treatment.11 Lactoferrin receptor (LfR) and the low-density lipoprotein receptor-related protein (LRP) are overexpressed on the blood-brain barrier cells and glioma cells surface, respectively.119,120 Besides, LRP is a kind of lactoferrin specific receptor with excellent selectivity.121,122 More than 60% of the drug in SHK/Lf-NPs experienced a continued release for more than 72 h. In Cytotoxicity assays, C6 cells were more responsive to Lf-modified SHK NPs. Further, higher brain tissues distribution and the brain/blood concentration ratios of SHK/Lf-NPs revealed that SHK/Lf-NPs possessed a significant brain targeting effect.
Lipid-Polymer Hybrid Nanoparticles
Lipid-polymer hybrid nanoparticles take the hydrophobic polymer as the core and the lipids and their hydrophilic corona as the shell, which integrate the advantages of polymeric nanoparticles and liposomes and overcome some of their shortcomings.
Nanoparticles co-loading SHK and PD-L1 knockdown siRNA (SHK/siR-NPs) were developed for cancer immunotherapy by easing PD-L1-regulated immune tolerance, inducing ICD, and repolarizing M2-TAMs.159 By inhibiting tumor glucose metabolism, SHK can reduce the production of lactate which is an important driving factor of TAM2 polarization.62 Decreased PD-L1 protein, declined PD-L1 mRNA, higher IFN-γ secretion and lower cell viability suggested that SHK/siR-NPs inhibited CT26 cell via blocking PD-1/PD-L1 pathway. Obvious extracellular release of HMGB1, secretion of ATP, secretion of CTR, and marked maturation of DCs suggested that SHK/siR-NPs induced a significant ICD effect. Reduced M2-TAM markers and raised M1-TAM markers verified SHK/siR-NPs effectively repolarized M2-TAMs. Moreover, SHK/siR-NPs treated group showed the highest tumor distribution and tumor suppression in comparison with SHK-NPs, siRNA-NPs, and free SHK. Li et al constructed tumor growth factor β small interference RNA (siTGF-β) and SHK co-delivery nanoparticles for TNBC chemo-immunotherapy.123 SHK-induced ICD exhibited a positive tumor inhibitory effect. Effective silencing of TGF- β by siTGF-β suppressed EMT, promoted CTL infiltration, inhibited regulatory T lymphocytes proliferation, and suppressed lung metastasis. Further, SHK/siTGF-β NPs exhibited a long-term immune memory to inhibit cancer recurrence.
ROS-sensitive prodrug nanomedicines are considered as promising platform for cancer therapy. But inefficient or incomplete prodrug activation caused by insufficient response or increasingly consumed ROS at the tumor site limits its efficiency.124,125 Herein, a self-amplifying ROS-responsive prodrug NP (Cu-SHK@DTC-PPB) was synthesized.126 DTC-PPB composed of two diethyldithiocarbamate (DTC) molecules binding by the ROS-sensitive phenylboronic ester bond. DTC or copper alone have no obvious cytotoxicity, but Cu (DTC)2 is highly cytotoxic. DTC-PPB in response to higher ROS concentration in tumor sites, DTC released. DTC can chelate Cu2+ from the Cu-SHK@DTC-PPB to generate Cu (DTC)2 and trigger SHK release. The released SHK can induce ROS generation, further promoting highly cytotoxic Cu (DTC)2 formation. Cu-SHK@DTC-PPB showed enhanced drug accumulation in tumor site with minimal side effect to normal cells. Moreover, Cu-SHK@DTC-PPB exhibited excellent anticancer effect in vitro and in vivo.
Biomimetic Nanoparticles
An ideal drug delivery system is supposed to be relatively stable in blood circulation and release drugs as it arrives at the target sites. However, exogenous NPs are easy to be identified and eliminated by the immune system. Using endogenous components (eg, proteins, polysaccharides, and cell membranes) as camouflage to evade the immune system and enhance tumor cell–specific uptake is a promising strategy.127
A mannosylated lactoferrin nanosystem (Man-LF NPs) co-delivering SHK and JQ1 was prepared to target colon cancer cells and TAM.62 JQ1 can effectively decrease PD-L1 expression in tumor cells when combined with other immune drugs.128 Characteristics of cancer cells undergoing ICD, repolarization of TAM2, and PD-L1 downregulation were observed in the Man-LF NPs treated CT26 cells. In vivo biodistribution assay, higher tumor sites accumulation in Man-LF NPs group might be owing to MR and LPR-1 act as double targeting receptors for cancer cells and TAM. In addition, the highest inhibition rate was seen in Man-LF NPs treated immunocompetent CT26 tumor mouse model. Zhao et al fabricated albumin/lactoferrin hybrid biomimetic nanoparticles (BSA/LFNP) to co-deliver SHK and DSF to target glioma energy metabolism.129 SHK, PKM2 inhibitor, can inhibit the glycolysis of tumor cells. Disulfiram (DSF) inhibits the ALDH1L1 enzyme in the 10-formyl-tetrahydrofolate-NADH-ATP metabolism axis, which can inhibit the supply of ATP and interfere with tumor energy metabolism. The results showed that BSA/LFNP possessed the advantages of BSA-mediated long circulation and LF-mediated blood-brain barrier penetration. DSF/SHK-loaded BSA/LFNP effectively inhibited GL261cell glycolysis and folic acid-NADH-ATP metabolic pathway, thus cutting off the energy supply of tumor cells. In addition, BSA/LFNP effectively suppressed tumor growth in the GL261 glioma model and increased the median survival time of mice.
In a study, SHK was loaded into hollow Prussian blue nanoparticles (HPB@SHK). PEI was decorated on the HPB@SHK, which then adsorbed ICG. Finally, the RBC-4T1 hybrid membrane was camouflaged ICG/PEI@HPB@SHK to prepare HMGPHS NPs.130 Decreased phagocytosis of macrophages in vitro and extended drug half-life in vivo suggested membrane coating has promoted immune evasion and prolonged the circulation time of HMGPHS NPs. Excellent homotypic targeting ability and anticancer activity in vitro and vivo may owe to the homing effect of 4T1 membrane and synergetic effect of photothermal therapy (PTT) and chemotherapy, respectively. Furthermore, SHK suppressed the 4T1 cells metastasis and reprogramed the TME via alleviating inflammation resulting from PTT.
Self-Assembled Nanoparticles
Most nano delivery carriers are usually exogenous materials without pharmacological activity. And biocompatibility, degradability, and toxicity of the materials need to be considered. The carrier-free nano-drug delivery system, a nanostructure assembled by the drug itself, with a superior drug-loading capability, and simple preparation method, has showed great potential in cancer therapy.161
A Fe(III)-SHK supramolecular nanomedicine (FSSNs) for tumor treatment through ferroptosis and necroptosis is designed.131 One mechanism by which tumor cells develop resistance to chemotherapy and radiotherapy is related to resistance to apoptosis.132 Designing novel medicines based on non-apoptotic cell death pathways is potential to achieve a long-term antitumor effect. The driving force of the preparation process is metal-polyphenol-coordinated interaction. SHK ethanol solution was added into FeCl3·6H2O deionized aqueous solution, stirring for an hour. After taken into tumor cells, FSSNs disassembled into Fe2+ and SHK under exposure to the high concentration of GSH in tumor sites. Due to the increase of Fe2+ level and the depletion of GSH, the LPO accumulated and the GPX4 downregulated, which led to cancer cell ferroptosis. Moreover, the released SHK strengthened the intracellular oxidative stress degree, inducing tumor cell death through necroptosis.
Polymeric nanoparticles can easily manipulate carrier properties by selecting the type of polymer and the mode of the carrier assembly. Antibody-decorated and ligand-decorated PLGA NPs were engineered to encapsulate SHK, showing great tumor site targeting ability, high drug accumulation, and continuous release. Lipid-polymer hybrid nanoparticles can carry various drugs in a certain proportion, controlling the release of drugs and maintaining biocompatibility. DTC-PPB and Cu-SHK loaded lipid-polymer hybrid nanoparticles achieved Cu (DTC)2 formation and SHK release by a self-amplifying positive feedback cycle via separating Cu2+ and DTC-PPB in nanostructure and lipid, respectively. Compared with liposomes and NPs, lipid-polymer hybrid nanoparticles possess higher stability and better biocompatibility, so they have a good prospect for gene delivery. SHK and siRNA play a synergistic effect in cancer immunotherapy via encapsulating in lipid-polymer hybrid nanoparticles. NPs are still facing challenges to regulate the in vivo fate of the drugs, biomimetic delivery may be a solution to this. Biomimetic SHK-loaded nanoparticles showed long circulation, enhanced immune evasion, excellent tumor-targeting ability, and superior antitumor efficacy. NPs assembled by SHK itself, with high drug loading capability and simple preparation method, exhibited antitumor effect via ferroptosis and necroptosis.
Nanoemulsions
Nanoemulsion (NE) is formed by water and oil phases, surfactants, and co-surfactants in appropriate proportions and different types of manufacture (low- or high-energy).133–135 NE has great potential in pharmaceutics due to its attractive property including suitable size, improved dispersion of hydrophobic drug, enhanced absorption, and the potential to be modified with different ligands to target components present in tumor cells’ surfaces.
SHK not only can induce apoptosis through multiple pathways in human glioma cells136 but also enhance the anti-glioma efficiency of certain chemotherapeutic agents such as docetaxel.137 Herein, a T7/AS1411 modified and superparamagnetic Fe3O4 nanoparticles-embedded nanoemulsion encapsulated with DTX&SHK (Fe3O4@T7/AS1411/DTX&SHK-M) was developed for glioma treatment.138 The highest drug distribution and glioma accumulation of DTX and SHK in Fe3O4@T7/AS1411/DTX&SHK-M group were seen compared with DTX+SHK, SHK&DTX-M, AS1411-DTX&SHK-M, and T7/AS1411-DTX&SHK-M. The luminescence (representing glioma) in Fe3O4@T7/AS1411/DTX&SHK-M- group was the lowest. Importantly, the bodyweight of mice treated with different nanoemulsions did not reduce obviously, indicating these nanoemulsions reduced systemic toxicity associated with chemotherapy drugs.
Nanogels
Nanogels are made from hydrogels with cross-linked polymer chains.139,140 Nanogels are with good properties such as good biocompatibility, high drug loading capacity, customizable surface properties, and adjustable morphology.
STP-modified disulfide bond-crosslinked nanogel (STP-NG/SHK) was prepared to target osteosarcoma.141 The ratio of SHK released from nanogels in PBS without GSH at 72 h was lower than 17%, compared with 98.4% in the presence of GSH, attributing to disulfide bond in STP-NG/SHK responding to high concentrations of GSH in tumor sites. STP-NG/SHK inhibited osteosarcoma growth and pulmonary metastasis in vitro and vivo in an effective way. Moreover, STP-NG/SHK reduced myocardial toxicity triggered by SHK.
Chimeric Advanced Drug Delivery Nano Systems
Chimeric advanced drug delivery nano system is defined as a hybrid nanosystem due to the binding process of nanomaterials, which can provide advantages as drug carriers. The binding of liposomes and dendrimers in liposomal locked-in dendrimers has been reported, which have been considered as Chi-aDDnSs.142 Dendrimer molecules will bring new pharmacokinetics and possible pharmacological properties.
Chi-aDDnSs were prepared by combining three hyperbranched polymers with liposomes to load SHK.143 LipoBs exhibited an impressive encapsulation efficiency compared with LipoAs because the non-complexed SHK can be encapsulated in the liposomal lipid bilayers in LipoBs rather than removed through centrifugation in LipoAs. Moreover, LipoBs experienced a continual 72h drug release, 95.51% for LipoB64--OH and 78.27% for LipoB-32-OH. No obvious increase was seen in the particle size under dark at 4°C for 5 days, suggesting LipoAs and LipoBs were stable under this condition. In another study, SHK-loaded liposomes, PEGylated SHK-loaded liposomes, SHK/hyperbranched polymers (HBP) complexes, conventional SHK-loaded chi-aDDnSs, and PEGylated SHK-loaded chi-aDDnSs were prepared.144 The results showed SHK partly damaged the liposomes, yet a more fluid liposome structure is beneficial to encapsulate SHK into the bilayer. And SHK avoided HBPs entering liposomes, making an ordered DSPC bilayer developed due to the interactions between HBP and liposome. In addition, PEG improved the stability of SHK inside of the liposomes and HBPs promoted the overall stability of chi-aDDnSs. In conclusion, Chi-aDDnSs generate a high potential for delivering SHK due to their excellent capability to encapsulate drugs, alter drug release rate, and enhance overall stability.
Challenges and Perspective
For the attractive properties of SHK in cancer therapy, an increasing number of rational, functional, and efficient SHK nanomedicines have been developed. Compared with free SHK, SHK nanomedicines possess good stability, improved bioavailability (Table 3), prolonged circulation time, active targeting ability, more tumor-site drug accumulation, efficient cellular uptake, tumor stimuli-responsive drug release, and enhanced cancer cell-killing efficiency (Figure 4). In addition, good biocompatibility and security has been indicated in studies. Moreover, nano drug delivery systems-mediated synergistic anticancer therapy based on SHK and other drugs including therapeutic genes, chemotherapeutic agents, immunotherapeutic drugs, and photosensitizers (Figure 5), has exhibited outstanding therapeutic efficacy and reduced systematic side effects. Although great progress and promising clinical translation prospects of SHK nanomedicines (Table 4), there remain restrictions to realizing the clinical translation of SHK nanomedicines. Several vital issues need to be considered.
Table 3.
Pharmacokinetics Properties and Bioavailability of Various SHK Nanomedicines
| SHK Nanomedicine | Dose (mg/kg) | Cmax (µg/ml)a | AUC (0-t) (µg*h/ml)b | CLobs (L/hr/kg)c | MRT0-∞ (hr)d | t1/2 (hr)e | Animal | Model | Ref |
|---|---|---|---|---|---|---|---|---|---|
| SHK in DMSO | 5 (i.p.) | 1889.921 ± 418.158 | 3952.351 ± 1345.323 | 1.399 ± 0.54 | – | 1.623 ± 0.232 | Female C57BL/6 mice | B16F10 melanoma cancer | [158] |
| LipSHK | 5 (i.v.) | 160651.069 ± 13766.262 | 587421.672 ± 73473.644 | 0.008 ± 0.001 | – | 4.537 ± 0.595 | |||
| SHK in PBS solution containing Tween 80 and PEG 300 | 10 (i.v.) | 1.05 ± 0.20 | 1.97 ± 0.38 | 2.21486 ± 826.39 | 1.74 ± 0.09 | – | Female ddY mice | – | [105] |
| ThTM/SHK@FP-RBCm | 7.46 ± 0.77 | 7.56 ± 0.92 | 1.19248 ± 159.35 | 1.72 ± 0.15 | – | ||||
| SHK | 10 (i.v.) | 0.94 ± 0.11 | 41.97 ± 5.15 | – | – | 15.15 ± 1.41 | SD rats | – | [11] |
| SHK-loaded Lf-NPs | 1.63 ± 0.08 | 75.41± 6.28 | – | – | 26.28 ± 2.38 | ||||
| SHK | 5 (i.v.) | – | ∼8 | – | – | ∼1.8 | SD rats | – | [159] |
| SHK/siR-NPs | – | ∼40 | – | – | ∼5.0 |
Abbreviations: aCmax, Peak plasma concentration, bAUC, Area under the plasma concentration; cCLobs, total body clearance, dMRT, Mean Resident time, et1/2 = half-life at biodistribution.
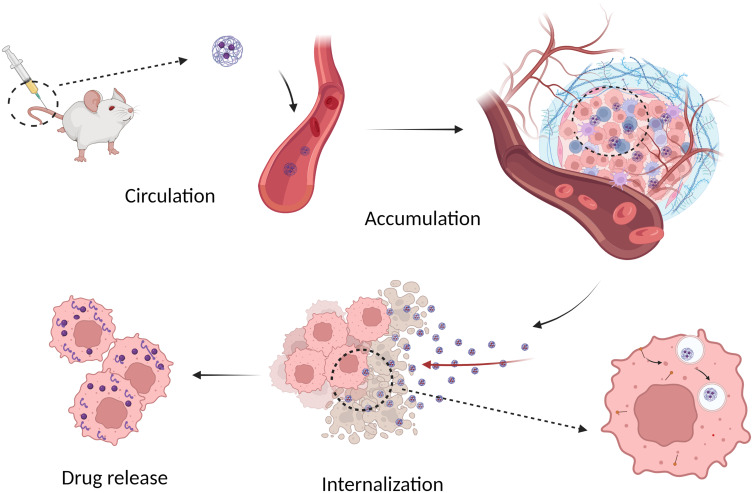
Figure 4.
A sketch of SHK nanomedicine to deliver drug into cancer cells: circulation in the blood, tumor accumulation, cellular internalization and intracellular drug release.
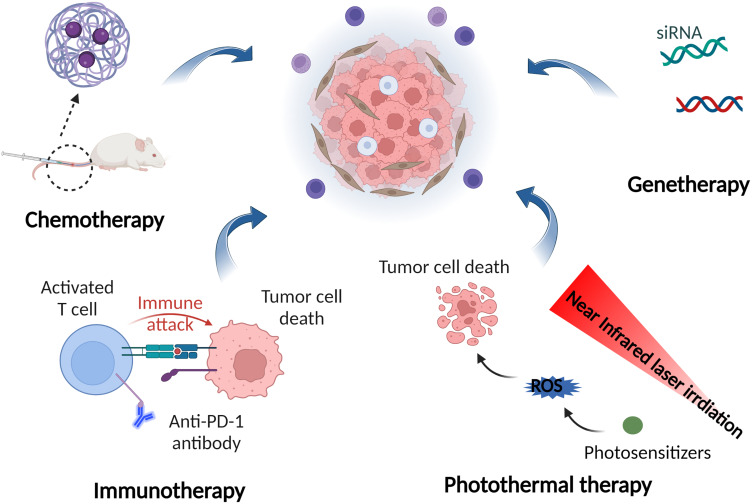
Figure 5.
Nano drug delivery systems-mediated synergistic anticancer therapy based on SHK and other drugs including therapeutic genes, chemotherapeutic agents, immunotherapeutic drugs, and photosensitizers.
Table 4.
The in vivo Application of SHK Nanomedicines for Cancer Therapy
| Formulation | Cancer Type | Animal Model | Dosage | Route | Therapeutic Outcomes | Ref |
|---|---|---|---|---|---|---|
| HA-SHK-Lip | Triple-negative breast cancer | Nude mice bearing MDA-MB-231 xenograft tumors | 20 mg/kg SHK, every 2 days for 18 days | IV | Smallest tumor volumes and tumor weight in HA-SHK-Lip group | [80] |
| Bio-C18 lipid containing RFC11 and SHK | Colon cancer | CT26 murine colon adenocarcinoma cells-bearing BALB/c mice | RFC11 (8 mg/kg) and SHK (0.75 mg/kg) every 2 days | IV | Effective tumor-regression ability | [87] |
| SHK and MIT dual-liposomes (LipMS) | Melanoma and prostatic cancer | B16F10 tumor-bearing female C57BL/6 mice and RM-1 cells tumor-bearing female C57BL/6 mice | 4 mg/kg MIT and SHK in total every 3 days | IV | Superior tumor suppression efficiency than single-loaded liposomes | [158] |
| LipHCQa and LipSHK | Colon cancer | CT26 cells-bearing female BALB/c mice | 15 mg/kg of LipSHK and LipHCQ every 4 days | IV | Outstanding anticancer efficacy without dampening the immune response | [96] |
| SHK-loaded thermosensitive nanomicelle (STN) | Breast cancer | MCF-7 cells-bearing BALB/c nude mice | 2 mg/kg of STN every 3 days | IV | Enhanced tumor accumulation and antitumor efficiency at the phase transition temperature | [99] |
| SHK/siIDO1-HMs | Colon cancer | CT26 cells-bearing female BALB/c mice | SHK, 5 mg/kg; siIDO1, 1 mg/ kg, every 2 days | IV | Outstanding antitumor efficacy via inducing ICD effect and mitigating the IDO-1-triggered immunosuppression | [160] |
| ThTM/SHK@FP-RBCm | Triple-negative breast cancer | MDA-MB-231 cells-bearing female NOD/SCID mice | 3 mg/kg SHK every other day seven times | IV | Superior mitochondrial targeting ability Inhibit tumor growth and lung metastasis Inhibit mitochondrial biogenesis |
[105] |
| SHK/siR-NPs | Colorectal cancer | CT26 cells-bearing BALB/c mice | SHK, 5 mg/kg; siRNA, 500 μg/kg, every 2 days | IV | Superior tumor suppression due to inducing ICD, modulating TAMs, and inhibiting PD-L1 | [159] |
| SHK/siTGF-β NPs | Triple-negative breast cancer | 4T1 cells female BALB/c mice | SHK, 5 mg/kg; siTGF-β, 1 mg/kg, every 3 days for five times | IV | Metastatic TNBC therapy efficiency by promoting the immune response and relieving the ITM | [123] |
| Cu-SHK@DTC-PPB | Breast cancer | 4T1 tumor-bearing mice | 0.197 mg/kg SHK, 0.885 mg/kg DTC-PPB every 2 days | IV | Improved anticancer therapeutic efficiency, ROS-induced self-amplifying prodrug | [126] |
| Man-LF NPs | Colon cancer | Colon cancer-bearing mice | SHK5 mg/kg, JQ1 15 mg/kg every 2 days | IV | Tumor inhibition rates for SHK, SHK+JQ, LF NPs and Man-LF NPs: 45%, 61%, 68% 84%. Induce ICD, modulate TAMs, and inhibit PD-L1 | [62] |
| BSA/LF NP | Glioma | Orthotopic glioma mice | SF + SHK (10 mg/kg + 5 mg/kg) | IV | Extend the survival of glioma mice via regulating energy metabolism and immune microenvironment | [129] |
| Hybrid membrane@ICG/PEI@HPB@SHK NPs | Triple-negative Breast cancer | 4T1 cells-bearing female BALB/c mice | SHK, 2 mg/kg; ICG, 5 mg/kg; HPB NPs, 5 mg/kg, every 2 days | IV | Significantly suppress the growth and metastasis of TNBC | [130] |
| NH2-PEG-cRGD-modified FSSNs (FSRSNs) | Breast cancer | 4T1 cells- bearing female BALB/c mice | – | IV | Stimulate the cancer cell death via ferroptosis and necroptosis | [131] |
| Fe3O4@T7/AS1411/DTX&SHK-M | Glioma | Luc-G422 glioma-bearing mice | DTX (or SHK) dose of 10 mg/kg every 2 days | IV | Prolong the overall survival period Triple glioma-targeted delivery |
[137] |
| STP-NG/SHK | Osteosarcoma | 143B osteosarcoma-bearing mice | SHK 2.0 mg/kg every 2 days | IV | Inhibit osteosarcoma growth and metastasis Reduced systemic toxicity |
[141] |
Abbreviation: IV, Intravenous.
Quality Control
The structure, particle size, polydispersity, surface properties, and stability of nanomedicines exert great effects on the pharmacokinetic property and in vivo therapeutic efficacy. Thus, the preparation process should be precise and repeatable to ensure the consistent properties of the prepared SHK nanomedicines. Furthermore, it is particularly critical to balance the relationship between large-scale preparation and intelligent design to minimize complexity and optimize efficacy.
Biosafety
Biosafety is one of the main obstacles hindering the clinical translation of SHK nanomedicines. Organic solvents are regularly introduced into the preparation process of SHK nanomedicines. Therefore, researchers should attach more importance to the possible solvent residue. In addition, the biocompatibility of carrier materials is also an essential scientific problem. In the design of SHK nanomedicine, biocompatible, natural, and food-derived carrier materials, especially FDA-approved materials, would be ideal options. Moreover, an in-depth understanding of the in vivo metabolic process of the carrier materials is a necessary condition for good biocompatibility. In particular, nanomedicine will combine with the surrounding protein molecules to form a protein crown in the blood circulation, affecting the toxicological properties of the nanomedicine.145 So, it is necessary to explore the biological mechanism of SHK nanomedicine protein crowns in the following study. Furthermore, long-term medication is the main treatment modality. But the long-term toxicity of SHK nanomedicines in vivo is neglected, which requires more attention.
Efficacy
The innovative design of a nano-drug delivery system to improve the efficacy of nanomedicine is an urgent problem to be solved. (1) Delivery efficiency. Nanomedicines are still facing challenges to pass through multiple biological barriers, including blood circulation, uptake by mononuclear phagocyte systems, intratumoral pressure, extracellular matrix, and cell membrane internalization. An ideal nanomedicine should possess stability transition (from being stable in the circulation, accumulation, penetration, and internalization steps to being disassembling in the release step), surface transition (from being neutral/pegylated/shielding in the circulation, accumulation, and penetration steps to being cationic/depegylated/exposin in the internalization step) and size transition properties (from being large in the circulation and accumulation steps to being small in the penetration step) to efficiently deliver drugs into cancer cells and exert high therapeutic efficacy with few side effects. A promising strategy might be integrating all needed nanoproperties into a SHK nanomedicine with stability, surface and size nanoproperty transitions to maximize the delivery efficiency.146 Due to the heterogeneity of the EPR effect, SHK nanomedicines based on the EPR effect may fail to deliver drugs to tumor cells far from blood vessels, resulting in poor clinical efficacy and final chemotherapy failure. Comparatively, transcytosis, is a vesicle-mediated transport utilized by molecules to pass through biological barriers without depending on the EPR effect, providing a new probability in developing novel nanoparticles for in vivo drug delivery and tumor targeting.147,148 Consequently, more attention should be paid to exploring transcytosis-mediated active delivery mechanism-based SHK nanomedicines. In addition, decorating nanomedicines with tumor penetrating peptides, camouflaging with cell membrane and modulating tumor extracellular matrix can improve the tumor targeting and penetration ability.149–151 (2) Drug resistance. Drug resistance is one of the leading causes of cancer chemotherapy failure. As the drug resistance study is ignored in SHK nanomedicines, more effort should be devoted to providing sufficient anti-resistant activity studies. (3) Tumor metastasis. Tumor metastasis, a multi-stage malignant progression process, significantly affects the prognosis of patients.152 Compared with orthotopic tumors, metastatic tumors show completely different metabolic characteristics, providing an opportunity for the prevention and treatment of tumor metastasis. But the advantages of SHK in regulating tumor growth, development, and metastasis have not been fully exploited in SHK nanomedicine. Especially, little attention has been paid to the effect of SHK on tumor cell glycolysis. Through the rational design of SHK nanomedicine, the comprehensive regulation and control of tumor occurrence, development, and metastasis may be achieved. (4) Combination therapy. The integration of chemotherapy with other therapies, such as immunotherapy, radiotherapy, PTT, or photodynamic therapy, can play a significant antitumor role, which presents us with a vital developmental direction for SHK nanomedicines. (5) Administration sequence. Nano drug delivery system-mediated combination therapy of SHK and other agents exhibited excellent antitumor efficacy. However, combination therapy can also have a negative effect because of the interaction between drugs or cycle-specific differences. Thus, administration timing and sequence are required to be carefully investigated. (6) Interspecies discrepancy. SHK nanomedicines are proven to be effective in the preclinical animal model, but this fails to sufficiently predict the clinical outcome due to the interspecies discrepancy between patients and animals. The practical application of SHK nanomedicines requires a research partnership among clinicians, the pharmaceutical industry, and the academy. (7) Patient compliance. Intravenous injection is the general administration route with less patient compliance. Hence, the development of oral SHK nanomedicines with ideal therapeutic efficacy is ought to be focused on in further study.
Conclusion
As a potential anticancer drug, more and more rational, efficient and functional SHK nanomedicines have been designed. Compared with free SHK, SHK nanomedicines possess improved bioavailability, prolonged circulation time, active targeting ability, more tumor-site drug accumulation, controllable drug release, efficient cellular uptake, and enhanced cancer cell-killing efficiency. Most notably, SHK-involved combination therapy has exhibited outstanding therapeutic efficacy and reduced systematic side effects. It is our sincere hope that SHK-involved combination cancer therapy based on well-designed nanomedicines with easy quality control, good biosafety, and excellent delivery efficiency can contribute to cancer treatment in the near future.
Funding Statement
This work was supported by the National Natural Science Foundation of China (grant number 82204633); the China Postdoctoral Science Foundation (grant number 2021M700550); the National Natural Science Foundation of Sichuan Province (grant numbers 2022NSFSC0634, 2023NSFSC1782); Traditional Chinese Medicine Scientific Research Project of Sichuan Provincial Administration of traditional Chinese Medicine (grant number 2020JC0038); Talent training quality and Teaching Reform Project of higher Education in Sichuan Province (grant number JG2021-787); Sichuan traditional Chinese Medicine Health Industry Development and Rural Revitalization Research Center Project “Research on the practice path of Intelligent Management of traditional Chinese Medicine under the background of Rural Revitalization” (grant number DJKYB202215); and the talent research promotion plan of “Xinglin scholar” discipline in Chengdu University of Traditional Chinese Medicine (grant number BSH2021024).
Author Contributions
All authors have made major contributions to this work; contributed to drafting, revising, and critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Bahrami A, Fereidouni M, Pirro M, et al. Modulation of regulatory T cells by natural products in cancer. Cancer Lett. 2019;459:72–85. doi: 10.1016/j.canlet.2019.06.001 [DOI] [PubMed] [Google Scholar]
- 3.Mann J. Natural products in cancer chemotherapy: past, present and future. Nat Rev Cancer. 2002;2:143–148. doi: 10.1038/nrc723 [DOI] [PubMed] [Google Scholar]
- 4.Mishra AP, Sharifi-Rad M, Shariati MA, et al. Bioactive compounds and health benefits of edible Rumex species-a review. Cell Mol Biol. 2018;64:27–34. doi: 10.14715/cmb/2018.64.8.5 [DOI] [PubMed] [Google Scholar]
- 5.Mokbel K, Wazir U, Mokbel K. Chemoprevention of prostate cancer by natural agents: evidence from molecular and epidemiological studies. Anticancer Res. 2019;39:5231–5259. doi: 10.21873/anticanres.13720 [DOI] [PubMed] [Google Scholar]
- 6.Guo C, He J, Song X, et al. Pharmacological properties and derivatives of shikonin—a review in recent years. Pharmacol Res. 2019;149:104463. doi: 10.1016/j.phrs.2019.104463 [DOI] [PubMed] [Google Scholar]
- 7.Lu Q, Tang HL, Shao YQ, et al. A new facile synthesis of shikalkin. Chin Chem Lett. 2008;19:172–174. doi: 10.1016/j.cclet.2007.11.031 [DOI] [Google Scholar]
- 8.Wu F-Y, Tang C-Y, Guo Y-M, et al. Transcriptome analysis explores genes related to shikonin biosynthesis in Lithospermeae plants and provides insights into Boraginales’ evolutionary history. Sci Rep. 2017;7:1–11. doi: 10.1038/s41598-016-0028-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C-S, Chen H-W, Lin T-Y, et al. Shikonin upregulates the expression of drug-metabolizing enzymes and drug transporters in primary rat hepatocytes. J Ethnopharmacol. 2018;216:18–25. doi: 10.1016/j.jep.2018.01.026 [DOI] [PubMed] [Google Scholar]
- 10.Shao -Y-Y, Yin Y, Lian B-P, et al. Synthesis and biological evaluation of novel shikonin-benzo [b] furan derivatives as tubulin polymerization inhibitors targeting the colchicine binding site. Eur J Med Chem. 2020;190:112105. doi: 10.1016/j.ejmech.2020.112105 [DOI] [PubMed] [Google Scholar]
- 11.Li H, Tong Y, Bai L, et al. Lactoferrin functionalized PEG-PLGA nanoparticles of shikonin for brain targeting therapy of glioma. Int J Biol Macromol. 2018;107:204–211. doi: 10.1016/j.ijbiomac.2017.08.155 [DOI] [PubMed] [Google Scholar]
- 12.Sun Q, Gong T, Liu M, et al. Shikonin, a naphthalene ingredient: therapeutic actions, pharmacokinetics, toxicology, clinical trials and pharmaceutical researches. Phytomedicine. 2022;94:153805. doi: 10.1016/j.phymed.2021.153805 [DOI] [PubMed] [Google Scholar]
- 13.Wei Q, Su J, Dong G, et al. Glycolysis inhibitors suppress renal interstitial fibrosis via divergent effects on fibroblasts and tubular cells. Am J Physiol Renal. 2019;316:F1162–F1172. doi: 10.1152/ajprenal.00422.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamashita K, Miyazaki H, Shinoda S, et al. Assessment of the skin sensitizing potential of chemicals, contained in foods and/or cosmetic ingredients, using a modified local lymph node assay with an elicitation phase (LLNA: DAE) method. J Toxicol Sci. 2018;43:513–520. doi: 10.2131/jts.43.513 [DOI] [PubMed] [Google Scholar]
- 15.Lupescu A, Bissinger R, Jilani K, et al. In vitro induction of erythrocyte phosphatidylserine translocation by the natural naphthoquinone shikonin. Toxins. 2014;6:1559–1574. doi: 10.3390/toxins6051559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang S, Chen A, Zhou X, et al. Assessment of the inhibition risk of shikonin on cytochrome P450 via cocktail inhibition assay. Toxicol Lett. 2017;281:74–83. doi: 10.1016/j.toxlet.2017.09.014 [DOI] [PubMed] [Google Scholar]
- 17.Adiseshaiah PP, Crist RM, Hook SS, et al. Nanomedicine strategies to overcome the pathophysiological barriers of pancreatic cancer. Nat Rev Clin Oncol. 2016;13:750–765. doi: 10.1038/nrclinonc.2016.119 [DOI] [PubMed] [Google Scholar]
- 18.Zhang H. Onivyde for the therapy of multiple solid tumors. OncoTargets Ther. 2016;9:3001. doi: 10.2147/OTT.S105587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barani M, Bilal M, Sabir F, et al. Nanotechnology in ovarian cancer: diagnosis and treatment. Life Sci. 2021;266:118914. doi: 10.1016/j.lfs.2020.118914 [DOI] [PubMed] [Google Scholar]
- 20.Norouzi M, Amerian M, Amerian M, et al. Clinical applications of nanomedicine in cancer therapy. Drug Discov Today. 2020;25:107–125. doi: 10.1016/j.drudis.2019.09.017 [DOI] [PubMed] [Google Scholar]
- 21.Seca C, Ferraresi A, Phadngam S, et al. Autophagy-dependent toxicity of amino-functionalized nanoparticles in ovarian cancer cells. J Mater Chem B. 2019;7:5376–5391. doi: 10.1039/C9TB00935C [DOI] [PubMed] [Google Scholar]
- 22.Wang F, Mayca Pozo F, Tian D, et al. Shikonin inhibits cancer through P21 upregulation and apoptosis induction. Front Pharmacol. 2020;11:861. doi: 10.3389/fphar.2020.00861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Y, Gao W, Tao S, et al. ER-mediated anti-tumor effects of shikonin on breast cancer. Eur J Pharmacol. 2019;863:172667. doi: 10.1016/j.ejphar.2019.172667 [DOI] [PubMed] [Google Scholar]
- 24.Wang W, Wu Y, Chen S, et al. Shikonin is a novel and selective IMPDH2 inhibitor that target triple‐negative breast cancer. Phytother Res. 2021;35:463–476. doi: 10.1002/ptr.6825 [DOI] [PubMed] [Google Scholar]
- 25.Zhang S, Gao Q, Li W, et al. Shikonin inhibits cancer cell cycling by targeting Cdc25s. BMC Cancer. 2019;19:1–9. doi: 10.1186/s12885-018-5219-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang, F, Mayca Pozo, F, Tian, D et al Shikonin inhibits cancer through P21 upregulation and apoptosis induction Frontiers in pharmacology 2020. 11 861 doi: 10.3389/fphar.2020.00861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li MY, Mi C, Wang KS, et al. Shikonin suppresses proliferation and induces cell cycle arrest through the inhibition of hypoxia-inducible factor-1α signaling. Chem Biol Interact. 2017;274:58–67. doi: 10.1016/j.cbi.2017.06.029 [DOI] [PubMed] [Google Scholar]
- 28.Matsuura K, Canfield K, Feng W, et al. Metabolic regulation of apoptosis in cancer. Int Rev Cell Mol Biol. 2016;327:43–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2000;21:485–495. doi: 10.1093/carcin/21.3.485 [DOI] [PubMed] [Google Scholar]
- 30.Gara RK, Srivastava VK, Duggal S, et al. Shikonin selectively induces apoptosis in human prostate cancer cells through the endoplasmic reticulum stress and mitochondrial apoptotic pathway. J Biomed Sci. 2015;22:1–12. doi: 10.1186/s12929-015-0127-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Kang X, Niu G, et al. Shikonin induces apoptosis and prosurvival autophagy in human melanoma A375 cells via ROS-mediated ER stress and p38 pathways. Artif Cells Nanomed Biotechnol. 2019;47:626–635. doi: 10.1080/21691401.2019.1575229 [DOI] [PubMed] [Google Scholar]
- 32.Kimura T, Nakazato T, Shimizu T, et al. Shikonin induces apoptosis by inhibiting phosphorylation of IGF-1 receptor in myeloma cells. Blood. 2004;104:4858. doi: 10.1182/blood.V104.11.4858.4858 [DOI] [Google Scholar]
- 33.Fidler IJ. Tumor heterogeneity and the biology of cancer invasion and metastasis. Cancer Res. 1978;38:2651–2660. [PubMed] [Google Scholar]
- 34.Veiseh O, Kievit FM, Ellenbogen RG, et al. Cancer cell invasion: treatment and monitoring opportunities in nanomedicine. Adv Drug Delivery Rev. 2011;63:582–596. doi: 10.1016/j.addr.2011.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson EW, Price JT. Mechanisms of tumour invasion and metastasis: emerging targets for therapy. Expert Opin Ther Targets. 2002;6:217–233. doi: 10.1517/14728222.6.2.217 [DOI] [PubMed] [Google Scholar]
- 36.Wang H, Wu C, Wan S, et al. Shikonin attenuates lung cancer cell adhesion to extracellular matrix and metastasis by inhibiting integrin β1 expression and the ERK1/2 signaling pathway. Toxicology. 2013;308:104–112. doi: 10.1016/j.tox.2013.03.015 [DOI] [PubMed] [Google Scholar]
- 37.Zhang -L-L, Zhan L, Jin Y-D, et al. SIRT2 mediated antitumor effects of shikonin on metastatic colorectal cancer. Eur J Pharmacol. 2017;797:1–8. doi: 10.1016/j.ejphar.2017.01.008 [DOI] [PubMed] [Google Scholar]
- 38.Klein G, Vellenga E, Fraaije M, et al. The possible role of matrix metalloproteinase (MMP)-2 and MMP-9 in cancer, eg acute leukemia. Crit Rev Oncol Hemat. 2004;50:87–100. doi: 10.1016/j.critrevonc.2003.09.001 [DOI] [PubMed] [Google Scholar]
- 39.Simpson‐Haidaris P, Rybarczyk B. Tumors and fibrinogen: the role of fibrinogen as an extracellular matrix protein. Ann N Y Acad Sci. 2001;936:406–425. doi: 10.1111/j.1749-6632.2001.tb03525.x [DOI] [PubMed] [Google Scholar]
- 40.Deng B, Qiu B. Shikonin inhibits invasiveness of osteosarcoma through MMP13 suppression. Tumor Biol. 2015;36:9311–9317. doi: 10.1007/s13277-015-3662-1 [DOI] [PubMed] [Google Scholar]
- 41.Chen Y, Zheng L, Liu J, et al. Shikonin inhibits prostate cancer cells metastasis by reducing matrix metalloproteinase-2/-9 expression via AKT/mTOR and ROS/ERK1/2 pathways. Int Immunopharmacol. 2014;21:447–455. doi: 10.1016/j.intimp.2014.05.026 [DOI] [PubMed] [Google Scholar]
- 42.Cannito S, Novo E, Di Bonzo LV, et al. Epithelial–mesenchymal transition: from molecular mechanisms, redox regulation to implications in human health and disease. Antioxid Redox Signal. 2010;12:1383–1430. doi: 10.1089/ars.2009.2737 [DOI] [PubMed] [Google Scholar]
- 43.Jeong H, Ryu Y, An J, et al. Epithelial–mesenchymal transition in breast cancer correlates with high histological grade and triple‐negative phenotype. Histopathology. 2012;60:E87–E95. doi: 10.1111/j.1365-2559.2012.04195.x [DOI] [PubMed] [Google Scholar]
- 44.Chen Y, Chen Z-Y, Chen L, et al. Shikonin inhibits triple-negative breast cancer-cell metastasis by reversing the epithelial-to-mesenchymal transition via glycogen synthase kinase 3β-regulated suppression of β-catenin signaling. Biochem Pharmacol. 2019;166:33–45. doi: 10.1016/j.bcp.2019.05.001 [DOI] [PubMed] [Google Scholar]
- 45.Najafov A, Chen H, Yuan J. Necroptosis and cancer. Trends Cancer. 2017;3:294–301. doi: 10.1016/j.trecan.2017.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Markowitsch SD, Juetter KM, Schupp P, et al. Shikonin reduces growth of docetaxel-resistant prostate cancer cells mainly through necroptosis. Cancers. 2021;13:882. doi: 10.3390/cancers13040882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu B, Gong X, Wang Z-Q, et al. Shikonin induces glioma cell necroptosis in vitro by ROS overproduction and promoting RIP1/RIP3 necrosome formation. Acta Pharmacol Sin. 2017;38:1543–1553. doi: 10.1038/aps.2017.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang A, Liu J, Yang Y, et al. Shikonin promotes ubiquitination and degradation of cIAP1/2-mediated apoptosis and necrosis in triple negative breast cancer cells. Chin Med. 2021;16:1–15. doi: 10.1186/s13020-021-00426-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li X, Fan -X-X, Jiang Z-B, et al. Shikonin inhibits gefitinib-resistant non-small cell lung cancer by inhibiting TrxR and activating the EGFR proteasomal degradation pathway. Pharmacol Res. 2017;115:45–55. doi: 10.1016/j.phrs.2016.11.011 [DOI] [PubMed] [Google Scholar]
- 50.Han W, Li L, Qiu S, et al. Shikonin circumvents cancer drug resistance by induction of a necroptotic death. Mol Cancer Ther. 2007;6:1641–1649. doi: 10.1158/1535-7163.MCT-06-0511 [DOI] [PubMed] [Google Scholar]
- 51.Li X, He S, Ma B. Autophagy and autophagy-related proteins in cancer. Mol Cancer. 2020;19:1–16. doi: 10.1186/s12943-019-1085-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Y, Wang L-X, Yang G, et al. Efficient cross-presentation depends on autophagy in tumor cells. Cancer Res. 2008;68:6889–6895. doi: 10.1158/0008-5472.CAN-08-0161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thorburn J, Horita H, Redzic J, et al. Autophagy regulates selective HMGB1 release in tumor cells that are destined to die. Cell Death Differ. 2009;16:175–183. doi: 10.1038/cdd.2008.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Twitty CG, Jensen SM, Hu H-M, et al. Tumor-derived autophagosome vaccine: induction of cross-protective immune responses against short-lived proteins through a p62-dependent mechanism. Clin Cancer Res. 2011;17:6467–6481. doi: 10.1158/1078-0432.CCR-11-0812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen H-M, Wang P-H, Chen -S-S, et al. Shikonin induces immunogenic cell death in tumor cells and enhances dendritic cell-based cancer vaccine. Cancer Immunol Immun. 2012;61:1989–2002. doi: 10.1007/s00262-012-1258-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Y, Lu H, Gu Y, et al. Enhancement of NK cells proliferation and function by Shikonin. Immunopharmacol Immunotoxicol. 2017;39:124–130. doi: 10.1080/08923973.2017.1299174 [DOI] [PubMed] [Google Scholar]
- 57.Baumeister SH, Freeman GJ, Dranoff G, et al. Coinhibitory pathways in immunotherapy for cancer. Annu Rev Immunol. 2016;34:539–573. doi: 10.1146/annurev-immunol-032414-112049 [DOI] [PubMed] [Google Scholar]
- 58.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730 [DOI] [PubMed] [Google Scholar]
- 59.Feng M, Xiong G, Cao Z, et al. PD-1/PD-L1 and immunotherapy for pancreatic cancer. Cancer Lett. 2017;407:57–65. doi: 10.1016/j.canlet.2017.08.006 [DOI] [PubMed] [Google Scholar]
- 60.Ruan Z, Liang M, Shang L, et al. Shikonin-mediated PD-L1 degradation suppresses immune evasion in pancreatic cancer by inhibiting NF-κB/STAT3 and NF-κB/CSN5 signaling pathways. Pancreatology. 2021;21:630–641. doi: 10.1016/j.pan.2021.01.023 [DOI] [PubMed] [Google Scholar]
- 61.Yang Q, Guo N, Zhou Y, et al. The role of tumor-associated macrophages (TAMs) in tumor progression and relevant advance in targeted therapy. Acta Pharm Sin B. 2020;10:2156–2170. doi: 10.1016/j.apsb.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang H, Tang Y, Fang Y, et al. Reprogramming tumor immune microenvironment (TIME) and metabolism via biomimetic targeting codelivery of shikonin/JQ1. Nano Lett. 2019;19:2935–2944. doi: 10.1021/acs.nanolett.9b00021 [DOI] [PubMed] [Google Scholar]
- 63.Chen J, Xie J, Jiang Z, et al. Shikonin and its analogs inhibit cancer cell glycolysis by targeting tumor pyruvate kinase-M2. Oncogene. 2011;30:4297–4306. doi: 10.1038/onc.2011.137 [DOI] [PubMed] [Google Scholar]
- 64.Zhao X, Zhu Y, Hu J, et al. Shikonin inhibits tumor growth in mice by suppressing pyruvate kinase M2-mediated aerobic glycolysis. Sci Rep. 2018;8:1–8. doi: 10.1038/s41598-017-17765-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu C, Li CY-T, Kong NT. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch Pharmacal Res. 2005;28:249–268. doi: 10.1007/BF02977789 [DOI] [PubMed] [Google Scholar]
- 66.Li H, Luo S, Zhou T. Studies on in vitro metabolism of shikonin. Phytother Res. 1999;13:236–238. doi: [DOI] [PubMed] [Google Scholar]
- 67.Meselhy MR, Kadota S, Tsubono K, et al. Biotransformation of shikonin by human intestinal bacteria. Tetrahedron. 1994;50:3081–3098. doi: 10.1016/S0040-4020(01)81108-X [DOI] [Google Scholar]
- 68.Xing H, Hwang K, Lu Y. Recent developments of liposomes as nanocarriers for theranostic applications. Theranostics. 2016;6:1336. doi: 10.7150/thno.15464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shi Y, Van der Meel R, Chen X, et al. The EPR effect and beyond: strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics. 2020;10:7921. doi: 10.7150/thno.49577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xia H, Tang C, Gui H, et al. Preparation, cellular uptake and angiogenic suppression of shikonin-containing liposomes in vitro and in vivo. Biosci Rep. 2013;33. doi: 10.1042/BSR20120065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pinto MP, Sotomayor P, Carrasco-Avino G, et al. Escaping antiangiogenic therapy: strategies employed by cancer cells. Int J Mol Sci. 2016;17:1489. doi: 10.3390/ijms17091489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ishihara A, Yamauchi M, Tsuchiya T, et al. A novel liposome surface modification agent that prolongs blood circulation and retains surface ligand reactivity. J Biomat Sci Polym E. 2012;23:2055–2068. doi: 10.1163/092050611X605933 [DOI] [PubMed] [Google Scholar]
- 73.Rahim S, Ghamsari MS, Radiman S. Surface modification of titanium oxide nanocrystals with PEG. Sci Iran. 2012;19:948–953. doi: 10.1016/j.scient.2012.03.009 [DOI] [Google Scholar]
- 74.Tsermentseli SK, Kontogiannopoulos KN, Papageorgiou VP, et al. Comparative study of PEGylated and conventional liposomes as carriers for shikonin. Fluids. 2018;3:36. doi: 10.3390/fluids3020036 [DOI] [Google Scholar]
- 75.Yan W, Leung SS, To KK. Updates on the use of liposomes for active tumor targeting in cancer therapy. Nanomedicine. 2020;15:303–318. doi: 10.2217/nnm-2019-0308 [DOI] [PubMed] [Google Scholar]
- 76.Cheng Y, Ji Y. RGD-modified polymer and liposome nanovehicles: recent research progress for drug delivery in cancer therapeutics. Eur J Pharm Sci. 2019;128:8–17. doi: 10.1016/j.ejps.2018.11.023 [DOI] [PubMed] [Google Scholar]
- 77.Mei L, Fu L, Shi K, et al. Increased tumor targeted delivery using a multistage liposome system functionalized with RGD, TAT and cleavable PEG. Int J Pharm. 2014;468:26–38. doi: 10.1016/j.ijpharm.2014.04.008 [DOI] [PubMed] [Google Scholar]
- 78.Wen X, Li J, Cai D, et al. Anticancer efficacy of targeted shikonin liposomes modified with RGD in breast cancer cells. Molecules. 2018;23:268. doi: 10.3390/molecules23020268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mattheolabakis G, Milane L, Singh A, et al. Hyaluronic acid targeting of CD44 for cancer therapy: from receptor biology to nanomedicine. J Drug Target. 2015;23:605–618. doi: 10.3109/1061186X.2015.1052072 [DOI] [PubMed] [Google Scholar]
- 80.Meng L, Ren J, Liu Z, et al. Hyaluronic acid-coated shikonin liposomes for the treatment of triple-negative breast cancer via targeting tumor cells and amplification of oxidative stress. J Drug Delivery Sci Technol. 2022;70:103193. doi: 10.1016/j.jddst.2022.103193 [DOI] [Google Scholar]
- 81.Xiao Y, Yu D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther. 2021;221:107753. doi: 10.1016/j.pharmthera.2020.107753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kiran AVR, Kumari GK, Krishnamurthy PT, et al. Tumor microenvironment and nanotherapeutics: intruding the tumor fort. Biomater Sci. 2021;9:7667–7704. doi: 10.1039/D1BM01127H [DOI] [PubMed] [Google Scholar]
- 83.Lei Y, Hamada Y, Li J, et al. Targeted tumor delivery and controlled release of neuronal drugs with ferritin nanoparticles to regulate pancreatic cancer progression. J Control Release. 2016;232:131–142. doi: 10.1016/j.jconrel.2016.03.023 [DOI] [PubMed] [Google Scholar]
- 84.Li Y, Chang Y, Lian X, et al. Silver nanoparticles for enhanced cancer theranostics: in vitro and in vivo perspectives. J Biomed Nanotechnol. 2018;14:1515–1542. doi: 10.1166/jbn.2018.2614 [DOI] [PubMed] [Google Scholar]
- 85.Larsson M, Huang W-T, Liu D-M, et al. Local co-administration of gene-silencing RNA and drugs in cancer therapy: state-of-the art and therapeutic potential. Cancer Treat Rev. 2017;55:128–135. doi: 10.1016/j.ctrv.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 86.Perciani CT, Liu LY, Wood L, et al. Enhancing immunity with nanomedicine: employing nanoparticles to harness the immune system. ACS nano. 2020;15:7–20. doi: 10.1021/acsnano.0c08913 [DOI] [PubMed] [Google Scholar]
- 87.Agarwalla P, Banerjee R. N-end rule pathway inhibition assists colon tumor regression via necroptosis. Mol Ther Oncolytics. 2016;3:16020. doi: 10.1038/mto.2016.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu T, Sun X, Cao Z. Shikonin-induced necroptosis in nasopharyngeal carcinoma cells via ROS overproduction and upregulation of RIPK1/RIPK3/MLKL expression. OncoTargets Ther. 2019;12:2605. doi: 10.2147/OTT.S200740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shahsavari Z, Karami-Tehrani F, Salami S. Targeting cell necroptosis and apoptosis induced by shikonin via receptor interacting protein kinases in estrogen receptor positive breast cancer cell line, MCF-7. Anti Cancer Agents Med Chem. 2018;18:245–254. doi: 10.2174/1871520617666170919164055 [DOI] [PubMed] [Google Scholar]
- 90.Bachmair A, Finley D, Varshavsky A. In vivo half-life of a protein is a function of its amino-terminal residue. Science. 1986;234:179–186. doi: 10.1126/science.3018930 [DOI] [PubMed] [Google Scholar]
- 91.Piatkov KI, Brower CS, Varshavsky A. The N-end rule pathway counteracts cell death by destroying proapoptotic protein fragments. Proc Natl Acad Sci USA Early. 2012;109:E1839–E1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tasaki T, Sriram SM, Park KS, et al. The N-end rule pathway. Annu Rev Biochem. 2012;81:261. doi: 10.1146/annurev-biochem-051710-093308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ahmed A, Tait SW. Targeting immunogenic cell death in cancer. Mol Oncol. 2020;14:2994–3006. doi: 10.1002/1878-0261.12851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gupta G, Borglum K, Chen H. Immunogenic cell death: a step ahead of autophagy in cancer therapy. J Cancer Immunol. 2021;3:47. doi: 10.33696/cancerimmunol.3.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.White E, Lattime EC, Guo JY. Autophagy regulates stress responses, metabolism, and anticancer immunity. Trends Cancer. 2021;7:778–789. doi: 10.1016/j.trecan.2021.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li J, Cai W, Yu J, et al. Autophagy inhibition recovers deficient ICD-based cancer immunotherapy. Biomaterials. 2022;287:121651. doi: 10.1016/j.biomaterials.2022.121651 [DOI] [PubMed] [Google Scholar]
- 97.Cagel M, Tesan FC, Bernabeu E, et al. Polymeric mixed micelles as nanomedicines: achievements and perspectives. Eur J Pharm Biopharm. 2017;113:211–228. doi: 10.1016/j.ejpb.2016.12.019 [DOI] [PubMed] [Google Scholar]
- 98.Hwang D, Ramsey JD, Kabanov AV. Polymeric micelles for the delivery of poorly soluble drugs: from nanoformulation to clinical approval. Adv Drug Delivery Rev. 2020;156:80–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Su Y, Huang N, Di Chen LZ, et al. Successful in vivo hyperthermal therapy toward breast cancer by Chinese medicine shikonin-loaded thermosensitive micelle. Int J Nanomed. 2017;12:4019. doi: 10.2147/IJN.S132639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Adams JL, Smothers J, Srinivasan R, et al. Big opportunities for small molecules in immuno-oncology. Nat Rev Drug Discov. 2015;14:603–622. doi: 10.1038/nrd4596 [DOI] [PubMed] [Google Scholar]
- 101.Mellor AL, Lemos H, Huang L. Indoleamine 2, 3-dioxygenase and tolerance: where are we now? Front Immunol. 2017;8:1360. doi: 10.3389/fimmu.2017.01360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mellor AL, Munn DH. Creating immune privilege: active local suppression that benefits friends, but protects foes. Nat Rev Immunol. 2008;8:74–80. doi: 10.1038/nri2233 [DOI] [PubMed] [Google Scholar]
- 103.Crosignani S, Bingham P, Bottemanne P, et al. Discovery of a novel and selective indoleamine 2, 3-dioxygenase (IDO-1) inhibitor 3-(5-fluoro-1 H-indol-3-yl) pyrrolidine-2, 5-dione (EOS200271/PF-06840003) and its characterization as a potential clinical candidate. J Med Chem. 2017;60:9617–9629. doi: 10.1021/acs.jmedchem.7b00974 [DOI] [PubMed] [Google Scholar]
- 104.Zhao H, Sun P, Guo W, et al. Discovery of indoleamine 2, 3-dioxygenase 1 (IDO-1) inhibitors based on ortho-naphthaquinone-containing natural product. Molecules. 2019;24:1059. doi: 10.3390/molecules24061059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Peng J, Hu X, Fan S, et al. Inhibition of mitochondrial biosynthesis using a “right‐side‐out” membrane‐camouflaged micelle to facilitate the therapeutic effects of shikonin on triple‐negative breast cancer. Adv Healthcare Mate. 2022;11:2200742. doi: 10.1002/adhm.202200742 [DOI] [PubMed] [Google Scholar]
- 106.Wiench B, Eichhorn T, Paulsen M, et al. Shikonin directly targets mitochondria and causes mitochondrial dysfunction in cancer cells. J Evid Based Integr Med. 2013;2012:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Qiu H-Y, Fu J-Y, Yang M-K, et al. Identification of new shikonin derivatives as STAT3 inhibitors. Biochem Pharmacol. 2017;146:74–86. doi: 10.1016/j.bcp.2017.10.009 [DOI] [PubMed] [Google Scholar]
- 108.Aghebati‐Maleki A, Dolati S, Ahmadi M, et al. Nanoparticles and cancer therapy: perspectives for application of nanoparticles in the treatment of cancers. J Cell Physiol. 2020;235:1962–1972. doi: 10.1002/jcp.29126 [DOI] [PubMed] [Google Scholar]
- 109.Desai N, Momin M, Khan T, et al. Metallic nanoparticles as drug delivery system for the treatment of cancer. Expert Opin Drug Deliv. 2021;18:1261–1290. doi: 10.1080/17425247.2021.1912008 [DOI] [PubMed] [Google Scholar]
- 110.AshaRani P, Low Kah Mun G, Hande MP, et al. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS nano. 2009;3:279–290. doi: 10.1021/nn800596w [DOI] [PubMed] [Google Scholar]
- 111.Jeong J-K, Gurunathan S, Kang M-H, et al. Hypoxia-mediated autophagic flux inhibits silver nanoparticle-triggered apoptosis in human lung cancer cells. Sci Rep. 2016;6:1–13. doi: 10.1038/srep21688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mukherjee S, Chowdhury D, Kotcherlakota R, et al. Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system). Theranostics. 2014;4:316. doi: 10.7150/thno.7819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sriram MI, Kanth SBM, Kalishwaralal K, et al. Antitumor activity of silver nanoparticles in Dalton’s lymphoma ascites tumor model. Int J Nanomed. 2010;5:753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fayez H, El‐Motaleb MA, Selim AA. Synergistic cytotoxicity of shikonin‐silver nanoparticles as an opportunity for lung cancer. J Labelled Compd Radiopharm. 2020;63:25–32. doi: 10.1002/jlcr.3818 [DOI] [PubMed] [Google Scholar]
- 115.Crucho CI, Barros MT. Polymeric nanoparticles: a study on the preparation variables and characterization methods. Mater Sci Eng C. 2017;80:771–784. doi: 10.1016/j.msec.2017.06.004 [DOI] [PubMed] [Google Scholar]
- 116.Anzar N, Mirza MA, Anwer K, et al. Preparation, evaluation and pharmacokinetic studies of spray dried PLGA polymeric submicron particles of simvastatin for the effective treatment of breast cancer. J Mol Liq. 2018;249:609–616. doi: 10.1016/j.molliq.2017.11.081 [DOI] [Google Scholar]
- 117.Matthaiou E-I, Barar J, Sandaltzopoulos R, et al. Shikonin-loaded antibody-armed nanoparticles for targeted therapy of ovarian cancer. Int J Nanomed. 2014;9:1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Marty C, Langer-Machova Z, Sigrist S, et al. Isolation and characterization of a scFv antibody specific for tumor endothelial marker 1 (TEM1), a new reagent for targeted tumor therapy. Cancer Lett. 2006;235:298–308. doi: 10.1016/j.canlet.2005.04.029 [DOI] [PubMed] [Google Scholar]
- 119.Elfinger M, Maucksch C, Rudolph C. Characterization of lactoferrin as a targeting ligand for nonviral gene delivery to airway epithelial cells. Biomaterials. 2007;28:3448–3455. doi: 10.1016/j.biomaterials.2007.04.011 [DOI] [PubMed] [Google Scholar]
- 120.Suzuki YA, Lönnerdal B. Baculovirus expression of mouse lactoferrin receptor and tissue distribution in the mouse. Biometals. 2004;17:301–309. doi: 10.1023/B:BIOM.0000027709.42733.e4 [DOI] [PubMed] [Google Scholar]
- 121.Demeule M, Currie JC, Bertrand Y, et al. Involvement of the low‐density lipoprotein receptor‐related protein in the transcytosis of the brain delivery vector Angiopep‐2. J Neurochem. 2008;106:1534–1544. doi: 10.1111/j.1471-4159.2008.05492.x [DOI] [PubMed] [Google Scholar]
- 122.Singh I, Swami R, Pooja D, et al. Lactoferrin bioconjugated solid lipid nanoparticles: a new drug delivery system for potential brain targeting. J Drug Target. 2016;24:212–223. doi: 10.3109/1061186X.2015.1068320 [DOI] [PubMed] [Google Scholar]
- 123.Li J, Zhao M, Liang W, et al. Codelivery of Shikonin and siTGF-β for enhanced triple negative breast cancer chemo-immunotherapy. J Control Release. 2022;342:308–320. doi: 10.1016/j.jconrel.2022.01.015 [DOI] [PubMed] [Google Scholar]
- 124.Ye M, Han Y, Tang J, et al. A tumor‐specific cascade amplification drug release nanoparticle for overcoming multidrug resistance in cancers. Adv Mater. 2017;29:1702342. doi: 10.1002/adma.201702342 [DOI] [PubMed] [Google Scholar]
- 125.Jiang Y, Chen M, Nie H, et al. PD-1 and PD-L1 in cancer immunotherapy: clinical implications and future considerations. Hum Vaccines Immunother. 2019;15:1111–1122. doi: 10.1080/21645515.2019.1571892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Huang Z, Ding Y, Luo Y, et al. ROS-triggered cycle amplification effect: a prodrug activation nanoamplifier for tumor-specific therapy. Acta Biomater. 2022;152:367–379. doi: 10.1016/j.actbio.2022.08.072 [DOI] [PubMed] [Google Scholar]
- 127.Asrorov AM, Gu Z, Li F, et al. Biomimetic camouflage delivery strategies for cancer therapy. Nanoscale. 2021;13:8693–8706. doi: 10.1039/D1NR01127H [DOI] [PubMed] [Google Scholar]
- 128.Zhu H, Bengsch F, Svoronos N, et al. BET bromodomain inhibition promotes anti-tumor immunity by suppressing PD-L1 expression. Cell Rep. 2016;16:2829–2837. doi: 10.1016/j.celrep.2016.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhao P, Qu J, Wu A, et al. Anti-alcoholism drug disulfiram for targeting glioma energy metabolism using BBB-penetrating delivery of fixed-dose combination. Nano Today. 2022;44:101448. doi: 10.1016/j.nantod.2022.101448 [DOI] [Google Scholar]
- 130.Liang J, Wang C, Fan J, et al. Hybrid membrane-camouflaged hollow Prussian blue nanoparticles for shikonin loading and combined chemo/photothermal therapy of metastatic TNBC. Mater Today Adv. 2022;14:100245. doi: 10.1016/j.mtadv.2022.100245 [DOI] [Google Scholar]
- 131.Feng W, Shi W, Liu S, et al. Fe (III)‐shikonin supramolecular nanomedicine for combined therapy of tumor via ferroptosis and necroptosis. Adv Healthcare Mate. 2022;11:2101926. doi: 10.1002/adhm.202101926 [DOI] [PubMed] [Google Scholar]
- 132.Gimenez-Bonafe P, Tortosa A, Perez-Tomas R. Overcoming drug resistance by enhancing apoptosis of tumor cells. Curr Cancer Drug Targets. 2009;9:320–340. doi: 10.2174/156800909788166600 [DOI] [PubMed] [Google Scholar]
- 133.Gorain B, Choudhury H, Nair AB, et al. Theranostic application of nanoemulsions in chemotherapy. Drug Discov Today. 2020;25:1174–1188. doi: 10.1016/j.drudis.2020.04.013 [DOI] [PubMed] [Google Scholar]
- 134.Lovelyn C, Attama AA. Current state of nanoemulsions in drug delivery. J Biomater Nanobiotechnol. 2011;2:626. doi: 10.4236/jbnb.2011.225075 [DOI] [Google Scholar]
- 135.Sánchez-López E, Guerra M, Dias-Ferreira J, et al. Current applications of nanoemulsions in cancer therapeutics. Nanomaterials. 2019;9:821. doi: 10.3390/nano9060821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen C-H, Lin M-L, Ong P-L, et al. Novel multiple apoptotic mechanism of shikonin in human glioma cells. Ann Surg Oncol. 2012;19:3097–3106. doi: 10.1245/s10434-012-2324-4 [DOI] [PubMed] [Google Scholar]
- 137.Wang H, Zhu Z, Zhang G, et al. AS1411 aptamer/hyaluronic acid-bifunctionalized microemulsion co-loading shikonin and docetaxel for enhanced antiglioma therapy. J Pharm Sci. 2019;108:3684–3694. doi: 10.1016/j.xphs.2019.08.017 [DOI] [PubMed] [Google Scholar]
- 138.Wang H, Chen W, Wu G, et al. A magnetic T7 peptide&AS1411 aptamer-modified microemulsion for triple glioma-targeted delivery of shikonin and docetaxel. J Pharm Sci. 2021;110:2946–2954. doi: 10.1016/j.xphs.2021.03.018 [DOI] [PubMed] [Google Scholar]
- 139.Ferreira SA, Pereira P, Sampaio P, et al. Supramolecular assembled nanogel made of mannan. J Colloid Interface Sci. 2011;361:97–108. doi: 10.1016/j.jcis.2011.05.020 [DOI] [PubMed] [Google Scholar]
- 140.Hashimoto Y, Mukai S, Sasaki Y, et al. Nanogel tectonics for tissue engineering: protein delivery systems with nanogel chaperones. Adv Healthcare Mate. 2018;7:1800729. doi: 10.1002/adhm.201800729 [DOI] [PubMed] [Google Scholar]
- 141.Li S, Zhang T, Xu W, et al. Sarcoma-targeting peptide-decorated polypeptide nanogel intracellularly delivers shikonin for upregulated osteosarcoma necroptosis and diminished pulmonary metastasis. Theranostics. 2018;8:1361. doi: 10.7150/thno.18299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Purohit G, Sakthivel T, Florence AT. Interaction of cationic partial dendrimers with charged and neutral liposomes. Int J Pharm. 2001;214:71–76. doi: 10.1016/S0378-5173(00)00635-9 [DOI] [PubMed] [Google Scholar]
- 143.Kontogiannopoulos KN, Assimopoulou AN, Hatziantoniou S, et al. Chimeric advanced drug delivery nano systems (chi-aDDnSs) for shikonin combining dendritic and liposomal technology. Int J Pharm. 2012;422:381–389. doi: 10.1016/j.ijpharm.2011.09.031 [DOI] [PubMed] [Google Scholar]
- 144.Kontogiannopoulos KN, Dasargyri A, Ottaviani MF, et al. Advanced drug delivery nanosystems for Shikonin: a calorimetric and electron paramagnetic resonance study. Langmuir. 2018;34:9424–9434. doi: 10.1021/acs.langmuir.8b00751 [DOI] [PubMed] [Google Scholar]
- 145.Cai R, Chen C. Protein Corona in vivo: dynamic complement proteins-mediated opsonization and immune modulation. Sci Bull. 2017;62:976–977. doi: 10.1016/j.scib.2017.07.002 [DOI] [PubMed] [Google Scholar]
- 146.Sun Q, Zhou Z, Qiu N, et al. Rational design of cancer nanomedicine: nanoproperty integration and synchronization. Adv Mater. 2017;29:1606628. doi: 10.1002/adma.201606628 [DOI] [PubMed] [Google Scholar]
- 147.de Lázaro I, Mooney DJ. A nanoparticle’s pathway into tumours. Nat Mater. 2020;19:486–487. doi: 10.1038/s41563-020-0669-9 [DOI] [PubMed] [Google Scholar]
- 148.Pandit S, Dutta D, Nie S. Active transcytosis and new opportunities for cancer nanomedicine. Nat Mater. 2020;19:478–480. doi: 10.1038/s41563-020-0672-1 [DOI] [PubMed] [Google Scholar]
- 149.Zhang Q, Liu N, Wang J, et al. The recent advance of cell-penetrating and tumor-targeting peptides as drug delivery systems based on tumor microenvironment. Mol Biopharm. 2023;20:789–809. doi: 10.1021/acs.molpharmaceut.2c00629 [DOI] [PubMed] [Google Scholar]
- 150.He X, Yang Y, Han Y, et al. Extracellular matrix physical properties govern the diffusion of nanoparticles in tumor microenvironment. Proc Natl Acad Sci. 2023;120(1):e2209260120. doi: 10.1073/pnas.2209260120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Luk BT, Zhang L. Cell membrane-camouflaged nanoparticles for drug delivery. J Control Release. 2015;220:600–607. doi: 10.1016/j.jconrel.2015.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li HX, Wang SQ, Lian ZX, et al. Relationship between tumor infiltrating immune cells and tumor metastasis and its prognostic value in cancer. Cells. 2023;12(1):64. doi: 10.3390/cells12010064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shilnikova K, Piao MJ, Kang KA, et al. Shikonin induces mitochondria-mediated apoptosis and attenuates epithelial-mesenchymal transition in cisplatin-resistant human ovarian cancer cells. Oncol Lett. 2018;15:5417–5424. doi: 10.3892/ol.2018.8065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wei P-L, Tu -C-C, Chen C-H, et al. Shikonin suppresses the migratory ability of hepatocellular carcinoma cells. J Agric Food Chem. 2013;61:8191–8197. doi: 10.1021/jf4009586 [DOI] [PubMed] [Google Scholar]
- 155.Wang Y, Hao F, Nan Y, et al. PKM2 inhibitor shikonin overcomes the cisplatin resistance in bladder cancer by inducing necroptosis. Int J Biol Sci. 2018;14:1883. doi: 10.7150/ijbs.27854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Huang S, Yu L and Shen P. (2010). Simultaneous Quantitative Analysis of Shikonin and Deoxyshikonin in Rat Plasma by Rapid LC–ESI–MS–MS. Chroma, 72(1–2), 63–69. 10.1365/s10337-010-1599-5 [DOI] [Google Scholar]
- 157.Li M, Chen X, Hu S, Wang R, Peng X and Bai X. (2018). Determination of blood concentrations of main active compounds in Zi-Cao-Cheng-Qi decoction and their total plasma protein binding rates based on hollow fiber liquid phase microextraction coupled with high performance liquid chromatography. Journal of Chromatography B, 1072 355–361. 10.1016/j.jchromb.2017.11.046 [DOI] [PubMed] [Google Scholar]
- 158.Li J, Zhou S, Yu J, et al. Low dose shikonin and anthracyclines coloaded liposomes induce robust immunogenetic cell death for synergistic chemo-immunotherapy. J Control Release. 2021;335:306–319. doi: 10.1016/j.jconrel.2021.05.040 [DOI] [PubMed] [Google Scholar]
- 159.Li J, Zhao M, Sun M, et al. Multifunctional nanoparticles boost cancer immunotherapy based on modulating the immunosuppressive tumor microenvironment. ACS Appl Mater Interfaces. 2020;12:50734–50747. doi: 10.1021/acsami.0c14909 [DOI] [PubMed] [Google Scholar]
- 160.Li J, Zhao M, Xu Y, Hu X, Dai Y and Wang D. (2021). Hybrid micelles codelivering shikonin and IDO-1 siRNA enhance immunotherapy by remodeling immunosuppressive tumor microenvironment. International Journal of Pharmaceutics, 597 120310 10.1016/j.ijpharm.2021.120310 [DOI] [PubMed] [Google Scholar]
- 161.Fu S, Li G, Zang W, Zhou X, Shi K and Zhai Y. (2022). Pure drug nano-assemblies: A facile carrier-free nanoplatform for efficient cancer therapy. Acta Pharmaceutica Sinica B, 12(1), 92–106. 10.1016/j.apsb.2021.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]