Abstract
Objective:
Blindsight is a disorder where brain injury causes loss of conscious but not unconscious visual perception. Prior studies have produced conflicting results regarding the neuroanatomical pathways involved in this unconscious perception.
Methods:
We performed a systematic literature search to identify lesion locations causing visual field loss in patients with blindsight (n = 34) and patients without blindsight (n = 35). Resting state functional connectivity between each lesion location and all other brain voxels was computed using a large connectome database (n = 1,000). Connections significantly associated with blindsight (vs no blindsight) were identified.
Results:
Functional connectivity between lesion locations and the ipsilesional medial pulvinar was significantly associated with blindsight (family wise error p = 0.029). No significant connectivity differences were found to other brain regions previously implicated in blindsight. This finding was independent of methods (eg, flipping lesions to the left or right) and stimulus type (moving vs static).
Interpretation:
Connectivity to the ipsilesional medial pulvinar best differentiates lesion locations associated with blindsight versus those without blindsight. Our results align with recent data from animal models and provide insight into the neuroanatomical substrate of unconscious visual abilities in patients.
Blindsight is a rare disorder where brain injury causes loss of conscious perception in part of the visual field yet unconscious perception in that area remains somewhat intact.1 For example, people with blindsight can accurately point to the location of a visual stimulus but still report that they are unable to consciously experience seeing the stimulus. Identifying the brain regions responsible for this unconscious perception has been challenging due to the rarity of the syndrome and because lesion location alone has not differentiated between patients with versus without blindsight.2 As such, different studies have implicated different brain regions, including the lateral geniculate nucleus (LGN),3–5 pulvinar,6–8 superior colliculus (SC),8 V1,9 and V5.3,4,10 For example, multiple studies in humans3,4 and earlier work in monkeys5 implicated the LGN, but recent work in monkeys points to the pulvinar.7,8
Lesion network mapping (LNM) is a new technique that accounts for lesion connectivity and can help link rare lesion-induced syndromes to neuroanatomy.11–20 The technique compares lesion locations to normalized resting state functional connectivity maps to determine group-level differences.20,21 Both blindsight patients and blindsight-negative controls have a brain injury causing conscious vision loss, but only blindsight patients have unconscious visual perception. As such, standard practice in the study of blindsight is to compare patients with blindsight to blindsight-negative controls; neuroanatomical differences between these groups are thought to be associated with the unconscious abilities.2–4 To identify the neural correlates of the unconscious visual perception in blindsight, we test whether lesion locations without versus with blindsight show differential connectivity to regions previously implicated in blindsight abilities. LNM is well suited for studying this rare syndrome, because it allows us to (1) interrogate lesion location from published images and (2) interrogate lesion connectivity without functional neuroimaging from the patients themselves.
Patients and Methods
Case Selection
The PubMed database was searched through October 2020 by combining the search terms “blindsight” or “blind-sight” or “Riddoch” with the terms “CT” or “MRI” or “imaging” or “neuroimaging” or “fMRI”. We identified all reported cases of blindsight where patients (1) had a focal brain lesion causing a unilateral hemianopia or quadrantanopia measured by perimetry; (2) denied subjective, conscious visual perception matching visual stimuli in the blind field during testing; (3) had structural images of sufficient quality for lesion tracing; and (4) performed better than chance in their blind visual field on a forced-choice paradigm. As a comparison group, we also identified patients who had been investigated specifically for the phenomena of blindsight and met the above Criteria 1–3 but did not perform better than chance on a forced-choice paradigm.
Blindsight Definition
To define blindsight for the purposes of this study, we included cases that employed a direct testing method where participants were required to point, direct a voluntary eye movement, or verbally report information regarding the location, direction of motion, or a specific simple feature of the stimulus in their blind field. Forced-choice paradigms, where participants are required to indicate a voluntary response regarding the features of a visual stimulus they deny experiencing seeing, were the earliest method used to detect blindsight and to define the phenomena.1,22 Although more complex techniques have been developed to assess for residual visual abilities,23 we focus on the forced-choice method, because it aligns with the classic definition of blindsight and has been used to define blindsight in the largest and most recent human studies.3,4,24
We also required denial of a conscious visual perception by participants during blindsight testing, although the studies we included assessed this conscious visual perception in different ways.25–27 We excluded cases if there was no assessment of conscious visual perception or if participants reported well-formed conscious perception, but we did not differentiate based on assessment method. We did not exclude participants for awareness that a stimulus was presented without a conscious visual perception (sometimes called blindsight type 2) or reports of vague, inconsistent experiences during a visual stimulus that did not clearly match the stimulus presented. Note that such reports are common among even the most famous and well-studied blindsight participants.28
Lesion Tracing
Lesion locations were traced in the 2-dimensional planes in which they were displayed in the published article, using neuroanatomical landmarks to allow accurate transfer onto a template brain. All brain lesions were traced from structural images in 3D Slicer (v4.10.2, https://www.slicer.org/)29 into normalized space on a standardized template brain (MNI152 T1 2mm brain, http://fsl.fmrib.ox.ac.uk/fsldownloads/). Mapping was performed by I.K. and reviewed for accuracy by C.A.A. or J.R.B., all board-certified neurologists. All images used are publicly available.
Lesion Network Mapping
LNM is a new, but extensively validated technique that compares structural lesion locations to normalized resting state functional connectivity maps to identify brain networks disrupted by a given lesion.11–20 In brief, resting state functional connectivity between each lesion location and all other brain voxels was computed using a large connectome database from healthy young individuals (n = 1,000, mean age = 21.3 years, range = 18–35 years, 42.7% male).30 Functional connectivity results were combined across the 1,000 subjects using a random effects analysis, producing a single “lesion network map” for each patient. Lesion network maps were grouped by blindsight status and compared using a voxelwise 2-sample t test masked to key regions previously implicated in blindsight (LGN,3–5 pulvinar,6–8 SC,5 V1,9 and V53,4). We controlled for multiple comparisons using a voxelwise family wise error (FWE) rate of p ≤ 0.05. To generate our region of interest mask, we used the DISTAL atlas31 for thalamic structures (LGN and inferior, lateral, and medial pulvinar) and the Julich atlas32 for cortical structures (V1 and V5). There are, unfortunately, no publicly available, high-quality, validated atlas masks of the superior colliculus, so a hand-drawn mask used in recent blindsight work was employed.33 Because all participants had unilateral field deficits, which localize unilaterally in the brain, images were flipped to one side (left) for analysis, as has been done in prior blindsight research.3,10 All analyses were repeated with images flipped to the right.
The strategy of Fox et al was employed to process resting state functional magnetic resonance imaging data.30,34 The functional connectivity data are available online through the Harvard Dataverse at https://doi.org/10.7910/DVN/ILXIKS. The lesions used in this study are publicly available and obtained from the medical literature (see Supplementary Tables S1 and S2). The pipeline used to prepare the functional connectivity data is available at https://github.com/bchcohenlab/BIDS_to_CBIG_fMRI_Preproc2016. Statistical analyses were performed in MATLAB (MathWorks, Natick, MA; v2019b) or SPSS (IBM, Armonk, NY; v27.0.1.0). The study was approved by mass general brigham (MGB)/Partners Institutional Review Board (protocol no. 2020P002987).
Results
We identified 34 unique blindsight patients (Fig 1 and Supplementary Table S1) and 35 blindsight-negative controls (Supplementary Table S2). The two cohorts had similar mechanisms of brain injury and types of field deficits; the control cohort was slightly older and had more males, and patients were tested closer to the time of injury (Table).
FIGURE 1:
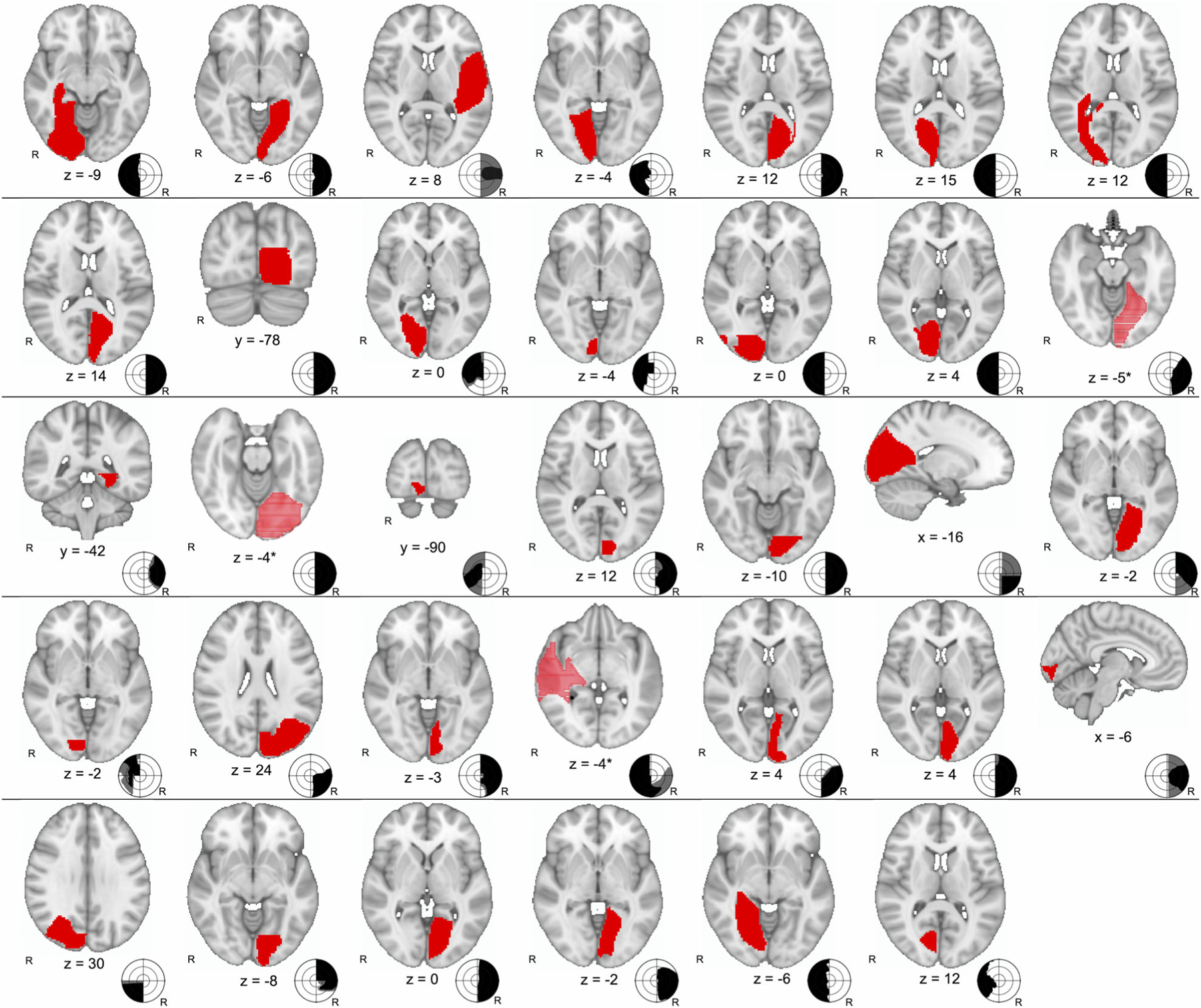
Traced lesion locations of 34 cases of blindsight (red) with schematic drawing of visual fields. By convention, visual fields are from perspective of viewer; black area represents dense visual field loss to even the brightest stimuli, whereas gray is relative loss. Participants were tested for blindsight within area of dense vision loss. *Coordinate provided is at center of the lesion; a nonconventional orientation was used to better illustrate the extent of the lesion. R = right.
TABLE.
Blindsight Participant and Blindsight-Negative Control Characteristics
| Characteristic | Blindsight, n = 34 | Blindsight-Negative, n = 35 | p |
|---|---|---|---|
| Mean age, yr (SD, range) | 49.2 (14.5, 19–77) | 55.8 (14.6, 24–78) | 0.064 |
| Women, n (%) | 13 (38.2) | 7 (20.0) | 0.098 |
| Time since injury when testing performed, mo (SD, range) | 103.5 (168.7, 1–780) | 37.9 (76.4, 3–384) | 0.040 |
| Laterality of field deficit, n (%) | 0.28 | ||
| Right | 20 (58.8) | 16 (45.7) | — |
| Left | 14 (41.2) | 19 (54.3) | — |
| Type of field deficit, n (%) | 0.94 | ||
| Hemianopia | 25 (73.5) | 26 (74.3) | — |
| Quadrantanopia or partial hemianopia | 9 (26.5) | 9 (25.7) | — |
| Laterality of brain lesion, n (%) | 0.28 | ||
| Right | 14 (41.2) | 19 (54.3) | — |
| Left | 20 (58.8) | 16 (45.7) | — |
| Primary reported pathology of brain injury leading to vision loss, n (%) | 0.73 | ||
| Stroke/hemorrhage | 26 (76.5) | 28 (80.0) | — |
| Other | 8 (23.5) | 7 (20.0) | — |
| Tumor | 3 (8.8) | 2 (5.7) | — |
| Postsurgical | 4 (11.8) | 3 (8.6) | — |
| Trauma | 3 (8.8) | 1 (2.9) | — |
| Developmental | 1 (2.9) | 2 (5.7) | — |
| Vascular malformation | 1 (2.9) | 2 (5.7) | — |
| Preexisting brain disease [prior to injury that caused vision loss], n (%) | 5 (14.7) | 6 (17.1) | 0.79 |
| Vascular malformation | 1 (2.9) | 2 (8.6) | — |
| Epilepsy | 1 (2.9) | 2 (5.7) | — |
| Tumor | 3 (8.8) | 2 (5.7) | — |
| Structural imaging used for mapping, n (%) | 0.25 | ||
| MRI | 32 (94.1) | 30 (85.7) | — |
| CT | 2 (5.9) | 5 (14.3) | — |
| Modality used in testing, n (%) | |||
| Moving | 18 (52.9) | 18 (51.4) | 0.90 |
| Static | 19 (55.9) | 22 (62.9) | 0.56 |
CT = computed tomography; MRI = magnetic resonance imaging; SD = standard deviation.
Lesion locations from blindsight-negative compared to blindsight patients showed a significant difference in functional connectivity to the ipsilesional medial pulvinar (left analysis peak [−8, −32, 2] FWE p = 0.029, uncorrected p = 0.0004; right analysis peak [10, −32, 4] FWE p = 0.041, uncorrected p = 0.0006; Fig 2). This difference was driven by more positive functional connectivity for blindsight-negative patients (T = 3.6, FzR = 0.02) compared to blindsight patients (T = −3.9, FzR = −0.02) (FzR is defined as Fisher-transformed R values). No voxels in the LGN, V1, V5, or SC demonstrated statistically significant differences in connectivity between blindsight and blindsight-negative subjects. No voxels outside of this a priori search space showed a significant difference in a whole-brain voxelwise analysis.
FIGURE 2:
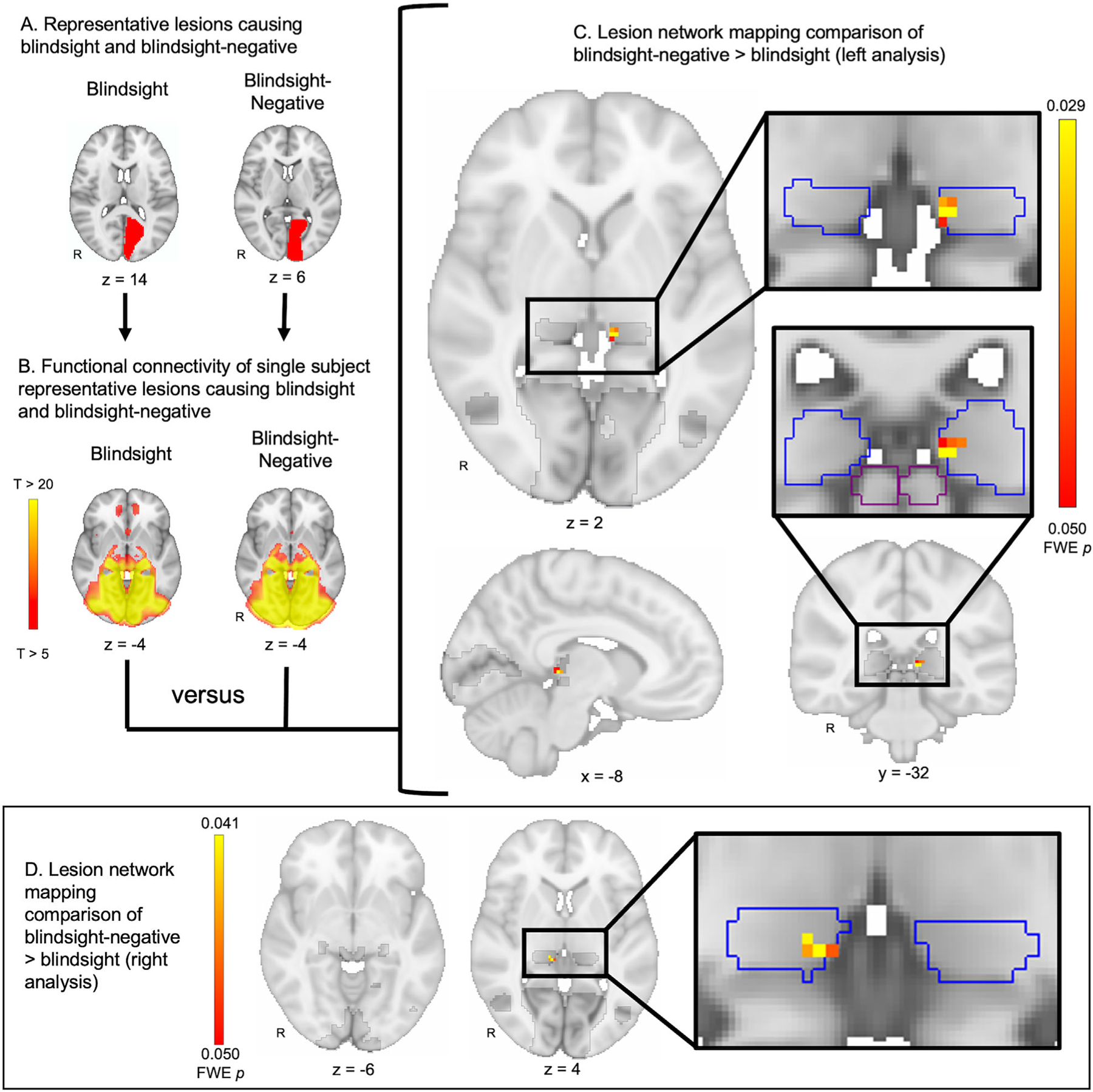
Lesion network mapping of blindsight-negative versus blindsight. (A) Representative lesion locations from a blindsight and blindsight-negative patient. Lesions were consolidated into a single hemisphere for analysis (left analysis shown). (B) Connectivity between each lesion location and the rest of the brain was computed using a normative database of resting state functional connectivity from 1,000 healthy subjects. Pictured are the connectivity patterns derived from the two representative lesion locations shown in A. (C) Connectivity differences between lesion locations from blindsight-negative (n = 35) versus blindsight patients (n = 34) were identified using a 2-sample, voxelwise t test within a mask of regions previously implicated in blindsight (dark gray). Lesions in blindsight-negative patients showed greater functional connectivity to the medial pulvinar (pulvinar in blue outline) compared to lesions in blindsight patients. No voxels in the lateral geniculate nucleus, V1, V5, or superior colliculus (outlined in purple) were identified. Images were corrected for multiple comparisons using a voxel-based family wise error (FWE) rate of p ≤ 0.05. (D) The analysis shown in A–C was repeated, but consolidating lesion locations onto the right hemisphere rather than the left hemisphere, with identical findings. R = right.
Connectivity to the same region of the medial pulvinar remained the key distinction in subgroup analyses when the cohort was divided by blindsight stimulus type including moving (left analysis peak [−10, −30, 2] FWE p = 0.17, uncorrected p = 0.0092; right analysis peak [14, −32, 10] FWE p = 0.24, uncorrected p = 0.0090) and static stimuli (left analysis peak [−8, −32, 2] FWE p = 0.095, uncorrected p = 0.0016; right analysis peak [10, −32, 4] FWE p = 0.124, uncorrected p = 0.0054).
Discussion
Using 69 lesion locations associated with visual field loss, we show that functional connectivity between the lesion location and the ipsilesional medial pulvinar best differentiates between patients with blindsight compared to patients without blindsight, suggesting that connectivity to this region is associated with the unconscious visual abilities. This result is consistent with recent monkey data,7,8 providing convergent support for the pulvinar as a critical node in supporting unconscious visual perception.
We show a group-level difference as in prior LNM work,20,21 but it is worth discussing how to interpret this group difference. Whereas LNM has been most extensively used to evaluate lesions that cause a new symptom13,15,16,18,20 or loss of function,19,21 here we employ it to study blindsight, which is preservation of function after brain injury. Conceptually, blindsight-negative patients (with loss of both conscious and unconscious visual perception) serve as the primary analysis group, whereas blindsight patients (loss of conscious but preserved unconscious visual perception) serve as the comparison, a contrast consistent with prior blindsight work.2–4 Our results show a statistically significant group-level difference in functional connectivity to the ipsilesional medial pulvinar, which is more positive in the blindsight-negative group compared to the blindsight group. Because both groups have similar visual field loss and differ only in the presence versus absence of blindsight, this suggests that pulvinar connectivity is associated with the unconscious abilities. Note that lesion network overlap, used in some prior LNM work, is not useful in the investigation of blindsight. We are not looking for connectivity common to all lesions associated with blindsight, but rather the contrast in connectivity between blindsight-positive and blindsight-negative lesions. This focus on differences in lesion connectivity between groups has been used in the most recent LNM studies from our group and others.20,21,35
Our results contradict some prior studies of blindsight in human patients,3,4 and earlier monkey work,5 both of which highlight the importance of the LGN. Prior human studies implicated the LGN and LGN-V5 connectivity3,4 in blindsight. However, these studies did not examine tracts to the pulvinar4 or focused on a region of interest in the ventrolateral pulvinar,3 which may be why they did not identify the medial pulvinar as we found here. Early work in monkeys also showed that lesions to the LGN disrupt blindsight.5 However, these LGN lesions also disrupt normal vision and conscious visual perception, leaving it unclear whether results were specific to blindsight. Recent double-dissociation monkey research found that only inactivation of the pulvinar is specific to disrupting blindsight abilities,7 aligning well with our findings in patients.
Role of Pulvinar in Blindsight
The pulvinar, which is an important association hub with rich connections to visual areas,36 is a compelling candidate for the unconscious visual abilities in blindsight. Previously considered enigmatic, the pulvinar has emerged as a relay essential for directing visual selective attention,37 calculating visual confidence, and identifying behaviorally relevant objects38 and has been implicated in other forms of unconscious visual perception.36 Earlier study of the role of the thalamus in vision focused on the lateral pulvinar, given its connections to V139; however, the medial pulvinar receives diverse corticocortical inputs from visual areas as well as connections relaying visual information via the superior colliculus and directly from the retina.7,40 In monkeys, the medial pulvinar is essential for directing visual attention, assigning visual confidence,41 visual salience, filtering distractors, and visually guided behavior.42 In humans, functional imaging studies have shown modulation in the medial pulvinar during selective visual attention tasks43 and filtering distractors.39,44 Pulvinar lesions cause contralesional neglect and impairments in visually guided behavior,39 which may explain why disruption of connectivity with this region is a fundamental difference distinguishing blindsight-negative from blindsight patients.
Limitations
Our study has several key limitations. First, although we used the largest collection of lesion locations associated with blindsight to date, small sample size due to the rarity of this phenomenon remains a limitation. Second, there is often a delay between the lesion and when blindsight is detected, raising questions as to whether the occurrence of the phenomenon primarily depends upon the lesion itself or brain reorganization following the lesion.45 Our results suggest that connectivity with lesion location is at least one important factor for blindsight and aligns with data showing that blindsight can be detected soon after injury.1,24,25,46 Moreover, LNM has yielded useful findings in syndromes that are known to emerge in a delayed fashion after injury, such as poststroke pain15 and dystonia,16 and has shown similar results independent of the delay between the lesion and symptoms.47 However, we cannot exclude the importance of brain reorganization or other factors that could be important for the development of blindsight.
A third limitation is that we did not explore all forms of blindsight and focused specifically on blindsight assessed using direct testing methods. Blindsight cases identified using indirect testing methods, such as assessing the priming effect or attentional cueing impact of unseen stimuli,48 may be less subjectively biased but could rely on different brain regions. Finally, there was heterogeneity in the methods used for blindsight testing across the included studies and the lack of full 3-dimensional neuroimaging data from each patient; both of these limitations, however, would bias toward the null hypothesis, rather than the results demonstrated here. In addition, prior work has demonstrated that single or multiple representative slices through a lesion volume can adequately approximate whole lesion connectivity patterns.15
Conclusions
In summary, by assessing for connectivity differences between lesions causing visual loss with or without blindsight, we found that preservation of connectivity with the ipsilesional medial pulvinar is associated with preserved unconscious visual abilities in blindsight.
Supplementary Material
Acknowledgments
A.L.C. was supported by NIH National Institute of Mental Health grant K23 MH120510 and the Shields Research Grant from the Child Neurology Foundation. R.R.D was supported by NIH National Institute on Aging grant K23 AG070320-01A1. M.D.F. was supported by the Nancy Lurie Marks Foundation, the Mather’s Foundation, the Ellison/Baszucki Foundation, the Kaye Family Research Endowment, and NIH National Institute of Mental Health grants R21 MH126271, R01 MH113929, R01 MH115949 and National Institute on Aging grants R56 AG069086, and R01 AG060987. We thank the many patients who participated in the included studies and the researchers for publicly sharing their results; and the lab members of the NimLab at Beth Israel Deaconess Medical Center and the Center for Brain Circuit Therapeutics at Brigham and Women’s Hospital who helped develop some of the code employed in this study.
Footnotes
Additional supporting information can be found in the online version of this article.
Potential Conflicts of Interest
Nothing to report
References
- 1.Weiskrantz L, Warrington EK, Sanders MD, Marshall J. Visual capacity in the hemianopic field following a restricted occipital ablation. Brain 1974;97:709–728. [DOI] [PubMed] [Google Scholar]
- 2.Blythe IM, Kennard C, Ruddock KH. Residual vision in patients with retrogeniculate lesions of the visual pathways. Brain 1987;110: 887–905. [DOI] [PubMed] [Google Scholar]
- 3.Ajina S, Bridge H. Blindsight relies on a functional connection between hMT+ and the lateral geniculate nucleus, not the pulvinar. PLoS Biol 2018;16:e2005769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ajina S, Pestilli F, Rokem A, et al. Human blindsight is mediated by an intact geniculo-extrastriate pathway. Elife 2015;20:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmid MC, Mrowka SW, Turchi J, et al. Blindsight depends on the lateral geniculate nucleus. Nature 2010;466:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barleben M, Stoppel CM, Kaufmann J, et al. Neural correlates of visual motion processing without awareness in patients with striate cortex and pulvinar lesions. Hum Brain Mapp 2015;36:1585–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takakuwa N, Isa K, Onoe H, et al. Contribution of the pulvinar and lateral geniculate nucleus to the control of visually guided saccades in blindsight monkeys. J Neurosci 2021;41:1755–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinoshita M, Kato R, Isa K, et al. Dissecting the circuit for blindsight to reveal the critical role of pulvinar and superior colliculus. Nat Commun 2019;10:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radoeva PD, Prasad S, Brainard DH, Aguirre GK. Neural activity within area V1 reflects unconscious visual performance in a case of blindsight. J Cogn Neurosci 2008;20:1927–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ajina S, Kennard C, Rees G, Bridge H. Motion area V5/MT+ response to global motion in the absence of V1 resembles early visual cortex. Brain 2015;138:164–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joutsa J, Shih LC, Fox MD. Mapping Holmes tremor circuit using the human brain connectome. Ann Neurol 2019;86:812–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fasano A, Laganiere SE, Lam S, Fox MD. Lesions causing freezing of gait localize to a cerebellar functional network. Ann Neurol 2017;81: 129–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen AL, Mulder BPF, Prohl AK, et al. Tuber locations associated with infantile spasms map to a common brain network. Ann Neurol 2021;89:726–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox MD. Mapping symptoms to brain networks with the human connectome. N Engl J Med 2018;379:2237–2245. [DOI] [PubMed] [Google Scholar]
- 15.Boes AD, Prasad S, Liu H, et al. Network localization of neurological symptoms from focal brain lesions. Brain 2015;138:3061–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corp DT, Joutsa J, Darby RR, et al. Network localization of cervical dystonia based on causal brain lesions. Brain 2019;142:1660–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darby RR, Laganiere S, Pascual-Leone A, et al. Finding the imposter: brain connectivity of lesions causing delusional misidentifications. Brain 2017;140:497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joutsa J, Horn A, Hsu J, Fox MD. Localizing parkinsonism based on focal brain lesions. Brain 2018;141:2445–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferguson MA, Lim C, Cooke D, et al. A human memory circuit derived from brain lesions causing amnesia. Nat Commun 2019;10: 3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Padmanabhan JL, Cooke D, Joutsa J, et al. A human depression circuit derived from focal brain lesions. Biol Psychiatry 2019;86: 749–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wawrzyniak M, Klingbeil J, Zeller D, et al. The neuronal network involved in self-attribution of an artificial hand: a lesion network-symptom-mapping study. Neuroimage 2018;166:317–324. [DOI] [PubMed] [Google Scholar]
- 22.Poppel E, Held R, Frost D. Letter: residual visual function after brain wounds involving the central visual pathways in man. Nature 1973; 243:295–296. [DOI] [PubMed] [Google Scholar]
- 23.Danckert J, Rossetti Y. Blindsight in action: what can the different sub-types of blindsight tell us about the control of visually guided actions? Neurosci Biobehav Rev 2005;29:1035–1046. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez-Lopez J, Cardobi N, Pedersini CA, et al. What cortical areas are responsible for blindsight in hemianopic patients? Cortex 2020; 132:113–134. [DOI] [PubMed] [Google Scholar]
- 25.Stoerig P, Zontanou A, Cowey A. Aware or unaware: assessment of cortical blindness in four men and a monkey. Cereb Cortex 2002;12: 565–574. [DOI] [PubMed] [Google Scholar]
- 26.Sahraie A, Trevethan CT, Weiskrantz L, et al. Spatial channels of visual processing in cortical blindness. Eur J Neurosci 2003;18:1189–1196. [DOI] [PubMed] [Google Scholar]
- 27.Ajina S, Rees G, Kennard C, Bridge H. Abnormal contrast responses in the extrastriate cortex of blindsight patients. J Neurosci 2015;35: 8201–8213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiskrantz L Is blindsight just degraded normal vision? Exp Brain Res 2009;192:413–416. [DOI] [PubMed] [Google Scholar]
- 29.Fedorov A, Beichel R, Kalpathy-Cramer J, et al. 3D slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging 2012;30:1323–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeo BT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 2011;106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ewert S, Plettig P, Li N, et al. Toward defining deep brain stimulation targets in MNI space: a subcortical atlas based on multimodal MRI, histology and structural connectivity. Neuroimage 2018;170: 271–282. [DOI] [PubMed] [Google Scholar]
- 32.Amunts K, Eickhoff SB, Caspers S, et al. Whole-brain parcellation of the Julich-brain cytoarchitectonic atlas (v1.18). 2019. https://search.kg.ebrains.eu/instances/Dataset/4ac9f0bc-560d-47e0-8916-7b24da9bb0ce. DOI: 10.25493/8EGG-ZAR. Accessed December 12, 2020. [DOI]
- 33.McFadyen J, Mattingley JB, Garrido MI. An afferent white matter pathway from the pulvinar to the amygdala facilitates fear recognition. Elife 2019;8:e40766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fox MD, Snyder AZ, Vincent JL, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 2005;102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cotovio G, Talmasov D, Barahona-Correa JB, et al. Mapping mania symptoms based on focal brain damage. J Clin Invest 2020;130: 5209–5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bridge H, Leopold DA, Bourne JA. Adaptive pulvinar circuitry supports visual cognition. Trends Cogn Sci 2016;20:146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LaBerge D, Buchsbaum MS. Positron emission tomographic measurements of pulvinar activity during an attention task. J Neurosci 1990;10:613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fischer J, Whitney D. Attention gates visual coding in the human pulvinar. Nat Commun 2012;3:1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benarroch EE. Pulvinar: associative role in cortical function and clinical correlations. Neurology 2015;84:738–747. [DOI] [PubMed] [Google Scholar]
- 40.Warner CE, Kwan WC, Wright D, et al. Preservation of vision by the pulvinar following early-life primary visual cortex lesions. Curr Biol 2015;25:424–434. [DOI] [PubMed] [Google Scholar]
- 41.Komura Y, Nikkuni A, Hirashima N, et al. Responses of pulvinar neurons reflect a subject’s confidence in visual categorization. Nat Neurosci 2013;16:749–755. [DOI] [PubMed] [Google Scholar]
- 42.Fiebelkorn IC, Kastner S. Functional specialization in the attention network. Annu Rev Psychol 2020;71:221–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kastner S, O’Connor DH, Fukui MM, et al. Functional imaging of the human lateral geniculate nucleus and pulvinar. J Neurophysiol 2004; 91:438–448. [DOI] [PubMed] [Google Scholar]
- 44.Arcaro MJ, Pinsk MA, Chen J, Kastner S. Organizing principles of pulvino-cortical functional coupling in humans. Nat Commun 2018;9: 5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perez C, Chokron S. Rehabilitation of homonymous hemianopia: insight into blindsight. Front Integr Neurosci 2014;8:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanders MD, Warrington EK, Marshall J, Wieskrantz L. “Blindsight”: vision in a field defect. Lancet 1974;1:707–708. [DOI] [PubMed] [Google Scholar]
- 47.Darby RR, Horn A, Cushman F, Fox MD. Lesion network localization of criminal behavior. Proc Natl Acad Sci USA 2018;115:601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Danckert J, Tamietto M, Rossetti Y. Definition: blindsight. Cortex 2019;119:569–570. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


