Abstract
Per- and polyfluoroalkyl substances (PFAS) are a set of synthetic chemicals which contain several carbon-fluorine (C–F) bonds and have been in production for the past eight decades. PFAS have been used in several industrial and consumer products including nonstick pans, food packaging, firefighting foams, and carpeting. PFAS require proper investigations worldwide due to their omnipresence in the biotic environment and the resulting pollution to drinking water sources. These harmful chemicals have been associated with adverse health effects such as liver damage, cancer, low fertility, hormone subjugation, and thyroid illness. In addition, these fluorinated compounds show high chemical, thermal, biological, hydrolytic, photochemical, and oxidative stability. Therefore, effective treatment processes are required for the removal and degradation of PFAS from wastewater, drinking water, and groundwater. Previous review papers have provided excellent summaries on PFAS treatment technologies, but the focus has been on the elimination efficiency without providing mechanistic understanding of removal/degradation pathways. The present review summarizes a comprehensive examination of various thermal and non-thermal PFAS destruction technologies. It includes sonochemical/ultrasound degradation, microwave hydrothermal treatment, subcritical or supercritical treatment, electrical discharge plasma technology, thermal destruction methods/incinerations, low/high-temperature thermal desorption process, vapor energy generator (VEG) technology and mechanochemical destruction. The background, degradation mechanisms/pathways, and advances of each remediation process are discussed in detail in this review.
Keywords: PFAS, Thermal degradation, Nonthermal degradation, Incineration, Mineralization
1. Introduction
Per- and polyfluoroalkyl substances (PFAS) are synthetic compounds with unique properties including high chemical and thermal stability, making them resistant to degradation and oxidation [1]. Since the 1940s, PFAS have been utilized in numerous consumer products, industrial applications, and aqueous film-forming foam deliveries operated for aerial firefighting [2,3]. The carbon-fluorine (C-F) bond in PFAS is thermodynamically robust and provides persistence, inertness, and stability to the perfluorinated molecule [4]. Therefore, PFAS are not readily biodegradable. These chemicals can enter the water cycle either via point sources (e.g., industrial, and municipal wastewater treatment plant sewage, industrial facilities, and firefighting training sites) or through atmospheric accumulation or nonpoint causes (e.g., groundwater and drainage penetration) (Fig. 1) [5,6]. Humans can be exposed to PFAS by consuming contaminated water and food, which can cause adverse health effects including thyroid disease, liver damage, and cancer [7]. The existence of perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) in groundwater have raised concerns including developmental effects to the fetus during pregnancy or nursing, liver effects, cancer, thyroid effects, and immune effects [8]. In response, the U.S. Environmental Protection Agency (U.S. EPA) has specified a new health advisory level of 0.02 ppt (ng/L) and 0.004 ppt for PFOS and PFOA in drinking water, respectively [9]. However, several water bodies near airports, military, and industrial sites exceed the U.S. EPA level [10,11].
Fig. 1.
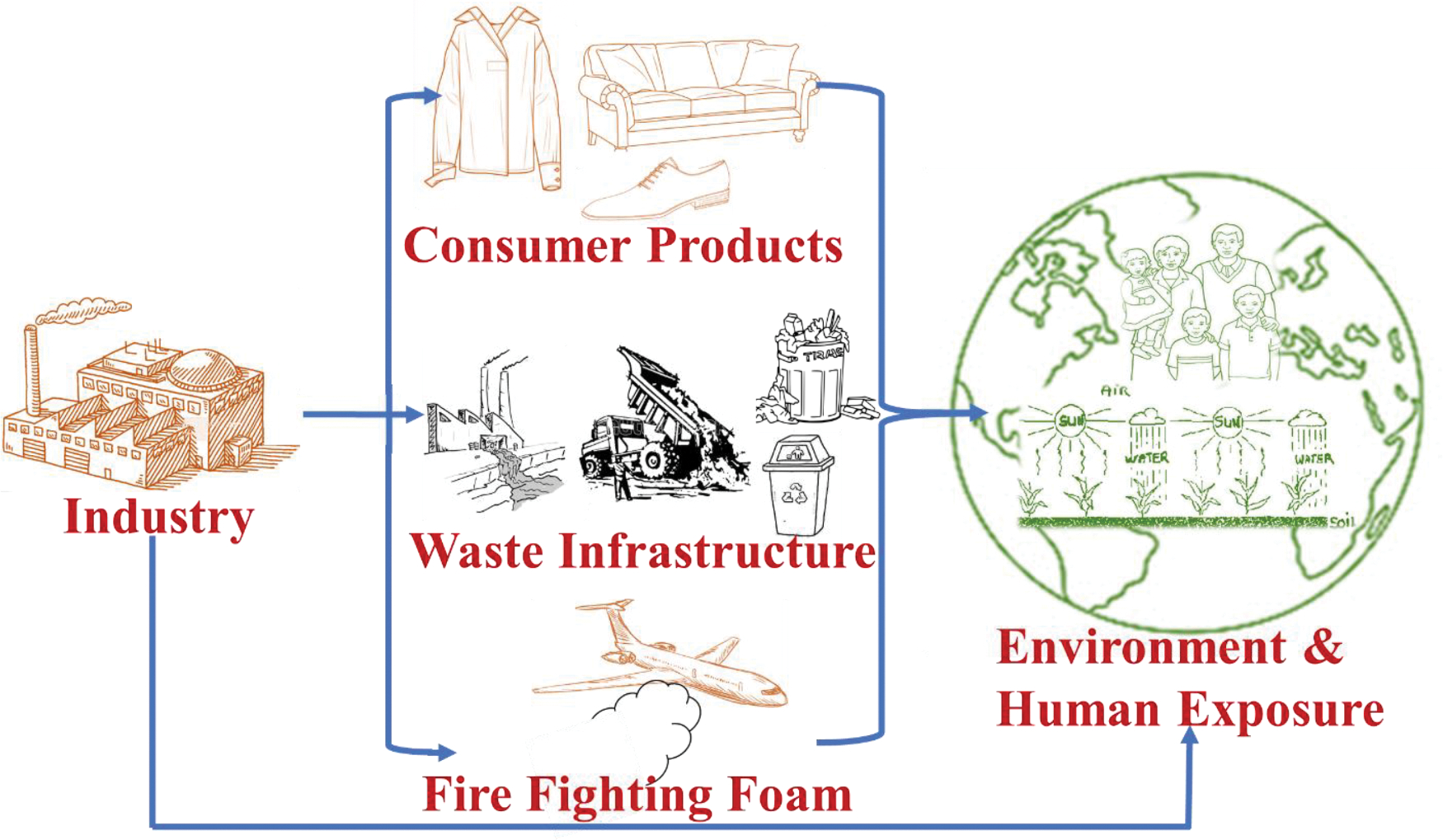
Understanding PFAS fate and transport.
Due to the persistence and documented toxic effects of PFAS, their elimination from water and wastewater is critical [12]. Various review articles have focused on PFAS occurrence, fate, transport, and treatment using various in-situ and ex-situ processes [5,6,11,13–19].The elimination of PFAS via adsorption, nanofiltration (NF) and reverse osmosis (RO), biological degradation, thermal degradation, photolysis, electrolysis, chemical oxidation, and reduction have been studied [14, 15,19]. PFAS treatment via adsorption has been studied using granular activated carbon (GAC), powdered activated carbon (PAC), anion exchange (AIX), molecularly imprinted polymers (MIP) and biocompatible materials [20]. Upon the different adsorbents, AIX has shown promise with achieving the highest adsorption capacity [21,22]. Yet, AIX is a costly process limiting its large-scale application. More importantly, adsorption processes require secondary treatment (i.e., regeneration of adsorbent) and waste management (i.e., disposal of spent adsorbent). Membrane separation processes including NF and RO have also shown promise in removing a wide range of PFAS, but their wider implementation is hampered by membrane fouling and high energy requirements [23]. Compared to physiochemical processes, the biological degradation of PFAS is challenged by the persistence of the C-F bond and the high negativity in F− [18]. From a technological perspective, advanced oxidation processes (e.g., chemical oxidation and reduction) have been successful in the complete mineralization of PFAS [24]. However, operational and technological requirements (e.g., slow reaction rates) have limited their large-scale application.
This review examines the literature for promising PFAS thermal and nonthermal treatment technologies including sonochemical/ultrasound degradation, microwave hydrothermal treatment, subcritical or supercritical treatment, electrical discharge plasma technology, thermal destruction methods/incinerations, low/high-temperature thermal desorption process, and vapor energy generator (VEG) technology.
2. Sonochemical/ultrasound degradation
In water treatment, sonochemistry involves the use of acoustic field to generate radicals to degrade contaminants in various aqueous media [25]. More specifically, acoustic cavitation (i.e., bubbles collapsing in solution due to sound waves) causes high temperature and pressure conditions resulting in the pyrolytic degradation of pollutants including PFAS at the bubble-water phase [13]. Most sonochemical studies for chemical pollutants’ degradation in aqueous media have been conducted at lab-scale (i.e., small volume) using ultrasonic irradiation at ambient pressures and temperatures [26–28]. In sonolysis, ultrasonic irradiation creates pressure waves generating small cavities in the aqueous medium [29,30]. More specifically, the soundwaves (i.e., sonowaves) induce localized areas of low- and high-pressure forming vapor bubbles (i.e., cavitation) that continue to grow and finally collapse causing a high temperature and pressure condition [31]. In these bubbles, the average internal vapor temperature increases to 4000 K, while bubble-water interface temperatures are generally between 600 K and 1000 K [32,33]. These momentary high temperatures assist in the in-situ pyrolysis of water into hydrogen atoms (H), oxygen atoms (O), and hydroxyl radicals (•OH) in the interfacial and vapor regions of each collapsing bubble [16]. The resulting radicals react quickly with organic molecules at the bubble interface or in the bubble interior gas-phase [34].
Ultrasonic dissociation has also been shown to help eliminate pollutants with high Henry’s Law constants that separate into the vapor phase or those pollutants that exist in the air-water interface [35,36]. Table 1 lists a variety of sonochemical treatment technologies that have been investigated for PFAS, primarily PFOA and PFOS. Moriwaki et al. investigated the sonolysis of PFOA (C0=10 ppm) and PFOS (C0=10 ppm) under an argon and oxygen atmosphere [37]. Under an argon atmosphere, this process showed promising results with pseudo-first-order rate constants of 0.16 and 0.32 min−1 for PFOS and PFOA, respectively. Liquid chromatography-mass spectroscopy (LC/MS) analysis revealed that most of the PFOS and PFOA molecules were decomposed at the interfacial area between the bulk solution and the cavitation bubbles [37]. In another study, the sonochemical degradation of groundwater beneath a landfill containing PFOA, PFOS, volatile organic compounds (VOCs), and dissolved organic matter (DOM) was explored. With organic components present, the sonolytic dissociation rate of PFOA and PFOS was reduced due to the competitive sorption at the bubble-water interface. However, the incorporation of ozonation with ultrasound increased the mineralization of PFOA and PFOS in landfill groundwater treatment [38]. Vecitis et al. also investigated sonolysis degradation of PFOA and PFOS in an aqueous solution. Their technology was determined to be effective for the complete mineralization of PFAS, ranging from 10 nM to 10 μM into carbon dioxide, carbon monoxide, fluoride, and sulfate [39]. The combination of the dual-transducer arrangement of ultrasonic and mega-sonic frequencies was also utilized for the dissociation of aqueous film-forming foam (AFFF) in bulk [40,41]. The presence of sulfate or bicarbonate ions diminished the sonolysis process, but perchlorate or nitrate present in solution increased the mineralization rate of PFAS under ultrasonic irradiation [42]. Additionally, the effect of co-surfactants such as anionic surfactant (e.g., sodium dodecyl sulfate (SDS)), non-ionic surfactant (e.g., octyl phenol ethoxylate (Triton X-110)) and cationic surfactant (e.g., hexadecyltrimethylammonium bromide (CTAB)) in the treatment of PFOA under ultrasonic irradiation was investigated [43]. CTAB enhanced the mineralization of PFOA at a lower pH, while SDS and Triton X-100 decreased the degree of degradation of PFOA. When a reaction mixture of PFOA (120 μM) and CTAB (0.12 mM) was treated under ultrasonic irradiation for 2 h at pH 4, 79% dissociation of PFOA was observed. Vecitis et al. studied the mineralization of PFOS in an aqueous dilution of FC-600 (an AFFF formulation) [44]. FC-600 is an AFFF formulation consisting of a mixture of hydrocarbon (HC) and fluorochemical components with co-solvents, anionic hydrocarbon surfactants, fluorinated amphiphilic surfactants, anionic fluorinated surfactants, and thickeners such as starch. PFOS was mineralized sonolytically in the scale of FC-600 aqueous dilutions, 65 ppb<[PFOS]<13100 ppb [44]. The degradation rate of the PFOS-AFFF system was found to be identical to PFOS-Milli-Q under the sonochemical condition. These studies showed that, initially, pyrolytic breakage of the carbon-sulfur (C-S) bond of PFOS occurred at the bubble-water interface [44]. Panchangam and his research group reported oxidative photodegradation of PFOA using TiO2 as a photocatalyst under sonication. This combination of photocatalyst and ultrasonic irradiation showed 65–70% degradation of PFOA (C0=50 ppm) within 7 h in relatively mild conditions such as ambient temperature and pressure and almost neutral pH [45].
Table 1.
Selected sonochemical PFAS degradation technologies.
| Type of solution | Type of PFAS and concentration | Atmosphere | Irradiation time (min), sonolytic frequency (kHz), and power density (W/L or W/cm2) | Degradation rate constant (min−1) | Yield | Ref. |
|---|---|---|---|---|---|---|
|
| ||||||
| Synthetic | PFOA (C0 = 10 ppm) and PFOS (C0 = 10 ppm) | air | 60 min | PFOA: 0.0155 | 63% | [37] |
| 200 kHz | PFOS: 0.0068 | 28% | ||||
| argon | PFOA: 0.032 | 85% | [37] | |||
| 200 W/L | PFOS: 0.016 | 60% | ||||
| Landfill/groundwater | PFOA (C0 = 100 ppb) and PFOS (C0 = 100 ppb) | argon | 120 min | PFOA: 0.021 | - | [38] |
| 354 kHz | ||||||
| 250 W/L | PFOS: 0.0094 | |||||
| Synthetic | PFOA (C0 = 30 ppb) and PFOS (C0 = 60 ppb) | argon | 180 min | PFOA: - | 44% | [39] |
| 358 kHz | PFOS: - | 39% | ||||
| 250 W/L | ||||||
| AFFF concentrate | PFOS (C0 = 65 ppb to 13,000 ppb) | argon | 120 min | - | 73% | [44] |
| 505 kHz | ||||||
| 188 W/L | ||||||
| Synthetic | PFOA (C0 = 0.24 μM) and PFOS (C0 = 0.2 μM) | argon | 120 min | PFOA: 0.041 | - | [46] |
| 358 kHz | PFOS: 0.027 | |||||
| 250 W/L | ||||||
| Synthetic | PFOS (C0 = 100 μM) | argon | 120 min | F− release rate of 3.58 | - | [40] |
| 500 kHz | μMmin−1 | |||||
| 8 W/cm2 | ||||||
| Synthetic | PFOA (C0 = 0.24 μM) and PFOS (C0 = 0.2 μM) | argon | 120 min | PFOA: 0.027 | - | [47] |
| 202 kHz | PFOS: 0.013 | |||||
| 250 W/L | ||||||
In summary, PFOA and PFOS can be sonochemically degraded via pyrolytic reactions at the water-bubble interface. Sonolytic PFAS mineralization is highly effective at a bench scale. However, PFAS treatment under sonication at a large scale has not been studied yet. Additionally, the proper optimization of parameters such as frequency and power should be considered in PFAS mineralization via sonication. Furthermore, the co-existence of other organic chemicals (e.g., humic substances) and inorganic chemicals (e.g., bicarbonate, sulfate) could also affect the sonochemical PFAS degradation. However, the integration of other techniques such as vacuum UV light irradiation, adsorption to sonochemical methods may enhance mineralization performance and support in reducing power requirements. For example, Zhao and his research group developed a combined technique of granular activated carbon (GAC) and ultrasound to treat PFAS effectively. The ultrasonic effect increased the adsorption of PFOS (C0=50 ppm) on GAC from 2.5 to 9 times [48]. Yang et al. also studied the combination of vacuum UV and ultrasonic irradiation for the mineralization of PFOS (10 ppm). This combined technology offered improved treatment of PFAS compared to sonolysis alone [49].
3. Microwave hydrothermal treatment
Microwave-hydrothermal processes have been widely used for their cost-effectiveness in preparing composite materials [50–52]. This process consumes up to 50% less energy and yields higher mineralization rates than conventional hydrothermal treatment methods [26,52,53]. Lee et al. investigated microwave-hydrothermal mineralization of PFOA in the presence of persulfate as an oxidant in the water at various temperatures: 60 °C, 90 °C, and 130 °C [54]. Table 2 summarizes the typical conditions and PFOA removal of various microwave hydrothermal treatment technologies. Persulfate generates active sulfate radicals (SO4•−) with high redox potential (2.6 V) and can degrade most organic pollutants (Eq. 1) [54–56]. PFOA was dissociated to non-measurable levels at 60 °C after 6 h of reaction. Microwave-hydrothermal treatment at higher temperatures increases the PFOA degradation rate. However, at exceedingly high temperatures such as 130 °C, persulfate generates a substantial number of active radicals that further react with residual persulfate (Eqs. 3 and 4), resulting in lower PFOA dissociation. The pH of solutions also affects the dissociation of PFOA. Solutions with higher pH have a slower reaction (Eq. 2) due to the formation of fewer active •OH radicals by the reaction of active sulfate radicals with −OH (Eq. 2). Hori et al. proposed the PFOA mineralization mechanism (Eqs. 5–(9). In this mechanism, sulfate-free radicals (from Eq. 1) oxidize PFOA (C7F15COOH) via hydrogen atom abstraction to form into the equivalent cationic radical i.e. [C7F15COOH]•+ (Eq. 5) which further generates an unstable perfluorinated alkyl radical •C7F15 (Eq. 6) [57]. This alkyl radical reacts with water to generate an unstable perfluorinated alcohol (C7F15OH; Eq. 7) and transforms into C6F13COF and HF (Eq. 8) [58]. Further, C6F13COF converts into perfluoroheptanoic acid (PFHpA, C6F13COOH; Eq. 9) via hydrolysis [59]. Other perfluorinated acids, such as perfluorohexanoic acid (PFHeA), perfluoropentanoic acid (PFPeA), and perfluorobutyric acid (PFBA), are observed by sequential oxidation of additional CF2 unit. At the end of the process, sulfate radicals completely mineralize the PFCAs into carbon dioxide (CO2) and fluoride (F−) [60].
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
| (9) |
Table 2.
Selected microwave hydrothermal treatment technologies for PFAS degradation.
| Type of solution | Type of PFAS and concentration | Conditions (additives, microwave energy and temperature) | Reaction time (hr) | Removal (%) | Defluorination efficiency (F− %) | Ref. |
|---|---|---|---|---|---|---|
|
| ||||||
| Synthetic | PFOA (C0 = 105 ppm) | 2.7 g/L Na2S2O8 70 W 90 °C |
4 hr | 85.7 % | 31.5 % | [56] |
| Synthetic | PFOA (C0 = 105 ppm) | 1.19 g/L Na2S2O8 70 W 90 °C |
4 hr | 79.1% | 70 % | [60] |
| Synthetic | PFOA (C0 = 100 ppm) | 1.19 g/L Na2S2O8 and 0.2 g/L zero-valent iron 70 W 90 °C |
2 hr | 67.6 % | 22.5 % | [61] |
A lab-scale approach of zero-valent iron (ZVI) and 5 Mm persulfate was examined for the microwave-hydrothermal degradation of PFOA at 60 °C and 90 °C [61]. This approach resulted in 67.6% decomposition of PFOA into short-chain PFCA and fluoride ions. ZVI not only degraded PFOA, but also generated Fe2+ (ferrous) ions under both anaerobic and aerobic conditions (Eqs. 10 and 11) [61,62]. These Fe2+ ions lower the activation energy of persulfate by generating sulfate free radicals at lower reaction temperature (Eq. 12). The synergetic effect of ZVI and persulfate increased the mineralization of PFOA and reduced the reaction time. Juxtaposing with conventional hydrothermal treatments, the microwave-hydrothermal method with ZVI and persulfate is quicker and a more energy-saving process for the degradation of perfluorinated carboxylic acids. However, scalability remains to be an obstacle of microwave-induced processes due to limited radiation depth and heat loss.
| (10) |
| (11) |
| (12) |
4. Subcritical or supercritical treatment
Subcritical or supercritical water treatments are eco-friendly and sustainable processes. Supercritical water occurs at temperatures >374 °C and at pressures >22.1 MPa. Alternatively, subcritical water is liquid water under pressure at temperatures between the boiling point, 100 °C, and 350 °C [63–65]. Previous studies have focused on hazardous waste destruction using subcritical and supercritical water [57,66–68]. Hori et al. examined the decomposition of PFOS and other short-chain (C2-C6) PFAS such as nonafluorobutanesulfonate, pentafluoroethanesulfonate, heptafluoropropanesulfonate, and perfluorohexanesulfonate in subcritical water [69]. They also studied the degradation of PFAS in the presence of metals including Al, Cu, Fe, and Zn powder in subcritical water [69]. PFOS showed minimal degradation in pure subcritical water, but the introduction of metal powder increased the PFOS mineralization in increasing order from no-metal ≈Al<Cu <Zn<<Fe (Table 3). The presence of iron supported the most effective PFOS dissociation. On the other hand, the order of redox potential over the series is Cu<Fe<Zn<Al. From these results, Hori et al. concluded that the metal surface and surface area play a greater role than its respective redox potential in the mineralization of PFOS. This phenomenon is applicable for ZVI and fluorinated species (PFOS) adsorbed on the iron surface even at room temperature. The adsorbed PFOS was then degraded into fluoride ions when the temperature was increased above 250°C. When the mixture of iron metal and an aqueous solution of PFOS (93–372 μM) was heated at 350°C for six hours, 46.2–51.4% PFOS degradation was obtained. This technique transformed PFOS into fluoride ions without any PFCA detection, though a small amount of fluoroform (CHF3) was observed [60]. Similar conditions were utilized for the elimination of perfluorohexanesulfonate (PFHS), an organic pollutant. In pure subcritical water at 350°C, little decomposition of PFHS was observed. On the other hand, in pure supercritical water at 380°C, it degraded into sulfate and fluoride ions. However, the incorporation of ZVI into the reaction process enhanced the degrdation of PFHS significantly [70]. Inspired by these studies, degradation of Nafion NRE-212, a model perfluorinated ion-exchange membrane applied for fuel cells, was explored in sub- and supercritical water in the presence of metal. The membrane demonstrated minimal decomposition in pure subcritical water, but the introduction of zero-valent metal enhanced the degradation of the membrane in the following order of Al< no metal<Zn<Cu<<Fe. When the mixture of membrane and ZVI were heated under the supercritical condition at 350°C for 17 h, 73.2% of the fluorine content of the membrane was converted into fluoride ions, and other intermediates including CF3COOH, HCF(CF3)OC2F4SO3, CO2, and HCF3 [71]. Hori et al. also investigated the mineralization of perfluorinated ionic liquid anions such as [(CF3SO2)2N]− and [(C4F9SO2)2N]− in subcritical and supercritical water to better understand the retrieval of the fluorine component. Similarly, the presence of ZVI enhanced the dissociation of perfluorinated ionic liquid anions. The mixture of [(CF3SO2)2N]− and ZVI yielded 69% fluoride ions at 344°C in six hours of reaction. This yield was 186 times higher than the yield without iron. Also, when the reaction time was increased to 18 h and the temperature was increased to 375°C, [(CF3SO2)2N]− converted 76.8% of the fluorine content into F− in the presence of ZVI [72].
Table 3.
Selected subcritical/supercritical treatment technologies for PFAS degradation.
| Type of solution | Type of PFAS and concentration | Qonditions (additives and temperature) | Reaction time (hr) | Removal (%) | Defluorination efficiency (F− %) | Ref. |
|---|---|---|---|---|---|---|
|
| ||||||
| Synthetic | PFOS (C0 = 186 ppm) | 54 g/L Fe 350 °C |
6 hr | >99 % | 51.4 % | [69] |
| Synthetic | PFOS (C0 = 186 ppm) | 62 g/L Zn 350 °C |
6 hr | 77 % | 18.5 % | [69] |
| Synthetic | PFOS (C0 = 186 ppm) | 61 g/L Cu 350 °C |
6 hr | 15.3 % | 6.8 % | [69] |
| Synthetic | PFOS (C0 = 186 ppm) | 25 g/L A1 350 °C |
6 hr | 6.4 % | 0.05 % | [69] |
| Synthetic | PFOA (C0 = 0.83 ppm) | 1008 g/L Nitric Acid 50 °C |
0.67 hr | 80 % | - | [73] |
| Synthetic | PFOS (C0 = 0.83 ppm) | 1008 g/L Nitric Acid and 20% Methanol 50 °C |
0.67 hr | 55 % | - | [73] |
Overall, sub- or supercritical treatment technology for PFAS mineralization could be an effective process for future applications. However, for industrial applications, additional studies at a large scale are required. Additionally, new methods should focus on improving the system design for low corrosion and salt build up.
5. Electrical discharge plasma technology
Plasma is a moderately or entirely ionized gas formed by electrical discharge [74,75]. It contains free neutrons, electrons, free radicals, ions, and atoms in heightened energy states. In terms of temperature and electron density, plasma systems can be characterized into two groups: nonthermal plasma process and thermal plasma process [76–79]. The nonthermal plasma process is associated with less power (i.e., dielectric barrier discharge, corona discharge, spark discharge, gliding arc discharge, and glow discharge). In the nonthermal generation process, energetic electrons collide with the oxygen (O2), nitrogen (N2), water (H2O) molecules and generate secondary electrons, ions, radicals, and photons [80–82]. Generation of plasma through thermal processes (typically torches or radiofrequency, arc discharge) is characterized by increased energy and plasma elements in thermal equilibrium [83–85]. High energy ions in plasma continuously degrade the carbon chains of PFAS [86]. Yasuoka et al. explored the decomposition of PFOA and PFOS in different plasmas using direct-current plasma produced within small gas bubbles in a solution [87]. The energy efficiency and degradation rate were estimated by determining the sulfate and fluoride ions isolated from PFOS/PFOA. The energy efficiency and concentration of F-ions in the PFOS were 26 mg kWh−1 and 17.7 mg/L, respectively, after 4 h of reaction [87]. Additionally, formic acid was introduced as a scavenger of hydrated electrons (e−aq) and phosphoric acid as a scavenger of hydroxyl radicals (•OH), but these demonstrated little effect on mineralization [87]. Another research group investigated PFOA decomposition using two processes: plasma treatment and sulfate radical anion treatment, where PFOA mineralized into carbon dioxide via interfacial reaction with the plasma [88].
Based on liquid chromatography-mass spectrometry studies, these authors proposed a PFOA decomposition scheme, as shown in Fig. 2 [88]. Firstly, PFOA acts as a surfactant and adsorbs to the gas-liquid interface during plasma treatment. Then, the sequential thermal cleavage of the PFOA carbon-carbon bonds occurs on the carbon chain edge, resulting in the generation of fluorocarbon radicals in the bubbles [88]. Further, fluorocarbon radicals react with plasma-produced H and •OH radicals and transform into CO, CO2, and HF via a redox reaction. Due to the high solubility of hydrogen fluoride gas, only carbon monoxide and carbon dioxide are released as the concluding products [88]. Another study explored the connection between the adsorbed amount of perfluorocarboxylic acids (PFCA) and the degree of dissociation by a direct-current plasma. The quantity of PFCA adsorbed at the gas-liquid interface increased for longer carbon chains, which subsequently improved the rate of mineralization [89]. Later, the same research group also investigated the complete degradation of PFOA (C0=41.4 ppm) and PFOS (C0=60 ppm) within oxygen bubbles by DC plasma after 3 h and 8 h of operation, respectively [90]. From LC/MS studies, the authors proposed plausible degradation pathways of PFOA (Eqs. 13–18) and PFOS (Eqs. 19–22) using DC plasma [90]. Initially, plasma-generated high-energy ions join with negatively charged ions of PFCAs on the surface of contaminated water. This process produces an electron and an unstable carboxyl radical (Eq. 13) [90]. Then, carbon dioxide and fluorocarbon radicals are formed via decarboxylation reaction (Eq. 14). The unstable fluorocarbon radical instantly reacts with water, and the carbon chain decreases by one, yielding PFCAs. In one study, Zhang et al. investigated the carbon-carbon bonds of PFOA cleaved by high energy vacuum ultraviolet light (184nm) [37]. Similarly, in this process, C-C bonds of higher energy ions could be broken (Eqs. 13–22) [90].
| (13) |
| (14) |
| (15) |
| (16) |
| (17) |
| (18) |
| (19) |
| (20) |
| (21) |
| (22) |
Fig. 2.
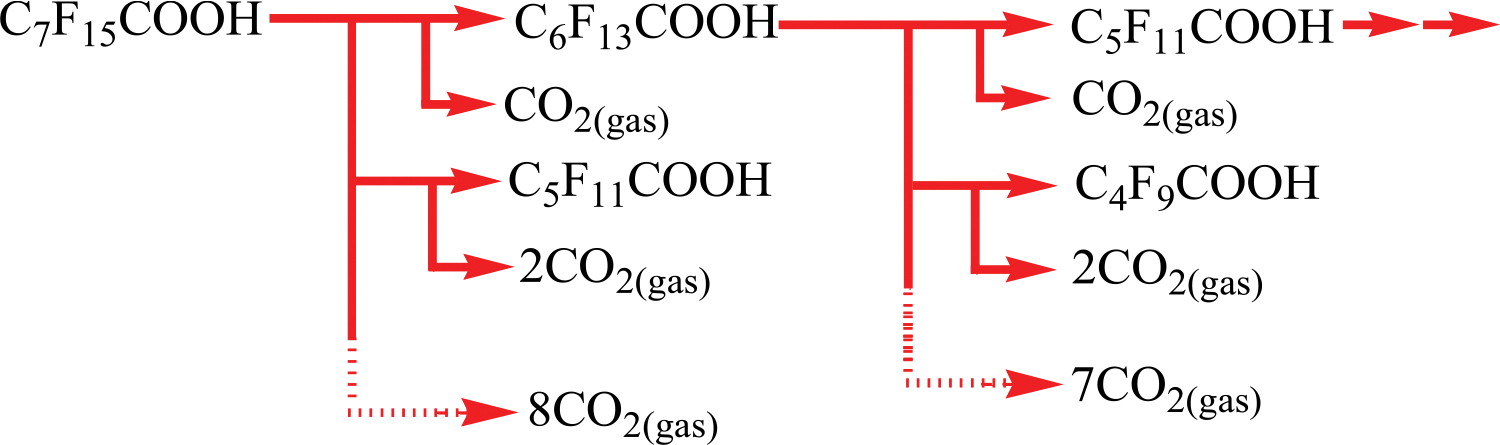
Mineralization processes of PFOA [78].
Singh and his research group used ultra-performance liquid chromatograph-quadrupole time-of-flight-high resolution mass spectrometry (UPLC-QTOF-HRMS) analysis to identify the by-products of PFAS mineralization by a plasma treatment process [91]. Based on their studies and the by-products quantified in the liquid phase, they proposed a mineralization mechanism for PFOA and PFOS (Fig. 3). Firstly, plasma-generated reactive species such as aqueous electrons, plasma electrons, and argon ions attack the carboxylic functional group (−COOH) of PFOA and generate unstable perfluoroalkyl radicals (•C7F15) [91]. This unstable •C7F15 radical reacts with •OH and converts into thermally unstable perfluoro alcohols (C7F15OH), which further transform into C6F13COF and HF by the attack of . The water reacts with C6F13COF and yields C6F13COOH and HF molecules. Therefore, chain propagation reactions, including oxidative and reductive species following hydrolysis, yield short chain perfluorinated carboxylic acid (PFCA). The mineralization pathway of PFOS seems to resemble that of PFOA. In the chain initiation reaction of PFOS, reactive species attack PFOS and form •C8F17 radicals by the cleavage of C-S bond [91]. Further, chain propagation reactions of •C8F17 lead to the generation of short-chain PFCA. The team identified 43 and 35 novel by-products of PFOA and PFOS, respectively, based on accurate mass measurements and isotopic profile [91].
Fig. 3.
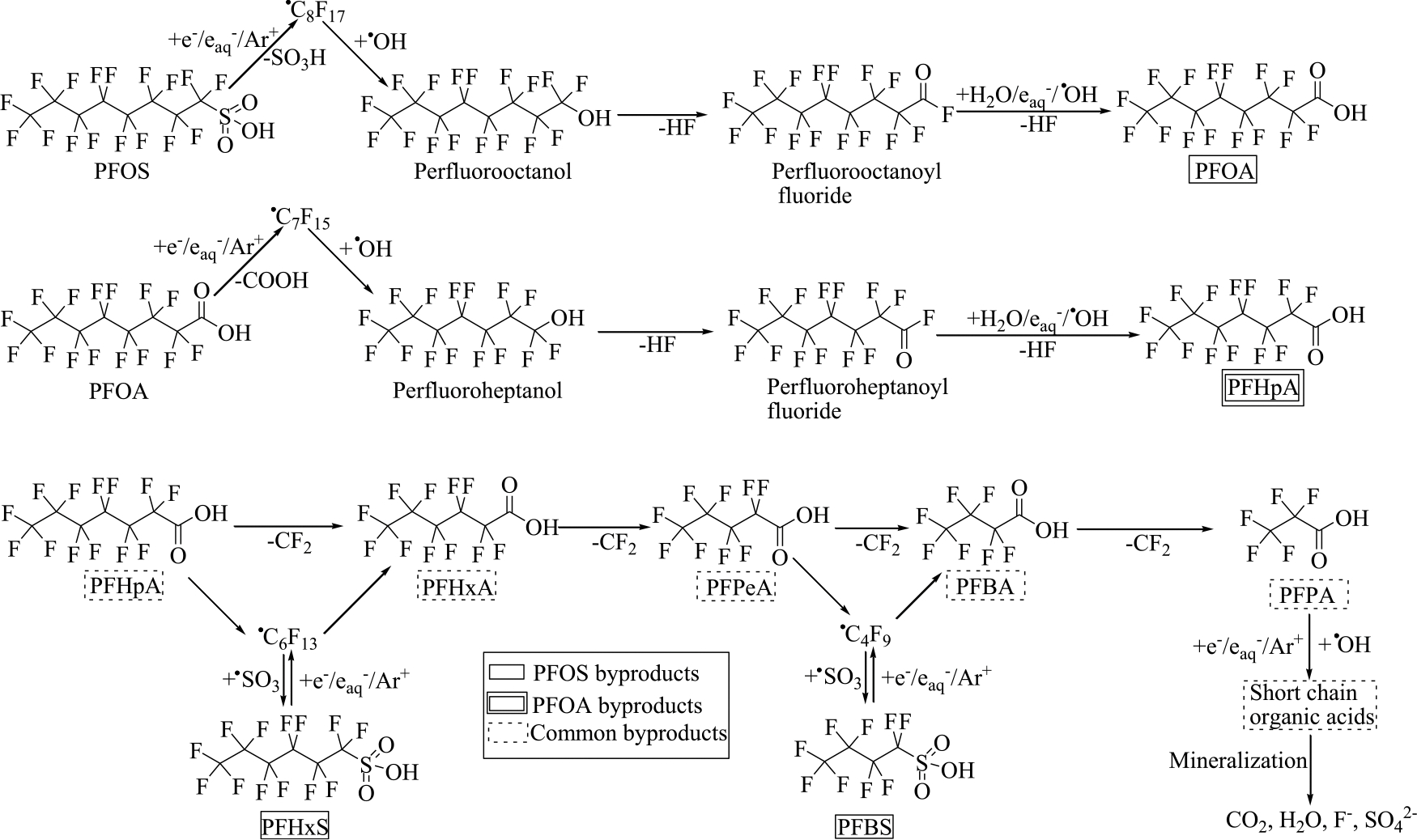
Proposed degradation pathway for PFOA and PFOS in plasma treatment [81].
Marouf-Khelifa et al. used TiO2 to catalyze the nonthermal process (NTP), Glidarc, for the degradation of perfluorinated non-ionic surfactant, Forafac 1110 (C6F13-C2H4(OC2H4)11.5OH) in aqueous solution [92]. Glidarc is characterized by the generation of an electric arc between two electrodes in a gaseous atmosphere. Reactive species such as NO• and •OH radicals are generated when the Glidarc is introduced to humid air plasma. Then, the NO• radical converts into NO2, and . These NO species show acidic properties and acidify the reaction, while hydroxyl radicals act as strong oxidizing agents to make Glidarc a robust oxidizer for the decomposition of PFAS [92]. The combination of heterogeneous catalysis (TiO2) with plasma-chemical treatment gave 96% mineralization of Forafac in one hour. Alternatively, six hours were required to accomplish the same degradation without the TiO2 catalyst [92].
| (23) |
| (24) |
| (25) |
| (26) |
| (27) |
| (28) |
Recently, nonthermal atmospheric plasma (NTAP) technology has been studied for the degradation of low concentrations (1 μg/L) of PFAS (mainly PFOA and PFOS) from polluted water samples taken from the soil cleaning process. The NTAP process can degrade 50% of the initial PFAS concentration in less than 200 s, and it can be utilized as an alternative tactic for the mineralization of PFAS [93].
Thus, plasma-based technologies are very efficient in the elimination of PFAS from both drinking water and groundwater. However, the co-existence of organic and inorganic contaminants affects the performance of plasma treatment processes. The assessment of by-products during plasma treatment should be considered for its practicability.
6. Thermal destruction (Incineration)
Incineration is a well-known mineralization pathway for the removal of harmful compounds, mostly toxic organic molecules, using heat [94–96]. Incineration is an energy intensive process, where high temperatures ranging from 600°C to 1000°C are applied to destroy harmful compounds [97,98]. Yet, there is an environmental tradeoff, where gaseous toxic substances can be released into the surrounding environment. Yamada et al. studied the thermal dissociation of a polyester/cellulose fabric substrate treated with a fluorotelomer-based acrylic polymer under conditions similar to a medical waste incinerator (MWI) and municipal waste combustor (MWC) processes in the US [99]. Thermal experiments were performed at non-flame reactor temperature ranging from 600°C to 1000°C. In this process, no detectible amount of PFOA was found using in-line gas chromatography/mass spectrometry (GC/MS). Hence, the burning of these wastes was not thought to be a source of PFOA to the environment [99]. Similarly, the kinetics of thermal degradation of ammonium perfluorooctanoate (APFO) were studied using high-temperature gas-phase nuclear magnetic resonance spectroscopy. However, in this process, volatile and toxic by-products such as 1-H-perfluoroheptane were observed [100]. Therefore, the burning of PFAS and foreign wastes was shown to release toxic substances including furan and dioxins [101,102].
In one study, the combustion of PFOS yielded greenhouse gases such as tetrafluoromethane (CF4) and hexafluoroethane (C2F6) [99]. These greenhouse gases show global warming potentials of 5,700 and 11,900, with long lifetimes of 50,000 and 10,000 years, respectively [103]. The fixation of these toxic by-products can be accomplished using certain additives such as calcium hydroxide [103].
The incineration approach was also utilized to evaluate the fate of PFAS during thermal regeneration of GAC [104,105]. PFOA, PFOS, and PFHxA adsorbed GAC were thermally treated in the nitrogen gas stream [104]. Volatile organic fluorine (VOF) measured 13.2, 5.9, and 4.8% for PFOA, PFOS, and PFHxA, respectively, at 700°C. However, VOF diminished to 0.1% at a higher temperature (1000°C). During reactivation of GAC via thermal regeneration, no PFAS were observed in GAC in the temperature range from 700 to 1000°C. Similarly, Xiao and his research group examined the thermal decomposition mechanism of seven perfluoroalkyl carboxylic acids (PFCA), three perfluoroalkyl sulfonic acids (PFSA), and one perfluoroalkyl ether carboxylic acid (PFECA) in different atmospheres (N2, O2, CO2, and air) on spent granular activated carbon (GAC) during thermal reactivation [106]. The proposed thermal decomposition pathways of PFOA based on the organic fluorine species identified by a thermal desorption-pyrolysis system (CDS Analytical) coupled to a gas chromatograph with an MS detector (TD-Pyr-GC-MS) are shown in Fig. 4 [106].
Fig. 4.
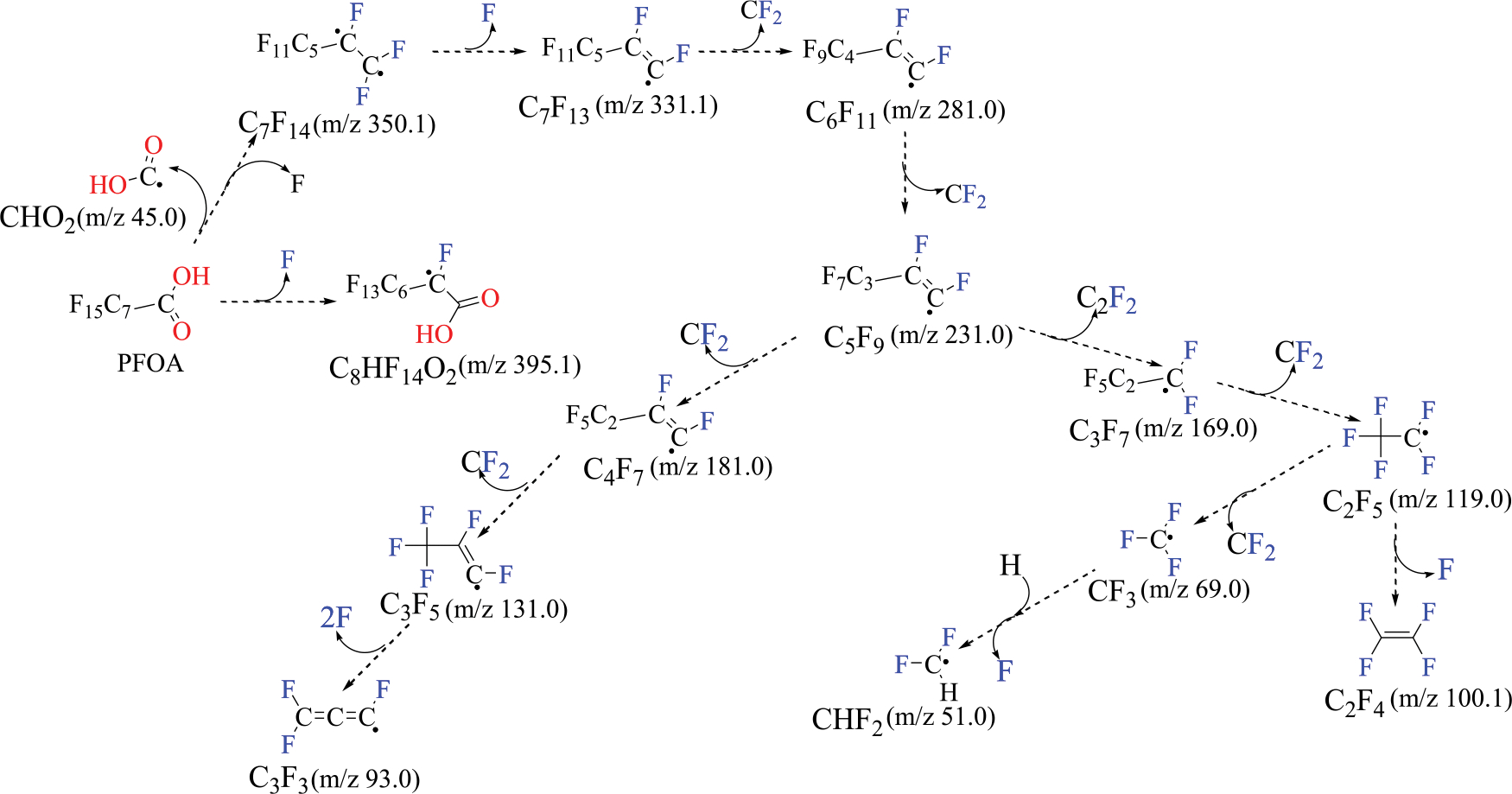
Proposed thermal decomposition pathways of PFOA [106].
Furthermore, PFAS have been detected in wastewater treatment plant effluent, influent and biosolids worldwide [107]. In one investigation on US biosolids, the major PFAS in biosolids were observed PFOA (34 ± 22 ppm dry weight) and PFOS (403 ± 127 ng/g dry weight) [108]. Research on the potential of pyrolysis and gasification processes to destroy PFAS in biosolids are extremely limited. Recently, in one study, >90% of PFOA and PFOS was safely removed from the biosolids via pyrolysis at the temperature range of 500°C - 600°C as part of a biochar generation process [109].
Ongoing investigations are currently exploring the thermal decomposition of PFAS, including catalytic destruction of PFAS at high temperatures [110]. Thermal treatment for PFAS elimination is under examination at the bench scale in Dandenong South, Victoria. Australia [111]. The environmental impact of incineration and thermal destruction methods for soils includes earth-moving equipment, transporting polluted soil, and storage in landfill. Incineration of contaminated soil is energy extensive. PFAS emissions and by-products from incinerators are currently not well understood. Therefore, further investigations are required to understand better the significance and viability of incineration in PFAS treatment and its generated by-products [111].
7. Low/high-temperature thermal desorption
Besides conventional thermal treatment (e.g., incineration), thermal desorption has been employed to heat contaminated soil ex situ or in situ, where the vaporized contaminants partition to the air phase. This thermal treatment process would require a polishing step with air filters to remove the vaporized contaminants. Thermal desorption process has been utilized extensively to treat soils contaminated with pesticides with comparable physicochemical properties to perfluorinated compounds [17,112]. Compared to incineration, this technique is less energy intensive and can still achieve high removal for most organic contaminants. For thermal desorption of PFAS, excavated soil has been treated at 500°C to 600°C in a rotary kiln to release PFAS into the gas stream [18, 113,114]. Then, PFAS have been mineralized at >1000°C via catalytic oxidation in the afterburner. The thermal desorption process looks to be a potential tactic for treatment of PFAS-contaminated soils. However, large scale studies have not been conducted on at PFAS specifically. At present, experimental information related to polyfluorinated precursor remediation is not available [18]. Also, for the thermal desorption process assessment, the mobilization cost of large rotary kilns and accompanying treatment rates should be measured. In another approach of thermal desorption, thermopiles have also been utilized. In this process, excavated soil is placed into shielded piles. These covered piles are heated in the range of 500°C-600°C using heater rods or diesel/gas burner to release PFAS into the vapor stream. These covered thermopiles are then secured under vacuum to extricate vapors and further subjected to condensers or thermal oxidizers to mineralize PFAS [18]. However, this method does not seem practical for PFAS mineralization, as the temperature of the soil should be kept between 500°C and 600°C for several weeks for efficient treatment [17,113].
Due to the high-temperature requirement, the thermal desorption process is expensive and requires a high preliminary investment in set-up.
8. Vapor energy generator (VEG) technology
Vapor energy generator (VEG) technology utilizes steam at 1100°C to degrade PFAS from contaminated soils in a chamber [115,116]. In this process, hydrogen gas is produced by the splitting water (H2O), and carbon monoxide (CO) is generated from the combustion of the organic fraction of soil. Then, the combination of hydrogen gas and carbon monoxide, which is known as syngas (H2+CO), burns and provides extra heat to the system. This process has a smaller functioning footprint, lower energy costs, and lesser organizational cost than thermal desorption systems. VEG technology was initially proposed by Endpoint Consulting Inc. for the bench-scale mineralization of PFAS in the soil [19]. Endpoint utilized VEG on spiked soil samples to study the treatment capability at 580°C, 595°C, and 950°C. VEG technology yielded 99% PFAS degradation within 30 min of treatment at 950°C. However, the company endorsed new bench-scale and scale-up tests of the VEG technology to establish the best treatment possibility [19]. The VEG process includes a compressed and high-efficiency steam generator patented by Endpoint Consulting Inc. (South San Francisco, CA). This is an ex-situ thermal desorption and mineralization method. Previously, VEG has been utilized for improved oil recovery for a range of intractable pollutants such as petroleum hydrocarbons, heavy-end oils, polychlorinated biphenyls, pesticides, selected metals (arsenic, zinc, and mercury), and polycyclic aromatic hydrocarbons, with ~45 full-size plants accomplished in the US [116].
To the best our knowledge, VEG’s full-scale application has not been studied precisely regarding PFAS. However, promising small-scale investigations on PFAS mineralization using VEG technology have been performed. VEG technology has been employed at several full-scale programs for non-PFAS toxins.
9. Mechanochemical destruction
Ball milling technology has been explored to treat PFAS-contaminated solid media, such as contaminated soils or residuals from desolvation of concentrated waste streams. The milling process is conducted at modest temperatures and pressures in the presence of co-milling reagents (e.g., potassium hydroxide [117], calcium oxide [118], alumina [119], sodium persulfate, and zero-valent iron [120]). The mechanochemical degradation of PFAS and the rapture of C–F and C–C bonds may be achieved either by amorphization of the crystal structure of PFAS and/or deforming valence bonds and angles under mechanical stress. Thus, the final milling powders would contain environmentally safe inorganic salts for disposal [120]. The exact destruction pathway is still unclear. Some earlier studies have suggested that PFAS molecules would first undergo decarboxylation or desulfonylation, then a sequential chain-shortening by one CF2 as each step, which is called the “flake-off” degradation mechanism [118]. However, a recent study has revealed new evidence using carbon and fluorine nuclear magnetic resonance spectroscopic (13C and 19F NMR) that the final milling products does not support the previously assumed pathway [121]. This might be attributed to the extreme conditions of localized high pressure and temperature during ball milling [122], which warrant further investigations to elucidate the destruction mechanisms.
10. Conclusions and future perspectives
PFAS are persistent in the environment due to their exceptional physical and chemical properties, so it is a challenge to eliminate them effectively from various environmental matrices. Significant efforts have focused on degradation of PFAS using thermal and non-thermal approaches. However, many of these techniques are still challenged with high energy consumption, low performance in the presence of competing ions and organic constituents typically present in real environmental matrices, and the generation of greenhouse gases and harmful by-products. In addition, most thermal and nonthermal treatment technologies have been utilized at the lab scale, and their large-scale applications have not been fully investigated precisely for PFAS mineralization.
The research path for future work should be focused on the efficient utilization of PFAS remediation processes in treatment trains. For the cost-efficient treatment of PFAS, combinations of different technologies should be examined in both the lab and large scale. For example, the incorporation of UV light irradiation or adsorption with the sonication process could enhance the elimination efficacy for PFAS and co-contaminants such as natural organic matter and humic acid. Perfluoroalkyl carboxylic acids (PFCAs) could be mineralized through a sodium hydroxide–mediated defluorination pathway. PFCA decarboxylation in polar aprotic solvents Dimethyl sulfoxide (DMSO) produced reactive perfluoroalkyl ion intermediates that degraded to fluoride ions (78 to ~100%) within 24 h [123]. Existing investigations have been restricted to realizing the elimination efficacies of one process under facile conditions. Preferably, the application of various research pathways will eventually deliver different treatment processes to removing PFAS at a reduced cost under practical field conditions.
11. Disclaimer
The United States Environmental Protection Agency through its Office of Research and Development funded and managed the research described here. It has been subjected to the Agency’s administrative review and approved for publication. The views expressed in this article are those of the author(s) and do not necessarily represent the U.S. Environmental Protection Agency’s views or policies. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Funding
No funding was received for this work.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- [1].Li Y, Oliver DP, Kookana RS, A critical analysis of published data to discern the role of soil and sediment properties in determining sorption of per and polyfluoroalkyl substances (PFASs), Sci. Total Environ. 628 (2018) 110–120. [DOI] [PubMed] [Google Scholar]
- [2].Gao K, Fu J, Xue Q, Li Y, Liang Y, Pan Y, Zhang A, Jiang G, An integrated method for simultaneously determining 10 classes of per-and polyfluoroalkyl substances in one drop of human serum, Anal. Chim. Acta 999 (2018) 76–86. [DOI] [PubMed] [Google Scholar]
- [3].Liu Y, Liu W, Xu Y, Zhao Y, Wang P, Yu S, Zhang J, Tang Y, Xiong G, Tao S, Characteristics and human inhalation exposure of ionic per-and polyfluoroalkyl substances (PFASs) in PM10 of cities around the Bohai Sea: Diurnal variation and effects of heating activity, Sci. Total Environ. 687 (2019) 177–187. [DOI] [PubMed] [Google Scholar]
- [4].Bentel MJ, Yu Y, Xu L, Li Z, Wong BM, Men Y, Liu J, Defluorination of per- and polyfluoroalkyl substances (PFASs) with hydrated electrons: structural dependence and implications to PFAS remediation and management, Environ. Sci. Technol. 53 (7) (2019) 3718–3728. [DOI] [PubMed] [Google Scholar]
- [5].Merino N, Qu Y, Deeb RA, Hawley EL, Hoffmann MR, Mahendra S, Degradation and removal methods for perfluoroalkyl and polyfluoroalkyl substances in water, Environ. Eng. Sci. 33 (9) (2016) 615–649. [Google Scholar]
- [6].Ahrens L, Bundschuh M, Fate and effects of poly-and perfluoroalkyl substances in the aquatic environment: a review, Environ. Toxicol. Chem. 33 (9) (2014) 1921–1929. [DOI] [PubMed] [Google Scholar]
- [7].Braun JM, Chen A, Romano ME, Calafat AM, Webster GM, Yolton K, Lanphear BP, Prenatal perfluoroalkyl substance exposure and child adiposity at 8 years of age: The HOME study, Obesity 24 (1) (2016) 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Verduzco R, Wong MS, Fighting PFAS with PFAS, ACS Publications, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Agency USEP, Drinking Water Health Advisory For Perfluorooctane Sulfonate (PFOS), Office of Water (4304T), Health and Ecological Criteria Division EPA, 2016. [Google Scholar]
- [10].Zhang X, Lohmann R, Dassuncao C, Hu XC, Weber AK, Vecitis CD, Sunderland EM, Source attribution of poly-and perfluoroalkyl substances (PFASs) in surface waters from rhode Island and the New York metropolitan area, Environ. Sci. Technol. Lett. 3 (9) (2016) 316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hu XC, Andrews DQ, Lindstrom AB, Bruton TA, Schaider LA, Grandjean P, Lohmann R, Carignan CC, Blum A, Balan SA, Detection of poly-and perfluoroalkyl substances (PFASs) in US drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants, Environ. Sci. Technol. Lett. 3 (10) (2016) 344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Flynn RW, Chislock MF, Gannon ME, Bauer SJ, Tornabene BJ, Hoverman JT, Sepúlveda MS, Acute and chronic effects of perfluoroalkyl substance mixtures on larval American bullfrogs (Rana catesbeiana), Chemosphere 236 (2019), 124350. [DOI] [PubMed] [Google Scholar]
- [13].Kucharzyk KH, Darlington R, Benotti M, Deeb R, Hawley E, Novel treatment technologies for PFAS compounds: a critical review, J. Environ. Manag. 204 (2017) 757–764. [DOI] [PubMed] [Google Scholar]
- [14].Ahmed MB, Alam MM, Zhou JL, Xu B, Johir MAH, Karmakar AK, Rahman MS, Hossen J, Hasan AK, Moni MA, Advanced treatment technologies efficacies and mechanism of per-and poly-fluoroalkyl substances removal from water, Process Safety and Environ. Protection 136 (2020) 1–14. [Google Scholar]
- [15].Crone BC, Speth TF, Wahman DG, Smith SJ, Abulikemu G, Kleiner EJ, Pressman JG, Occurrence of per-and polyfluoroalkyl substances (PFAS) in source water and their treatment in drinking water, Critical Rev. Environ. Sci. Technol. 49 (24) (2019) 2359–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Espana VAA, Mallavarapu M, Naidu R, Treatment technologies for aqueous perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA): A critical review with an emphasis on field testing, Environ. Technol. Innovation 4 (2015) 168–181. [Google Scholar]
- [17].Sznajder-Katarzyńska K, Surma M, Cieślik I, A review of perfluoroalkyl acids (PFAAs) in terms of sources, applications, human exposure, dietary intake, toxicity, legal regulation, and methods of determination, J. Chem. 2019 (2019). [Google Scholar]
- [18].Ross I, McDonough J, Miles J, Storch P, Thelakkat Kochunarayanan P, Kalve E, Hurst J, Dasgupta SS, Burdick J, A review of emerging technologies for remediation of PFASs, Remediation J. 28 (2) (2018) 101–126. [Google Scholar]
- [19].Nzeribe BN, Crimi M, Mededovic Thagard S, Holsen TM, Physico-chemical processes for the treatment of per-and polyfluoroalkyl substances (PFAS): a review, Critical Rev. Environ. Sci. Technol. 49 (10) (2019) 866–915. [Google Scholar]
- [20].Appleman TD, Dickenson ER, Bellona C, Higgins C.P.J.J.o.h.m., Nanofiltration and granular activated carbon treatment of perfluoroalkyl acids, 260 (2013) 740–746. [DOI] [PubMed] [Google Scholar]
- [21].Appleman TD, Higgins CP, Quiñones O, Vanderford BJ, Kolstad C, Zeigler-Holady JC, Dickenson E.R.J.W.r., Treatment of poly-and perfluoroalkyl substances in US full-scale water treatment systems, 51 (2014) 246–255. [DOI] [PubMed] [Google Scholar]
- [22].Yu Q, Zhang R, Deng S, Huang J, Yu G.J.W.r., Sorption of perfluorooctane sulfonate and perfluorooctanoate on activated carbons and resin: kinetic and isotherm study, 43(4) (2009) 1150–1158. [DOI] [PubMed] [Google Scholar]
- [23].Dickenson E, Higgins C, Treatment mitigation strategies for poly-and perfluoroalkyl substances, Water Res. Foundation Web Report 4322 (2016). [Google Scholar]
- [24].Wanninayake DM, Comparison of currently available PFAS remediation technologies in water: a review, J. Environ. Manag. 283 (2021), 111977. [DOI] [PubMed] [Google Scholar]
- [25].Rayaroth MP, Aravind UK, Aravindakumar CT, Degradation of pharmaceuticals by ultrasound-based advanced oxidation process, Environ. Chem. Lett. 14 (3) (2016) 259–290. [Google Scholar]
- [26].Khataee A, Soltani RDC, Karimi A, Joo SW, Sonocatalytic degradation of a textile dye over Gd-doped ZnO nanoparticles synthesized through sonochemical process, Ultrasonics Sonochem. 23 (2015) 219–230. [DOI] [PubMed] [Google Scholar]
- [27].Areerob Y, Cho JY, Jang WK, Oh W-C, Enhanced sonocatalytic degradation of organic dyes from aqueous solutions by novel synthesis of mesoporous Fe3O4-graphene/ZnO@ SiO2 nanocomposites, Ultrasonics Sonochem. 41 (2018) 267–278. [DOI] [PubMed] [Google Scholar]
- [28].Wei Z, Spinney R, Ke R, Yang Z, Xiao R, Effect of pH on the sonochemical degradation of organic pollutants, Environ. Chem. Lett. 14 (2) (2016) 163–182. [Google Scholar]
- [29].Ince N, Tezcanli G, Belen R, Apikyan IG, Ultrasound as a catalyzer of aqueous˙ reaction systems: the state of the art and environmental applications, Appl. Catalysis B: Environ. 29 (3) (2001) 167–176. [Google Scholar]
- [30].Sajjadi B, Raman AAA, Ibrahim S, Influence of ultrasound power on acoustic streaming and micro-bubbles formations in a low frequency sono-reactor: Mathematical and 3D computational simulation, Ultrasonics Sonochem. 24 (2015) 193–203. [DOI] [PubMed] [Google Scholar]
- [31].Rayaroth MP, Aravindakumar CT, Shah NS, Boczkaj G, Advanced oxidation processes (AOPs) based wastewater treatment-unexpected nitration side reactions-a serious environmental issue: a review, Chem. Eng. J. 430 (2022), 133002. [Google Scholar]
- [32].Ohl S-W, Klaseboer E, Khoo BC, Bubbles with shock waves and ultrasound: a review, Interface Focus 5 (5) (2015), 20150019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Stricker L, Acoustic Cavitation and Sonochemistry, Universiteit Twente, 2013. [Google Scholar]
- [34].Ciawi E, Rae J, Ashokkumar M, Grieser F, Determination of temperatures within acoustically generated bubbles in aqueous solutions at different ultrasound frequencies, The J. Phys. Chem. B 110 (27) (2006) 13656–13660. [DOI] [PubMed] [Google Scholar]
- [35].Henglein A, Kormann C, Scavenging of OH radicals produced in the sonolysis of water, Int. J. Radiation Biol. Related Stud. Phys. Chem. Med. 48 (2) (1985) 251–258. [DOI] [PubMed] [Google Scholar]
- [36].Yang L, Sostaric JZ, Rathman JF, Kuppusamy P, Weavers LK, Effects of pulsed ultrasound on the adsorption of n-alkyl anionic surfactants at the gas/solution interface of cavitation bubbles, The J. Phys. Chem. B 111 (6) (2007) 1361–1367. [DOI] [PubMed] [Google Scholar]
- [37].Moriwaki H, Takagi Y, Tanaka M, Tsuruho K, Okitsu K, Maeda Y, Sonochemical decomposition of perfluorooctane sulfonate and perfluorooctanoic acid, Environ. Sci. Technol. 39 (9) (2005) 3388–3392. [DOI] [PubMed] [Google Scholar]
- [38].Cheng J, Vecitis CD, Park H, Mader BT, Hoffmann MR, Sonochemical degradation of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in landfill groundwater: environmental matrix effects, Environ. Sci. Technol. 42 (21) (2008) 8057–8063. [DOI] [PubMed] [Google Scholar]
- [39].Vecitis CD, Park H, Cheng J, Mader BT, Hoffmann MR, Kinetics and mechanism of the sonolytic conversion of the aqueous perfluorinated surfactants, perfluorooctanoate (PFOA), and perfluorooctane sulfonate (PFOS) into inorganic products, The J. Phys. Chem. A 112 (18) (2008) 4261–4270. [DOI] [PubMed] [Google Scholar]
- [40].Rodriguez-Freire L, Balachandran R, Sierra-Alvarez R, Keswani M, Effect of sound frequency and initial concentration on the sonochemical degradation of perfluorooctane sulfonate (PFOS), J. Hazardous Mater. 300 (2015) 662–669. [DOI] [PubMed] [Google Scholar]
- [41].Keswani M, Complete mineralization of fluorochemicals in aqueous fire-fightin foams using a novel dual-frequency based sonochemical process.
- [42].Cheng J, Vecitis CD, Park H, Mader BT, Hoffmann MR, Sonochemical degradation of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in groundwater: kinetic effects of matrix inorganics, Environ. Sci. Technol. 44 (1) (2010) 445–450. [DOI] [PubMed] [Google Scholar]
- [43].Lin J-C, Hu C-Y, Lo S-L, Effect of surfactants on the degradation of perfluorooctanoic acid (PFOA) by ultrasonic (US) treatment, Ultrasonics Sonochem. 28 (2016) 130–135. [DOI] [PubMed] [Google Scholar]
- [44].Vecitis CD, Wang Y, Cheng J, Park H, Mader BT, Hoffmann MR, Sonochemical degradation of perfluorooctanesulfonate in aqueous film-forming foams, Environ. Sci. Technol. 44 (1) (2010) 432–438. [DOI] [PubMed] [Google Scholar]
- [45].Panchangam SC, Lin AY-C, Tsai J-H, Lin C-F, Sonication-assisted photocatalytic decomposition of perfluorooctanoic acid, Chemosphere 75 (5) (2009) 654–660. [DOI] [PubMed] [Google Scholar]
- [46].Campbell TY, Vecitis CD, Mader BT, Hoffmann MR, Perfluorinated surfactant chain-length effects on sonochemical kinetics, The J. Phys. Chem. A 113 (36) (2009) 9834–9842. [DOI] [PubMed] [Google Scholar]
- [47].Campbell T, Hoffmann MR, Sonochemical degradation of perfluorinated surfactants: power and multiple frequency effects, Separation and Purification Technol. 156 (2015) 1019–1027. [Google Scholar]
- [48].Zhao D, Cheng J, Vecitis CD, Hoffmann MR, Sorption of perfluorochemicals to granular activated carbon in the presence of ultrasound, The J. Phys. Chem. A 115 (11) (2011) 2250–2257. [DOI] [PubMed] [Google Scholar]
- [49].Yang SW, Sun J, Hu YY, Cheng JH, Liang XY, Effect of vacuum ultraviolet on ultrasonic defluorination of aqueous perfluorooctanesulfonate, Chem. Eng. J. 234 (2013) 106–114. [Google Scholar]
- [50].Andjelkovic I, Jovic B, Jovic M, Markovic M, Stankovic D, Manojlovic D, Roglic G, Microwave-hydrothermal method for the synthesis of composite materials for removal of arsenic from water, Environ. Sci. Pollut. Res. 23 (1) (2016) 469–476. [DOI] [PubMed] [Google Scholar]
- [51].Murugan AV, Muraliganth T, Manthiram A, One-pot microwave-hydrothermal synthesis and characterization of carbon-coated LiMPO4 (M= Mn, Fe, and Co) cathodes, J. Electrochem. Soc. 156 (2) (2008) A79. [Google Scholar]
- [52].Yang G, Park SJ, Conventional and microwave hydrothermal synthesis and application of functional materials: a review, Materials 12 (7) (2019) 1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Jones DA, Lelyveld T, Mavrofidis S, Kingman S, Miles N, Microwave heating applications in environmental engineering—a review, Resour. Conserv. Recycl. 34 (2) (2002) 75–90. [Google Scholar]
- [54].Lee YC, Lo SL, Kuo J, Lin YL, Persulfate oxidation of perfluorooctanoic acid under the temperatures of 20–40 C, Chem. Eng. J. 198 (2012) 27–32. [Google Scholar]
- [55].Huang KC, Zhao Z, Hoag GE, Dahmani A, Block PA, Degradation of volatile organic compounds with thermally activated persulfate oxidation, Chemosphere 61 (4) (2005) 551–560. [DOI] [PubMed] [Google Scholar]
- [56].Lee Y, Lo S, Kuo J, Hsieh C, Decomposition of perfluorooctanoic acid by microwaveactivated persulfate: Effects of temperature, pH, and chloride ions, Front. Environ. Sci. Eng. 6 (1) (2012) 17–25. [Google Scholar]
- [57].Hori H, Saito H, Sakai H, Kitahara T, Sakamoto T, Efficient decomposition of a new fluorochemical surfactant: Perfluoroalkane disulfonate to fluoride ions in subcritical and supercritical water, Chemosphere 129 (2015) 27–32. [DOI] [PubMed] [Google Scholar]
- [58].Nohara K, Toma M, Kutsuna S, Takeuchi K, Ibusuki T, Cl atom-initiated oxidation of three homologous methyl perfluoroalkyl ethers, Environ. Sci. Technol. 35 (1) (2001) 114–120. [DOI] [PubMed] [Google Scholar]
- [59].de Bruyn WJ, Shorter JA, Davidovits P, Worsnop DR, Zahniser MS, Kolb CE, Uptake of haloacetyl and carbonyl halides by water surfaces, Environ. Sci. Technol. 29 (5) (1995) 1179–1185. [DOI] [PubMed] [Google Scholar]
- [60].Lee Y-C, Lo S-L, Chiueh P-T, Chang D-G, Efficient decomposition of perfluorocarboxylic acids in aqueous solution using microwave-induced persulfate, Water Res. 43 (11) (2009) 2811–2816. [DOI] [PubMed] [Google Scholar]
- [61].Lee Y-C, Lo S-L, Chiueh P-T, Liou Y-H, Chen M-L, Microwave-hydrothermal decomposition of perfluorooctanoic acid in water by iron-activated persulfate oxidation, Water Res. 44 (3) (2010) 886–892. [DOI] [PubMed] [Google Scholar]
- [62].Furukawa Y, Kim J.-w., Watkins J, Wilkin RT, Formation of ferrihydrite and associated iron corrosion products in permeable reactive barriers of zero-valent iron, Environ. Sci. Technol. 36 (24) (2002) 5469–5475. [DOI] [PubMed] [Google Scholar]
- [63].Möller M, Nilges P, Harnisch F, Schröder U, Subcritical water as reaction environment: fundamentals of hydrothermal biomass transformation, Chem. Sus Chem. 4 (5) (2011) 566–579. [DOI] [PubMed] [Google Scholar]
- [64].Hanim SS, Norsyabilah R, Suhaila MN, Noraishah A, Kartina AS, Effects of temperature, time and pressure on the hemicelluloses yield extracted using subcritical water extraction, Procedia Eng. 42 (2012) 562–565. [Google Scholar]
- [65].Tavakoli O, Yoshida H, Effective recovery of harmful metal ions from squid wastes using subcritical and supercritical water treatments, Environ. Sci. Technol. 39 (7) (2005) 2357–2363. [DOI] [PubMed] [Google Scholar]
- [66].Peterson AA, Vogel F, Lachance RP, Fröling M, Antal MJ Jr, Tester JW, Thermochemical biofuel production in hydrothermal media: a review of sub-and supercritical water technologies, Energy & Environ. Sci. 1 (1) (2008) 32–65. [Google Scholar]
- [67].Cocero MJ, Supercritical water processes: Future prospects, The J. Supercritical Fluids 134 (2018) 124–132. [Google Scholar]
- [68].Takahashi F, Sun Z, Fukushi K, Oshima Y, Yamamoto K, Catalytic oxidation of acetic acid over sodium titanate synthesized hydrothermally in supercritical water, The J. Supercritical Fluids 61 (2012) 126–133. [Google Scholar]
- [69].Hori H, Nagaoka Y, Yamamoto A, Sano T, Yamashita N, Taniyasu S, Kutsuna S, Osaka I, Arakawa R, Efficient decomposition of environmentally persistent perfluorooctanesulfonate and related fluorochemicals using zerovalent iron in subcritical water, Environ. Sci. Technol. 40 (3) (2006) 1049–1054. [DOI] [PubMed] [Google Scholar]
- [70].Hori H, Nagaoka Y, Sano T, Kutsuna S, Iron-induced decomposition of perfluorohexanesulfonate in sub-and supercritical water, Chemosphere 70 (5) (2008) 800–806. [DOI] [PubMed] [Google Scholar]
- [71].Hori H, Murayama M, Sano T, Kutsuna S, Decomposition of perfluorinated ion-exchange membrane to fluoride ions using zerovalent metals in subcritical water, Ind. Eng. Chem. Res. 49 (2) (2010) 464–471. [Google Scholar]
- [72].Hori H, Noda Y, Takahashi A, Sakamoto T, Decomposition of perfluorinated ionic liquid anions to fluoride ions in subcritical and supercritical water with iron-based reducing agents, Ind. Eng. Chem. Res. 52 (38) (2013) 13622–13628. [Google Scholar]
- [73].Chen H-Y, Liao W, Wu B-Z, Nian H, Chiu K, Yak H-K, Removing perfluorooctane sulfonate and perfluorooctanoic acid from solid matrices, paper, fabrics, and sand by mineral acid suppression and supercritical carbon dioxide extraction, Chemosphere 89 (2) (2012) 179–184. [DOI] [PubMed] [Google Scholar]
- [74].Cui Y, Cheng J, Chen Q, Yin Z, The types of plasma reactors in wastewater treatment, in: IOP Conference Series: Earth and Environmental Science, IOP Publishing, 2018, 012002. [Google Scholar]
- [75].Horikoshi S, Serpone N, In-liquid plasma: A novel tool in the fabrication of nanomaterials and in the treatment of wastewaters, RSC Adv. 7 (75) (2017) 47196–47218. [Google Scholar]
- [76].Foster JE, Plasma-based water purification: Challenges and prospects for the future, Phys. Plasmas 24 (5) (2017), 055501. [Google Scholar]
- [77].Attri P, Tochikubo F, Park JH, Choi EH, Koga K, Shiratani M, Impact of Gamma rays and DBD plasma treatments on wastewater treatment, Sci. Reports 8 (1) (2018) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Reddy PMK, Subrahmanyam C, Green approach for wastewater treatment degradation and mineralization of aqueous organic pollutants by discharge plasma, Ind. Eng. Chem. Res. 51 (34) (2012) 11097–11103. [Google Scholar]
- [79].Chang J, Thermal plasma solid waste and water treatments: a critical review, Int. J. Plasma Environ. Sci. Technol. 3 (2) (2009) 67–84. [Google Scholar]
- [80].Rezaei F, Vanraes P, Nikiforov A, Morent R, De Geyter N, Applications of plasma-liquid systems: a review, Materials 12 (17) (2019) 2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].López M, Calvo T, Prieto M, Múgica-Vidal R, Múro-Fraguas I, Alba-Elías F, Alvarez-Ordóñez A, A review on non-thermal atmospheric plasma for food preservation: Mode of action, determinants of effectiveness, and applications, Front. Microbiol. 10 (2019) 622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].De Geyter N, Morent R, Nonthermal plasma sterilization of living and nonliving surfaces, Ann. Rev. Biomed. Eng. 14 (2012) 255–274. [DOI] [PubMed] [Google Scholar]
- [83].Taylor PR, Pirzada SA, Thermal plasma processing of materials: A review, Adv. Performance Mater. 1 (1) (1994) 35–50. [Google Scholar]
- [84].Samal S, Thermal plasma technology: The prospective future in material processing, J. Clean. Prod. 142 (2017) 3131–3150. [Google Scholar]
- [85].Tanaka Y, Fujino T, Iwao T, Review of thermal plasma simulation technique, IEEJ Trans. Electric. Electron. Eng. 14 (11) (2019) 1582–1594. [Google Scholar]
- [86].Stratton GR, Dai F, Bellona CL, Holsen TM, Dickenson ER, Mededovic Thagard S, Plasma-based water treatment: efficient transformation of perfluoroalkyl substances in prepared solutions and contaminated groundwater, Environ. Sci. Technol. 51 (3) (2017) 1643–1648. [DOI] [PubMed] [Google Scholar]
- [87].Yasuoka K, Sasaki K, Hayashi R, An energy-efficient process for decomposing perfluorooctanoic and perfluorooctane sulfonic acids using dc plasmas generated within gas bubbles, Plasma Sources Sci.Technol. 20 (3) (2011), 034009. [Google Scholar]
- [88].Takeuchi N, Kitagawa Y, Kosugi A, Tachibana K, Obo H, Yasuoka K, Plasma–liquid interfacial reaction in decomposition of perfluoro surfactants, J. Phys. D: Appl. Phys. 47 (4) (2013), 045203. [Google Scholar]
- [89].Matsuya Y, Takeuchi N, Yasuoka K, Relationship between reaction rate of perfluorocarboxylic acid decomposition at a plasma–liquid interface and adsorbed amount, Electric. Eng. Japan 188 (2) (2014) 1–8. [Google Scholar]
- [90].Hayashi R, Obo H, Takeuchi N, Yasuoka K, Decomposition of perfluorinated compounds in water by DC plasma within oxygen bubbles, Electric. Eng. Japan 190 (3) (2015) 9–16. [Google Scholar]
- [91].Singh RK, Fernando S, Baygi SF, Multari N, Thagard SM, Holsen TM, Breakdown products from perfluorinated alkyl substances (PFAS) degradation in a plasma-based water treatment process, Environ. Sci. Technol. 53 (5) (2019) 2731–2738. [DOI] [PubMed] [Google Scholar]
- [92].Marouf-Khelifa K, Abdelmalek F, Khelifa A, Addou A, TiO2-assisted degradation of a perfluorinated surfactant in aqueous solutions treated by gliding arc discharge, Chemosphere 70 (11) (2008) 1995–2001. [DOI] [PubMed] [Google Scholar]
- [93].Jovicic V, Khan MJ, Zbogar-Rasic A, Fedorova N, Poser A, Swoboda P, Delgado A, Degradation of low concentrated perfluorinated compounds (PFCS) from water samples using non-thermal atmospheric plasma (NTAP), Energies 11 (5) (2018) 1290. [Google Scholar]
- [94].Aleksandrov K, Gehrmann H-J, Hauser M, Mätzing H, Pigeon D, Stapf D, Wexler M, Waste incineration of Polytetrafluoroethylene (PTFE) to evaluate potential formation of per-and poly-fluorinated alkyl substances (PFAS) in flue gas, Chemosphere 226 (2019) 898–906. [DOI] [PubMed] [Google Scholar]
- [95].Lind A-S, An assessment of thermal desorption as a remediation technique for per-and polyfluoroalkyl substances (PFASs) in contaminated soil, 2018.
- [96].Stockenhuber S, Weber N, Dixon L, Lucas J, Grimison C, Bennett M, Stockenhuber M, Mackie J, Kennedy E, Thermal degradation of perfluorooctanoic acid (PFOA), 16th internationl conferenc on environmental science and technololgy rhodes, Greece (2019). [Google Scholar]
- [97].Arkenbout A, Long-Term Sampling Emission of PFOS and PFOA of a Waste-to-Energy Incinerator, NGO ToxicoWatch Foundation, Harlingen, The Netherlands, 2018. [Google Scholar]
- [98].Lundin L, Jansson S, A desktop study on destruction of persistent organic compounds in combustion systems, 2017.
- [99].Yamada T, Taylor PH, Buck RC, Kaiser MA, Giraud RJ, Thermal degradation of fluorotelomer treated articles and related materials, Chemosphere 61 (7) (2005) 974–984. [DOI] [PubMed] [Google Scholar]
- [100].Krusic PJ, Roe DC, Gas-phase NMR technique for studying the thermolysis of materials: thermal decomposition of ammonium perfluorooctanoate, Analytical Chem. 76 (13) (2004) 3800–3803. [DOI] [PubMed] [Google Scholar]
- [101].Tuppurainen K, Halonen I, Ruokojärvi P, Tarhanen J, Ruuskanen J, Formation of PCDDs and PCDFs in municipal waste incineration and its inhibition mechanisms: a review, Chemosphere 36 (7) (1998) 1493–1511. [Google Scholar]
- [102].McKay G, Dioxin characterisation, formation and minimisation during municipal solid waste (MSW) incineration, Chem. Eng. J. 86 (3) (2002) 343–368. [Google Scholar]
- [103].Song Z, Tang H, Wang N, Zhu L, Reductive defluorination of perfluorooctanoic acid by hydrated electrons in a sulfite-mediated UV photochemical system, J. Hazardous Mater. 262 (2013) 332–338. [DOI] [PubMed] [Google Scholar]
- [104].Watanabe N, Takemine S, Yamamoto K, Haga Y, Takata M, Residual organic fluorinated compounds from thermal treatment of PFOA, PFHxA and PFOS adsorbed onto granular activated carbon (GAC), J. Mater. Cycles and Waste Manag. 18 (4) (2016) 625–630. [Google Scholar]
- [105].Watanabe N, Takata M, Takemine S, Yamamoto K, Thermal mineralization behavior of PFOA, PFHxA, and PFOS during reactivation of granular activated carbon (GAC) in nitrogen atmosphere, Environ. Sci. Pollut. Res. 25 (8) (2018) 7200–7205. [DOI] [PubMed] [Google Scholar]
- [106].Xiao F, Sasi PC, Yao B, Kubátová A, Golovko SA, Golovko MY, Soli D, Thermal stability and decomposition of perfluoroalkyl substances on spent granular activated carbon, Environ. Sci. Technol. Lett. 7 (5) (2020) 343–350. [Google Scholar]
- [107].Coggan TL, Moodie D, Kolobaric A, Szabo D, Shimeta J, Crosbie ND, Lee E, Fernandes M, Clarke BO, An investigation into per-and polyfluoroalkyl substances (PFAS) in nineteen Australian wastewater treatment plants (WWTPs), Heliyon 5 (8) (2019) e02316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Venkatesan AK, Halden RU, National inventory of perfluoroalkyl substances in archived US biosolids from the 2001 EPA national sewage sludge survey, J. Hazardous Mater. 252 (2013) 413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Kundu S, Patel S, Halder P, Patel T, Marzbali MH, Pramanik BK, Paz-Ferreiro J, de Figueiredo CC, Bergmann D, Surapaneni A, Removal of PFASs from biosolids using a semi-pilot scale pyrolysis reactor and the application of biosolids derived biochar for the removal of PFASs from contaminated water, Environ. Sci. Water Res. Technol. 7 (3) (2021) 638–649. [Google Scholar]
- [110].Wang J, Lin Z, He X, Song M, Westerhoff P, Doudrick K, Hanigan D, Critical review of thermal decomposition of per-and polyfluoroalkyl substances: mechanisms and implications for thermal treatment processes, Environ. Sci. Technol. 56 (9) (2022) 5355–5370. [DOI] [PubMed] [Google Scholar]
- [111].Yu J, Nickerson A, Li Y, Fang Y, Strathmann TJ, Fate of per-and polyfluoroalkyl substances (PFAS) during hydrothermal liquefaction of municipal wastewater treatment sludge, Environ. Sci. Water Res. Technol. 6 (5) (2020) 1388–1399. [Google Scholar]
- [112].Sörengård M, Lindh A, Ahrens L, Thermal desorption as a high removal remediation technique for soils contaminated with per-and polyfluoroalkyl substances (PFASs), PloS one 15 (6) (2020), e0234476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Aly YH, Enhanced Adsorption of Perfluoro Alkyl Substances in Groundwater: Development of a Novel in-situ Groundwater Remediation Method, University of Minnesota, 2019. [Google Scholar]
- [114].Ok YS, Rinklebe J, Hou D, Tsang DC, Tack FM, Soil and Groundwater Remediation Technologies: a practical guide, CRC Press, 2020. [Google Scholar]
- [115].Longpré D, Lorusso L, Levicki C, Carrier R, Cureton P, PFOS PFOA, LC-PFCAS, and certain other PFAS: A focus on Canadian guidelines and guidance for contaminated sites management, Environ. Technol. Innovation 18 (2020), 100752. [Google Scholar]
- [116].Endpoint, Bench-scale VEG research & development study: Implementation memorandum for ex-situ thermal desorption of perfluoroalkyl compounds (PFCs) in soils, (2017).
- [117].Zhang K, Huang J, Yu G, Zhang Q, Deng S, Wang B, Destruction of perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) by ball milling, Environ. Sci. Technol. 47 (12) (2013) 6471–6477. [DOI] [PubMed] [Google Scholar]
- [118].Cagnetta G, Robertson J, Huang J, Zhang K, Yu G, Mechanochemical destruction of halogenated organic pollutants: a critical review, J. Hazardous Mater. 313 (2016) 85–102. [DOI] [PubMed] [Google Scholar]
- [119].Deng S, Bao Y, Cagnetta G, Huang J, Yu G, Mechanochemical degradation of perfluorohexane sulfonate: Synergistic effect of ferrate (VI) and zero-valent iron, Environ. Pollut. 264 (2020), 114789. [DOI] [PubMed] [Google Scholar]
- [120].Lu M, Cagnetta G, Zhang K, Huang J, Yu G, Mechanochemical mineralization of “very persistent” fluorocarbon surfactants–6: 2 fluorotelomer sulfonate (6: 2FTS) as an example, Sci. Reports 7 (1) (2017) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Ateia M, Skala LP, Yang A, Dichtel WR, Product analysis and insight into the mechanochemical destruction of anionic PFAS with potassium hydroxide, J. Hazardous Mater. Adv. 3 (2021), 100014. [Google Scholar]
- [122].Dubinskaya AM, Transformations of organic compounds under the action of mechanical stress, Russian Chem. Rev. 68 (8) (1999) 637–652. [Google Scholar]
- [123].Trang B, Li Y, Xue X-S, Ateia M, Houk K, Dichtel WR, Low-temperature mineralization of perfluorocarboxylic acids, Science 377 (6608) (2022) 839–845. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


