Abstract
Background
This systematic review provides updated insights, from the published literature in the past 5 years, based on the 2017 European Association of Neuro-Oncology (EANO) guidelines for palliative care in adults with malignant brain tumors. It provides an overview of palliative care options, including during the end-of-life phase for patients with malignant brain tumors.
Methods
A systematic literature search was conducted from 2016 to 2021 focusing on four main topics: (1) symptom management, (2) caregiver needs, (3) early palliative care, and (4) care in the end-of-life phase. An international panel of palliative care experts in neuro-oncology synthesized the literature and reported the most relevant updates. A total of 140 articles were included.
Results
New insights include that: Hippocampal avoidance and stereotactic radiosurgery results in a lower risk of neurocognitive decline in patients with brain metastases; levetiracetam is more efficacious in reducing seizures than valproic acid as first-line monotherapy antiseizure drug (ASD) in glioma patients; lacosamide and perampanel seem well-tolerated and efficacious add-on ASDs; and a comprehensive framework of palliative and supportive care for high-grade glioma patients and their caregivers was proposed. No pharmacological agents have been shown in randomized controlled trials to significantly improve fatigue or neurocognition.
Conclusions
Since the 2017 EANO palliative care guidelines, new insights have been reported regarding symptom management and end-of-life care, however, most recommendations remain unchanged. Early palliative care interventions are essential to define goals of care and minimize symptom burden in a timely fashion. Interventional studies that address pain, fatigue, and psychiatric symptoms as well as (the timing of) early palliative care are urgently needed.
Keywords: brain metastases, brain tumor, end of life, glioma, palliative care
Patients with a primary or metastatic brain tumor present with high morbidity and symptom burden throughout their disease trajectory. The life-limiting nature of brain tumors and the presence of specific symptoms caused by the tumor itself or by treatment require continuous assessment and adjustment of symptom management. Treatment is therefore twofold: To improve survival as well as maintain health-related quality of life (HRQoL) throughout the disease trajectory and during the end-of-life phase. Palliative care, sometimes also called supportive care, focuses on pro-active symptom assessment and relief as well as improving function and other aspects of HRQoL. Palliative care encompasses attention to physical, mental, social, and spiritual needs of both patients and their families. Unlike hospice care, it is not limited to end-of-life care, but open to any patient with a life-threatening disease and may be offered concurrently with any life-prolonging treatment at any point during the disease course. Palliative care encompasses a multidisciplinary team approach and is therefore uniquely positioned to address the many different and evolving symptoms of patients and their caregivers encountered along the disease trajectory.
Studies in oncology showed that patients derived the most benefit when being introduced early to palliative care while still undergoing life-prolonging treatment.1 Specifically, lung cancer patients had less depression and anxiety, better HRQoL, and improved survival and coping when seeing specialized palliative care teams compared to usual oncology care alone. However, it is not fully established how the early introduction of palliative care might affect patients with brain tumors.2 The European Association for Neuro-Oncology (EANO) guidelines for palliative care in adults with glioma provided a first overview on how to approach patients with malignant brain tumors. This systematic review provides updated insights based on these guidelines and aims to effectuate an overview of palliative care and end-of-life care interventions for adult patients with malignant brain tumors.
Methods
Topics of Interest
A panel composed of international experts in neuro-oncology and palliative care/end-of-life care defined four main topics, comprising (1) symptom management, (2) caregiver needs, (3) early palliative care, and (4) care in the end-of-life phase. For symptom management, we specifically focused on interventions addressing cognition, seizures, fatigue, pain, headaches, edema, and psychiatric symptoms. For the other three topics, all relevant articles were considered, with the focus on needs of caregivers and advance care planning (ACP). Each subsection was written by the most suitable expert(s) (eg, patient representatives/former caregivers took the lead in writing the section on caregivers), and critically reviewed by the other authors.
Search Strategy and Selection Procedures
We performed a systematic literature search using the same search strategy as used for the EANO guidelines. We searched the electronic databases PubMed, Embase, Emcare, PsycINFO, Cochrane Library, Web of Science, and Academic Search Premier from January 25th, 2016 (final date of previous search) up to July 12th, 2021. The search strategy consisted of two search strings, one related to malignant brain tumor and the other to the topics of interest (see Supplemental File 1 for the full search strategy).
Based on predefined criteria, four independent reviewers screened all titles and abstracts for eligibility, and subsequently full texts. Disagreements in both phases were resolved by consensus. In addition, important articles that were not identified by the search (eg, evidence from other disease groups) but known by the authors, were added. Predefined inclusion criteria were original studies in English involving patients with glioma or brain metastasis from systemic cancer (n ≥ 10 malignant brain tumors or in mixed population of n ≥ 20 at least 50% malignant brain tumors), and information on the management of symptoms, caregiver needs, early palliative care, or care in the end-of-life phase.
Results
Search Results
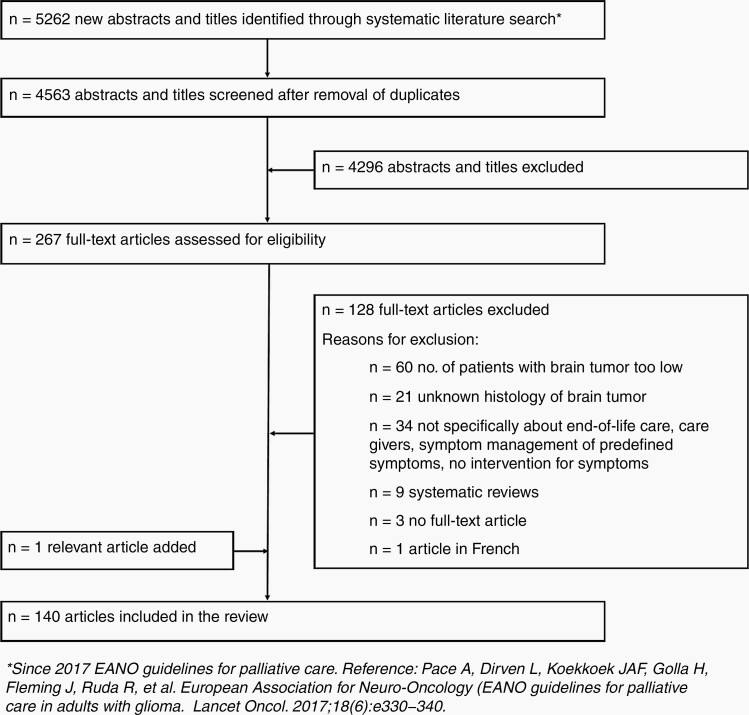
The literature search yielded 5262 unique articles published since 2017, of which 140 were considered eligible according to the predefined inclusion and exclusion criteria (see Figure 1 for the results of the selection procedure). Four main topics will be discussed in the subsequent sections: (1) symptom management, (2) caregiver needs, (3) early palliative care, and (4) care in the end-of-life phase (See Table 1 for summary of previous recommendations and new insights).3-30
Figure 1.
Flow chart demonstrating results of the selection procedure.
Table 1.
Summary of Previous Recommendations and New Insights Regarding Symptom Management, Caregiver Needs, Early Palliative Care, and Care in the End-Of-Life Phase
| Symptom Management | Recommendation in the 2017 EANO Guidelinea | New Insights | Additional Practical Recommendations |
|---|---|---|---|
| Cognition | Pharmacological treatment to treat or prevent neurocognitive decline is not routinely recommended, while neurocognitive rehabilitation seems to have modest positive effects (in young patients with a relatively favorable prognosis). | (Awake) Surgical resection of the tumor seems to have a beneficial effect on neurocognitive functioning.3 Stereotactic radiosurgery or hippocampal avoidance during whole-brain radiotherapy results in a lower risk of neurocognitive decline in patients with brain metastases.4–7 There are no new insights regarding (non-)pharmacologic interventions.8–10 |
No pharmacological agents have been shown to prevent or treat neurocognitive decline. Hippocampal avoidance in patients with brain metastases who plan to receive whole brain radiotherapy with no metastases in the hippocampal region is considered the new standard of care. |
| Epilepsy | In patients with swallowing difficulties, intranasal midazolam or buccal clonazepam are treatment options for seizures in the end-of-life phase. | Levetiracetam is a more efficacious first-line monotherapy antiseizure drug compared to valproic acid, with similar tolerability.11 Perampanel and lacosamide seem well tolerated and efficacious add-on antiseizure drugs.12–17 | In case of no contraindications, levetiracetam is the first-line antiseizure medication of choice in patients experiencing a first seizure. Intranasal midazolam or buccal clonazepam can be prescribed in the end-of-life phase in dysphagic patients with active seizures. |
| Fatigue | There is no high-level evidence of efficacy for (non-)pharmacologic interventions for fatigue. | No pharmacological interventions are indicated,18,19 but non-pharmacological interventions (eg, aerobic training sessions) might improve fatigue.10,20 | Pharmacological agents should not be prescribed on a routine basis to treat fatigue, but patients should be encouraged to try non-pharmacological interventions. |
| Pain, headaches, and edema | Dexamethasone is the mainstay of treatment for headaches in glioma patients, but (co-)analgesics could also be considered. | Bevacizumab might be a treatment option as an alternative to dexamethasone in reducing cerebral edema associated with radiation treatment.21 | Dexamethasone is the medication of choice in case of edema and headaches due to its widespread availability and low costs, but short- and long-term adverse effects are common. |
| Psychiatric symptoms | Pharmacological interventions (eg, donepezil) for psychiatric symptoms are limited, but a non-pharmacologic intervention might improve depressive symptoms. | No new insights.9,22–24 | No specific (non-)pharmacological interventions are recommended. Provider should screen for medications that might provoke psychiatric symptoms (eg, steroids, levetiracetam). |
| Caregiver needs | Non-pharmacological interventions (ie, psycho-education and cognitive behavioral therapy) could increase the sense of mastery in caregivers. Distress and anxiety of caregivers can be mitigated by medical professionals through actively engaging with them throughout the disease trajectory and providing them with information about treatment and symptom management. | Use of social support is directly related to higher levels of HRQoL.25 Caregiver support needs may be addressed by targeting their social networks.26 Interventions targeting caregivers need to be aligned with their needs using a palliative care approach throughout the illness trajectory.27–29 | Caregivers benefit from social support, and active engagement by medical professionals during the disease trajectory. |
| Early palliative care | Due to neurocognitive deficits, medical decision-making might start to decline soon after diagnosis. Dying with dignity should be improved by enhancing caregiver satisfaction with the care in the last week of life and avoiding transitions between health-care settings. | A framework of palliative and supportive care in high-grade glioma patients has been proposed with five different phases defining the disease trajectory; each with specific patient and caregiver needs that should be proactively addressed.30 | Early palliative care interventions and advance care planning should be part of each disease phase, proactively addressing different patient and caregiver needs. |
| End-of-life care | Quality of perceived care in the end-of-life phase could be enhanced by effective symptom control, satisfaction with information received, and adherence to the preferred place of death, while the type of care (eg, hospice, home care) is less determinative for quality. | No new insights. | Symptoms should be effectively controlled in the end-of-life phase and the preferred place of death of the patient should be adhered to. Where possible, aggressive care in the end-of-life phase, including hospitalization, should be avoided. |
a Expert opinion recommendation for symptom management in the 2017 EANO guideline not included.
Symptom Management
Cognition
Neurocognitive functioning is of major concern for patients with brain tumors and for their families. It refers to mental processes involved in acquiring and processing information. Neurocognitive domains most typically affected in glioma patients include attention, executive functioning, language, and memory. At diagnosis, already ~90% of patients with a malignant brain tumor have neurocognitive deficits in at least one neurocognitive domain, impacting everyday functioning.31,32 Neurocognitive deficits in patients with brain tumors can have many causes, such as the tumor itself, antitumor treatments (eg, radiotherapy), or supportive treatments (eg, antiseizure drugs [ASDs]), but most likely it is due to a combination of these factors.33 A meta-analysis demonstrated a positive effect of tumor resection on attention, language, learning, and memory, but a negative effect on executive functioning in the immediate postoperative period and at 6-month follow-up. Additionally, awake surgeries resulted in positive effects on these neurocognitive domains.3
Information from randomized trials on how radiation might affect the cognition of patients with primary brain tumors is limited. For patients with brain metastases, whole brain radiotherapy (WBRT) has been studied frequently as neurocognitive toxicity is of great concern, but high-level evidence on mitigation of such side effects is evolving.34 In a randomized controlled trial (RCT) in patients with one resected brain metastasis and up to three unresected brain metastases, stereotactic radiosurgery (SRS) to the surgical cavity resulted in significantly less neurocognitive deterioration at 6 months compared to WBRT, while overall survival was similar.4 A trial comparing SRS to the surgical cavity alone versus in combination with WBRT, resulted in significantly less neurocognitive deterioration at 3 months with SRS compared to the combination treatment, with no difference in overall survival in patients with 1–3 brain metastases.5 Icotinib, an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, was compared to WBRT in EGFR-mutant nonsmall-cell lung cancer patients with ≥3 brain metastases and demonstrated a significantly longer progression-free survival, but no difference in neurocognition.35 Hippocampal avoidance during WBRT (HA-WBRT) with the goal of preserving neurocognitive functioning, combined with memantine, led to a significantly lower risk of neurocognitive deterioration compared to traditional WBRT combined with memantine, while overall survival was similar.6 In a single-arm phase II trial, HA-WBRT with a simultaneous integrated boost resulted in significantly decreased decline of neurocognitive functioning, and similar intracranial control, compared to a historical control group of patients treated with WBRT combined with SRS.7 Despite the hypothesis of better long-term neurocognitive outcomes with proton compared to photon therapy, published data remains scarce. In a recent phase II trial of newly diagnosed glioblastoma patients, proton therapy did not prevent cognitive failure after a median follow-up of 48 months.36 A multicenter trial comparing these modalities in low-grade gliomas is ongoing (NCT03180502).
Several trials assessed the ability of pharmacological agents to prevent neurocognitive decline or to improve neurocognitive functioning in patients with brain tumors. Donepezil, an acetylcholinesterase inhibitor, was compared to placebo in a phase III trial. While some domains of neurocognitive functioning improved in patients with more severe neurocognitive deficits, the primary endpoint (an overall neurocognitive composite score) did not differ significantly in patients treated with donepezil or placebo.8 In a randomized phase 3 trial, dexamphetamine, a stimulant, did not improve neurocognition in patients with brain tumors either.9 Non-pharmacologic interventions such as exercise or yoga might be an option for selected patients, but the evidence for an effect on neurocognition is limited.10
Epilepsy
Seizures are a well-recognized symptom in brain tumors and the incidence varies mainly according to subtype, with the incidence ranging from ~15% to 30% in brain metastases to ~80% in grade 2 glioma patients during the course of the disease.37,38 Current guidelines discourage primary prophylaxis with ASDs in seizure-naïve patients with brain tumors.39 The use of nonenzyme-inducing ASDs after a first seizure is strongly recommended, due to their lack of or limited interactions with other drugs, including corticosteroids and systemic therapies.40 Levetiracetam, a nonenzyme inducing ASD, is the most frequently prescribed first-line ASD in patients with brain tumors.41 In a recent systematic review evaluating the efficacy of ASDs in glioma patients with epilepsy, phenytoin, pregabalin, and levetiracetam had the highest efficacy as ASD monotherapy agents, with the latter showing the lowest treatment failure rate compared to the other agents studied.42 In a retrospective study comparing first-line monotherapy levetiracetam with valproic acid in 1435 glioma patients with epilepsy, levetiracetam had a significantly better efficacy than valproic acid, while the level of toxicity was similar.11 Almost half of intolerable adverse effects due to levetiracetam in glioma patients are of psychiatric origin.11 As a consequence, levetiracetam is not recommended in patients with a history of psychiatric disorders, such as anxiety or depression.43 Brivaracetam is an analog of levetiracetam, but seems less likely to induce psychiatric adverse effects.44 In patients with brain tumors brivaracetam has been shown to be well tolerated and effective in reducing seizure frequency.45 About a third of glioma patients with epilepsy need more than one ASD due to uncontrolled seizures on ASD monotherapy.11 Based on rational polytherapy, combining ASDs with different mechanisms of action is recommended.46 Several studies have evaluated lacosamide or perampanel as add-on in patients with brain tumors with uncontrolled seizures, and showed good efficacy and tolerability.12–17 In low-grade glioma patients with long-term seizure freedom after antitumor treatment, ASD discontinuation can be considered using a shared decision-making approach involving both patients and their clinicians.47 In the end-of-life phase seizures are thought to become more prevalent and are a common reason for hospitalization.48 Seizure medication should be continued and in patients with swallowing difficulties, oral solution or subcutaneous levetiracetam, buccal clonazepam, or intranasal or subcutaneous midazolam can be considered.49
Fatigue
Fatigue is among the most prevalent symptoms and reported in up to ~90% of malignant glioma patients at some point during the disease trajectory.50 Fatigue in patients with brain tumors likely has a multifactorial origin, including the tumor itself, antitumor treatments (eg, radiotherapy), and symptomatic treatments (eg, ASDs). A 2016 Cochrane systematic review did not find any evidence that (non-)pharmacologic interventions might improve fatigue in patients with brain tumors.18 Since then, several interventions have been evaluated. In a pilot RCT, glioma patients receiving a home-based, remotely coached, three times weekly aerobic training session of 20–45 min had less fatigue compared to the waitlist control group.10 A comprehensive nursing program based on cognitive behavioral interventions showed improved fatigue symptoms in glioma patients compared to patients in the control group who received routine nursing follow-up for 3 months.20 Non-pharmacologic interventions such as yoga,51 an educational program,52 and an online problem-solving therapy course (a low-intensity form of cognitive behavioral therapy),22 did not significantly improve fatigue in patients with brain tumors. In recent years, armodafinil has been studied in glioma patients in a phase II (n = 54) and phase III (n = 328) double-blind placebo-controlled trial, but did not result in an improvement of fatigue.19,53 Other pharmacological interventions such as donepezil or dexamphetamine were also ultimately unsuccessful in improving fatigue.9,54
Pain and headaches
Headaches are a common symptom in patients with brain tumors, with 4%–62% being affected.55 In some patients headaches can be explained by increased intracranial pressure due to mass effect or edema, which can be alleviated by reducing the intracranial pressure with medical treatment or surgical intervention.56,57
Brain tumors and their treatment can disrupt the blood-brain barrier, causing extravasation of plasma fluid and proteins, which leads to vasogenic edema and can result in increased intracranial pressure. Cerebral edema causes significant morbidity in patients with brain tumors and results in symptoms such as focal neurological deficits, seizures, nausea, vomiting, and headaches.58 Corticosteroids can be used in the treatment of cerebral edema. Dexamethasone has become the corticosteroid of choice in neuro-oncology, because of its high potency, the long half-life, and limited mineralocorticoid effects, but can have significant long-term adverse effects such as adrenal insufficiency, diabetes, immune suppression and resultant opportunistic infections, and myopathy, as well as neuropsychiatric effects, and therefore should be limited as much as possible.56 The angiotensin II receptor blocker losartan was compared to dexamethasone in a double-blind, placebo-controlled, randomized phase III trial in 75 newly-diagnosed glioblastoma patients during radiotherapy. However, no significant difference was found between the two treatment arms.59 A potentially more effective alternative to corticosteroids in reducing cerebral edema is bevacizumab, a VEGF inhibitor, although large prospective trials are lacking.21
In addition to headaches, about 13%–25% of patients with brain tumors suffer from bodily pain. Its management is multimodal and the approach taken similar to pain management in patients with systemic cancers, including the World Health Organization's sequential three-step analgesic ladder.55,60 In a survey among glioma patients, about 85% reported pain relief by using cannabis-based therapies,61 but medicinal cannabis failed to demonstrate improvement of pain in glioma patients in another prospective trial.62 There have been few studies examining the management of either headaches or bodily pain in malignant brain tumors in recent years.
Psychiatric symptoms
Neurobehavioral symptoms are common in patients with brain tumors. Patient-reported prevalence of depression is ~15% in glioma patients,43,63 while the prevalence of anxiety is ~25%.43 In a systematic review, reported prevalence rates of changes in personality and/or behavior varied widely, ranging between 8% and 67% in glioma patients.64 Psychosis has been studied less extensively in patients with brain tumors and prevalence is currently unknown.65 Several non-pharmacologic interventions to treat psychiatric symptoms have been studied in recent years in patients with brain tumors. In an RCT of 115 glioma patients with depressive symptoms, an online problem-solving therapy (a low-intensity form of cognitive behavioral therapy) did not result in less depression compared to the waitlist control group.22 However, in an RCT including a total of 150 glioma patients, patients randomized into the reminiscence therapy-based care program group (ie, the discussion of past activities, events, and experiences in a group of glioma patients) had less depression and anxiety compared to the control group.23 A comprehensive nursing program evaluating cognitive behavioral interventions in an RCT resulted in lower levels of depression and anxiety in glioma patients.20 A 2020 Cochrane systematic review concluded that no high-quality studies have been conducted investigating pharmacological treatment of depression specifically in patients with a brain tumor.24 An RCT studying the role of dexamphetamine to improve neurocognition, HRQoL, and mood in patients with brain tumors did not result in improvement of affective symptoms.9
Caregiver Needs
Family caregivers not only provide care for people with a brain tumor, but their support improves patients’ level of HRQoL.66 However, caring for a loved one with a brain tumor can be a traumatic and life-changing event due to the patient’s altered behavior and changed personality leading to caregivers having “mixed feelings of right and wrong, patience and guilt, hope and despair.” 67 As the brain tumor caregiver burden is multidimensional, neuro-oncology caregivers have many unmet needs.68 Among the significant challenges caregivers face are: (1) fear of recurrence of their loved one’s brain tumor which highlights the need for caregivers to be included in psychotherapeutic support as appropriate,69 (2) insufficient tools for dealing with caregiver burnout, patients’ end-of-life care and caregiver psychological and bereavement needs,70 (3) high levels of distress, anxiety, and depression,71–75 (4) low levels of HRQoL76, and (5) lack of information.77
Qualitative research involving small caregiver cohorts (in the United States and the Netherlands) revealed that caregiver issues were agnostic to culture- and country-specific differences. Most care issues were experienced early, prompting more need for support.78 Evaluations of coping in high-grade glioma patient–caregiver dyads indicated that coping strategies directly impacted HRQoL aspects.25 Early in the disease trajectory, caregivers’ use of social support was directly related to higher levels of HRQoL and use of avoidance was linked to lower HRQoL. Patients’ HRQoL was also higher when caregivers used social support coping.26 Assessment of caregiver social networks indicated an opportunity to address support needs by targeting their social networks.79 Web-based apps may be purposeful tools to enable social support interventions.80 Interventions assessing exercise, yoga, and meditation for caregivers and patients within small feasibility studies have yielded mixed results; each intervention was favorably reviewed by participants and was deemed feasible by the authors.27,51,81 Preliminary interventions using a palliative care approach delivered by a nurse or a cancer care coordinator to support caregivers throughout the illness trajectory have been reported.28,29 A palliative care framework to support glioma patients and caregivers aligned with their needs across transition points, including bereavement, has been proposed.30
Early Palliative Care
In 2017, a framework of palliative and supportive care in high-grade glioma patients was developed and identified five different phases in the disease, each with its specific patient and caregiver needs: (1) time of diagnosis, (2) conclusion of initial radiotherapy treatment, (3) tumor recurrence, (4) deterioration to death, and (5) after the patient’s death.30 ACP can contribute to meeting patient and caregiver needs during the different phases of the disease trajectory in a timely manner. The European Association for Palliative Care defines ACP as: “enabling individuals to define goals and preferences for future medical treatment and care, to discuss these goals and preferences with family and healthcare providers, and to record and review these preferences if appropriate.” 82 ACP is of particular importance for patients with a malignant brain tumor, because the disease can lead to decreased neurocognitive functioning, communication difficulties, loss of consciousness, and other neurological and psychiatric symptoms hampering patients’ decision-making capacities. Several studies report that most patients with a glioma and their caregivers may have an inaccurate appreciation of the prognosis of their illness and of treatment goals.83 Therefore, communication strategies with brain tumor patients and their families should be tailored to patients’ cognitive deficits to facilitate their participation and to obtain their preferences about goals and treatment choices. For glioblastoma patients, a disease-specific ACP program has been developed, consisting of different topics to be discussed, including the current situation of the patient (eg, current health issues), worries and fears (eg, concerns with respect to performing household tasks), (supportive) treatment (eg, withdrawal and withholding of treatment), and preferred place of care and death, and its implementation in clinical practice is currently being evaluated.84
The American Society of Clinical Oncology (ASCO) developed clinical practice guidelines for the integration of palliative care into standard oncology care for all patients diagnosed with cancer. One of these recommendations is early palliative care involvement within 8 weeks of diagnosis in newly diagnosed patients with advanced cancer.85 In a retrospective study among patients with brain metastases, only about half of the patients received palliative care consultation.86 ACP in glioblastoma patients was evaluated in another study at three separate points in the illness, prior to diagnosis, within 6 months of diagnosis, and at last follow-up, revealing that ACP was documented in 11%, 29%, and 54%, respectively.87 Despite high-level evidence of early palliative care improving HRQoL, reducing depression, improving care satisfaction, reducing chemotherapy near the end-of-life phase, and improving survival in oncology patients, it is frequently not implemented in systemic cancer and not systematically established or routinely utilized for patients with brain tumors.85,88,89
End-of-Life Needs and Care
Patients with a malignant brain tumor have significant and often unmet care needs as they approach the end of life. Data suggest that these patients experience a multitude of active symptoms in the terminal phase of their illness, including high rates of drowsiness; communication challenges, which may be related to cognitive decline, aphasia and speech impairment; seizures; motor dysfunction, including focal weakness and dysphagia; and pain, most often in the form of headaches.2,55,90,91 Patients also may struggle with psychological and existential distress.88,91,92 Importantly, the end-of-life care needs of patients with brain tumors are unique, as the symptoms these patients most commonly experience are largely related to the neurological impact of their illness, and thus differ from the symptoms observed in patients with cancer not involving the central nervous system.90,91,93 These findings underscore the need for population-specific approaches to end-of-life care for patients with brain tumors.
While there remains significant variability across countries in the metrics and standards used to assess the quality of end-of-life care, there is general agreement amongst physicians that aggressive care at the end of life—including hospitalization and particularly intensive care unit (ICU) admission, as well as the administration of chemotherapy and radiation in the last weeks of life—may compromise HRQoL aspects without significant benefit.2,94,95 Despite this, rates of late hospitalization and ICU admission among patients with malignant brain tumors remain high, with single-center studies reporting that more than one-third of patients with glioblastoma are hospitalized in the final month of life.94,96 Reported rates of chemotherapy administration in the last few weeks of life, on the other hand, are reassuringly low, ranging from 6% to 18%.88,96,97 In the United States, where hospice referral and timely hospice enrollment are considered important aspects of end-of-life care for patients with cancer, 24%–37% of patients with a malignant brain tumor are nonetheless enrolled in hospice late (ie, within the last 3 days of life) or not at all.96,98,99 Rates of hospice enrollment are considerably lower in Europe (19%) and Asia (8%) than in the United States, although this may reflect regional differences in healthcare delivery and access to hospice services.100 Several studies have demonstrated higher rates of hospice enrollment for patients receiving formal palliative care consultation services.92,101 As palliative care services remain underutilized in neuro-oncology, efforts aimed at increasing palliative care involvement may improve the timing and rates of hospice enrollment for patients with brain tumors.
Conclusion
Patients with malignant brain tumors experience a high and increasing symptom burden throughout the course of the disease that requires specific attention and management to maintain HRQoL, optimal functioning, and well-being. We performed a systematic literature search with updated insights based on the 2017 EANO palliative care guidelines with a focus on interventions to address the most common and disabling symptoms. Some new insights are reported in the prevention of neurocognitive deterioration by refinement of radiation therapy modalities, and in the evidence of ASD selection in brain tumor patients with epilepsy. Early palliative care interventions and implementation of an ACP program are essential to define goals of care and minimize symptoms in a timely way before patients lose their decision-making capacity due to communication difficulties caused by progressive neurological deficits. An ACP program may help facilitate targeted care in line with patients’ and caregivers’ needs, which may in turn reduce hospitalization and avoid unnecessary aggressive care in the end-of-life phase. Nonetheless, there is still limited evidence from the literature to support the introduction of early palliative care interventions in patients with malignant brain tumors. Certain palliative and end-of-life topics are more extensively studied in other cancers or neurological diseases. A systematic comparison between brain tumor patients and patients with other diseases should be performed in the future to enrich our understanding of key differences and similarities in different diseases. To further improve the quality of care in patients with a malignant brain tumor, studies on the early integration of palliative care, the added value and timing of ACP in this population, as well as interventional studies that address the most common symptoms, such as pain, seizures, brain edema, fatigue, and neurobehavioral symptoms, are urgently needed.
Supplementary Material
Contributor Information
Johan A F Koekkoek, Department of Neurology, Leiden University Medical Center, Leiden, The Netherlands; Department of Neurology, Haaglanden Medical Center, The Hague, The Netherlands.
Pim B van der Meer, Department of Neurology, Leiden University Medical Center, Leiden, The Netherlands.
Andrea Pace, Neuro-Oncology Unit, IRCCS Regina Elena National Cancer Institute, Rome, Italy.
Caroline Hertler, Competence Center Palliative Care, Department of Radiation Oncology, University Hospital Zurich and University of Zurich, Zurich, Switzerland.
Rebecca Harrison, Division of Medical Oncology, BC Cancer, The University of British Colombia, Vancouver, Canada.
Heather E Leeper, Neuro-Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA.
Deborah A Forst, Division of Neuro-Oncology, Department of Neurology, Massachusetts General Hospital Cancer Center, Boston, Massachusetts, USA.
Rakesh Jalali, Department of Radiation Oncology, Apollo Proton Cancer Center, Chennai, India.
Kathy Oliver, International Brain Tumour Alliance, Tadworth, UK.
Jennifer Philip, Department of Medicine, St. Vincent’s Hospital Melbourne, University of Melbourne, Victoria, Australia.
Martin J B Taphoorn, Department of Neurology, Leiden University Medical Center, Leiden, The Netherlands; Department of Neurology, Haaglanden Medical Center, The Hague, The Netherlands.
Linda Dirven, Department of Neurology, Leiden University Medical Center, Leiden, The Netherlands; Department of Neurology, Haaglanden Medical Center, The Hague, The Netherlands.
Tobias Walbert, Department of Neurology and Neurosurgery, Henry Ford Health System and Department of Neurology Wayne State University, Detroit, Michigan, USA.
Funding
This review was developed without financial support.
Conflict of interest statement
KO: As Co-Director of the International Brain Tumor Alliance (IBTA), Kathy Oliver declares that, as far as she is aware, she has no real or perceived conflicts of interest regarding coauthorship of this paper. But for the sake of completeness and transparency, please see www.theibta.org for details of the IBTA’s sponsorship policy and funding organisations which include a range of pharmaceutical and device companies as well as other non-industry funders. DAF: Minority stock ownership in Eli Lilly, LLC. TW: Consulting work for NovoCure, Advisory Board AstraZeneca, Advisory Board Orbus. JAFK, PBM, AP, CH, RH, HEL, RJ, JP, MJBT, and LD report no conflicts of interest.
Authorship statement.
JAFK, PBM, LD, and TW: study concept and design, acquisition of data, analysis and interpretation of data, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content. MJBT: study concept and design, analysis and interpretation of data, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content. AP, CH, RH, HEL, DAF, RJ, KO, and JP: analysis and interpretation of data, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content.
References
- 1. Temel JS, Greer JA, Muzikansky A, et al. . Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010; 363(8):733–742. [DOI] [PubMed] [Google Scholar]
- 2. Pace A, Dirven L, Koekkoek JAF, et al. . European Association for Neuro-Oncology (EANO) guidelines for palliative care in adults with glioma. Lancet Oncol. 2017; 18(6):e330–e340. [DOI] [PubMed] [Google Scholar]
- 3. Ng JCH, See AAQ, Ang TY, et al. . Effects of surgery on neurocognitive function in patients with glioma: a meta-analysis of immediate post-operative and long-term follow-up neurocognitive outcomes. J Neurooncol. 2019; 141(1):167–182. [DOI] [PubMed] [Google Scholar]
- 4. Brown PD, Ballman KV, Cerhan JH, et al. . Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC.3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017; 18(8):1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown PD, Jaeckle K, Ballman KV, et al. . Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016; 316(4):401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown PD, Gondi V, Pugh S, et al. . Hippocampal avoidance during whole-brain radiotherapy plus memantine for patients with brain metastases: phase III trial NRG oncology CC001. J Clin Oncol. 2020; 38(10):1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Westover KD, Mendel JT, Dan T, et al. . Phase II trial of hippocampal-sparing whole brain irradiation with simultaneous integrated boost for metastatic cancer. Neuro Oncol. 2020; 22(12):1831–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rapp SR, Case LD, Peiffer A, et al. . Donepezil for irradiated brain tumor survivors: a phase III randomized placebo-controlled clinical trial. J Clin Oncol. 2015; 33(15):1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Laigle-Donadey F, Ducray F, Boone M, et al. . A phase III double-blind placebo-controlled randomized study of dexamphetamine sulfate for fatigue in primary brain tumors patients: an ANOCEF trial (DXA). Neurooncol Adv. 2019; 1(1):vdz2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gehring K, Stuiver MM, Visser E, et al. . A pilot randomized controlled trial of exercise to improve cognitive performance in patients with stable glioma: a proof of concept. Neuro Oncol. 2020; 22(1):103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van der Meer PB, Dirven L, Fiocco M, et al. . First-line antiepileptic drug treatment in glioma patients with epilepsy: levetiracetam vs valproic acid. Epilepsia. 2021; 62(5):1119–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maschio M, Zarabla A, Maialetti A, et al. . Quality of life, mood and seizure control in patients with brain tumor related epilepsy treated with lacosamide as add-on therapy: a prospective explorative study with a historical control group. Epilepsy Behav. 2017; 73:83–89. [DOI] [PubMed] [Google Scholar]
- 13. Villanueva V, Saiz-Diaz R, Toledo M, et al. . NEOPLASM study: real-life use of lacosamide in patients with brain tumor-related epilepsy. Epilepsy Behav. 2016; 65:25–32. [DOI] [PubMed] [Google Scholar]
- 14. Coppola A, Zarabla A, Maialetti A, et al. . Perampanel confirms to be effective and well-tolerated as an add-on treatment in patients with brain tumor-related epilepsy (PERADET study). Front Neurol. 2020; 11(592). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rudà R, Pellerino A, Franchino F, et al. . Lacosamide in patients with gliomas and uncontrolled seizures: results from an observational study. J Neurooncol. 2018; 136(1):105–114. [DOI] [PubMed] [Google Scholar]
- 16. Toledo M, Molins A, Quintana M, et al. . Outcome of cancer-related seizures in patients treated with lacosamide. Acta Neurol Scand. 2018; 137(1):67–75. [DOI] [PubMed] [Google Scholar]
- 17. Motomura K, Chalise L, Shimizu H, et al. . Intraoperative seizure outcome of levetiracetam combined with perampanel therapy in patients with glioma undergoing awake brain surgery. J Neurosurg. 2021;135(4):998–1007. [DOI] [PubMed] [Google Scholar]
- 18. Day J, Yust-Katz S, Cachia D, et al. . Interventions for the management of fatigue in adults with a primary brain tumour. Cochrane Database Syst Rev. 2016;4(4):Cd011376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Porter AB, Liu H, Kohli S, et al. . Efficacy of treatment with armodafinil for cancer-related fatigue in patients with high-grade glioma: a phase 3 randomized clinical trial. JAMA Oncol. 2022;8(2):259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao Y-H, Xu Y. Effect of comprehensive nursing based on cognitive behavior on psychological function of glioma patients. Neuropsychiatr Dis Treat. 2021; 17:777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khan M, Zhao Z, Arooj S, Liao G. Bevacizumab for radiation necrosis following radiotherapy of brain metastatic disease: a systematic review & meta-analysis. BMC Cancer. 2021; 21(1):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boele FW, Klein M, Verdonck-de Leeuw IM, et al. . Internet-based guided self-help for glioma patients with depressive symptoms: a randomized controlled trial. J Neurooncol. 2018; 137(1):191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao X. Reminiscence therapy-based care program for reducing anxiety and depression in glioma survivors: a randomized controlled trial. Medicine (Baltimore). 2021; 100(5):e23056–e23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beevers Z, Hussain S, Boele FW, Rooney AG. Pharmacological treatment of depression in people with a primary brain tumour. Cochrane Database Syst Rev. 2020; 7(7):CD006932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baumstarck K, Leroy T, Hamidou Z, et al. . Coping with a newly diagnosed high-grade glioma: patient-caregiver dyad effects on quality of life. J Neurooncol. 2016; 129(1):155–164. [DOI] [PubMed] [Google Scholar]
- 26. Baumstarck K, Chinot O, Tabouret E, et al. . Coping strategies and quality of life: a longitudinal study of high-grade glioma patient-caregiver dyads. Health Qual Life Outcomes. 2018; 16(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Milbury K, Weathers SP, Durrani S, et al. . Online couple-based meditation intervention for patients with primary or metastatic brain tumors and their partners: results of a pilot randomized controlled trial. J Pain Symptom Manage. 2020; 59(6):1260–1267. [DOI] [PubMed] [Google Scholar]
- 28. Dionne-Odom JN, Williams GR, Warren PP, et al. . Implementing a clinic-based telehealth support service (familystrong) for family caregivers of individuals with grade IV brain tumors. J Palliat Med. 2021; 24(3):347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Philip J, Collins A, Staker J, Murphy M. I-CoPE: a pilot study of structured supportive care delivery to people with newly diagnosed high-grade glioma and their carers. Neurooncol Pract. 2019; 6(1):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Philip J, Collins A, Brand C, et al. . A proposed framework of supportive and palliative care for people with high-grade glioma. Neuro Oncol. 2018; 20(3):391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tucha O, Smely C, Preier M, Lange KW. Cognitive deficits before treatment among patients with brain tumors. Neurosurgery. 2000; 47(2):324–333; discussion 333. [DOI] [PubMed] [Google Scholar]
- 32. Meyers CA, Smith JA, Bezjak A, et al. . Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol. 2004; 22(1):157–165. [DOI] [PubMed] [Google Scholar]
- 33. Taphoorn MJB, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004; 3(3):159–168. [DOI] [PubMed] [Google Scholar]
- 34. Brown PD, Ahluwalia MS, Khan OH, et al. . Whole-brain radiotherapy for brain metastases: evolution or revolution? J Clin Oncol. 2018; 36(5):483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang JJ, Zhou C, Huang Y, et al. . Icotinib versus whole-brain irradiation in patients with EGFR-mutant non-small-cell lung cancer and multiple brain metastases (BRAIN): a multicentre, phase 3, open-label, parallel, randomised controlled trial. Lancet Respir Med. 2017; 5(9):707–716. [DOI] [PubMed] [Google Scholar]
- 36. Brown PD, Chung C, Liu DD, et al. . A prospective phase II randomized trial of proton radiotherapy vs intensity-modulated radiotherapy for patients with newly diagnosed glioblastoma. Neuro Oncol. 2021; 23(8):1337–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jo J, Nevel K, Sutyla R, et al. . Predictors of early, recurrent, and intractable seizures in low-grade glioma. Neurooncol Pract. 2020; 8(1):40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chan V, Sahgal A, Egeto P, Schweizer T, Das S. Incidence of seizure in adult patients with intracranial metastatic disease. J Neurooncol. 2017; 131(3):619–624. [DOI] [PubMed] [Google Scholar]
- 39. Walbert T, Harrison RA, Schiff D, et al. . SNO and EANO practice guideline update: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Neuro Oncol. 2021; 23(11):1835–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weller M, van den Bent M, Preusser M, et al. . EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18(3):170–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van der Meer PB, Dirven L, van den Bent MJ, et al. . Prescription preferences of antiepileptic drugs in brain tumor patients: an international survey among EANO members. Neurooncol Pract. 2021; 9(2):105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. de Bruin ME, van der Meer PB, Dirven L, Taphoorn MJB, Koekkoek JAF. Efficacy of antiepileptic drugs in glioma patients with epilepsy: a systematic review. Neurooncol Pract. 2021; 8(5):501–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van der Meer PB, Koekkoek JAF, van den Bent MJ, Dirven L, Taphoorn MJB. Effect of antiepileptic drugs in glioma patients on self-reported depression, anxiety, and cognitive complaints. J Neurooncol. 2021; 153(1):89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Steinhoff BJ, Staack AM. Levetiracetam and brivaracetam: a review of evidence from clinical trials and clinical experience. Ther Adv Neurol Disord. 2019; 12(12):1756286419873518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maschio M, Maialetti A, Mocellini C, et al. . Effect of brivaracetam on efficacy and tolerability in patients with brain tumor-related epilepsy: a retrospective multicenter study. Front Neurol. 2020; 11:813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. St Louis EK. Truly “rational” polytherapy: maximizing efficacy and minimizing drug interactions, drug load, and adverse effects. Curr Neuropharmacol. 2009; 7(2):96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kerkhof M, Koekkoek JAF, Vos MJ, et al. . Withdrawal of antiepileptic drugs in patients with low grade and anaplastic glioma after long-term seizure freedom: a prospective observational study. J Neurooncol. 2019; 142(3):463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Koekkoek JAF, Dirven L, Reijneveld JC, et al. . Epilepsy in the end of life phase of brain tumor patients: a systematic review. Neurooncol Pract. 2014; 1(3):134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Koekkoek JA, Postma TJ, Heimans JJ, Reijneveld JC, Taphoorn MJ. Antiepileptic drug treatment in the end-of-life phase of glioma patients: a feasibility study. Support Care Cancer. 2016; 24(4):1633–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Osoba D, Brada M, Prados MD, Yung WK. Effect of disease burden on health-related quality of life in patients with malignant gliomas. Neurooncol. 2000; 2(4):221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Milbury K, Li J, Weathers SP, et al. . Pilot randomized, controlled trial of a dyadic yoga program for glioma patients undergoing radiotherapy and their family caregivers. Neurooncol Prac.t 2019; 6(4):311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bigatão Mdos R, Peria FM, Tirapelli DP, Carlotti Junior CG. Educational program on fatigue for brain tumor patients: possibility strategy? Arq Neuropsiquiatr. 2016; 74(2):155–160. [DOI] [PubMed] [Google Scholar]
- 53. Lee EQ, Muzikansky A, Drappatz J, et al. . A randomized, placebo-controlled pilot trial of armodafinil for fatigue in patients with gliomas undergoing radiotherapy. Neuro Oncol. 2016; 18(6):849–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Naughton MJ, Case LD, Peiffer A, et al. . Quality of life of irradiated brain tumor survivors treated with donepezil or placebo: results of the WFU CCOP research base protocol 91105. Neurooncol Pract. 2018; 5(2):114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Walbert T, Khan M. End-of-life symptoms and care in patients with primary malignant brain tumors: a systematic literature review. 2014/04/01. J Neurooncol. 2014; 117(2):217–224. [DOI] [PubMed] [Google Scholar]
- 56. Dietrich J, Rao K, Pastorino S, Kesari S. Corticosteroids in brain cancer patients: benefits and pitfalls. Expert Rev Clin Pharmacol. 2011; 4(2):233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nelson S, Taylor LP. Headaches in brain tumor patients: primary or secondary? Headache J Head Face Pain. 2014; 54(4):776–785. [DOI] [PubMed] [Google Scholar]
- 58. Zoccarato M, Nardetto L, Basile AM, Giometto B, Zagonel V, Lombardi G. Seizures, edema, thrombosis, and hemorrhages: an update review on the medical management of gliomas. Front Oncol. 2021; 11(483):617966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ursu R, Thomas L, Psimaras D, et al. . Angiotensin II receptor blockers, steroids and radiotherapy in glioblastoma-a randomised multicentre trial (ASTER trial). An ANOCEF study. Eur J Cancer. 2019; 109:129–136. [DOI] [PubMed] [Google Scholar]
- 60. Fallon M, Giusti R, Aielli F, et al. . Management of cancer pain in adult patients: ESMO clinical practice guidelines. Ann Oncol. 2018; 29(Suppl 4):iv166–iv191. [DOI] [PubMed] [Google Scholar]
- 61. Reblin M, Sahebjam S, Peeri NC, et al. . Medical cannabis use in glioma patients treated at a comprehensive cancer center in Florida. J Palliat Med. 2019; 22(10):1202–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schloss J, Lacey J, Sinclair J, et al. . A phase 2 randomised clinical trial assessing the tolerability of two different ratios of medicinal cannabis in patients with high grade gliomas. Front Oncol. 2021; 11(1687). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rooney AG, Carson A, Grant R. Depression in cerebral glioma patients: a systematic review of observational studies. J Natl Cancer Inst. 2011; 103(1):61–76. [DOI] [PubMed] [Google Scholar]
- 64. Zwinkels H, Dirven L, Vissers T, et al. . Prevalence of changes in personality and behavior in adult glioma patients: a systematic review. Neurooncol Pract. 2015; 3(4):222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Boele FW, Rooney AG, Grant R, Klein M. Psychiatric symptoms in glioma patients: from diagnosis to management. Neuropsychiatr Dis Treat. 2015; 11:1413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bayen E, Laigle-Donadey F, Prouté M, et al. . The multidimensional burden of informal caregivers in primary malignant brain tumor. Support Care Cancer. 2017; 25(1):245–253. [DOI] [PubMed] [Google Scholar]
- 67. Francis SR, Hall EOC, Delmar C. Spouse caregivers’ experiences of suffering in relation to care for a partner with brain tumor: a qualitative study.. Cancer Nurs. 2022; 45(2):E320–E328. [DOI] [PubMed] [Google Scholar]
- 68. Chen C, Wang H, Zhang L, et al. . Clinical study of preoperative psychological distress and its related factors in the primary caregivers of patients with glioma. Clin Neurol Neurosurg. 2021; 200(January):106364. [DOI] [PubMed] [Google Scholar]
- 69. Braun SE, Aslanzadeh FJ, Thacker L, Loughan AR. Examining fear of cancer recurrence in primary brain tumor patients and their caregivers using the Actor-Partner Interdependence Model. Psychooncology. 2021; 30(7):1120–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fortunato JT, Van Harn M, Haider SA, Phillips J, Walbert T. Caregiver perceptions of end-of-life care in patients with high-grade glioma. Neurooncol Pract. 2021; 8(2):171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Long A, Halkett GKB, Lobb EA, et al. . Carers of patients with high-grade glioma report high levels of distress, unmet needs, and psychological morbidity during patient chemoradiotherapy. Neurooncol Pract. 2016; 3(2):105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Halkett GK, Lobb EA, Shaw T, et al. . Distress and psychological morbidity do not reduce over time in carers of patients with high-grade glioma. Support Care Cancer 2017; 25(3):887–893. [DOI] [PubMed] [Google Scholar]
- 73. Halkett GKB, Lobb EA, Shaw T, et al. . Do carer’s levels of unmet needs change over time when caring for patients diagnosed with high-grade glioma and how are these needs correlated with distress? Support Care Cancer. 2018; 26(1):275–286. [DOI] [PubMed] [Google Scholar]
- 74. Reinert C, Gerken M, Rathberger K, et al. . Single-institution cross-sectional study to evaluate need for information and need for referral to psychooncology care in association with depression in brain tumor patients and their family caregivers. BMC Psychol. 2020; 8(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Stieb S, Fischbeck S, Wagner W, Appels J, Wiewrodt D. High psychosocial burden in relatives of malignant brain tumor patients. Clin Neurol Neurosurg. 2018; 170:1–6. [DOI] [PubMed] [Google Scholar]
- 76. Ståhl P, Fekete B, Henoch I, et al. . Health-related quality of life and emotional well-being in patients with glioblastoma and their relatives. J Neurooncol. 2020; 149(2):347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Reinert C, Rathberger K, Klinkhammer-Schalke M, et al. . Information needs and requirements in patients with brain tumours and their relatives. J Neurooncol. 2018; 138(2):407–415. [DOI] [PubMed] [Google Scholar]
- 78. Boele FW, van Uden-Kraan CF, Hilverda K, et al. . Neuro-oncology family caregivers’ view on keeping track of care issues using eHealth systems: it’s a question of time. J Neurooncol. 2017; 134(1):157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ketcher D, Reblin M. Social networks of caregivers of patients with primary malignant brain tumor. Psychol Health Med. 2019; 24(10):1235–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Reblin M, Ketcher D, Forsyth P, et al. . Feasibility of implementing an electronic social support and resource visualization tool for caregivers in a neuro-oncology clinic. Support Care Cancer. 2018; 26(12):4199–4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Halkett GKB, Cormie P, McGough S, et al. . Patients and carers’ perspectives of participating in a pilot tailored exercise program during chemoradiotherapy for high grade glioma: a qualitative study. Eur J Cancer Care (Engl). 2021; 30(5):e13453. [DOI] [PubMed] [Google Scholar]
- 82. Rietjens JAC, Sudore RL, Connolly M, et al. . Definition and recommendations for advance care planning: an international consensus supported by the European Association for Palliative Care. Lancet Oncol. 2017; 18(9):e543–e551. [DOI] [PubMed] [Google Scholar]
- 83. Diamond EL, Prigerson HG, Correa DC, et al. . Prognostic awareness, prognostic communication, and cognitive function in patients with malignant glioma. Neuro Oncol. 2017; 19(11):1532–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Fritz L, Zwinkels H, Koekkoek JAF, et al. . Advance care planning in glioblastoma patients: development of a disease-specific ACP program. Support Care Cancer.. 2020; 28(3):1315–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ferrell BR, Temel JS, Temin S, et al. . Integration of palliative care into standard oncology care: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol Off J Am Soc Clin Oncol. 2017; 35(1):96–112. [DOI] [PubMed] [Google Scholar]
- 86. McDermott DM, Seldomridge A, Maniar A, Mattes MD. Patterns of palliative care consultation among patients with brain metastasis: an opportunity for radiation oncologists to facilitate earlier referral. Ann Palliat Med. 2020; 9(5):3513–3521. [DOI] [PubMed] [Google Scholar]
- 87. Pollom EL, Sborov KD, Soltys SG, et al. . Advance care planning needs in patients with glioblastoma undergoing radiotherapy. J Pain Symptom Manage. 2018; 56(6):e6–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hemminger LE, Pittman CA, Korones DN, et al. . Palliative and end-of-life care in glioblastoma: defining and measuring opportunities to improve care. Neurooncol Pract. 2017; 4(3):182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wang Y, Zhang Y, Hong Y, et al. . Advance directives and end-of-life care: knowledge and preferences of patients with brain tumours from Anhui, China. BMC Cancer. 2021; 21(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Walbert T. Integration of palliative care into the neuro-oncology practice: patterns in the United States. Neurooncol Pract. 2014; 1(1):3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. IJzerman-Korevaar M, Snijders TJ, de Graeff A, Teunissen S, de Vos FYF. Prevalence of symptoms in glioma patients throughout the disease trajectory: a systematic review. J Neurooncol. 2018; 140(3):485–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Crooms RC, Lin HM, Neifert S, et al. . Palliative care consultation for hospitalized patients with primary and secondary brain tumors at a single academic center. J Palliat Med. 2021; 24(10):1550–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Koekkoek JA, Chang S, Taphoorn MJ. Palliative care at the end-of-life in glioma patients. Handb Clin Neurol. 2016; 134:315–326. [DOI] [PubMed] [Google Scholar]
- 94. Diamond EL, Panageas KS, Dallara A, et al. . Frequency and predictors of acute hospitalization before death in patients with glioblastoma. J Pain Symptom Manage. 2017; 53(2):257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Crawford GB, Dzierzanowski T, Hauser K, et al. . Care of the adult cancer patient at the end of life: ESMO Clinical Practice Guidelines. ESMO Open. 2021; 6(4):100225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kuchinad KE, Strowd R, Evans A, Riley WA, Smith TJ. End of life care for glioblastoma patients at a large academic cancer center. J Neurooncol. 2017; 134(1):75–81. [DOI] [PubMed] [Google Scholar]
- 97. Dover LL, Dulaney CR, Williams CP, et al. . Hospice care, cancer-directed therapy, and Medicare expenditures among older patients dying with malignant brain tumors. Neuro Oncol. 2018; 20(7):986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Mehanna EK, Catalano PJ, Cagney DN, et al. . Hospice utilization in elderly patients with brain metastases. J Natl Cancer Inst. 2020; 112(12):1251–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Forst D, Adams E, Nipp R, et al. . Hospice utilization in patients with malignant gliomas. Neuro Oncol. 2018; 20(4):538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Walbert T, Puduvalli VK, Taphoorn MJB, Taylor AR, Jalali R. International patterns of palliative care in neuro-oncology: a survey of physician members of the Asian Society for Neuro-Oncology, the European Association of Neuro-Oncology, and the Society for Neuro-Oncology. Neurooncol Pract. 2015; 2(2):62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Rosenberg J, Massaro A, Siegler J, et al. . Palliative care in patients with high-grade gliomas in the neurological intensive care unit. Neurohospitalist. 2020; 10(3):163–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.