Significance
A historically abundant sea urchin has been struck by two mass mortalities separated by 40 y. The 1983 event was across the Western Atlantic and populations never fully recovered. The 2022 event has thus far has reduced population densities in many regions of the Caribbean. On St. John, US Virgin Islands, the geographical epicenter of the 2022 event, these sequential mortality events transitioned the distribution of D. antillarum from highly abundant prior to 1984, to a locally patchy species associated with small areas of high coral recruitment, to a rare species in 2022, eliminating their characteristic grazing that once promoted coral recruitment and survival.
Keywords: mass mortality, echinoid, coral reef, Diadema
Abstract
In 1983 to 1984, a mass mortality event caused a Caribbean-wide, >95% population reduction of the echinoid grazer, Diadema antillarum. This led to blooms of algae contributing to the devastation of scleractinian coral populations. Since then, D. antillarum exhibited only limited and patchy population recovery in shallow water, and in 2022 was struck by a second mass mortality reported over many reef localities in the Caribbean. Half-a-century time-series analyses of populations of this sea urchin from St. John, US Virgin Islands, reveal that the 2022 event has reduced population densities by 98.00% compared to 2021, and by 99.96% compared to 1983. In 2021, coral cover throughout the Caribbean was approaching the lowest values recorded in modern times. However, prior to 2022, locations with small aggregations of D. antillarum produced grazing halos in which weedy corals were able to successfully recruit and become the dominant coral taxa. The 2022 mortality has eliminated these algal-free halos on St. John and perhaps many other regions, thereby increasing the risk that these reefs will further transition into coral-free communities.
On Caribbean coral reefs prior to the early 1980s, Diadema antillarum was an abundant grazer that had a major role controlling algae and clearing space to promote coral recruitment (1–3). The importance of these grazing effects was confirmed over 1983 to 1984 when an unidentified, species-specific, water-borne pathogen caused a mass mortality of D. antillarum that reduced population densities by 93 to 99% throughout the tropical Western Atlantic (4); within 6 mo, coral reefs became dominated by algae (e.g., 3,000% increase in biomass in St. John, ref. 5). Persistent low population densities of D. antillarum, together with multiple stressors affecting coral reefs (6), have caused coral cover throughout the region to decline (6), leading to coral cover below the predicted threshold needed to maintain positive reef accretion (7). The loss of coral cover and subsequent flattening of reef structure (8) has occurred in concert with profound changes in coral reef community structure in the pelagic and benthic realms (9).
In February 2022, a second mass mortality of D. antillarum was first observed in the US Virgin Islands and has spread to many regions across the Caribbean (10–12). The progression and signs of the current unidentified pathogen are similar to the 1983 event; >50% mortality of D. antillarum populations within a week from the first observations of spine loss and reduced movement (10). It remains to be determined whether the spatial extent and severity of the present event will match the scope of the 1983 event (12). As of March 2022, reports of mortality were primarily recorded in the Lesser and Greater Antilles and these locations were not uniformly affected (10), unlike the 1983 event that ultimately was more pervasive. With the 2022 event, so far, there have been fewer observations of Diadema mortality in Florida and both Central and South America (10). The difference between island and more mainland locations with respect to recent D. antillarum mortality could be due to mainland regions having lower abundances before 1984 (13, 14) and 2021 (15), which might have reduced the likelihood of transmission of the disease among individuals, or made mortality events more difficult to detect. Alternately, regions more distant from the site of first detection in the recent mortality event in the Virgin Islands may yet to encounter the putative pathogen or source of mortality.
Our study, at the geographic epicenter of the 2022 mortality of D. antillarum, finds that this event is equally lethal to the 1983 event. The reduction of D. antillarum densities to even lower values than reported following the first mortality event in 1983 will have further negative impacts on these degraded reefs. The loss of D. antillarum on reefs throughout islands within the northeastern Caribbean has diminished the scant remaining capacity to create space available for the recruitment of stony corals. In the last few decades, the assemblage of recruiting corals has been reduced to include virtually only weedy coral species that dominate most Caribbean reefs (16), and now this relic coral community will be further challenged to persist.
Results and Discussion
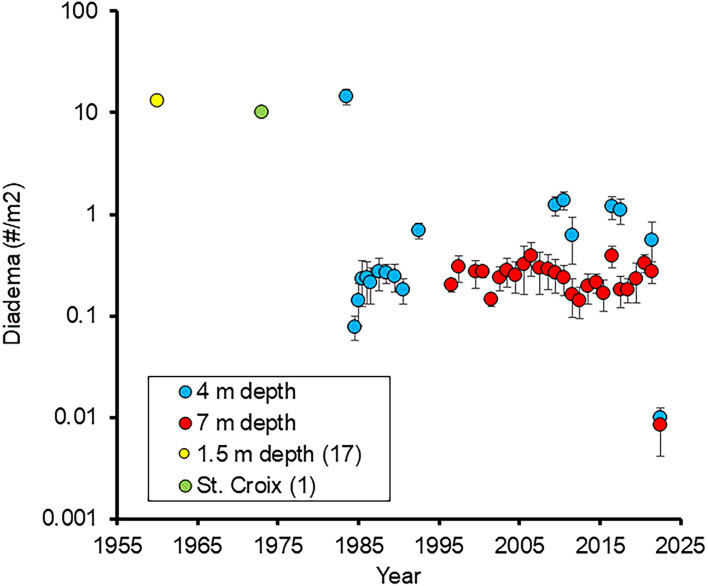
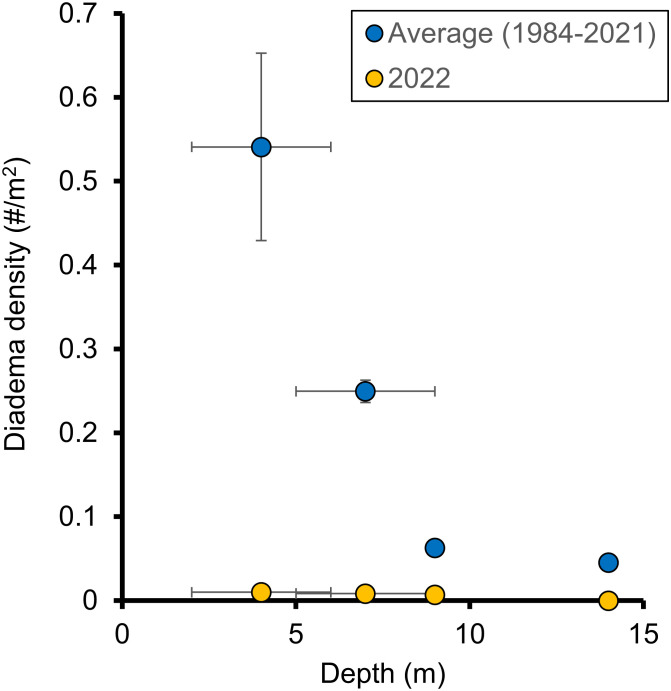
Surveys of D. antillarum along the south shore of St. John, US Virgin Islands, over five decades provide an opportunity to quantify the scope and implications of the first and second mass mortality events affecting D. antillarum. The average population density of D. antillarum in Greater Lameshur Bay, St. John (≤ 1.5 m depth), over 1958 to 1961 was 13.4/m2 (17), which is similar to densities noted in other regions of the US Virgin Islands in the 1970s (2), and nearly identical to the densities noted in Little and Greater Lameshur Bays in 1983 (14.394/m2 at 2 to 6 m depth) when our surveys began. Six months after the 1983 to 1984 mortality event, the population density of D. antillarum at these sites was depressed by 99.4% (to 0.080/m2) compared with the summer of 1983. Over the next 8 y, their density increased 10-fold, but did not further recover up to 2021 (Fig. 1 and Table 1). In 1996, 22 additional transects at 5 to 14 m depth were added to the present surveys. Although these surveys found decreasing population densities with depth (Fig. 2 and Table 2), they did not reveal a significant increase in population density from 1996 to 2021 (Fig. 1 and Table 1).
Fig. 1.
D. antillarum population densities at shallow sites (mean and standard error). Data from 4 m and 7 m from current study, data from 1.5 m from Randall et al. (17) in Greater and Little Lameshur Bays, St. John, US Virgin Islands. St. Croix (1) is a shallow patch reef. Note log scale. N = 5 sites at 2 to 6 m (4 m) and 6 sites at 5 to 9 m (7 m).
Table 1.
Multiple regression of population density (#/m2) as a function of year (1992 through 2021) and depth (mean depth of each survey site; 4, 7, 9, and 14 m). Independent regressions of population density as a function of year at each depth
| Source | DF | Type III SS | Mean square | F | P |
|---|---|---|---|---|---|
| Year | 1 | 0.0059 | 0.0059 | 0.09 | 0.7583 |
| Depth | 1 | 13.1529 | 13.1529 | 210.28 | <0.0001 |
| Error | 583 | 36.4659 | 0.0625 | ||
| Corrected total | 585 | 49.6955 | |||
| Depth = 4 m | |||||
| Year | 1 | 0.0213 | 0.0213 | 0.06 | 0.8128 |
| Error | 33 | 12.3308 | 0.3737 | ||
| Corrected total | 34 | 12.3521 | |||
| Depth = 7 m | |||||
| Year | 1 | 0.0116 | 0.0116 | 0.29 | 0.5882 |
| Error | 140 | 5.5030 | 0.0393 | ||
| Corrected total | 141 | 5.5146 | |||
| Depth = 9 m | |||||
| Year | 1 | 0.0269 | 0.0269 | 2.41 | 0.1217 |
| Error | 331 | 3.6976 | 0.0112 | ||
| Corrected total | 332 | 3.7245 | |||
| Depth = 14 m | |||||
| Year | 1 | 0.0065 | 0.0065 | 2.52 | 0.1167 |
| Error | 74 | 0.1897 | 0.0026 | ||
| Corrected total | 75 | 0.1961 |
DF = degrees of freedom; SS = sums of squares, F = F statistic, P = probability level.
Fig. 2.
Diadema population density (mean, standard error) prior to the 2022 mortality event decreased with depth (mean, range). Mortality in 2022 leveled D. antillarum densities at all depths to near zero. N = 5, 6, 13, and 3 for each depth each year.
Table 2.
Analysis of covariance of population density as a function of year (main effect), depth (covariate), and the interaction of depth and year
| Source | DF | Type III SS | Mean square | F | P |
|---|---|---|---|---|---|
| Year | 1 | 0.8666 | 0.8666 | 19.54 | <0.0001 |
| Depth | 1 | 0.5751 | 0.5751 | 12.96 | 0.0007 |
| Depth × year | 1 | 0.5562 | 0.5562 | 12.54 | 0.0009 |
| Error | 50 | 2.2178 | 0.0444 | ||
| Corrected total | 53 | 3.8410 |
DF = degrees of freedom, SS = sums of squares, F = F statistic, P = probability level.
In the summer of 2022, 5 mo after the second D. antillarum mortality event was first recorded off the US Virgin Islands (10), the average density of D. antillarum was 0.005/m2 at 4 m depth, 0.008/m2 at 7 m, and 0.004/m2 at 9 m. D. antillarum were not detected at 14 m depth. Overall, population densities of D. antillarum were two orders of magnitude less than those in 2021, and over three orders of magnitude less than those in 1983 (Fig. 1 and Table 2).
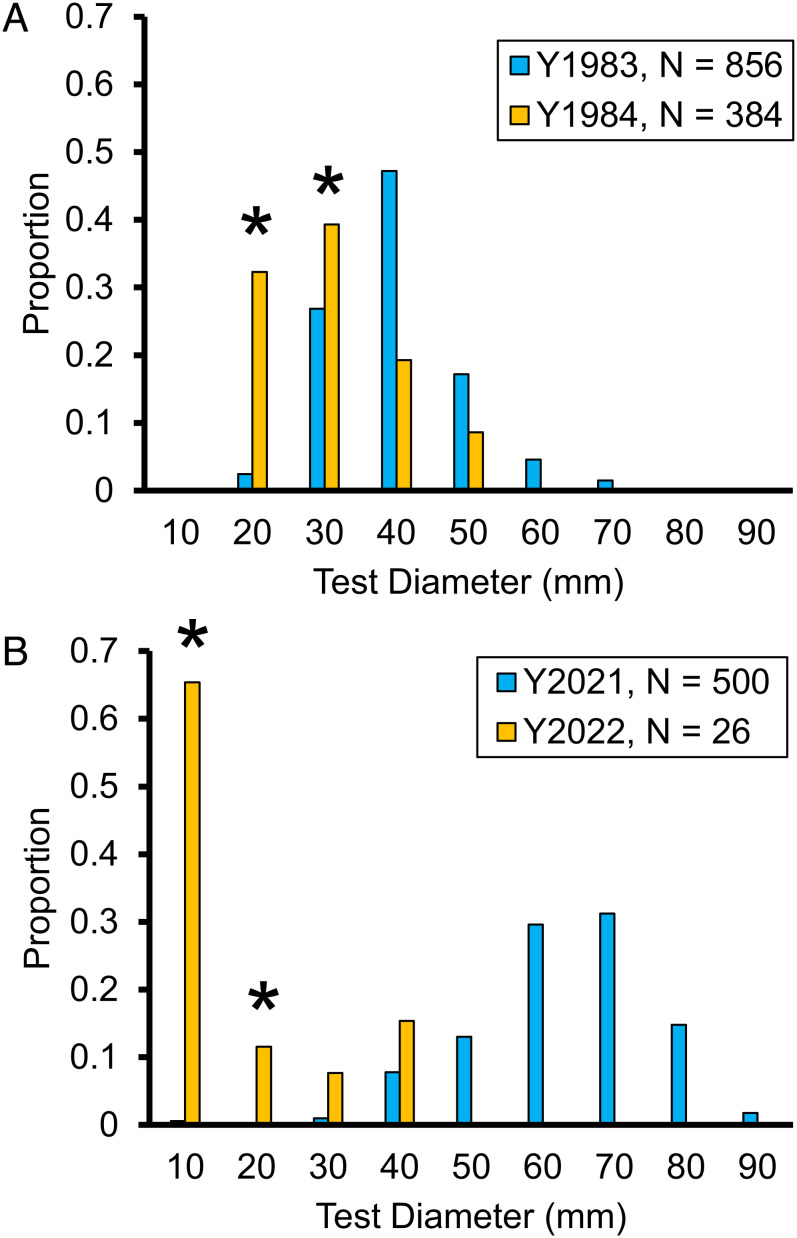
The surveys of D. antillarum during 2022 revealed mostly small individuals (test diameter < 20 mm) that likely recruited to the reef after the recent mortality event began (Materials and Methods). Only six adults (test diameter > 20 mm, Fig. 3) were found in 5,000 m2 of reef, indicating a per capita mortality of 0.998 over 2021 to 2022, compared with a per capita mortality of 0.996 over 1983 to 1984. The average density of recruiting D. antillarum in the summer of 2022 was 0.004/m2, around an order of magnitude less than in the months following the first mortality event (summer 1984, 0.026/m2) and in the years leading up to the current mortality event (average of 0.051/m2 2009 to 2021, Table 3).
Fig. 3.
Size–frequency distribution of D. antillarum test diameter pre- and post- the first (A) and second (B) mass mortality events. The 1984 and 2022 surveys were 7- and 5-mo postmortality, respectively. Most individuals following the 2022 event were likely recruited following the mortality event (asterisks). Proportions are plotted to visualize shifts in size distribution and do not reflect density differences among years.
Table 3.
Number of adult (≥20 mm test diameter, mean, standard error) and new recruits (<20 mm test diameter, mean, standard error) of D. antillarum at the five original sites in Lameshur Bay censused in summertime. New recruits grow rapidly and the 20-mm test diameter cutoff allowed for comparisons of recruitment across years, regardless of whether there was a wintertime mass mortality in the prior winter (1983 to 1984 and 2022)
| Adult | Recruit | |||
|---|---|---|---|---|
| Year | Mean | SE | Mean | SE |
| 1984 | 0.054 | 0.013 | 0.026 | 0.010 |
| 2009 | 0.984 | 0.290 | 0.116 | 0.042 |
| 2010 | 1.076 | 0.300 | 0.070 | 0.031 |
| 2011 | 0.678 | 0.310 | 0.068 | 0.019 |
| 2016 | 1.208 | 0.306 | 0.018 | 0.013 |
| 2017 | 1.088 | 0.303 | 0.008 | 0.004 |
| 2021 | 0.626 | 0.287 | 0.024 | 0.010 |
| 2022 | 0.001 | 0.001 | 0.004 | 0.001 |
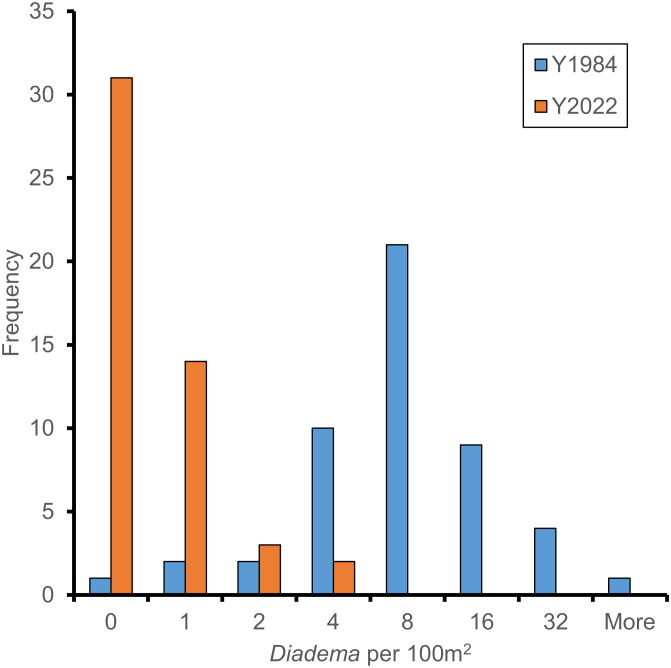
In contrast to the first mortality event, in the summer of 2022, individual sea urchins were almost always isolated on the reef (i.e., there were no clusters of this sea urchin). In the summer of 1984, 94% of 100 m2 quadrats (n = 50) contained at least two D. antillarum, with up to 37 individuals in one quadrat, whereas in the summer of 2022, only 10% of the same-size quadrats (n = 50) contained at least two D. antillarum, and only one contained two mature individuals, which was the greatest number recorded in any quadrat (Fig. 4). D. antillarum, like many marine taxa, reproduce by external fertilization, and their fertilization becomes unlikely when spawning neighbors are >5 m apart (18). Although lunar periodicity and phytoplankton blooms can influence sperm release in male sea urchins, males and females tend to spawn when they detect conspecific sperm in the seawater (19). After the 1983 to 1984 mortality, spawning of D. antillarum tended to be asynchronous among individuals, likely because of reduced availability of conspecific cues, further depressing the probability of fertilization below that predicted by low densities alone (20). Low per capita fertilization success reduces larval production and settlement at the population scale, and these effects are likely to be exacerbated among the highly isolated D. antillarum remaining in the summer of 2022.
Fig. 4.
Distribution of D. antillarum among 100 m2 quadrats (N = 50 each year) 7 (1984) and 5 (2022) mo following the two mass mortality events. Almost all individuals in the summer of 2022 were isolated on the reef.
The lack of recovery of D. antillarum since 1984 likely stems from a lethal disease striking quickly over the full species range of this sea urchin (4, 21). Without unaffected source populations to supply larvae, the 1983 to 1984 mortality represented a species-wide marine event from which population recovery relied solely on larval reproduction from regionally depleted populations. The 1983-84 mortality of D. antillarum, therefore, provided a glimpse of the consequences of a historically common species (9) suddenly exposed to conditions typically faced by much less common species that might be adapted to successfully reproduce at very low population densities (21). The 2022 mortality event places D. antillarum in a precarious demographic future, the outcome of which ultimately will depend on the spatial extent and severity of the current mortality. If the current mortality event differentially affects high-density populations, it will also differentially target the likely source populations producing the larval supply that might fuel a recovery. An Allee threshold (low-density boundary at which population growth becomes negative—ref. 22) was not evident following the 1983 to 1984 mortality event, as populations did not further decline over the following decades (23). If the 2022 event continues to spread throughout the Caribbean, with effects similar to those documented here for the reefs of St. John, it might push population densities to the threshold where per capita population growth is negative, making recovery of D. antillarum populations even less likely than after 1984.
Although mean D. antillarum densities throughout the Caribbean (23) have remained ≤1/m2 since 1984, periodic recruitment and aggregations of adults in crevices in mostly shallow water (i.e., <5 m depth) created a mosaic of higher density patches of D. antillarum (21, 24–26). In the early 2000s, these aggregations produced distinct algal-free halos in St. John (21), Jamaica (24), and other Caribbean locations in the Greater and Lesser Antilles (15, 25). This is in contrast to more mainland locations such as Central America (15) and Florida (27), where D. antillarum densities remained more consistently low. Aggregations of D. antillarum provide important loci for coral recruitment (24, 28) and in both cohesive zones of dense D. antillarum individuals and smaller halos around clusters of sea urchins, corals recruit in greater density, grow faster, and survive better than that in algal-dominated portions of the reef (29). The limitation of settlement space for coral recruits has intensified over the last few years as shallow areas of the reefs of St. John (and elsewhere) have become densely populated by peyssonnelid algal crusts (28).
The corals recorded as settling in these algal-free zones on the Caribbean reefs (16, 30) have mostly been taxa with rapid early growth to maturity, small adult colony size, and a brooding life history strategy (31). Brooded eggs are fertilized internally, a reproductive mode that enhances fertilization success at low densities (32), particularly when selfing or producing asexual larval has been implicated (33, 34). Larvae produced by brooding corals are large compared to those produced by broadcasting species, and typically are competent to settle immediately after release and are provisioned with maternal resources that enhance growth and survivorship. The environmental features favoring brooding corals are the conditions prevailing on many Caribbean reefs where coral populations have been decimated, and the substratum available for coral settlement is spatially dominated by algae (35). In contrast, broadcasters—which include most of the iconic Caribbean corals such as Orbicella spp. and Acropora spp.—release numerous small eggs into the seawater for external fertilization, followed by the development of larvae to settlement competency. Many broadcast spawning corals in the Caribbean have great ecological importance (36), yet they are rarely recorded as recruits on the present-day reefs (16). The reasons for this trend are unknown, but it could arise from reduced reproductive success at low population densities (37), or inimical conditions on the benthos created by profuse growth of macroalgae (38) that could select for the more maternally provisioned brooding taxa.
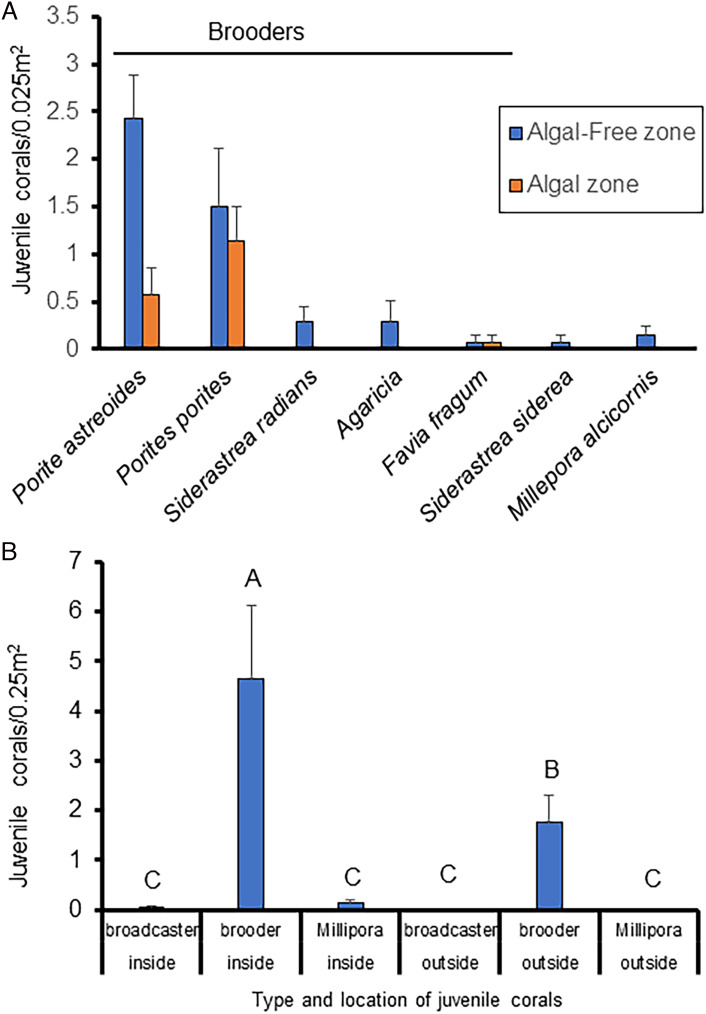
These conditions are evident on St. John. In the summer of 2021, we examined juvenile coral (<4 cm diameter) abundance within and surrounding 10 halo sites characterized by a small algal-free halo (1 to 12 m2 in area) around discrete clusters of 5 to 38 D. antillarum; the halo sites were distributed along a 100-m transect. The average area of these algal-free halos was 5.47 m2 (SE 0.90) containing 3.93 D. antillarum/m2 (SE 0.54). Coral density was 2.8 times higher within the algal-free halos. Overall, brooding species made up 96% of the individuals inside the halo and 100% outside the halo (Fig. 5). The brooders were Porites astreoides (45%), Porites porites (42%), Agaricia spp. (5%), Siderastrea radians (3%), and Favia fragum (2%). The remainder comprised the medusa producing hydrozoan Millepora alcornis (2%), and the broadcasting Siderastrea siderea was represented by one colony (<1%). Most juvenile corals outside the halos were P. porites, which is a brooding species with an erect branching morphology. A two-way analysis of variance revealed a significant effect of reproductive mode (P < 0.0001), location with respect to halo (in vs. out, P < 0.0004), and their interaction (P < 0.0001). On return to these sites in the summer of 2022, all evidence for these halos and the D. antillarum that produced them was gone.
Fig. 5.
The average number (and standard error) of corals per 0.25 m (N = 28) quadrats within and outside of D. antillarum algae-free halos. (A) individual species; P. astreoides, P. porites, S. radians, Agaricia spp., F. fragum, S. siderea (broadcaster), and M. alcornis (Hydrozoan). (B) Corals grouped by reproductive mode (brooders, broadcaster or the medusa spawning hydrozoan Millepora). Letters indicate significant differences from Tukey’s pairwise tests; A > B&C, P < 0.001; B > C, P < 0.015.
The difference between the population collapse of D. antillarum and the numerous other stresses affecting the present-day coral reefs (6) is that the loss of D. antillarum directly affects coral recruitment, while most other stressors mediate mortality of the existing coral colonies. Caribbean coral reefs have been experiencing multidecadal transition from corals characterized by slow growth, infrequent recruitment, high survivorship, and great longevity [e.g., Orbicella spp. (39)], to corals that recruit in high numbers and are characterized by rapid growth to maturity (e.g., P. astreoides, ref. 16). In St. John, and other locations similarly affected by the 2022 mortality of D. antillarum, this ecological transition may now be entering a phase of persistent recruitment failure by scleractinian corals, regardless of taxon, as scant algal-free zones suitable for their settlement disappear following the 2022 mortality of D. antillarum. The brooding functional group of corals that has dominated many Caribbean reefs over the last few decades (39) might be the next group to fade into functional extinction.
Materials and Methods
Long-Term Monitoring.
D. antillarum have been monitored across five long-term sites and 22 transects in Greater and Little Lameshur Bays since 1983. Five sites were established at 2 to 6 m depth in 1983 prior to the first mass mortality, and 22 transects were established in 1996 at 5 to 14 m depth. The five sites at 2 to 6 m depth were surveyed using two methods. From the summer of 1983 through 1990, 1992, and 2022, D. antillarum density and test diameter were measured using 40 quadrats (5 × 5 m) along a 100-m transect, for a total area of 1,000-m2 per site (for details of sites and methods, see ref. 21). From 2009 through 2011 and 2021, each plot was surveyed using 100 1-m2 quadrats placed haphazardly along the 100-m transect. Size distribution was measured across all years by randomly selecting two points within the site and measuring 50 D. antillarum closest to each point for a total of 100 individuals. If there were <100 individuals within a site, all individuals were measured. Additionally, juveniles, defined as having banded spines and a test diameter <20 mm diameter, were counted in each quadrat. The 22 transects established in 1996 were surveyed for D. antillarum density, but not size distribution, using a 2-m wide band transect. These transects range from 10 to 40 m in length and have been surveyed annually until present. For details on these transect surveys, see ref. 21.
Diadema Growth Rate and Size in the First Year.
The larval duration of D. antillarum in larval cultures is approximately 35 d (40). At 70 d postsettlement (estimated 105 d postfertilization), their test diameter is ~6 mm test diameter, under laboratory conditions (41). At 112 d postsettlement (147 d postfertilization), the test diameter is between 10 and 12 mm under laboratory conditions (41), and between 12 and 16 mm in cage enclosures in the field (17). The time from the first report of mass mortalities in the US Virgin Islands in mid-February 2022 until the start of the summer census in 2022 is 160 d. Based on the above estimates of larval duration and postsettlement growth, the test diameter of individuals fertilized on day one following the mass mortality (using 14 February) would be between 13 and 19 mm test diameter. We use these estimates to establish the cutoff of 20 mm test diameter as the maximum size of individuals fertilized and recruited into the population following the 2022 mass mortality censused in the summer of 2022. A lower cutoff size would reflect the lower bound of estimated growth, while a higher cutoff size would reflect individuals fertilized earlier, but escaping the pathogen while in the pelagic environment. In natural (uncaged) populations following the mass mortality in 1983 to 1984, D. antillarum increased in test diameter from 10 to >50 mm in 1 y (21). A 50-mm threshold would better reflect annual recruitment (23), but we use the 20-mm threshold to more accurately compare postmortality recruitment in years with and without wintertime mass mortality events (December 1983 and February 2022).
Juvenile Coral Abundance In and Out of D. antillarum Algal-Free Zones.
Juvenile corals were defined as colonies ≤4 cm diameter, and colonies were identified to species or genera if ambiguous and counted in quadrats (0.5 × 0.5 m). At each of the 10 halo sites, quadrats were haphazardly placed within the halo and then a few meters outside of the halo. For six halos, one quadrat was surveyed inside and outside of the halo, and for the remaining four halos, two quadrats were surveyed inside and outside the halo. To maintain a balanced design, halos with replicate counts were averaged to one value inside and one outside the halo.
Statistical Methods.
Prior studies have documented the decline in population density following the 1983 to 1984 event and the modest levels of recovery from 1984 to 1992. Here, we examine trends of changing density from 1992 to 2021 in the five sites initiated in 1983 and the 22 transects initiated in 1996. The general linear model tested population density as a function of year and depth (multiple regression). Independent regressions were used to examine changes across years at each depth independently. We used analysis of covariance to test for changes in population density before (summer 2021) vs. after (summer 2022) the second mass mortality (main effect), using depth as a covariate (Table 2). We used an analysis of variance to examine differences in coral abundance in and out of D. antillarum halos as a function of reproductive mode.
Acknowledgments
We thank the Virgin Islands National Park and the Virgin Island Ecological Research Station. This work was funded by the US National Science Foundation Division of Ocean Sciences 2019992 (P.J.E.). This is contribution 376 of the California State University, Northridge marine biology program.
Author contributions
D.R.L. and P.J.E. designed research; D.R.L., R.M.B., and P.J.E. performed research; D.R.L. analyzed data; and D.R.L., R.M.B., and P.J.E. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
Data repository is located in Dryad; https://doi.org/10.5061/dryad.51c59zwcm (42).
References
- 1.Ogden J. C., Brown R. A., Salesky N., Grazing by the echinoid Diadema antillarum Philippi: Formation of Halos around West Indian Patch Reefs. Science 182, 715–717 (1973). [DOI] [PubMed] [Google Scholar]
- 2.Sammarco P. W., Echinoid grazing as a structuring force in coral communities; whole reef manipulations. J. Exp. Mar. Biol. Ecol. 61, 31–55 (1982). [Google Scholar]
- 3.Carpenter R. C., Grazing by Diadema antillarum (Philippi) and its effects on the benthic algal community. J. Mar. Res. 39, 749–765 (1981). [Google Scholar]
- 4.Lessios H. A., Mass mortality of Diadema antillarum in the Caribbean: What have we learned? Annu. Rev. Ecol. Syst. 19, 371–393 (1988). [Google Scholar]
- 5.Levitan D. R., Algal-urchin biomass responses following the mass mortality of the sea urchin Diadema antillarum Philippi at Saint John, US Virgin Islands. J. Exp. Mar. Biol. Ecol. 119, 167–178 (1988a). [Google Scholar]
- 6.Jackson J., Donovan M., Cramer K., Lam V., Eds., “Status and trends of Caribbean coral reefs: 1970-2012”, (Glob. Coral Reef Monit. Network, IUCN, Gland. Switz., 2014). Available at http://wedocs.unep.org/bitstream/handle/20.500.11822/9230. [Google Scholar]
- 7.Perry C. T., et al. , Caribbean-wide decline in carbonate production threatens coral reef growth. Nat. Commun. 4, 1–7 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alvarez-Filip L., Dulvy N. K., Gill J. A., Côté I. M., Watkinson A. R., Flattening of Caribbean coral reefs: Region-wide declines in architectural complexity. Proc. R. Soc. B. 276, 3019–3025 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson J. B. C., Reefs since Columbus. Coral Reefs. 16, 23–32 (1997). [Google Scholar]
- 10.Ocean Research & Education Foundation, Diadema Response Network https://www.agrra.org/sea-urchin-die-off/. Accessed 5 January 2023.
- 11.Villalpando M. F., Guendulain-García S. D., Valdez-Trinidad A., Croquer A., Sellares-Blasco R. I., Coral reefs of southeastern Dominican Republic hit by two simultaneous epizootic events. Bull. Mar. Sci. 98, 507–508 (2022). [Google Scholar]
- 12.Hylkema A., et al. , The 2022 Diadema antillarum die-off event: comparisons with the 1983–1984 mass mortality. Front. Mar. Sci. 9, 2654 (2022). [Google Scholar]
- 13.Hay M. E., Patterns of fish and urchin grazing on Caribbean coral reefs: Are previous results typical? Ecol. Res. 65, 446–454 (1984). [Google Scholar]
- 14.Levitan D. R., Community structure in times past: Influence of human fishing pressure on algal-urchin interactions. Ecology 73, 1597–1605 (1992). [Google Scholar]
- 15.Lessios H. A., The great Diadema antillarum die-off: 30 years later. Ann. Rev. Mar. Sci. 8, 267–283 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Edmunds P. J., The hidden dynamics of low coral cover communities. Hydrobiologia 818, 193–209 (2018). [Google Scholar]
- 17.Randall J. E., Schroeder R. E., Starck W. A. I., Notes on the biology of the echinoid Diadema antillarum. Caribb. J. Sci. 421–433 (1964). [Google Scholar]
- 18.Levitan D. R., Density-dependent selection on gamete traits in three congeneric sea urchins. Ecology 8, 464–479 (2002). [Google Scholar]
- 19.Reuter K. E., Levitan D. R., Influence of sperm and phytoplankton on spawning in the echinoid Lytechinus variegatus. Biol. Bull. 219, 198–206 (2010). [DOI] [PubMed] [Google Scholar]
- 20.Levitan D. R., Asynchronous spawning and aggregative behavior in the sea urchin Diadema antillarum (Philippi). Echinoderm. Biol. 181–186 (1988b). [Google Scholar]
- 21.Levitan D. R., Edmunds P. J., Levitan K. E., What makes a species common? No evidence of density-dependent recruitment or mortality of the sea urchin Diadema antillarum after the 1983–1984 mass mortality. Oecologia 175, 117–128 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Berec L., Angulo E., Courchamp F., Multiple allee effects and population management. Trends Ecol. Evol. 22, 185–191 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Hughes T. P., Graham N. A. J., Jackson J. B. C., Mumby P. J., Steneck R. S., Rising to the challenge of sustaining coral reef resilience. Trends Ecol. Evol. 25, 633–642 (2010). [DOI] [PubMed] [Google Scholar]
- 24.Edmunds P. J., Carpenter R. C., Recovery of Diadema antillarum reduces macroalgal cover and increases abundance of juvenile corals on a Caribbean reef. Proc. Natl. Acad. Sci. U.S.A. 98, 5067–5071 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carpenter R. C., Edmunds P. J., Local and regional scale recovery of Diadema promotes recruitment of scleractinian corals. Ecol. Lett. 9, 268–277 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Tuohy E., Wade C., Weil E., Lack of recovery of the long-spined sea urchin Diadema antillarum Philippi in Puerto Rico 33 years after the Caribbean-wide mass mortality. PeerJ. 8, e8428 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiappone M., Rutten L. M., Swanson D. W., Miller S. L., “Population status of the urchin Diadema antillarum in the Florida Keys 25 years after the Caribbean mass mortality” in Proc. 11th Int. Coral Reef Symp. (Ft, Lauderdale, FL, 2008), pp. 706–710. [Google Scholar]
- 28.Stockton L., Edmunds P. J., Spatially aggressive peyssonnelid algal crusts (PAC) constrain coral recruitment to Diadema grazing halos on a shallow Caribbean reef. J. Exp. Mar. Bio. Ecol. 541, 151569 (2021). [Google Scholar]
- 29.Idjadi J. A., Haring R. N., Precht W. F., Recovery of the sea urchin Diadema antillarum promotes scleractinian coral growth and survivorship on shallow Jamaican reefs. Mar. Ecol. Prog. Ser. 403, 91–100 (2010). [Google Scholar]
- 30.Smith S. R., Patterns of coral recruitment and post-settlement mortality on bermuda’s reefs: Comparisons to Caribbean and Pacific reefs. Integr. Comp. Biol. 32, 663–673 (1992). [Google Scholar]
- 31.Darling E. S., Alvarez-Filip L., Oliver T. A., Mcclanahan T. R., Côté I. M., Evaluating life-history strategies of reef corals from species traits. Ecol. Lett. 15, 1378–1386 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Pemberton A. J., Hughes R. N., Manríquez P. H., Bishop J. D. D., Efficient utilization of very dilute aquatic sperm: Sperm competition may be more likely than sperm limitation when eggs are retained. Proc. R. Soc. B Biol. Sci. 270, 223–226 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brazeau D. A., Gleason D. F., Morgan M. E., Self-fertilization in brooding hermaphroditic Caribbean corals: Evidence from molecular markers. J. Exp. Mar. Bio. Ecol. 231, 225–238 (1998). [Google Scholar]
- 34.Vollmer A. A., Rare parthenogenic reproduction in a common reef coral, Porites astreoides (Nova Southeastern University, 2018). Available at https://nsuworks.nova.edu/occ_stuetd/464. [Google Scholar]
- 35.Roff G., Mumby P. J., Global disparity in the resilience of coral reefs. Trends Ecol. Evol. 27, 404–413 (2012). [DOI] [PubMed] [Google Scholar]
- 36.Alvarez-Filip L., Dulvy N. K., Côteé I. M., Watkinson A. R., Gill J. A., Coral identity underpins architectural complexity on Caribbean reefs. Ecol. Appl. 21, 2223–2231 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Levitan D. R., et al. , Mechanisms of reproductive isolation among sympatric broadcast-spawning corals of the Montastraea annularis species complex. Evolution. 58, 308–323 (2004). [PubMed] [Google Scholar]
- 38.Contreras-Silva A. I., et al. , A meta-analysis to assess long-term spatiotemporal changes of benthic coral and macroalgae cover in the Mexican Caribbean. Sci. Rep. 10, 1–12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hughes T. P., Tanne J. E., Recruitment failure, life histories, and long-term decline of Caribbean corals. Ecology 81, 2250–2263 (2000). [Google Scholar]
- 40.Eckert G. L., Larval development, growth and morphology of the sea urchin Diadema antillarum. Bull. Mar. Sci. 63, 443–451 (1998). [Google Scholar]
- 41.Hassan M. M., et al. , Growth and foraging behavior of hatchery propagated long-spined sea urchins, Diadema antillarum: Implications for aquaculture and restocking. Aqua Rep. 26, 101298 (2022). [Google Scholar]
- 42.Levitan D. R., Best R. M., Edmunds P. E., Data for Sea urchin mass mortalities 40 years apart further threaten Caribbean coral reefs, Dryad, Dataset, 10.5061/dryad.51c59zwcm Deposited 31 January 2023. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data repository is located in Dryad; https://doi.org/10.5061/dryad.51c59zwcm (42).