Abstract
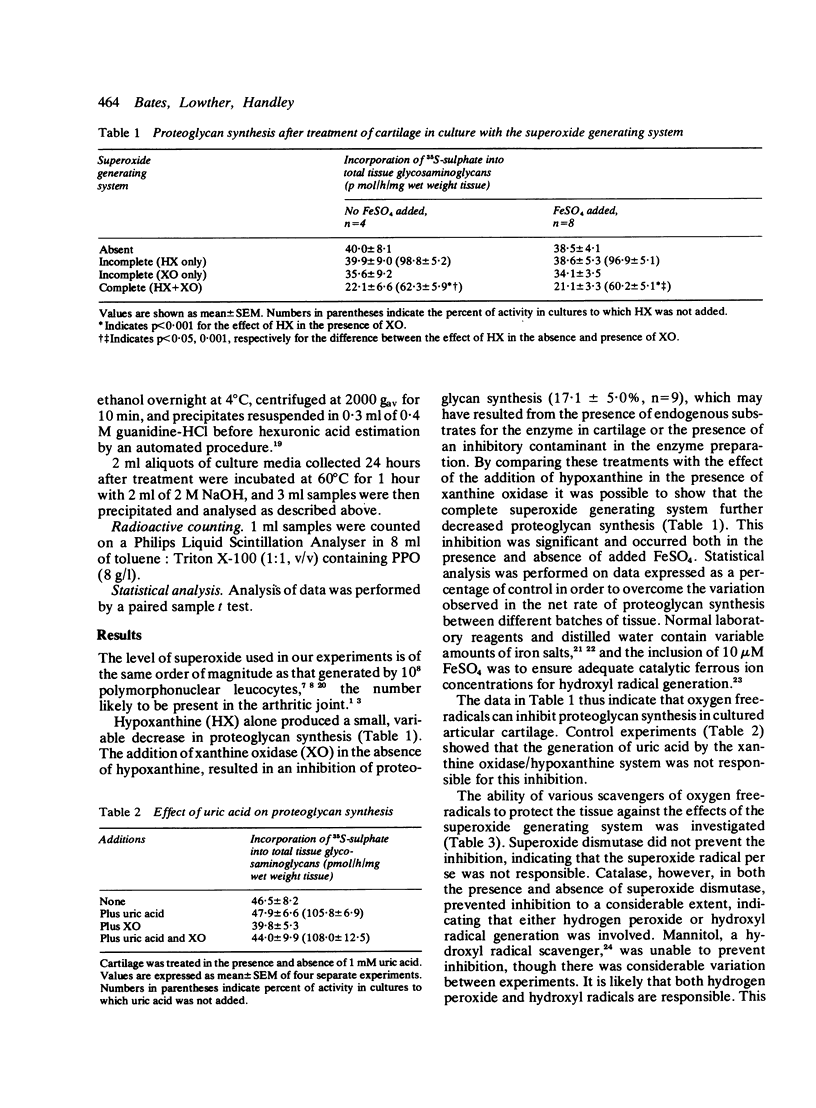
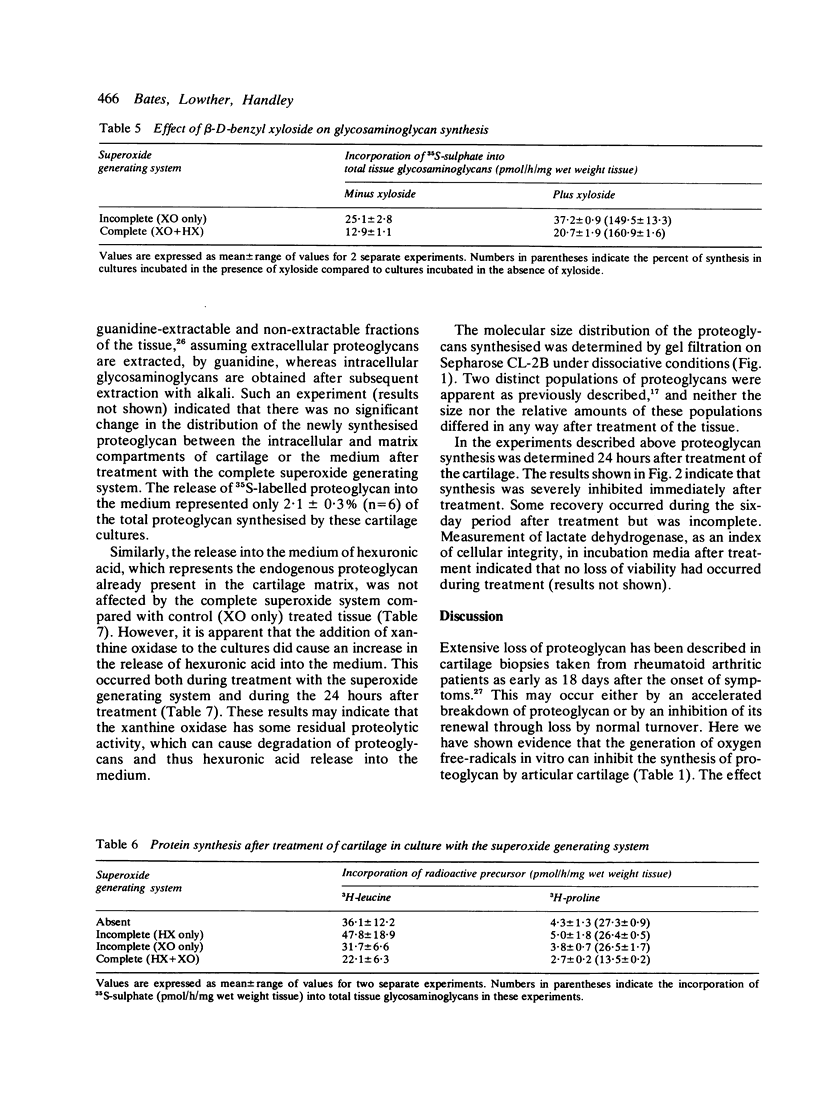
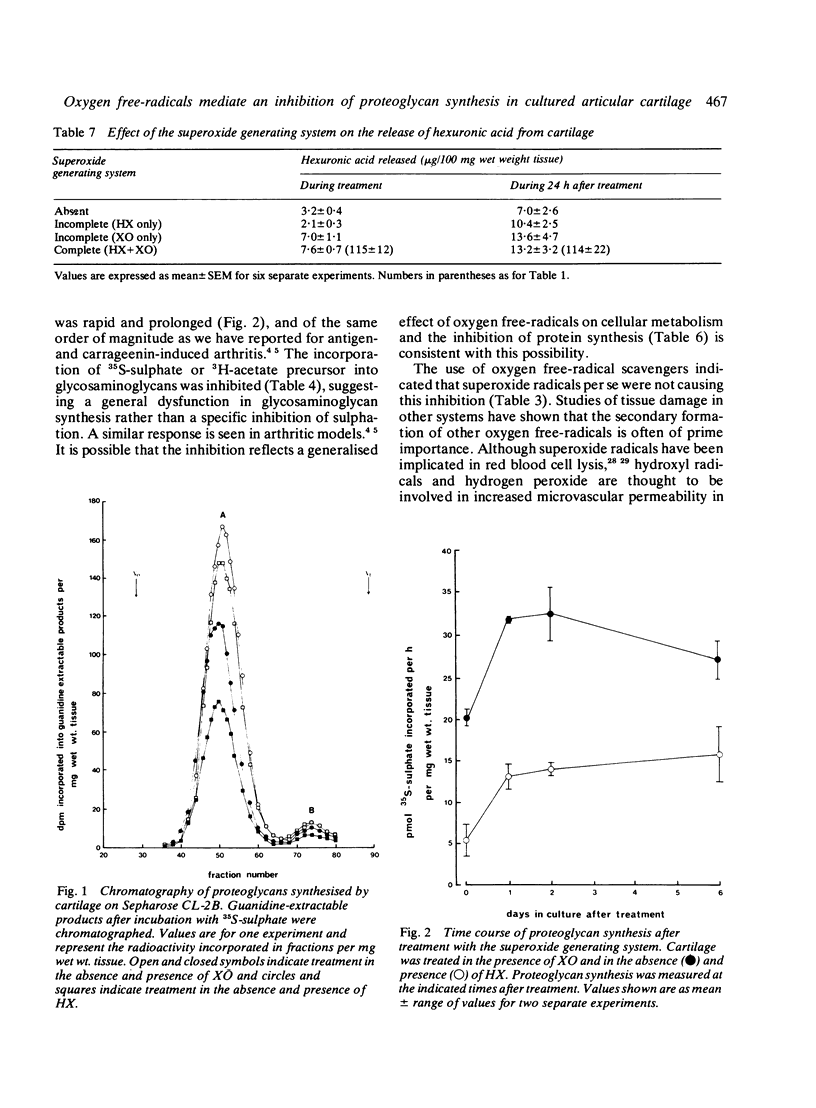
Superoxide radical, generated enzymatically by the action of xanthine oxidase on hypoxanthine, significantly inhibited proteoglycan synthesis by cultured bovine articular cartilage. This inhibition was not due to the generation of uric acid or to the generation of superoxide per se. It was immediate in onset and still evident after six days in culture. The inhibition was similar for both 35S-sulphate and 3H-acetate incorporation into glycosaminoglycans and could not be reversed by addition of beta-D-benzyl xyloside. Protein synthesis was also inhibited.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Barrett A. J. The possible role of neutrophil proteinases in damage to articular cartilage. Agents Actions. 1978 Jan;8(1-2):11–18. doi: 10.1007/BF01972396. [DOI] [PubMed] [Google Scholar]
- Carp H., Janoff A. In vitro suppression of serum elastase-inhibitory capacity by reactive oxygen species generated by phagocytosing polymorphonuclear leukocytes. J Clin Invest. 1979 Apr;63(4):793–797. doi: 10.1172/JCI109364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M. Biological defense mechanisms. The effect of bacteria and serum on superoxide production by granulocytes. J Clin Invest. 1974 Jun;53(6):1662–1672. doi: 10.1172/JCI107717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Maestro R. F. An approach to free radicals in medicine and biology. Acta Physiol Scand Suppl. 1980;492:153–168. [PubMed] [Google Scholar]
- Del Maestro R., Thaw H. H., Björk J., Planker M., Arfors K. E. Free radicals as mediators of tissue injury. Acta Physiol Scand Suppl. 1980;492:43–57. [PubMed] [Google Scholar]
- Gillard G. C., Lowther D. A. Carrageenin-induced arthritis. II. Effect of intraarticular injection of carrageenin on the synthesis of proteoglycan in articular cartilage. Arthritis Rheum. 1976 Sep-Oct;19(5):918–922. doi: 10.1002/art.1780190513. [DOI] [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald R. A., Moy W. W. Effect of oxygen-derived free radicals on hyaluronic acid. Arthritis Rheum. 1980 Apr;23(4):455–463. doi: 10.1002/art.1780230408. [DOI] [PubMed] [Google Scholar]
- Greenwald R. A., Moy W. W. Inhibition of collagen gelation by action of the superoxide radical. Arthritis Rheum. 1979 Mar;22(3):251–259. doi: 10.1002/art.1780220307. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Rowley D. A., Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts. Detection of 'free' iron in biological systems by using bleomycin-dependent degradation of DNA. Biochem J. 1981 Oct 1;199(1):263–265. doi: 10.1042/bj1990263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts. Its role in degradation of hyaluronic acid by a superoxide-generating system. FEBS Lett. 1978 Dec 15;96(2):238–242. doi: 10.1016/0014-5793(78)80409-8. [DOI] [PubMed] [Google Scholar]
- Handley C. J., Lowther D. A. Extracellular matrix metabolism by chondrocytes. III. Modulation of proteoglycan synthesis by extracellular levels of proteoglycan in cartilage cells in culture. Biochim Biophys Acta. 1977 Nov 7;500(1):132–139. doi: 10.1016/0304-4165(77)90053-8. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Biosynthesis of proteoglycans in cartilage slices. Fractionation by gel chromatography and equilibrium density-gradient centrifugation. Biochem J. 1972 Feb;126(4):791–803. doi: 10.1042/bj1260791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C., Handley C. J., McQuillan D. J., Hascall G. K., Robinson H. C., Lowther D. A. The effect of serum on biosynthesis of proteoglycans by bovine articular cartilage in culture. Arch Biochem Biophys. 1983 Jul 1;224(1):206–223. doi: 10.1016/0003-9861(83)90205-9. [DOI] [PubMed] [Google Scholar]
- Igari T., Kaneda H., Horiuchi S., Ono S. A remarkable increase of superoxide dismutase activity in synovial fluid of patients with rheumatoid arthritis. Clin Orthop Relat Res. 1982 Jan-Feb;(162):282–287. [PubMed] [Google Scholar]
- Janis R., Hamerman D. Articular cartilage changes in early arthritis. Bull Hosp Joint Dis. 1969 Oct;30(2):136–152. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Lehmeyer J. E. Elaboration of toxic oxygen by-products by neutrophils in a model of immune complex disease. J Clin Invest. 1976 Apr;57(4):836–841. doi: 10.1172/JCI108359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. S., Piette L. H. Spin-trapping studies of hydroxyl radical production involved in lipid peroxidation. Arch Biochem Biophys. 1978 Sep;190(1):27–38. doi: 10.1016/0003-9861(78)90250-3. [DOI] [PubMed] [Google Scholar]
- Lowther D. A., Gillard G. C. Carrageenin-induced arthritis. I. The effect of intraarticular carrageenin on the chemical composition of articular cartilage. Arthritis Rheum. 1976 Jul-Aug;19(4):769–776. doi: 10.1002/1529-0131(197607/08)19:4<769::aid-art1780190419>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Lowther D. A., Sandy J. D., Santer V. B., Brown H. L. Antigen-induced arthritis. Decreased proteoglycan content and inhibition of proteoglycan synthesis in articular cartilage. Arthritis Rheum. 1978 Jul-Aug;21(6):675–680. doi: 10.1002/art.1780210611. [DOI] [PubMed] [Google Scholar]
- Lunec J., Halloran S. P., White A. G., Dormandy T. L. Free-radical oxidation (peroxidation) products in serum and synovial fluid in rheumatoid arthritis. J Rheumatol. 1981 Mar-Apr;8(2):233–245. [PubMed] [Google Scholar]
- Lynch R. E., Fridovich I. Effects of superoxide on the erythrocyte membrane. J Biol Chem. 1978 Mar 25;253(6):1838–1845. [PubMed] [Google Scholar]
- McCord J. M. Free radicals and inflammation: protection of synovial fluid by superoxide dismutase. Science. 1974 Aug 9;185(4150):529–531. doi: 10.1126/science.185.4150.529. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Mccord J. M., Wong K., Stokes S. H., Petrone W. F., English D. Superoxide and inflammation: a mechanism for the anti-inflammatory activity of superoxide dismutase. Acta Physiol Scand Suppl. 1980;492:25–30. [PubMed] [Google Scholar]
- Perez H. D., Weksler B. B., Goldstein I. M. Generation of a chemotactic lipid from a arachidonic acid by exposure to a superoxide-generating system. Inflammation. 1980 Sep;4(3):313–328. doi: 10.1007/BF00915032. [DOI] [PubMed] [Google Scholar]
- Rister M., Bauermeister K. Superoxid-Dismutase und Superoxid-Radikal-Freisetzung bei juveniler rheumatoider Arthritis. Klin Wochenschr. 1982 Jun 1;60(11):561–565. doi: 10.1007/BF01724212. [DOI] [PubMed] [Google Scholar]
- Robinson H. C., Brett M. J., Tralaggan P. J., Lowther D. A., Okayama M. The effect of D-xylose, beta-D-xylosides and beta-D-galactosides on chondroitin sulphate biosynthesis in embryonic chicken cartilage. Biochem J. 1975 Apr;148(1):25–34. doi: 10.1042/bj1480025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandy J. D., Brown H. L., Lowther D. A. Control of proteoglycan synthesis. Studies on the activation of synthesis observed during culture of articular cartilages. Biochem J. 1980 Apr 15;188(1):119–130. doi: 10.1042/bj1880119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandy J. D., Lowther D. A., Brown H. L. Antigen-induced arthritis. Studies on the inhibition of proteoglycan synthesis observed in articular cartilage during short-term joint inflammation. Arthritis Rheum. 1980 Apr;23(4):433–447. doi: 10.1002/art.1780230406. [DOI] [PubMed] [Google Scholar]
- Sandy J. D., Sriratana A., Brown H. L., Lowther D. A. Evidence for polymorphonuclear-leucocyte-derived proteinases in arthritic cartilage. Biochem J. 1981 Jan 1;193(1):193–202. doi: 10.1042/bj1930193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simchowitz L., Spilberg I. Evidence for the role of superoxide radicals in neutrophil-mediated cytotoxicity. Immunology. 1979 Jun;37(2):301–309. [PMC free article] [PubMed] [Google Scholar]
- Simon R. H., Scoggin C. H., Patterson D. Hydrogen peroxide causes the fatal injury to human fibroblasts exposed to oxygen radicals. J Biol Chem. 1981 Jul 25;256(14):7181–7186. [PubMed] [Google Scholar]
- Zvaifler N. J. The immunopathology of joint inflammation in rheumatoid arthritis. Adv Immunol. 1973;16(0):265–336. doi: 10.1016/s0065-2776(08)60299-0. [DOI] [PubMed] [Google Scholar]


