Abstract
Introduction
Understanding racial/ethnic differences in patients with acute myocardial infarction (AMI) lays the foundation for more equitable health care. This study evaluated racial/ethnic differences in risk factors, treatment, and outcomes in patients with AMI.
Methods
This retrospective study included patients aged 18–50 years hospitalized for AMI between 2006 and 2016. Cox regression models were used to evaluate the association of race/ethnicity with all-cause mortality.
Results
Among 1753 patients hospitalized for type 1 AMI (median age 44 years, 85% male), 35.8% self-identified as White, 9.4% non-Hispanic Black, 37.6% Hispanic, 14.5% Asian, and 2.6% as other. Compared to White patients, Black patients were more likely to have hypertension (53.1% vs 32.2%, p < 0.001) and Hispanic patients were more likely to have diabetes (28.2% vs 15.5%, p < 0.001) and obesity (23.9% vs 17.7%, p = 0.008). There were no substantial differences in revascularization rates or initial medical treatment. However, adherence to statin therapy was lower among Black and Hispanic patients (50.3% and 58.6% for Black and Hispanic vs 67.4% and 72.3% for White and Asian patients, respectively). Over a median follow-up of 7.5 years, Black patients had higher all-cause mortality (unadjusted hazard ratio = 1.88, 95% confidence interval = 1.09–3.24) compared to White patients, but this difference was no longer significant after adjustments (adjusted hazard ratio = 1.32, 95% confidence interval = 0.74–2.36).
Discussion and Conclusion
There are racial/ethnic differences in risk factors and medication adherence patterns in adults with AMI. To achieve equitable care, programs with tailored intervention addressing needs of different groups should be developed.
Keywords: myocardial infarction, health disparities, race and ethnicity
Introduction
Cardiovascular disease is the leading cause of death in the United States, and ischemic heart disease accounts for most of these deaths. 1 It has been well established that members of racial and ethnic minority groups endure a disproportionate burden of morbidity and mortality from ischemic heart disease. 2,3 Studies have reported disparities in treatment among patients presenting with acute coronary syndrome, with patients from racial and ethnic minority groups being less likely to receive guideline-directed medical therapies or coronary revascularization. 4,5 These differences lead to increased risks for adverse outcomes, including death. 6,7 What is less well established is how ischemic heart disease affects younger members of racial and ethnic minority groups. The long-term impact of ischemic heart disease is particularly high among young adults, given the potential cost of lifetime health care utilization and loss of productivity.
Coronary artery disease is a chronic process that progresses over time. Identifying the specific risk factors that predispose racial and ethnic minorities to early manifestations of ischemic heart disease will help highlight the primary drivers of the atherosclerotic disease process for each group. This understanding can help target interventions to counter these primary risk factors earlier in the process.
This study aimed to evaluate racial and ethnic differences in risk factors, treatment, and clinical outcomes among patients ages 18 to 50 hospitalized for acute myocardial infarction (AMI) in a large integrated health care system in the United States.
Methods
Study design and data source
This retrospective observational study included patients ages 18 to 50 years who were hospitalized with AMI within the Kaiser Permanente Southern California health care system. Kaiser Permanente Southern California is a large, integrated health care delivery system with more than 4 million members in the United States. Members enroll through the Kaiser Foundation Health Plan for comprehensive insurance, including pharmaceutical benefits. The Kaiser Permanente Southern California health care system serves an ethnically and socioeconomically diverse population that is representative of the racial and ethnic groups within Southern California. 8 Comprehensive medical information, which includes demographics, administrative, pharmacy, laboratory, and health care utilization data from ambulatory and inpatient encounters, is prospectively captured electronically through a centralized data warehouse. The present study was approved by the Kaiser Permanente Southern California institutional review board. A waiver of informed consent was obtained due to the study’s observational nature.
Study population
Consecutive patients ages 18 to 50 years hospitalized with a principal diagnosis of AMI between January 1, 2006 and December 31, 2016 were identified using International Classification of Diseases, 9th Revision (ICD-9) or International Classification of Diseases, 10th Revision (ICD-10) codes. For patients with multiple admissions for AMI during the study period, only the first admission was included in the study. Index date was defined as the date of admission. Patients who were not Kaiser Permanente Southern California members or had been members for less than 1 year were excluded. Patients who did not undergo cardiac catheterization were excluded. Detailed chart review and data abstraction was performed by at least 2 physicians (MN, BH, SC, KG) to confirm the diagnosis of AMI, adjudicate AMI type (type 1 myocardial infarction, type 2 myocardial infarction, and myocardial injury), define coronary anatomy, identify comorbidities, and determine treatment provided. AMI type was adjudicated according to the fourth universal definition of myocardial infarction. 9
Covariates
Race and ethnicity were based on patient self-identification and categorized as White, Black, Hispanic, Asian, and other (comprising unknown/declined and patients indicating their race as multiple or “other”). Insurance enrollment records were used to identify patient demographics (age, sex). The electronic health records were used to identify medical comorbidities. Household income was estimated using the provided home address and the corresponding neighborhood information. Smoking status was based on patient self-report and categorized as active, passive, quit, never, or missing. Medication use was identified using outpatient pharmacy dispensing databases. Baseline medications at time of index hospitalization as well as medication use within 1 year postdischarge were identified. Procedures performed (percutaneous coronary intervention or coronary artery bypass graft surgery) were determined using electronic health records.
Outcomes
The primary outcome was all-cause death. Mortality data were extracted from a mortality data file that included integrated death information from multiple sources, including insurance plan administrative records, California state death master files, Social Security Administration death master files, and hospital death records. Patients were followed until they reached the study endpoint (all-cause death) or the end of the study period (December 31, 2019).
Medication adherence was measured during the 365 days following hospital discharge. Medication use was extracted from outpatient pharmacy dispensing records. The proportion of days covered (PDC) was calculated over 365 days using dates and days of supply of the prescription filled. Adherence level was classified as high when PDC was 80% or higher. 10,11 PDC is used by the Centers for Disease Control and Prevention and the Centers for Medicare and Medicaid Services as a quality metric. It is also the preferred adherence method of the Pharmacy Quality Alliance. 11
Statistical Analysis
Descriptive statistics were used to examine covariate distribution. Continuous variables were summarized and reported in medians with 25th and 75th percentiles. Categorical variables were reported as counts and percentages. Differences in categorical data were compared by a Chi-square test. Differences in continuous data were compared by the Wilcoxon rank-sum test. Kaplan–Meier survival curves were created to evaluate overall survival among the different racial and ethnic groups. Cox proportional hazard regression analyses were performed to evaluate the association between race/ethnicity and all-cause mortality. The proportional hazard assumption was assessed using Schoenfeld residuals. Multivariable adjustments were performed, including the following variables: age, sex, race/ethnicity, comorbidities (hypertension, atrial fibrillation, heart failure, history of stroke/transient ischemic attack, diabetes, lung disease, hypothyroidism, liver disease, obesity, and history of smoking), and medications (statin, angiotensin-converting enzyme inhibitor [ACEI], angiotensin receptor blocker [ARB], and P2Y12 receptor blockers). Hazard ratios (HRs) with corresponding 95% confidence intervals (CI) were reported. A 2-sided p value of < 0.05 was considered statistically significant. Statistical analyses were performed using STATA 14 (Stata-Corp, College Station, TX).
Results
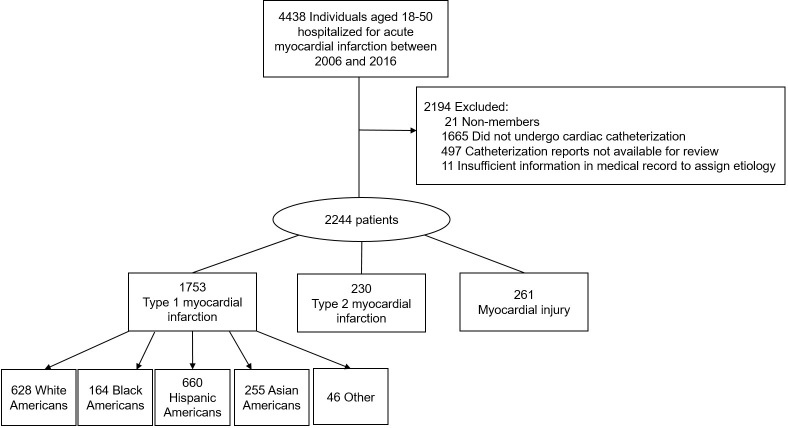
Between 2006 and 2016, 1753 patients ages 18 to 50 years were hospitalized for type 1 myocardial infarction (Figure 1). In this group, 628 (35.8%) self-identified as White, 164 (9.4%) non-Hispanic Black, 660 (37.6%) Hispanic, 255 (14.5%) Asian, and 46 (2.6%) were multiracial or did not identify as belonging to a racial or ethnic category.
Figure 1:
Study flowchart.
Racial and ethnic differences in baseline characteristics
Table 1 shows the baseline characteristics of the cohort. The median age was 44.3 years (interquartile range 41.8 to 48.1 years), and 1455 (83.0%) were men. There was a higher proportion of men among Asian Americans (91.8%) compared to Black Americans (65.2%). Household income was lower among Black Americans, with 54.9% of Black Americans from households with an annual income of less than $45,000, compared to 18.5% White Americans and 16.1% Asian Americans.
Table 1:
Baseline characteristics of patients with type 1 myocardial infarction
| Characteristic | White (n = 628) | Black (n = 164) | Hispanic (n = 660) | Asian (n = 255) |
p value |
|---|---|---|---|---|---|
| Age, yrs | 44.9 ± 4.5 | 43.8 ± 5.4 | 44.1 ± 4.6 | 44.3 ± 4.7 | 0.02 |
| Male | 539 (85.8) a | 107 (65.2) | 575 (87.1) | 234 (91.8) | < 0.001 |
| Income | |||||
| < $45,000 | 116 (18.5) | 90 (54.9) | 252 (38.2) | 41 (16.1) | < 0.001 |
| 45,000–80,000 | 304 (48.4) | 56 (34.1) | 317 (48.0) | 128 (50.2) | |
| < 80,000 | 189 (30.1) | 17 (10.4) | 74 (11.2) | 73 (28.6) | |
| Unknown | 19 (3.0) | 1 (0.6) | 17 (2.6) | 13 (5.1) | |
| Comorbidities | |||||
| Hypertension | 202 (32.2) | 87 (53.1) | 238 (36.1) | 96 (37.7) | < 0.001 |
| Hyperlipidemia | 241 (38.4) | 63 (38.4) | 298 (45.2) | 112 (43.9) | 0.06 |
| Diabetes | 97 (15.5) | 43 (26.2) | 186 (28.2) | 55 (21.6) | < 0.001 |
| Obesity | 111 (17.7) | 40 (24.4) | 158 (23.9) | 42 (16.5) | 0.008 |
| Atrial fibrillation | 2 (0.3) | 0 (0) | 4 (0.6) | 1 (0.4) | 0.70 |
| Heart failure | 8 (1.3) | 8 (4.9) | 14 (2.1) | 5 (2.0) | 0.038 |
| Chronic kidney disease | 37 (4.3) | 16 (9.8) | 49 (7.4) | 18 (7.1) | 0.029 |
| CVA/TIA | 21 (3.3) | 5 (3.1) | 22 (3.3) | 3 (1.2) | 0.33 |
| COPD/asthma | 59 (9.4) | 19 (11.6) | 51 (7.7) | 29 (11.4) | 0.23 |
| Liver disease | 22 (3.5) | 2 (1.2) | 23 (3.5) | 7 (2.8) | 0.45 |
| Hypothyroidism | 30 (4.8) | 2 (1.2) | 25 (3.8) | 5 (2.0) | 0.07 |
| Depression | 70 (11.2) | 15 (9.2) | 52 (7.9) | 12 (4.7) | 0.015 |
| Smoking status | |||||
| Active | 159 (25.3) | 46 (28.1) | 104 (15.8) | 45 (17.7) | < 0.001 |
| Passive | 3 (0.5) | 2 (1.2) | 3 (0.5) | 4 (1.6) | |
| Quit | 96 (15.3) | 27 (16.5) | 108 (16.4) | 48 (18.8) | |
| Never | 191 (30.4) | 54 (32.9) | 290 (43.9) | 99 (38.8) | |
| Missing | 179 (28.5) | 35 (21.3) | 155 (23.5) | 59 (23.1) | |
| Family history of coronary artery disease | 156 (24.8) | 30 (18.3) | 152 (23.0) | 53 (20.8) | 0.27 |
| Baseline medications | |||||
| Statin | 158 (25.2) | 44 (26.8) | 179 (27.1) | 87 (34.1) | 0.06 |
| Beta-blockers | 119 (19.0) | 40 (24.4) | 103 (15.6) | 53 (20.8) | 0.038 |
| ACEI/ARB | 155 (24.7) | 53 (32.3) | 186 (28.2) | 78 (30.6) | 0.13 |
| P2Y12 inhibitors | 27 (4.3) | 10 (6.1) | 27 (4.1) | 11 (4.3) | 0.73 |
Values are median (interquartile range) or n (%).
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; P2Y12, P2Y12 receptor; TIA, transient ischemic attack; Yrs, years.
The distribution of cardiovascular risk factors varied between racial and ethnic groups. A higher proportion of Black Americans had hypertension (53.1%) compared to White patients (32.2%). Obesity primarily affected Black (24.4%) and Hispanic patients (23.9%) but was less of a contribution among Asian (16.5%) and White patients (17.7%). Similarly, diabetes was present in 28.2% of Hispanic patients and 26.2% of Black patients but only observed in 15.5% of White patients.
Active smoking was an important modifiable risk factor, with 25.3% of White and 28.1% of Black patients endorsing active smoking. Active smoking was also seen among Hispanic (15.8%) and Asian patients (17.7%). The rate of depression was highest among White patients (11.2%) and lowest among Asian patients (4.7%).
Coronary anatomy
Patients were stratified according to the extent of obstructive coronary artery disease: 1-, 2-, 3-vessel, or left main disease (Table 2). Asian patients had the highest disease burden, with 25.1% having left main or 3-vessel disease. In contrast, 11.6% of Black patients and 14.7% of White patients had left main or 3-vessel disease.
Table 2:
Number of obstructive coronary arteries among patients with type 1 myocardial infarction (N = 1707)
| Coronary arteries | White (n = 628) | Black (n = 164) | Hispanic (n = 660) | Asian (n = 255) |
p value |
|---|---|---|---|---|---|
| Number of arteries with obstructive coronary artery disease a b | < 0.001 | ||||
| 1 | 376 (59.9) | 99 (60.4) | 355 (53.8) | 108 (42.4) | |
| 2 | 170 (27.1) | 48 (29.3) | 193 (29.2) | 89 (34.9) | |
| 3 | 82 (13.1) | 16 (9.8) | 112 (17.0) | 58 (22.8) | |
| Left main or 3-vessel disease | 92 (14.7) | 19 (11.6) | 124 (18.8) | 64 (25.1) | < 0.001 |
| Coronary arteries involved | |||||
| Left main | 14 (2.2) | 3 (1.8) | 16 (2.4) | 10 (3.9) | 0.46 |
| Left anterior descending | 376 (59.9) | 98 (59.8) | 433 (65.6) | 170 (66.7) | 0.08 |
| Left circumflex | 255 (40.6) | 62 (37.8) | 297 (45.0) | 127 (49.8) | 0.03 |
| Right coronary artery | 326 (51.9) | 81 (49.4) | 340 (51.5) | 160 (62.8) | 0.01 |
Obstructive left main disease was counted as 2-vessel.
One patient presented with ST-elevation myocardial infarction and was treated with thrombolytics. Coronary angiography showed a 30% lesion in one of the coronary arteries.
Revascularization and medication use
Most patients (81.7%) underwent revascularization with either percutaneous coronary intervention or coronary artery bypass graft surgery (Table 3). No substantial differences in revascularization rates were observed between racial and ethnic groups.
Table 3:
Treatment a
| Treatment | White (n = 628) | Black (n = 164) | Hispanic (n = 660) | Asian (n = 255) |
p value |
|---|---|---|---|---|---|
| Revascularization | |||||
| PCI | 490 (78.0) | 116 (70.7) | 466 (70.6) | 186 (72.9) | 0.02 |
| CABG | 59 (9.4) | 19 (11.6) | 80 (12.1) | 39 (15.3) | 0.09 |
| PCI/CABG | 524 (83.4) | 130 (79.3) | 524 (79.4) | 216 (84.7) | 0.12 |
| Medications (filled at least 1 prescription postdischarge) |
|||||
| Statin | 594 (94.6) | 148 (90.2) | 618 (93.6) | 240 (94.1) | 0.24 |
| Beta-blockers | 591 (94.1) | 153 (93.3) | 620 (93.9) | 246 (93.9) | 0.43 |
| ACEI/ARB | 536 (85.4) | 135 (82.3) | 559 (84.7) | 219 (85.9) | 0.76 |
| P2Y12 inhibitors | 575 (91.6) | 144 (87.8) | 578 (87.6) | 229 (89.8) | 0.12 |
| Medication adherence (among patients with a last 1 year of follow-up) |
(n = 622) | (n = 159) | (n = 654) | (n = 249) | |
| Statin PDC ≥ 80% | 419 (67.4) | 80 (50.3) | 383 (58.6) | 180 (72.3) | < 0.001 |
| Beta-blockers PDC ≥ 80% | 417 (67.0) | 99 (62.3) | 394 (60.2) | 189 (75.9) | < 0.001 |
| ACEI/ARB PDC ≥ 80% | 367 (59.0) | 90 (56.6) | 382 (58.4) | 154 (61.9) | 0.73 |
| P2Y12 inhibitors PDC ≥ 80% | 429 (69.0) | 93 (58.5) | 412 (63.0) | 187 (75.1) | < 0.001 |
The measurement period used in this study was the first year after discharge from hospitalization for myocardial infarction.
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention; PDC, proportion of days covered, with PDC ≥ 80% indicating good adherence to medication during the measurement period; P2Y12, P2Y12 receptor.
Initial treatment with statin, beta-blockers, and P2Y12 receptor blockers was comparable between racial and ethnic groups (Table 2). However, over the course of 1 year after discharge, differences in medication adherence were observed between racial and ethnic groups, with medication adherence lower among Black and Hispanic patients. A PDC of 80% or above was used to define high adherence to medications. Using this definition, 50.3% of Black patients and 58.5% of Hispanic patients had high adherence to statin therapy, compared to 67.4% of White patients and 72.3% of Asian patients. Similarly, high adherence to P2Y12 inhibitor therapy was only observed in 58.5% of Black patients, compared to 69% of White patients and 75.1% of Asian patients.
All-cause mortality
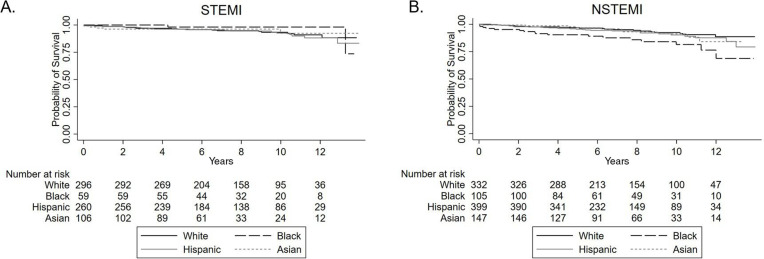
Over a median follow-up of 7.5 years (interquartile range of 4.9 to 10.4 years), 126 (7.4%) patients died. Death occurred in 40 (6.4%) White patients, 19 (11.6%) Black patients, 50 (7.6%) Hispanic patients, and 17 (6.7%) Asian patients. Unadjusted Kaplan–Meier survival curves stratified by racial and ethnic groups are shown for patients with ST-elevation myocardial infarction (Figure 2A) and non-ST-elevation myocardial infarction (Figure 2B).
Figure 2:
Kaplan–Meier overall survival curves according to race/ethnicity for patients who presented with (A) STEMI and (B) NSTEMI. NSTEMI = non-ST-elevation myocardial infarction; STEMI = ST-elevation myocardial infarction.
Unadjusted Cox proportional hazard analyses showed higher mortality associated with Black patients (unadjusted HR = 1.88, 95% CI = 1.09–3.24) when using White patients as reference (Table 4). This difference was driven primarily by the non-ST-elevation myocardial infarction population. After adjusting for demographic factors, comorbidities, and medications, no difference was observed between Black and White patients (adjusted HR = 1.32, 95% CI = 0.74–2.36). There was no substantial difference in long-term survival observed for Hispanic and Asian patients compared to White patients.
Table 4:
Hazard ratios of mortality according to race/ethnicity
| Population | White | Black | Hispanic | Asian | ||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value | |
| All patients with type 1 MI (N = 1707) | ||||||||
| Unadjusted | reference | 1.88 (1.09–3.24) | 0.024 | 1.25 (0.82–1.90) | 0.29 | 1.05 (0.58–1.91) | 0.86 | |
| Adjusted for demographics a | reference | 0.74 (0.99–3.05) | 0.052 | 1.28 (0.84–1.94) | 0.25 | 1.08 (0.60–1.97) | 0.60 | |
| Adjusted for demographics and comorbidities b | reference | 1.40 (0.80–2.50) | 0.24 | 1.02 (0.66–1.57) | 0.94 | 0.83 (0.44–1.53) | 0.54 | |
| Adjusted for demographics and comorbidities and medications c | reference | 1.32 (0.74–2.36) | 0.35 | 0.99 (0.64–1.53) | 0.96 | 0.79 (0.42–1.48) | 0.46 | |
| STEMI (n = 723) | ||||||||
| Unadjusted | reference | 0.50 (0.12–2.14) | 0.35 | 1.12 (0.59–2.11) | 0.74 | 0.84 (0.31–2.24) | 0.72 | |
| Adjusted for demographics a | reference | 0.46 (0.10–2.02) | 0.30 | 1.10 (0.58–2.10) | 0.77 | 0.79 (0.29–2.13) | 0.64 | |
| Adjusted for demographics and comorbidities b | reference | 0.41 (0.09–1.87) | 0.25 | 0.90 (0.46–1.76) | 0.76 | 0.54 (0.18–1.65) | 0.28 | |
| Adjusted for demographics and comorbidities and medications c | reference | 0.35 (0.08–1.59) | 0.18 | 0.81 (0.41–1.61) | 0.55 | 0.56 (0.18–1.73) | 0.31 | |
| NSTEMI (n = 984) | ||||||||
| Unadjusted | reference | 2.78 (1.46–5.27) | 0.002 | 1.35 (0.77–2.37) | 0.29 | 1.18 (0.56–2.51) | 0.66 | |
| Adjusted for demographics a | reference | 2.06 (1.08–3.93) | 0.03 | 1.28 (0.73–2.25) | 0.39 | 1.16 (0.54–2.48) | 0.70 | |
| Adjusted for demographics and comorbidities b | reference | 1.87 (0.95–3.69) | 0.07 | 1.21 (0.67–2.19) | 0.53 | 0.97 (0.43–2.19) | 0.94 | |
| Adjusted for demographics and comorbidities and medications c | reference | 1.85 (0.92–3.70) | 0.08 | 1.20 (0.66–2.18) | 0.54 | 0.94 (0.41–2.18) | 0.89 | |
Demographics: age, sex, race/ethnicity.
Comorbidities: hypertension, atrial fibrillation, heart failure, history of stroke/transient ischemic attack, diabetes, lung disease, hypothyroidism, liver disease, obesity, history of smoking.
Medications: statin, ACEI/ARB, beta-blockers, P2Y12 inhibitors.
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CI, Confidence interval; MI, myocardial infarction; NSTEMI, non-ST-elevation myocardial infarction; STEMI, ST-elevation myocardial infarction.
Discussion
This population-based cohort study reports racial and ethnic differences in risk factors, treatment, and clinical outcomes among patients 18 to 50 years of age hospitalized for AMI. The principal findings of the study are as follows: 1) cardiovascular risk factor profiles differ between racial and ethnic groups, with hypertension and smoking being key risk factors for Black patients; diabetes and obesity being key risk factors for Hispanic patients; and smoking being an important risk factor for White patients; 2) no differences were observed between revascularization rate and initial medical treatment between racial and ethnic groups, but long-term medication adherence differed, with Black and Hispanic patients having lower adherence to cardiac medications; 3) survival was lowest among Black patients; however, no difference in survival was observed between Black and White patients after adjusting for comorbidities and medications.
Coronary artery disease manifests from the culmination of risk factors over time. Risk factors that contribute the most to atherosclerotic cardiovascular disease are different among people from different racial and ethnic groups. 12 Understanding the risk profiles of adults presenting with AMI helps establish which risk factors contribute to the early manifestation of coronary artery disease in each group and allows targeted efforts to mitigate these risk factors. The importance of modifiable risk factors was highlighted in a study that utilized the United States Healthcare Cost and Utilization Project National Inpatient Sample. 13 This study showed that 92% of patients had at least 1 modifiable risk factor, and the prevalence of modifiable risk factors increased with the age of the patient, demonstrating the progressive nature of risk factor burden in patients with coronary artery disease.
Disparities in outcomes after myocardial infarction, particularly between Black and White patients, have been shown to be directly related to differences in risk factor burden. 14,15 After accounting for differences in risk factor burden, some studies showed that differences in outcomes after myocardial infarction between White and Black patients disappear. 15 These findings call attention to the importance of identifying which particular risk factors predispose different racial and ethnic groups to myocardial infarction as these specific risk factors present opportunities for efforts to halt the progression of atherosclerotic disease early on.
The increased prevalence of hypertension among Black patients has been described, and these findings are supported by prior research demonstrating hypertension to be a major risk factor for ischemic heart disease among Black individuals. 16 Previous studies demonstrated smoking to be a major contributor to coronary artery disease among older Black patients. 14,17 The findings of this study extend this association to a much younger cohort, underscoring the importance of smoking cessation programs and hypertension control in younger Black individuals. Among Black barbershop patrons with uncontrolled hypertension, the combination of medication management in the barbershop by pharmacists and health education by barbers resulted in a substantial reduction in blood pressure, 18 underscoring the importance of culturally responsive interventions. Further research to understand better how to implement a barbershop-type intervention on a broader scale, especially among young adults, will be important.
This study found diabetes and obesity to be major contributors to the risk of myocardial infarction among Hispanic patients. Hispanic individuals have been shown to live longer than non-Hispanic White people and non-Hispanic Black people and have lower rates of cardiovascular events. 19,20 Irrespective of these findings, cardiovascular disease remains a major contributor to mortality among Hispanic individuals. 21 Although research is still needed to explain the “Hispanic paradox” and elucidate protective factors in this community, known risk factors for early cardiovascular events must continue to be addressed.
No substantial difference in revascularization rates or initial use of guideline-directed medical therapy was observed in this cohort. This contrasts prior studies showing lower use of coronary revascularization and medications in patients from racial and ethnic minority groups. 4,5 This cohort is different from other studies in that all patients had access to the same type of comprehensive health insurance with equal access to primary and subspecialty care. Lack of access to health care services may have partially contributed to disparities observed in other studies.
During the first year after discharge, medication adherence was lower among Black patients and Hispanic patients. Socioeconomic factors, including lack of community resources, and individual factors, such as lack of education regarding the benefits of medication, all contribute. 7,22 Because the benefit of medical therapy depends not only on initiation, but also on long-term adherence, understanding factors that contribute to disparities in medication adherence is important. 23
There are a few limitations to this study. First, only patients who underwent coronary angiography were included, so these findings do not apply to patients with medically managed AMI. However, including coronary angiogram information allowed adjudication of type 1 myocardial infarction and confirmation that the etiology of AMI is related to atherosclerosis. The pathophysiology of type 2 myocardial infarction and myocardial injury differs from type 1 myocardial infarction, and the risk factors and clinical profiles of these populations are different. Second, grouping patients into broad racial and ethnic categories does not account for the spectrum of possible ancestral backgrounds within each group. Third, cases were identified using ICD-9 and ICD-10 codes. It is possible that these codes failed to capture all cases of AMI. On the other hand, because all cases were manually reviewed and adjudicated by physicians, the cases included in this study were all confirmed cases of AMI. Fourth, medication adherence was assessed using outpatient pharmacy dispense information. As such, it is not a direct measurement of whether a patient took the medication, and detailed information to explain differences in adherence was not collected. Fifth, as an observational study, there were biases inherent to the design that could not be accounted for despite statistical analytical efforts. Sixth, because this cohort came from an insured population with access to health care, findings may not be generalizable to patients who lack health insurance.
Conclusions
In conclusion, cardiovascular risk factor profiles differ among adults from various racial and ethnic groups with AMI. Hypertension and smoking are key risk factors for Black patients, and diabetes and obesity are key risk factors for Hispanic patients. Although no difference was observed with revascularization and initial medical treatment, Black and Hispanic patients had lower long-term adherence to cardiac medications. Black patients experienced the highest rate of long-term, all-cause mortality, a difference that was attenuated after adjusting for comorbidities and medications. Clinicians and health systems should continue to focus attention on eliminating disparities in care. Research to measure and understand the problem allows the development of programs to improve cultural competency and tailor interventions to address the needs of different communities.
Footnotes
Funding: None declared
Conflicts of Interest: None declared
Author Contributions: Bryant Hammershaimb, MD, participated in data curation, conceptualization, writing (review and editing); Jesse Goitia, MD, participated in writing (original draft), conceptualization; Karo Gyurjian, DO, participated in data curation, writing (review and editing); Sarah Chiu, MD, participated in data curation, writing (review and editing); Malini Nadadur, MD, participated in data curation, writing (review and editing); Aiyu Chen, MPH, participated in data curation, formal analysis, methodology; Ming-Sum Lee MD, PhD, participated in conceptualization, resources, formal analysis, methodology, supervision, writing (review and editing).
References
- 1.Nowbar AN, Gitto M, Howard JP, Francis DP, Al-Lamee R. Mortality from ischemic heart disease. Circ Cardiovasc Qual Outcomes . 2019;12(6):e005375. 10.1161/CIRCOUTCOMES.118.005375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graham G. Disparities in cardiovascular disease risk in the United States. Curr Cardiol Rev . 2015;11(3):238–245. 10.2174/1573403x11666141122220003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2022 update: A report from the American Heart Association. Circulation . 2022;145(8):e153–e639. 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 4.Edmund Anstey D, Li S, Thomas L, Wang TY, Wiviott SD. Race and sex differences in management and outcomes of patients after ST-elevation and non-ST-elevation myocardial infarct: Results from the NCDR. Clin Cardiol . 2016;39(10):585–595. 10.1002/clc.22570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canto JG, Allison JJ, Kiefe CI, et al. Relation of race and sex to the use of reperfusion therapy in medicare beneficiaries with acute myocardial infarction. N Engl J Med . 2000;342(15):1094–1100. 10.1056/NEJM200004133421505 [DOI] [PubMed] [Google Scholar]
- 6.Joseph L, Chan PS, Bradley SM, et al. Temporal changes in the racial gap in survival after in-hospital cardiac arrest. JAMA Cardiol . 2017;2(9):976–984. 10.1001/jamacardio.2017.2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goitia JJ, Phan DQ, Schweis F, et al. Neighborhood resources and racial/ethnic differences in survival after myocardial infarction. J Am Coll Cardiol . 2021;78(6):632–633. 10.1016/j.jacc.2021.06.007 [DOI] [PubMed] [Google Scholar]
- 8.Derose SF, Contreras R, Coleman KJ, Koebnick C, Jacobsen SJ. Race and ethnicity data quality and imputation using U.S. Census data in an integrated health system: The Kaiser Permanente Southern California experience. Med Care Res Rev . 2013;70(3):330–345. 10.1177/1077558712466293 [DOI] [PubMed] [Google Scholar]
- 9.Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). Circulation . 2018;138(20):e618–e651. 10.1161/CIR.0000000000000617 [DOI] [PubMed] [Google Scholar]
- 10.Choudhry NK, Shrank WH, Levin RL, et al. Measuring concurrent adherence to multiple related medications. Am J Manag Care . 2009;15(7):457–464. [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Q, Chang A, Ritchey MD, Loustalot F. Antihypertensive medication adherence and risk of cardiovascular disease among older adults: A population-based cohort study. J Am Heart Assoc . 2017;6(6):e006056. 10.1161/JAHA.117.006056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meadows TA, Bhatt DL, Cannon CP, et al. Ethnic differences in cardiovascular risks and mortality in atherothrombotic disease: Insights from the reduction of atherothrombosis for continued health (REACH) registry. Mayo Clin Proc . 2011;86(10):960–967. 10.4065/mcp.2011.0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yandrapalli S, Nabors C, Goyal A, Aronow WS, Frishman WH. Modifiable risk factors in young adults with first myocardial infarction. J Am Coll Cardiol . 2019;73(5):573–584. 10.1016/j.jacc.2018.10.084 [DOI] [PubMed] [Google Scholar]
- 14.Blackston JW, Safford MM, Mefford MT, et al. Cardiovascular disease events and mortality after myocardial infarction among black and white adults: Regards study. Circ Cardiovasc Qual Outcomes . 2020;13(12):e006683. 10.1161/CIRCOUTCOMES.120.006683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham GN, Jones PG, Chan PS, Arnold SV, Krumholz HM, Spertus JA. Racial disparities in patient characteristics and survival after acute myocardial infarction. JAMA Netw Open . 2018;1(7):e184240. 10.1001/jamanetworkopen.2018.4240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carnethon MR, Pu J, Howard G, et al. Cardiovascular health in African Americans: A scientific statement from the American Heart Association. Circulation . 2017;136(21):e393–e423. 10.1161/CIR.0000000000000534 [DOI] [PubMed] [Google Scholar]
- 17.Oshunbade AA, Kassahun-Yimer W, Valle KA, et al. Cigarette smoking, incident coronary heart disease, and coronary artery calcification in black adults: The jackson heart study. J Am Heart Assoc . 2021;10(7):e017320. 10.1161/JAHA.120.017320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Victor RG, Lynch K, Li N, et al. A cluster-randomized trial of blood-pressure reduction in black barbershops. N Engl J Med . 2018;378(14):1291–1301. 10.1056/NEJMoa1717250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balfour PC, Ruiz JM, Talavera GA, Allison MA, Rodriguez CJ. Cardiovascular disease in hispanics/latinos in the United States. J Lat Psychol . 2016;4(2):98–113. 10.1037/lat0000056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruiz JM, Steffen P, Smith TB. Hispanic mortality paradox: A systematic review and meta-analysis of the longitudinal literature. Am J Public Health . 2013;103(3):e52–e60. 10.2105/AJPH.2012.301103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daviglus ML, Pirzada A, Durazo-Arvizu R, et al. Prevalence of low cardiovascular risk profile among diverse hispanic/latino adults in the United States by age, sex, and level of acculturation: The hispanic community health study/study of latinos. J Am Heart Assoc . 2016;5(8):e003929. 10.1161/JAHA.116.003929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baroletti S, Dell’Orfano H. Medication adherence in cardiovascular disease. Circulation . 2010;121(12):1455–1458. 10.1161/CIRCULATIONAHA.109.904003 [DOI] [PubMed] [Google Scholar]
- 23.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA . 2007;297(2):177–186. 10.1001/jama.297.2.177 [DOI] [PubMed] [Google Scholar]