Significance
Adoptive transfer of antigen-specific T cells has shown impressive clinical success in cancer and infectious diseases. However, due to constant stimulation, the transferred cells can switch into dysfunctional states marked by the expression of programmed cell death protein 1 (PD-1). Recent breakthroughs in cell engineering enabled the precise genetic ablation of this inhibitory receptor via CRISPR/Cas9 technology. First studies have shown benefits of PD-1 knockout for T cell functionality in short-term experiments but potential loss of fitness at later stages. Here, we could show that PD-1 ablation is not necessarily detrimental for long-term functionality and persistence of engineered T cells. These findings are of particular importance since the first PD-1 KO T cell products have recently entered into the clinics.
Keywords: adoptive T cell therapy, genetic engineering, PD-1, CRISPR/Cas9
Abstract
Engagement of the inhibitory T cell receptor programmed cell death protein 1 (PD-1) associates with dysfunctional states of pathogen- or tumor-specific T cells. Accordingly, systemic antibody-mediated blockade of PD-1 has become a central target for immunotherapies but is also associated with severe toxicities due to loss of peripheral tolerance. Therefore, selective ablation of PD-1 expression on adoptively transferred T cells through direct genetic knockout (KO) is currently being explored as an alternative therapeutic approach. However, since PD-1 might also be required for the regulation of physiological T cell function and differentiation, the suitability of PD-1 as an engineering target is controversial. In this study, we systematically investigated the maintenance of T cell functionality after CRISPR/Cas9-mediated PD-1 KO in vivo during and after acute and chronic antigen encounter. Under all tested conditions, PD-1 ablation preserved the persistence, differentiation, and memory formation of adoptively transferred receptor transgenic T cells. Functional PD-1 KO T cells expressing chimeric antigen receptors (CARs) targeting CD19 could be robustly detected for over 390 d in a syngeneic immunocompetent mouse model, in which constant antigen exposure was provided by continuous B cell renewal, representing the longest in vivo follow-up of CAR-T cells described to date. PD-1 KO CAR-T cells showed no evidence for malignant transformation during the entire observation period. Our data demonstrate that genetic ablation of PD-1 does not impair functionality and longevity of adoptively transferred T cells per se and therefore may be pursued more generally in engineered T cell-based immunotherapy to overcome a central immunosuppressive axis.
Functional T cell responses are regulated by a network of costimulatory and inhibitory signals. Indeed, T cell activation is followed immediately by T cell suppression, in order to prevent overshooting inflammatory toxicities and ensure protective immunity with minimal immunopathology (1). Programmed cell death protein 1 (PD-1) is one of the most prominent inhibitory receptors, especially in cytotoxic T cells. While PD-1 is transiently upregulated after acute antigen-specific stimulation to tune down T cell receptor (TCR) signaling cascades (2, 3), extensive activation and consequently long-term overexpression of PD-1 during chronic stimulation can drive T cells into a dysfunctional and exhausted state (4, 5).
A major breakthrough in the field of immunotherapy was the discovery that tumors mediate immune evasion through the expression of inhibitory receptor ligands (i.e., PD-L1); consequently, tumor-infiltrating lymphocytes become suppressed due to continuous engagement of inhibitory receptors, including PD-1, within the tumor environment (6, 7). This evidence turned into the development of the so-called checkpoint inhibitors, which have been already approved as a first-line treatment in several malignancies (8–10). Interestingly, these therapies are currently under investigation also for chronic infections, for which the PD-1 axis was shown to drive T cell exhaustion (11–14). While the “rejuvenation” of exhausted antigen-specific CD8+ T cells showed remarkable clinical success, these therapies rely on the presence of a highly functional preexisting immunity (15), resulting in limited benefits in patients with the absence of high-avidity T cells. Furthermore, as PD-1 plays a crucial role in peripheral immune tolerance (16–18), systemic blockade is associated with toxicities due to enhanced T cell activation and autoreactivity, which can ultimately lead to severe immune-related adverse events in patients (19–21).
Adoptive transfer of antigen-specific T cells or autologous T cells engineered with highly defined antigen-specific receptors has proven to be capable of inducing potent, clinically relevant anti-tumor and anti-viral immune responses (22). Nevertheless, such transferred T cells are still susceptible to immune inhibition and can be driven into dysfunction or exhaustion within the tumor microenvironment as well as during chronic infections (23–25). Therefore, intrinsic impairment of PD-1 expression in therapeutic T cells, rather than combination with PD-1 blockage (26–28), has become an attractive strategy for improving the function of adoptively transferred antigen-specific T cells while limiting immune-related toxicities of checkpoint inhibitors at the same time.
Proof-of-concept for this strategy was shown by CRISPR/Cas9-mediated disruption of the endogenous Pdcd1 locus (29–33) or PD-1 knockdown (34) in primary human chimeric antigen receptor (CAR)-T cells, which resulted in enhanced anti-tumor responses in preclinical xenograft models. Interestingly, PD-1–ablated CAR-T cells showed also greater persistence and tumor infiltration (32–34). Similarly, the abrogation of PD-1/PD-L1 signaling in syngeneic mouse models of acute infections by the use of either anti-PD-1 blocking antibody (2) or PD-1 knockout (KO) mice (35) led to superior activity. While broad consensus on the short-term functional benefits of PD-1 KO in T cells has been achieved, the influence of PD-1 ablation on long-term functionality and safety is still controversial. In the context of cell-intrinsic PD-1 gene disruption, short antigen exposure (tumor models and acute infections) preserved memory formation (34, 35). Only the concomitant abrogation of multiple inhibitor receptors seemed to destabilize the long-term protection of antigen-specific T cells (36). However, PD-1 loss was found detrimental for T cell homeostatic maintenance at later stages both in acute (37) and in chronic (38) infections. Finally, PD-1 was recently identified as a haploinsufficient tumor suppressor in vivo (39), suggesting that PD-1–deficient cells could have an increased risk for unwanted malignant transformation also in adoptively transferred T cells.
Regardless of these concerns, the superior (even if perhaps short) functionality consequent to PD-1 deletion guided the opening of several clinical trials with transgenic receptor-engineered PD-1 KO T cells (40). A first clinical study exploring the adoptive transfer of transgenic TCR-engineered T cells carrying triple endogenous TCR and PD-1 KO has reported on the persistence of the transferred cells without major acute toxicities. Even though PD-1 KO therapeutic T cells seemed to decline in the peripheral circulation over time, a causal connection to a potential loss of fitness has not been demonstrated yet (41), thus leaving the persistence of PD-1–deficient T cells an important unanswered question. Therefore, deeper investigations of the long-term fate of PD-1 KO T cells in both clinical settings as well as preclinical syngeneic mouse models are urgently needed.
Seemingly contradicting results from in vivo experiments with PD-1 inhibition likely raise from the use of different approaches (blocking antibodies, PD-1 KO mice, or genetic gene disruption) as well as different models of antigen stimulation. Here, we systematically investigated the persistence, differentiation capacity, and functionality of adoptively transferred T cells after ex vivo CRISPR/Cas9-mediated PD-1 KO in settings of increasingly longer antigen exposure, from acute to chronic antigen stress. Our data clearly demonstrate that adoptively transferred TCR-transgenic PD-1 KO T cells can differentiate into highly functional effector and memory T cell subsets, the latter supporting long-term immunity. Furthermore, the combination of genetic disruption of inhibitory PD-1 together with retroviral transduction of a CD19-targeting CAR allowed the in vivo tracking of engineered T cells over 13 mo in a syngeneic immunocompetent mouse model with continuous antigen exposure due to B cell renewal. Even in this model, constitutively functional PD-1 KO CAR-T cells could be robustly followed for more than 1 y.
Results
PD-1 KO T Cells Persist and Maintain Functionality after Acute, Nonchronic Infection.
Since it is still controversial whether PD-1 is required for functional long-term T cell memory, we first explored if the genetic ablation of PD-1 in adoptively transferred antigen-specific CD8+ T cells would impair their functionality, maintenance, or memory formation upon acute, nonchronic infection.
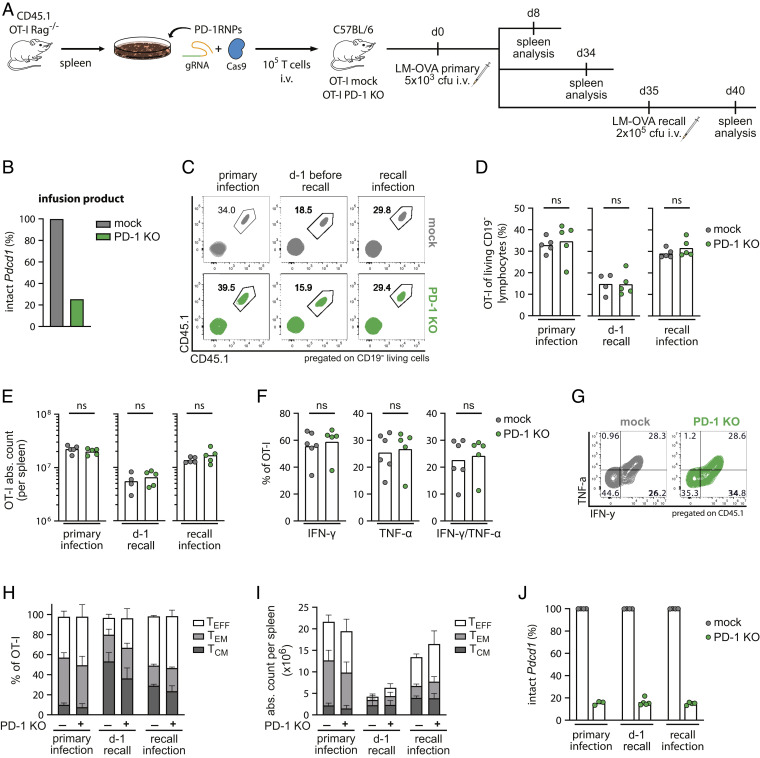
To this purpose, ovalbumin (OVA)-specific TCR-transgenic CD8+ T cells (OT-I T cells) were harvested from spleens of OT-I Rag−/− mice and modified via CRISPR/Cas9 gene editing to disrupt the Pdcd1 locus (Fig. 1A). Congenically labeled (CD45.1+) unedited or edited T cells, the latter consisting of a bulk population with approximately 75% of PD-1 KO (Fig. 1B), were adoptively transferred into immunocompetent mice, followed by infection with Listeria monocytogenes overexpressing recombinant OVA (LM-OVA). Mice were analyzed 8 and 34 d after infection, representing the peak and memory phase of the primary immune response. To investigate the development of T cell memory, a third cohort of mice was rechallenged with LM-OVA at day 35 and their spleens were analyzed after additional 5 d (Fig. 1A).
Fig. 1.
PD-1 KO T cells persist after acute infection. (A) Schematic overview of the experimental setup (n = 8). (B) Next-generation sequencing (NGS) results of T cell infusion products. (C) Representative flow cytometry plots, (D) frequencies, and (E) absolute counts of transferred cells from spleens of mice treated with unedited (mock—grey) or PD-1 edited (PD-1 KO—green) OT-I T cells. Transferred cells were identified by double staining of the congenic marker CD45.1. Statistical testing by Mann–Whitney test, ns P > 0.05. (F) Functional ex vivo cytokine response of OT-I T cells on day 8 post LM-OVA infection (n = 5 to 6 mice) after stimulation with 10−6 M OVA (SIINFEKL) peptide. Statistical testing by Mann–Whitney test, ns P > 0.05. (G) Representative flow cytometry plots of intracellular staining of IFN-γ and TNF-α quantified in (F). (H and I) Phenotypic analysis of transferred OT-I T cells by flow cytometry after primary and recall infection. TCM (CD62L+ CD27+), TEM (CD62L− CD27+), TEFF (CD62L− CD27−). Percentages (H) and absolute counts (I) of T cell subsets expressed as mean + SD. (J) NGS results of CD45.1+ OT-I T cells after flow cytometry-based cell sorting from spleens.
PD-1 KO T cells expanded (day 8), contracted (day 34), and re-expanded (day 40) comparable to control (mock) T cells, with no significant differences in the frequencies or total numbers of antigen-specific OT-I CD8+ T cells (Fig. 1 C–E). Accordingly, we did not observe statistically significant differences in the spleen size between the two groups (SI Appendix, Fig. S1A), consistent with results seen by others during acute Lymphocytic choriomeningitis virus (LCMV) infection (38).
Despite sustained proliferative ability during acute infection, it was unclear whether these OT-I T cells remained functional after PD-1 ablation. We, therefore, tested the ability of PD-1 KO OT-I T cells to produce cytokines after ex vivo peptide restimulation. At day 8 postinfection, both mock and PD-1 KO T cells were highly capable of producing interferon-gamma (IFN-y) as well as tumor necrosis factor alpha (TNF-α) and showed comparable levels of polyfunctionality (Fig. 1 F and G).
Long-term protective CD8+ T cell immunity further relies on diversification and maintenance of different T cell subsets, such as less differentiated central memory (TCM) and effector memory (TEM) T cells. Therefore, we next determined subset diversification in PD-1 competent and deficient T cells based on expression levels of the phenotypic markers CD62L and CD27 (SI Appendix, Fig. S1B). No significant differences were observed in the phenotypic composition as well as in total cell numbers of the different T cell subsets between PD-1 KO and mock OT-I T cells during the observation period (Fig. 1 H and I and SI Appendix, Fig. S1 C and D). Importantly, PD-1–ablated T cells were capable of preserving a stable population of CD62L+ TCM, which is crucial for immune protection in case of reinfection (42–44) and can explain the comparable immune response observed after LM-OVA rechallenge.
Because PD-1 edited T cells were transferred as a bulk population, we could also check for persistence and putative in vivo selection of PD-1 edited or nonedited T cells within the same mouse. Interestingly, genomic analyses revealed constant levels of Pdcd1 editing in PD-1 KO transferred cells after primary and recall infection (Fig. 1J), which were similar to the infusion T cell product (Fig. 1B), indicating that PD-1 KO T cells persisted over time. On the contrary, flow cytometry analysis of PD-1 protein expression was not useful to properly assess the persistence of genetically altered OT-I T cells within the PD-1 KO group but rather showed very similar frequencies of PD-1+ cells in mice treated with PD-1 KO and mock OT-I T cells (SI Appendix, Fig. S2 A and B). A reason for this observation could be the absence of persisting antigen stimulation during LM-OVA infection together with transient PD-1 expression after acute TCR engagement, which resulted in comparable basal expression levels at the time of analyses. For the PD-1 KO group, considering the knockout frequency (around 75%), all OT-I cells with intact PDCD1 loci should express PD-1. Still, the absolute numbers as well as the PD-1 mean fluorescence intensities (MFIs) within the fraction of PD-1+ cells were similar when comparing transferred or endogenous CD8+ T cell populations from PD-1 KO or mock T cell treated mice, suggesting that no compensatory mechanisms occurred in unedited or endogenous OVA-specific CD8+ T cells in the PD-1 KO group (SI Appendix, Fig. S2 B and C).
In summary, these data demonstrate that antigen-specific T cells with PD-1 genetic ablation maintain functionality, differentiation ability, and long-term persistence in this model of acute, nonchronic infection.
Preserved Memory Formation and Maintenance of PD-1 KO T Cells during Chronic Latent Infection.
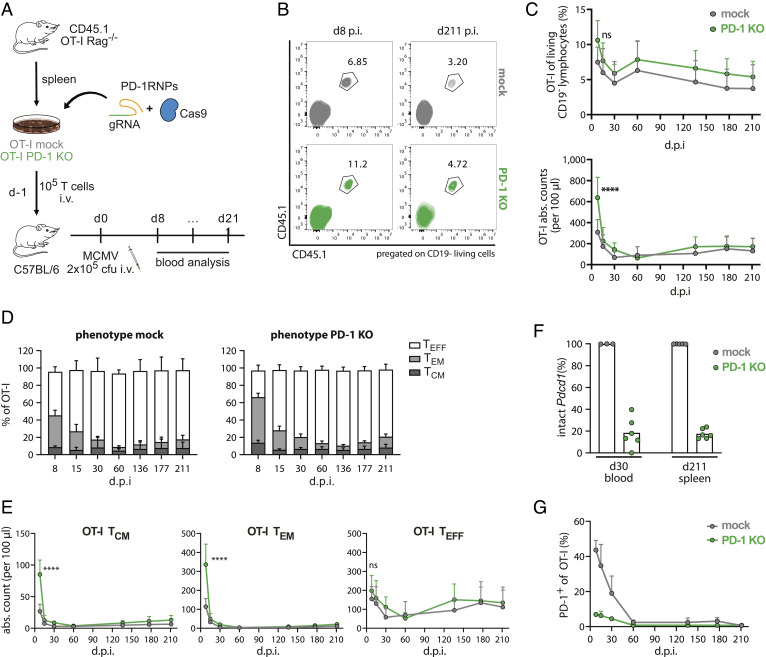
We next examined the effect of PD-1 ablation on longevity and functionality of antigen-specific T cells during murine Cytomegalovirus (MCMV) infection and whether any differences to responses after acute infection could be detected. MCMV is a model for chronic latent infection in which MCMV-specific T cells can be followed over very long periods; thereby, intermittent transcriptional events can trigger an inflationary CD8+ T cell response (45).
For that purpose, T cell products similarly engineered as for the acute LM-OVA setting (mock and PD-1 KO OT-I T cells, Fig. 1B) were simultaneously transferred into BL/6 recipient mice, followed by infection with MCMV overexpressing recombinant OVA (MCMV-OVA) 1 d after T cell infusion. Antigen-specific OT-I T cell responses were monitored in blood over 211 d postinfection (Fig. 2A).
Fig. 2.
PD-1 KO T cells persist during chronic latent infection. (A) Schematic overview of the experimental setup (n = 8). (B) Representative flow cytometry plots of transferred cells on an early (day 8 post-infusion (p.i.)) and late (day 211 p.i.) time point. (C) Kinetics of transferred OT-I T cells in the blood depicted as frequencies (Top row) and absolute counts (Lower row). Data represent the mean + SD. Statistical testing by two-way ANOVA, ns P > 0.05, ****P ≤ 0.0001. (D) Phenotypic analysis of transferred OT-I T cells by flow cytometry for each time point of blood analysis. TCM (CD62L+ CD27+), TEM (CD62L− CD27+), TEFF (CD62L− CD27−). Data are expressed as mean + SD. (E) Absolute counts of T cell subsets over time. Statistical testing by two-way ANOVA, ns P > 0.05, ****P ≤ 0.0001. (F) NGS results of Pdcd1 locus disruption analysis of CD45.1+ OT-I T cells after flow cytometry-based cell sorting. (G) Flow cytometry analyses of PD-1-expressing OT-I T cells. Cells were pregated on living CD45.1 congenic marker.
Transferred OT-I T cells (CD45.1+) robustly expanded in response to the infection (Fig. 2 B and C). Intriguingly, we observed stronger OT-I expansion on day 8 postinfection (both in terms of frequencies and absolute counts) in mice receiving PD-1 KO T cells, even though PD-1 edited transferred cells contracted to levels similar to mock OT-I T cells already 1 wk later (Fig. 2C). It was reported in chronic LCMV infections that PD-1 KO mediates a transient proliferative advantage in antigen-specific T cells due to increased TCR signaling, which eventually leads to accelerated cell death and thereby long-term detrimental effects on longevity and functionality (38). However, unlike the chronic LCMV model, we observed that during MCMV infection PD-1 KO T cells were maintained at stable detectable levels in the blood after an initial faster contraction similarly to mock OT-I T cells. Indeed, frequencies and total cell numbers of circulating OT-I T cells stayed globally comparable in both T cell-treated groups (Fig. 2C and SI Appendix, Fig. S3), indicating long-term durability of antigen-specific CD8+ T cell responses even after PD-1 ablation during chronic, latent MCMV infection. It could be speculated that this divergent behavior between the two infection models may be linked to the different persistence and strength of antigen exposure (45).
Since it was recently shown that continuous antigen stimulation can lead to impaired memory formation in PD-1–ablated T cells (38), we further analyzed whether PD-1 KO could affect memory differentiation of OT-I T cells after MCMV-OVA infection. In line with other reports (35), we observed a significant accumulation of memory (TCM and TEM) cells in mice treated with PD-1 KO OT-I T cells during the acute phase of the infection (day 8), even though this difference was transient and already vanished within 1 wk, presumably due to accelerated cell death (Fig. 2 D and E and SI Appendix, Fig. S4) (37, 38, 46). After contraction, ablation of PD-1 had no influence on phenotypic differentiation as same frequencies and cell numbers of TCM, TEM, and effector (TEFF) T cell subsets were detected in PD-1 KO and mock T cells over time.
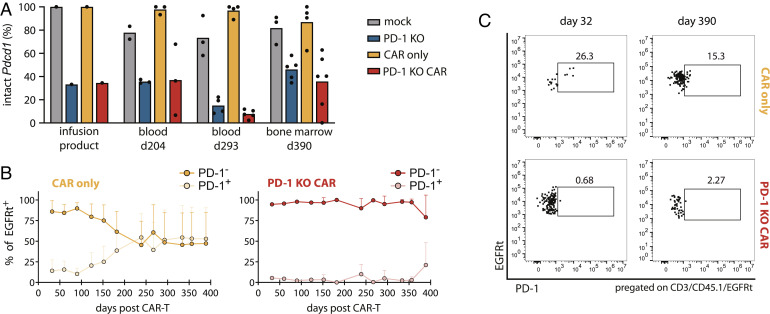
Finally, we analyzed if any PD-1-dependent population shift occurred within mice treated with bulk PD-1 KO T cells. Next-generation sequencing (NGS) analysis revealed maintained levels of Pdcd1 locus disruption (on average 80%) among the infusion product and the transferred PD-1 KO T cells up to day 211 postinfection (Fig. 2F), thereby demonstrating that PD-1 ablation does not lead to positive or negative in vivo selection of antigen-specific T cells during MCMV infection at later stages. Interestingly, also the analysis of PD-1 expression by flow cytometry showed a clear difference in the percentage of PD-1 expressing cells between PD-1 KO and mock OT-I T cells, in particular during the first month after infection (Fig. 2G). These differences in PD-1+ cells could be associated with the frequency of Pdcd1 locus ablation (SI Appendix, Fig. S5A), reflecting prolonged antigen exposure in chronic MCMV infection in comparison to the acute LM-OVA infection.
Similar to the acute LM-OVA model, chronic MCMV infection also did not result in observable differences in the PD-1 MFI of PD-1+ OT-I or endogenous CD8+ T cells between the two groups (SI Appendix, Fig. S5B). This observation suggests again that PD-1-KO T cells provide similar and sufficient functionality as unedited T cells since no compensatory PD-1 upregulation seemed to have occurred.
Taken together, our data clearly show that genetically PD-1–ablated antigen-specific T cells are capable of developing into long-living memory T cells during chronic latent MCMV-OVA infection, demonstrating that loss of PD-1 per se does not impair memory formation in this model. Furthermore, levels of PD-1 surface expression can be used as a surrogate of genomic analyses in settings of persistent antigen exposure.
PD-1 KO CAR-T Cells Maintain Long-Term Functionality Even after 390 d.
During MCMV infection, antigen encounter is believed to occur infrequently and only with low intensity. Consequently, it cannot be excluded that these special characteristics of the MCMV system could be the reason for the high and long-term functionality of PD-1 edited T cells. In order to analyze the influence of PD-1 KO in a model in which triggering via the antigen-specific receptor occurs more stringently and continuously, we assessed T cell functionality after PD-1 ablation in a model of adoptive CAR-T cell transfer targeting mouse CD19+ B cells. Comparable to patients receiving anti-CD19 CAR-T cells, this treatment causes long-lasting B cell aplasia (47–49), which depends on the constitutive recruitment of highly functional CAR-T cells to deplete newly generated CD19+ B cells.
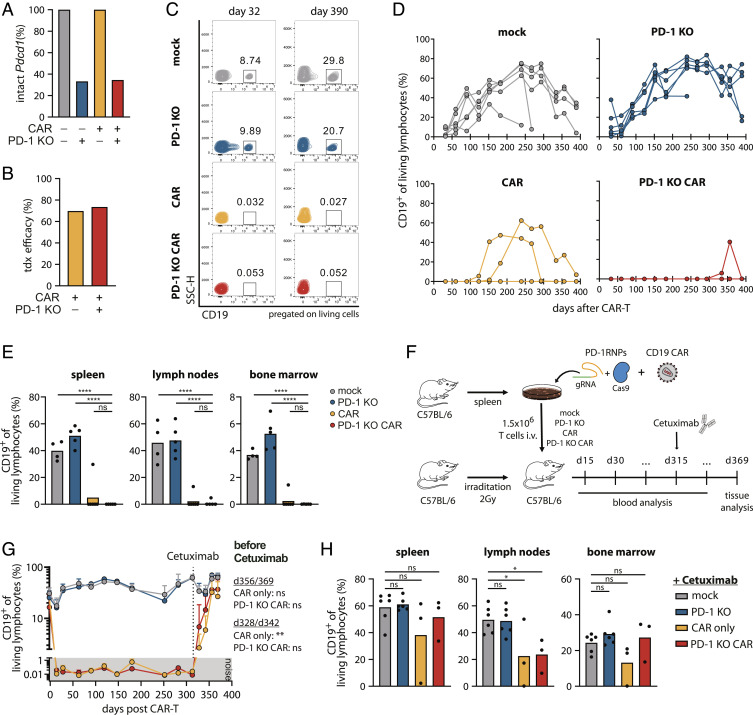
For the generation of PD-1 KO CAR-T cells, we used a combined protocol of Cas9 ribonucleoprotein (RNP) gene editing and retroviral transduction. Briefly, primary murine T cells were in vitro stimulated, followed by nucleofection with RNPs targeting the genetic locus of Pdcd1 and subsequent retroviral transduction of the anti-CD19 CAR. We utilized a retroviral vector of a second-generation murine anti-CD19 CAR in combination with a sequence for truncated epidermal growth factor receptor (EGFRt), as previously described (50). Coexpression of EGFRt functions as a marker of CAR-transduced cells. 70% of the cell population showed efficient genomic disruption of the Pdcd1 locus (Fig. 3A), whereas 70% of cells with or without PD-1 were successfully transduced with the CAR, measured by coexpression of EGFRt (Fig. 3B and SI Appendix, Fig. S6A). Similar MFIs for EGFRt expression confirmed comparable CAR surface expression levels of CAR and PD-1 KO CAR-T cells (SI Appendix, Fig. S6B).
Fig. 3.
PD-1 KO CAR-T cells maintain long-term functionality over 390 d. CD45.1+ splenocytes were edited for PD-1 knockout and anti-CD19 CAR expression, and transferred bulk in C57BL/6 mice (n = 6). (A) NGS analysis of Pdcd1 locus disruption of all four T cell infusion products [mock (gray), PD-1 KO (blue), CAR (yellow), PD-1 KO CAR (red)]. (B) Transduction efficacies of CAR-T cell products measured by flow cytometry as percentage of coexpressed EGFRt+ of living lymphocytes. (C) Representative flow cytometry plots of B cell frequencies in the peripheral blood after CAR-T cell injection. (D) Monthly blood analysis of B cells, depicted as CD19+ cells of living lymphocytes. (E) Mice were killed on day 390 after T cell transfer and remaining B cells were quantified in organs. Statistical testing by one-way ANOVA, ns P > 0.05, ****P ≤ 0.0001 using PD-1 KO CAR group as reference control. (F) Engineered PD-1 KO CAR-T cells were generated and infused as described before. On day 315 post T cell infusion, 1 mg Cetuximab was administered via i.p. injection. (G) B cell kinetics in the peripheral blood (percentage of CD19+ cells of living lymphocytes). The dotted line depicts the time point of Cetuximab administration. Statistical testing by two-way ANOVA, ns P > 0.05, ****P ≤ 0.0001 using mock group as reference control. (H) Mice were killed on day 369 post CAR-T cell injection and tissues were analyzed for B cells by surface staining of CD19. Statistical testing by one-way ANOVA, ns P > 0.05, *P ≤ 0.05 using mock group as reference control.
Especially “life-long” effects of PD-1 ablation on CAR-T cell functionality and safety are still largely unexplored but of particular importance for clinical use. Therefore, we tracked CAR-T cells, B cell depletion, and the role of PD-1 KO for over 390 d (SI Appendix, Fig. S6C), representing to our knowledge the longest in vivo follow-up of CAR-T cells described to date. While two mouse groups received CAR-T cells edited or nonedited for PD-1, two control groups were injected with either completely unedited mock T cells or PD-1 KO T cells without a CAR. All four groups were employed in bulk and without enrichment for PD-1 ablation or CAR expression. Therefore, each mouse of the PD-1 KO CAR group was injected with a defined mixture of four different subpopulations (+/− CAR and +/− PD-1, SI Appendix, Fig. S6 A and C), which allowed additional analysis of in vivo selection within the same recipient mouse.
While B cells were stably circulating in the two control groups, CAR-T cells with and without PD-1 KO showed effective B cell depletion during the entire observation period. Two mice of the CAR group had transient B cell reappearances at 100 and 150 d after CAR-T cell treatment, which were resolved some weeks later. In the PD-1 KO CAR group, a very low level of B cells arose in a single mouse during the last analyzed time points (Fig. 3 C and D). Effective B cell aplasia was also observed in all analyzed lymphoid organs on day 390 (Fig. 3E), emphasizing that the long-term functionality and tissue infiltration of CAR-T cells were not impaired by genetic KO of PD-1 expression.
We next performed CAR-T cell depletion experiments to demonstrate that the transferred CAR-T cells indeed needed to stay constitutively active to maintain B cell aplasia in this long-term model (Fig. 3F). As the engineered CAR-T cells coexpress EGFRt as a safety-switch on their surface, selective depletion was possible by antibody-dependent cell-mediated cytotoxicity via injection of the monoclonal antibody Cetuximab (50, 51). Freshly produced CAR-T cells with 75% transduction efficacy and 90% PD-1 editing (SI Appendix, Fig. S7 A and B) showed again stable long-term B cell aplasia over 40 wk. After Cetuximab treatment, rapid recovery of B cells could be observed in the blood (Fig. 3G and SI Appendix, Fig. S7C), as well as 1 mo later in all lymphoid organs (Fig. 3H). Only one mouse in the CAR group did not respond to Cetuximab treatment for unknown reasons. B cell reconstitution after CAR-T cell depletion further confirmed continuous B cell renewal in the bone marrow and, thereby, constant stimulation of the transferred CAR-T cells.
In summary, these results demonstrate that disruption of PD-1 preserves long-term functionality in a model of constitutively active CAR-T cells.
Memory Subsets in PD-1 KO CAR-T Cells Are Maintained over 1 Y In Vivo.
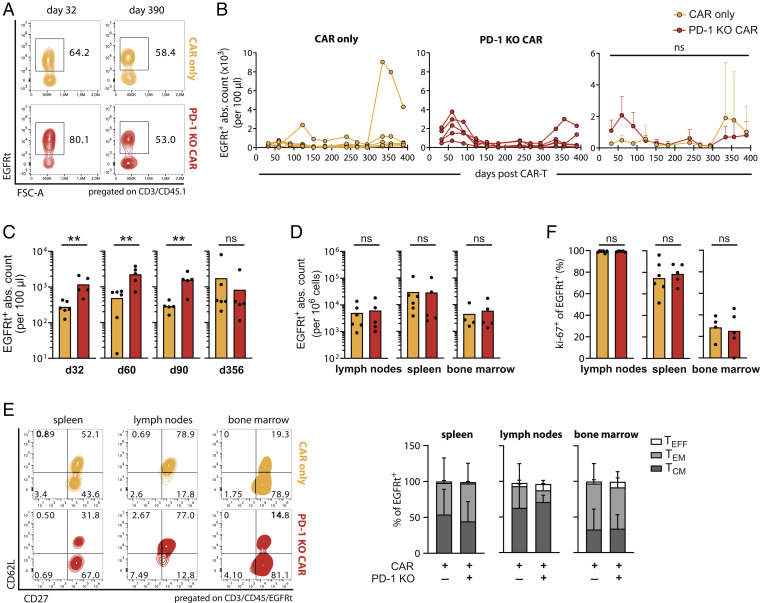
In order to gain insights into the quality of memory subset generation in PD-1 KO CAR-T cells, we performed further analyses on the persistence and phenotype of the transferred cells.
Congenically labeled CAR-T cells with and without PD-1 editing were robustly detected in the peripheral circulation during the entire observation period (Fig. 4 A–C), as well as in lymphoid organs 390 d post infusion (Fig. 4D). Intriguingly, PD-1 KO CAR-T cells showed a stronger expansion than normal CAR-T cells during the first 100 d, as previously described (33, 34, 40). Moreover, we found similar to other reports (38) an accelerated contraction during the chronic phase, which ultimately resulted in similar persistence of the transferred cells between the CAR and PD-1 KO CAR groups at late time points. Interestingly, we observed a strong expansion of transferred CAR-T cells in one particular mouse showing a transient relapse of B cells in the CAR group, indicating recall capacity, a hallmark of memory T cell responses.
Fig. 4.
Persistence and differentiation of CAR-T cells over 390 d. (A) Representative flow cytometry plots showing CAR-T cell (EGFRt+) frequencies of transferred cells (CD3+CD45.1+) in the peripheral blood at the indicated time points after T cell injection. Mice belong to the experiment of Fig. 3 A–E. (B) Longitudinal quantification of CAR-T cell counts (EGFRt+) in individual mice (Left and Middle) and as mean + SD (Right). (C and D) Quantification of CAR-T cell counts (EGFRt+) in blood at the indicated time points (C) and in lymphoid tissues on day 390 (D) after T cell transfer. (E) Phenotypic analysis of tissue-recovered CAR-T cells (EGFRt+) on day 390. TCM (CD62L+ CD27+), TEM (CD62L− CD27+), TEFF (CD62L− CD27−). Representative flow cytometry plots (Left) and relative quantification of subpopulations in organs as mean + SD (Right). (F) Proliferative capacity of tissue-recovered CAR-T cells (EGFRt+). Statistical testing by two-way ANOVA (B) and Mann–Whitney test (C, D, and F), ns P > 0.05, **P ≤ 0.005.
As a next step, we analyzed the subset composition of remaining transferred CAR-T cells in lymphoid organs on day 390 after infusion. CAR-T cells stably engrafted and successfully differentiated independent of their PD-1 status. Frequencies as well as cell numbers of both memory (TCM + TEM) and effector T cell (TEFF) subsets were comparable in the PD-1 KO CAR and CAR group across all lymphoid organs (Fig. 4E and SI Appendix, Fig. S8). Typical memory subset phenotypes made up the biggest part of CAR-T cells, in frequency, as well as in cell numbers, while only few effector phenotype CAR-T cells were detected.
Despite preferential differentiation into memory T cells, dividing CAR-T cells were still observed, as a consequence of constant B cell renewal. The proliferation marker Ki-67 was expressed on nearly 100% of CAR and PD-1 KO CAR-T cells in the lymph nodes and at comparable, but lower, levels in the two other lymphoid tissues (Fig. 4F), indicating considerable divisions of both engineered T cell products among different organs. This phenotype indirectly reflects preserved functionality as limited proliferative ability was observed in functionally impaired PD-1 KO T cells at later stages after chronic LCMV infection (38).
In summary, these results reinforce again that ablation of PD-1 has no negative impact on the persistence and engraftment of CAR-T cells, even 390 d after injection, as well as on efficient differentiation into long-living memory subsets, which are probably crucial for stable B cell aplasia in this model.
We found similar evidence in a syngeneic tumor model, where the leukemic murine Eµ-acute lymphoblastic leukemia (ALL) cell line was inoculated in immunocompetent mice prior to the transfer of either CAR or PD-1 KO CAR-T cells (SI Appendix, Fig. S9A). As expected, PD-1 KO CAR-T cell-treated mice showed longer survival rates despite subsequent tumor relapse in this model (SI Appendix, Fig. S9 B and C), confirming the superior antitumor functionality induced by the removal of the inhibitory receptor (30, 34). Throughout the observation time, PD-1–ablated CAR-T cells remained detectable in both the peripheral circulation and lymphoid organs (SI Appendix, Fig. S9D); indeed, while PD-1 upregulation was observed in most of the conventional CAR-T cells as an indication of antigen-driven stimulation, we found an abundant fraction of PD-1− cells in the PD-1 KO CAR-T cell product at similar levels compared to the infusion product (SI Appendix, Fig. S9E). Within the observable time frame in this model, PD-1 KO CAR-T cells persisted at comparable levels than conventional CAR-T cells (SI Appendix, Fig. S9 F and G) and efficiently established phenotypic changes associated with memory formation (SI Appendix, Fig. S9H). However, further experiments on long-term memory should be performed in settings where tumors do not relapse.
PD-1 KO CAR-T Cells Show Long-Term Persistence but No In Vivo Selection under Constant Stimulation.
The safety profile of CAR-T cells with PD-1 ablation may raise some concerns, due to the fact that expression of PD-1 plays a crucial role in peripheral immune tolerance. Indeed, it is known that the use of PD-1 checkpoint inhibitors can lead to autoimmunity (8, 21) and that disruption of the PD-1 locus, concomitantly with enforcement of oncogenic TCR signaling, can lead to malignant T cell transformation (39). Therefore, we investigated in more detail if adoptively transferred CAR-T cells with intrinsic PD-1 KO could persist long-term and lead to the development of uncontrollable autoreactive T cells under constitutive stimulation in vivo.
Genomic NGS analysis at specific time points supported that no consistent negative or positive in vivo selection of PD-1–ablated T cells occurred. We measured stable frequencies of Pdcd1 disruption in both PD-1 KO groups (with and without CAR) at day 204 and day 293 after T cell injection in the blood as well as on day 390 in the bone marrow (Fig. 5A). In support of that, we also tracked the progression of PD-1 edited and unedited CAR-T cell populations in blood via flow cytometry (Fig. 5 B and C), as PD-1 expression could be used as a surrogate marker of genetic Pdcd1 ablation in models of constant stimulation as previously shown for the chronic MCMV infection model. PD-1+ CAR-T cells (EGFRt+) were reliably detected in the CAR group for over one year. On the contrary, EGFRt+ T cells also expressing PD-1 were barely detectable in mice treated with PD-1 KO CAR-T cells.
Fig. 5.
Absence of in vivo selection of PD-1 edited CAR-T cells. (A) NGS analysis of infused T cell products and transferred CD45.1+ flow cytometry-sorted T cells, belonging to the experiment of Fig. 3 A–E. (B) Longitudinal blood analysis of PD-1-expression in CAR- and PD-1 KO CAR-T cells by flow cytometry. (C) Representative flow cytometry plots showing PD-1 frequencies of EGFRt+ transferred cells in the peripheral blood at the indicated time points after T cell injection.
Consistent with genomic analysis, these results demonstrate that ablation of PD-1 expression does not lead to in vivo selection of potentially autoreactive CAR-T cells during constant stimulation.
Enhanced Expansion of PD-1–Ablated T Cells without CAR Expression.
While KO of PD-1 in antigen-stimulated CAR-T cells did not lead to uncontrolled proliferation, malignant transformation of polyclonal PD-1–ablated T cells without a CAR could not be excluded at this point. Using the previously described bulk transfer method, we tracked potential in vivo competition of all four engineered T cell subpopulations (EGFRt+/− and PD-1+/−) within a single mouse over time.
Overall, we detected persistently higher numbers of total transferred cells in mice receiving PD-1 KO CAR-T cells compared to mice of the CAR group in both blood and lymphoid tissues (SI Appendix, Fig. S10 A–D). As no differences in the total numbers of antigen-specific CAR-T cells were detected beyond the initial phase (Fig. 4B), we were wondering which subpopulation was responsible for this increase. Interestingly, the same trend was observed comparing the total numbers of transferred cells between the PD-1 KO and mock control groups (SI Appendix, Fig. S10 A–D). These results already pointed toward a stronger proliferation of PD-1–ablated T cells without an additional antigen-specific transgenic receptor.
Evaluation of the four subpopulations in blood revealed PD-1 KO T cells without CAR expression (EGFRt−/PD-1−) to be the clear dominators in both CAR groups. This population constituted over 90% of transferred cells in mice treated with PD-1 KO CAR-T cells but only 50% of transferred cells in the CAR group (SI Appendix, Fig. S10E). Furthermore, while only low numbers of EGFRt−/PD-1− engineered T cells were detected within the CAR group, this population immensely overgrew the whole PD-1 KO CAR group in the blood over time (SI Appendix, Fig. S10F). Similar dominance, though on a lower level, was also observed in all screened lymphoid organs (SI Appendix, Fig. S10 G and H). Consistent with the results before, the same trend was observed in the PD-1 KO control group compared to mock T cells (SI Appendix, Fig. S10 E–H). In summary, higher numbers of persisting and engrafting transferred T cells could be explained by a stronger expansion of PD-1–ablated T cells without expression of an additional CAR.
Although the observed differences were not statistically significant at any time point and despite the lack of evidence for more severe toxicities in mice receiving PD-1 KO T cells, the results indicate potential unpredictable risks for the long-term usage of PD-1–ablated polyclonal T cells without selection for a specific antigen receptor, as PD-1 KO is known to reduce thresholds, especially for autoreactive T cell proliferation.
Discussion
In this manuscript, we addressed the question whether genetic ablation of PD-1 in physiologically matured, adoptively transferred T cells compromises in vivo establishment of functional immune responses, memory formation, and maintenance. Thereby, the basic engineering approach for PD-1 ablation was similar to recently reported first-in-man clinical applications (41), despite differences in the cell-type composition of the products (mainly naive T cells in this study compared to more differentiated T cells from autologous patient-derived peripheral T cells). Since differences in genetic engineering procedures can affect T cell performance, we generated T cell products with a highly standardized CRISPR/Cas9-mediated protocol (for several experiments, the same T cell product was even comparatively used in different models, like LM-OVA and MCMV-OVA). Subsequently, PD-1 KO T cells were tested side-by-side in a diverse range of in vivo models, from acute to chronic (but mild) antigen exposure, to obtain highly comparable results.
We showed in models for acute and chronic antigen encounter that for T cell populations expressing a highly defined antigen-specific receptor (either a TCR or a CAR), PD-1 ablation does not negatively impair effector and memory responses per se. In addition, during constitutive long-term recruitment of genetically engineered T cells, PD-1 ablation permitted maintenance of effective immunity and no evidence for malignant transformation was observed. Since PD-1 ablation in normal polyclonal T cells (not expressing a recombinant CAR or TCR) was found to lead to some organ-specific infiltrates, potentially related to some degree of autoreactivity, our data also indicate that PD-1–ablated T cell products should be highly enriched for cells expressing the therapeutically desired antigen-targeting receptor.
Adoptive T cell therapy is rapidly developing and adapting to emerging hurdles. A better understanding of mechanisms of immune suppression like PD-1 has opened the way for a new generation of treatment approaches against cancer and viral infections based on T cell coengineering. Similarly to the success of combinatorial therapy of PD-1 checkpoint inhibitors with adoptively transferred T cells (26, 27), PD-1 KO in engineered T cells, mainly with CAR-T cells, led to superior in vivo anti-tumor efficacy (29, 33, 52). However, these studies reported only on short-term observations, as being conducted in xenograft models. Considering reports on possible long-term negative effects of PD-1 gene disruption on homeostatic maintenance (37, 38), we here provided a clinically relevant model for monitoring of adoptively transferred cells over almost the entire life span of a mouse. Our syngeneic anti-CD19 CAR-T cell system ensures chronic antigen stress by the constant renewal of CD19+ B cells, as demonstrated by the recovery of B cells after CAR-T cell depletion and expression of Ki-67 in CAR-T cells infiltrating the bone marrow. Importantly, we observed long-term functionality of PD-1 KO CAR-T cells, as indicated by stable B cell aplasia, but not an overall functional advantage. Reasonably, conventional CD19 CAR-T cells already suffice for the elimination of CD19+ target cells in our model, as complete B cell relapses were not observed. Instead, in previous studies, PD-1 competent CAR-T cells were challenged against PD-L1-expressing tumors (29, 30, 33, 53) or transferred at relatively low doses (34), which resulted in a suboptimal in vivo functionality. Further studies combining our approach of long-term monitoring with tumor models providing initially high antigen exposure but subsequently complete tumor clearance are necessary to eventually also conclude on the role of PD-1 KO on long-term memory formation under such conditions.
Besides functionality, we also observed sustained persistence, lymphoid tissue infiltration, and memory formation of PD-1 KO T cells regardless of the antigen model. The kinetics of expansion and contraction of PD-1 KO CAR-T cells in our models of chronicity (MCMV and anti-CD19 CAR) were similar to chronic LCMV infections (38) but, differently, PD-1 KO CAR-T cells did not vanish or become dysfunctional at later stages. Sustained antigen expression in LCMV is known to rapidly induce a state of virus-specific T cell exhaustion; on the contrary, MCMV-specific adaptive immunity still preserves functionality over long periods of time because of a discontinuous virus activation and T cell engagement (45). Furthermore, as discussed before, also the anti-CD19 syngeneic CAR-T cell model does not induce rapid and strong features of T cell exhaustion. Taken together, these data suggest that not only chronicity but also the strength and frequency of the antigen stress might be an important trigger for unleashing potentially detrimental effects of PD-1 KO. Therefore, further systematic analyses in suitable mouse models, e.g., using murine tumor cell lines with low to high (persistent) antigen expression, should be performed to elucidate a potential threshold from where on fitness and functionality of PD-1–ablated T cells start to be impaired.
Our work demonstrates that functionality and memory of PD-1 KO T cells are maintained during acute LM-OVA infection, which is in line with previous studies performed also in other models of acute infections (2, 35, 54). However, it was recently reported that T cells with germline PD-1 KO showed a disrupted T cell fitness at later points also after nonchronic stimulations (37, 46). Intriguingly, the temporary inhibition of PD-1 via blocking antibodies during priming did not lead to the same outcome (46), which was interpreted as a dependency of CD8+ T cells responses from the timing and duration of PD-1 inhibition. Despite being plausible, it cannot be excluded that the absence of PD-1 signaling during T cell maturation would already predispose to a lower fitness. Thereby, stringent comparisons between germline PD-1 KO and PD-1 gene disruption in mature T cells are necessary to better understand the contribution of PD-1 loss during the different phases of maturation and priming.
Next to functionality, the safety profile of immunotherapies is a crucial aspect for their clinical application. As PD-1 is broadly expressed in different cell types and involved in the regulation of diverse mechanisms to maintain immune tolerance (16–18), it is not surprising that systemic blockage of PD-1 via checkpoint inhibitors is associated with diverse immune-related adverse events, including autoimmunity (8, 19, 20). In this context, PD-1 ablation on adoptively transferred antigen-specific T cells (29, 30, 52) could be a clever strategy to avoid such systemic side effects and to maintain peripheral tolerance by targeting only the therapeutically relevant T cells.
Nevertheless, some toxicities could still derive from the engineered cell product itself. A more general threat for adoptive cell therapy is that polyclonal populations of PD-1 deficient T cells could still express autoreactive TCRs, which could, without PD-1 as an immunosuppressive receptor, lead to autoimmunity. Our approach to adoptively transfer bulk populations without previous enrichment allowed us to evaluate the effects of the polyclonal endogenous TCR repertoire on the safety profile of antigen-specific and bystander PD-1 KO T cells, in particular in the CAR-T cell model. We did not observe the outgrowing of PD-1 KO CAR-T cells. Even after 13 mo of longitudinal in vivo tracking of continuously stimulated CAR-T cells, there was no evidence of autoimmunity due to genomic PD-1 ablation. It has been demonstrated before that the introduction of a dominant CAR into normal donor T cells can overrule endogenous TCR stimulation and avoid allo- or auto-reactivity (55), which may explain our observations.
In contrast, higher frequencies and cell numbers of PD-1 KO T cells without an anti-CD19 CAR persisted in two independent groups (PD-1 KO and PD-1 KO CAR) throughout the examination period. Even though these PD-1 disrupted T cells did not excessively proliferate, they dominated over CAR-T cells not only in blood but also in organs, which could indicate a potential risk for autoimmunity. This could be overcome by the enrichment of cells expressing the therapeutically desired antigen-targeting receptors prior to adoptive T cell transfer or by additional genetic ablation of endogenous TCRs. The recently described approach of orthotopic TCR replacement combines recombinant TCR engineering with endogenous TCR ablation (56), which could be an elegant way to increase the safety of additionally PD-1 KO-engineered T cell products.
PD-1 was also reported to function as a haploinsufficient tumor suppressor in mouse T cells (39). These data caution that loss of PD-1 could lead to unwanted malignant development of T cells in case of additionally occurring oncogenic mutations during constant receptor triggering. Therefore, we were concerned that in our long-term in vivo model of constitutively active anti-CD19 CAR-T cells in combination with PD-1 ablation, at some point malignant transformation of engineered T cells could occur. However, so far we did not observe any T cell-derived malignancy after PD-1 ablation in any of our experiments, not even after 390 d. These observations suggest that the risk for malignant transformation of PD-1 KO in adoptively transferred CAR- or TCR-engineered T cells is rather low. This is in line with so far published data on patients treated with PD-1 KO TCR-engineered T cells, who also showed no signs of T cell genotoxicity during the monitoring time (41). However, in light of the different quality of T cell products in terms of cell composition between clinical and preclinical studies, more extensive data in syngeneic mouse models as well as clinical patient data are required, to come to more conclusive statements regarding oncogenic risks.
In summary, our data demonstrate that PD-1 ablation via CRISPR/Cas9 does not impair long-term T cell functions per se. Although this was shown unequivocally in different mouse models of acute and chronic antigen encounter, it needs to be emphasized that our findings do not exclude the possibility that under certain conditions, especially with much stronger or more persistent antigen exposure, the outcome could be different. Nevertheless, as no long-term in vivo toxicities were observed upon adoptive transfer of T cells with highly defined antigen-specificity, genomic editing of PD-1 might represent a more broadly applicable approach for protecting engineered T cells from PD-1 mediated inhibitory effects.
Materials and Methods
Study Design.
This study was conducted to evaluate the long-term effects of CRISPR/Cas9-mediated PD-1 KO on the persistence, functionality, and safety of adoptively transferred antigen-specific T cells in vivo. Therefore, PD-1–ablated adoptively transferred T cells were monitored in an acute and chronic infection model with LM-OVA and MCMV-OVA, respectively, and in an acute and long-term therapeutic setting of adoptive CAR-T cell transfer in syngeneic mice. T cell products were either generated by CRISPR-mediated KO of Pdcd1 on transgenic OT-I T cells, recognizing the SIINFEKL antigen, for usage in infection models or on OT-I/wild-type T cells followed by retroviral transduction of an anti-murine CD19 CAR to assess long-term maintenance. Adoptively transferred T cells were tracked by congenic labeling and CAR-T cells were marked by coexpression of a truncated version of epidermal growth factor receipt (EGFR). While genomic PD-1 ablation was studied by next-generation sequencing, expansion kinetics, maintenance of in vivo and ex vivo functionality, phenotypic evolution, and PD-1 expression signatures of adoptively transferred T cells were followed by flow cytometry.
All primary data are reported in SI Appendix.
Animal Models and Cell Lines.
C57BL/6JOlaHsd (female, 8 to 11 wk) were purchased from Envigo. CD45.1+/+ congenic C57BL/6 (female, 7 to 8 wk) were purchased from Charles Rivers, CD45.1 congenic C57BL/6 and SIINFEKL-specific TCR transgenic OT-I RAG1−/− donor mice were derived from in-house breeding under specific pathogen-free conditions at our mouse facility at the Technical University Munich, Institute for Medical Microbiology, Immunology and Hygiene. All animal experiments were approved by the district government of Upper Bavaria (Department 5—Environment, Health and Consumer Protection ROB-55.2-2532.Vet_02-17-138).
The Eμ-ALL cell line was kindly provided by the lab of Riddell at the Fred Hutchinson Cancer Research Center and was derived from an Eμ-myc transgenic mouse as previously described (48).
Infection Models.
Primary and secondary infections with recombinant LM-OVA were performed by intravenous injection of 5 × 103 and 2 × 105 cfu, respectively. For recombinant MCMVIE2-OVA, 5 × 102 pfu was injected intravenously. For additional details, see SI Appendix, Materials and Methods.
CRISPR/Cas9-Mediated PD-1 Knockout.
Cas9 ribonucleoproteins were generated and nucleofected in murine splenocytes as previously described (56). For additional details, see SI Appendix, Materials and Methods.
Retroviral Transduction.
Retroviral particles were produced from PlatE cells and spinoculated on RetroNectin-coated plates prior to the addition of CRISPR/Cas9-edited, stimulated murine splenocytes. Cells were cultured in complete Roswell Park Memorial Institute (RPMI) with 25 ng/mL recombinant human interleukin-15 (rhIL-15) for 3 d prior to intravenous (i.v.) transfer into sublethally irradiated C57BL/6 mice. For additional details, see SI Appendix, Materials and Methods.
Adoptive T Cell Transfer.
For adoptive transfer experiments, murine splenocytes edited for PD-1 knockout and/or CAR expression on a unique congenic marker background (CD45) were used. For additional details, see SI Appendix, Materials and Methods.
Flow Cytometry and Cell Sorting.
Single-cell suspensions were generated from blood, bone marrow, lymph nodes, and spleen, followed by surface marker staining with fluorophore-conjugated antibodies. For additional details, see SI Appendix, Materials and Methods.
Antigen-Specific Ex Vivo Stimulation and Intracellular Cytokine Staining.
Splenocytes from T-cell treated, LM-OVA infected mice were stimulated with 10−6 M SIINFEKL peptide and a positive control (phorbol-12-myristat-13-acetat/ionomycin), followed by intracellular cytokine staining. For additional details, see SI Appendix, Materials and Methods.
Analysis of CRISPR/Cas9-Mediated Pdcd1 Disruption.
Genomic DNA was extracted from flow cytometry-sorted CD45.1+ OT-I T cells or EGFRt+ CAR-T cells. The region surrounding the guide RNA (gRNA) cutting site into the PD-1 locus was amplified via PCR and sequenced. For additional details, see SI Appendix, Materials and Methods.
Statistical Analysis.
All statistical tests were performed using GraphPad Prism8 (GraphPad Software Inc.). Statistical details are indicated in the respective figure legends. Levels of significance were defined as the following: ns P > 0.05, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001. Data are presented as mean + SD if not stated otherwise specifically.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We thank our flow cytometry unit for cell sorting, especially I. Andrä, C. Angerpointner, D. Raju, and M. Schiemann. We also thank members of the Busch and Buchholz laboratories for experimental help and critical discussions. We are also grateful to Steven Broadley for critically reading the manuscript. This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), SFB 1321/1—329628492 (project P17), SFB 1054/3—210592381 (project B09), SFB-TRR 338/1 2021—452881907 (project A01), and FOR2830 (project 5).
Author contributions
S.D., K.S., E.D., and D.H.B. designed research; S.D., M.S., M.H., A.W., F.G., L.W., and J.B. performed research; L.C.-S. contributed new reagents/analytic tools; S.D., M.S., S.J., L.W., J.B., E.D., and D.H.B. analyzed data; and S.D., E.D., and D.H.B. wrote the paper.
Competing interests
D.H.B. has a consulting contract with and receives sponsored research support from Juno Therapeutics/BMS. The remaining authors declare no competing interests.
Footnotes
This article is a PNAS Direct Submission. P.G. is a guest editor invited by the Editorial Board.
Contributor Information
Elvira D’Ippolito, Email: elvira.dippolito@tum.de.
Dirk H. Busch, Email: dirk.busch@tum.de.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Attanasio J., Wherry E. J., Costimulatory and coinhibitory receptor pathways in infectious disease. Immunity 44, 1052–1068 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn E., et al. , Role of PD-1 during effector CD8 T cell differentiation. Proc. Natl. Acad. Sci. U.S.A. 115, 4749–4754 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agata Y., et al. , Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int. Immunol. 8, 765–772 (1996). [DOI] [PubMed] [Google Scholar]
- 4.Mueller S. N., Ahmed R., High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. U.S.A. 106, 8623–8628 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wherry E. J., Kurachi M., Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 15, 486–499 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwai Y., et al. , Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. U. S. A. 99, 12293–12297 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taube J. M., et al. , Colocalization of inflammatory response with B7–H1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci. Transl. Med. 4, 127ra37 (2012), 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topalian S. L., et al. , Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brahmer J. R., et al. , Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366, 2455–2465 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamid O., et al. , Safety and tumor responses with lambrolizumab (anti–PD-1) in melanoma. N. Engl. J. Med. 369, 134–144 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Day C. L., et al. , PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443, 350–354 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Boni C., et al. , Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J. Virol. 81, 4215 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urbani S., et al. , PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J. Virol. 80, 11398–11403 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooks D. G., McGavern D. B., Oldstone M. B. A., Reprogramming of antiviral T cells prevents inactivation and restores T cell activity during persistent viral infection. J. Clin. Invest. 116, 1675–1685 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tumeh P. C., et al. , PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishimura H., et al. , Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 291, 319–322 (2001). [DOI] [PubMed] [Google Scholar]
- 17.Nishimura H., Nose M., Hiai H., Minato N., Honjo T., Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11, 141–151 (1999). [DOI] [PubMed] [Google Scholar]
- 18.Fife B. T., Pauken K. E., The role of the PD-1 pathway in autoimmunity and peripheral tolerance. Ann. N. Y. Acad. Sci. 1217, 45–59 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Wong E., et al. , Nivolumab for relapsed or residual haematological malignancies after allogeneic haematopoietic stem cell transplantation (NIVALLO). Blood 132, 4633–4633 (2018). [Google Scholar]
- 20.Boutros C., et al. , Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combinationNat. Rev. Clin. Oncol. 13, 473–486 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Michot J. M., et al. , Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 54, 139–148 (2016). [DOI] [PubMed] [Google Scholar]
- 22.D’Ippolito E., Schober K., Nauerth M., Busch D. H., T cell engineering for adoptive T cell therapy: Safety and receptor avidity. Cancer Immunol. Immunother. 68, 1701–1712 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feucht J., et al. , T-cell responses against CD19+ pediatric acute lymphoblastic leukemia mediated by bispecific T-cell engager (BiTE) are regulated contrarily by PD-L1 and CD80/CD86 on leukemic blasts. Oncotarget 7, 76902–76919 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joyce J. A., Fearon D. T., T cell exclusion, immune privilege, and the tumor microenvironment. Science 348, 74–80 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Moon E. K., et al. , Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human T cells in solid tumors. Clin. Cancer Res. 20, 4262–4273 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.John L. B., et al. , Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin. Cancer Res. 19, 5636–5646 (2013). [DOI] [PubMed] [Google Scholar]
- 27.Kato D., et al. , GPC1 specific CAR-T cells eradicate established solid tumor without adverse effects and synergize with anti-PD-1 ab. Elife 9, 1–20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu J., et al. , Current status and future prospects of the strategy of combining CAR-T with PD-1 blockade for antitumor therapy (review). Mol. Med. Rep. 17, 2083–2088 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Rupp L. J., et al. , CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci. Rep. 7, 1–10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren J., et al. , Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clin. Cancer Res. 23, 2255–2266 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X., et al. , CRISPR-Cas9-mediated multiplex gene editing in CAR-T cells. Cell Res. 27, 154–157 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo X., et al. , Disruption of PD-1 enhanced the anti-tumor activity of chimeric antigen receptor T cells against hepatocellular carcinoma. Front. Pharmacol. 9, 1–15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu W., et al. , CRISPR/Cas9-mediated PD-1 disruption enhances human mesothelin-targeted CAR T cell effector functions. Cancer Immunol. Immunother. 68, 365–377 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou F., et al. , Engineered triple inhibitory receptor resistance improves anti-tumor CAR-T cell performance via CD56. Nat. Commun. 10, 1–14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allie S. R., Zhang W., Fuse S., Usherwood E. J., Programmed death 1 regulates development of central memory CD8 T cells after acute viral infection. J. Immunol. 186, 6280–6286 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnnidis J. B., et al. , Inhibitory signaling sustains a distinct early memory CD8+ T cell precursor that is resistant to DNA damage. Sci. Immunol. 6, eabe3702 (2021), 10.1126/SCIIMMUNOL.ABE3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalia V., et al. , Metabolic regulation by PD-1 signaling promotes long-lived quiescent CD8 T cell memory in mice. Sci. Transl. Med. 13, 1–33 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Odorizzi P. M., Pauken K. E., Paley M. A., Sharpe A., John Wherry E., Genetic absence of PD-1 promotes accumulation of terminally differentiated exhausted CD8+ T cells. J. Exp. Med. 212, 1125–1137 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wartewig T., et al. , PD-1 is a haploinsufficient suppressor of T cell lymphomagenesis. Nature 552, 121–125 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGowan E., et al. , PD-1 disrupted CAR-T cells in the treatment of solid tumors: Promises and challenges. Biomed. Pharmacother. 121, 109625 (2020). [DOI] [PubMed] [Google Scholar]
- 41.Stadtmauer E. A., et al. , CRISPR-engineered T cells in patients with refractory cancer. Science 367, eaba7365 (2020), 10.1126/science.aba7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graef P., et al. , Serial transfer of single-cell-derived immunocompetence reveals stemness of CD8+ central memory T cells. Immunity 41, 116–126 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Grassmann S., et al. , Early emergence of T central memory precursors programs clonal dominance during chronic viral infection. Nat. Immunol. 21, 1563–1573 (2020). [DOI] [PubMed] [Google Scholar]
- 44.Pais Ferreira D., et al. , Central memory CD8+ T cells derive from stem-like Tcf7hi effector cells in the absence of cytotoxic differentiation. Immunity 53, 985–1000.e11 (2020). [DOI] [PubMed] [Google Scholar]
- 45.Cicin-Sain L., Arens R., Exhaustion and inflation at antipodes of T cell responses to chronic virus infection. Trends Microbiol. 26, 498–509 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Pauken K. E., et al. , The PD-1 pathway regulates development and function of memory CD8+ T cells following respiratory viral infection. Cell Rep. 31, 107827 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maude S. L., et al. , Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 371, 1507–1517 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davila M. L., Kloss C. C., Gunset G., Sadelain M., CD19 CAR-targeted T cells induce long-term remission and B cell aplasia in an immunocompetent mouse model of B cell acute lymphoblastic leukemia. PLoS One 8, e61338 (2013), 10.1371/journal.pone.0061338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kochenderfer J. N., et al. , Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 116, 4099 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paszkiewicz P. J., et al. , Targeted antibody-mediated depletion of murine CD19 CAR T cells permanently reverses B cell aplasia. J. Clin. Invest. 126, 4262–4272 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang X., et al. , A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood 118, 1255–1263 (2011), 10.1182/blood-2011-02-337360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao Z., et al. , CRISPR knock out of programmed cell death protein 1 enhances anti-tumor activity of cytotoxic T lymphocytes. Oncotarget 9, 5208–5215 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi B. D., et al. , CRISPR-Cas9 disruption of PD-1 enhances activity of universal EGFRvIII CAR T cells in a preclinical model of human glioblastoma. J. Immunother. Cancer 7, 304 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu D., et al. , A potential new pathway for PD-L1 costimulation of the CD8-T cell response to listeria monocytogenes infection. PLoS One 8, 7–14 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghosh A., et al. , Donor CD19 CAR T cells exert potent graft-versus-lymphoma activity with diminished graft-versus-host activity. Nat. Med. 23, 242–249 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schober K., et al. , Orthotopic replacement of T-cell receptor α- and β-chains with preservation of near-physiological T-cell function. Nat. Biomed. Eng. 3, 974–984 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.