Abstract
Neisseria gonorrhoeae (Gc) is a human-specific pathogen that causes the sexually transmitted infection gonorrhea. Gc survives in neutrophil-rich gonorrheal secretions, and recovered bacteria predominantly express phase-variable, surface-expressed opacity-associated (Opa) proteins (Opa+). However, expression of Opa proteins like OpaD decreases Gc survival when exposed to human neutrophils ex vivo. Here, we made the unexpected observation that incubation with normal human serum, which is found in inflamed mucosal secretions, enhances survival of Opa+ Gc from primary human neutrophils. We directly linked this phenomenon to a novel complement-independent function for C4b-binding protein (C4BP). When bound to the bacteria, C4BP was necessary and sufficient to suppress Gc-induced neutrophil reactive oxygen species production and prevent neutrophil phagocytosis of Opa+ Gc. This research identifies for the first time a complement-independent role for C4BP in enhancing the survival of a pathogenic bacterium from phagocytes, thereby revealing how Gc exploits inflammatory conditions to persist at human mucosal surfaces.
Author summary
Gonorrhea is considered an urgent threat to public health with an estimated 87 million cases occurring annually worldwide, growing antimicrobial resistance, and the absence of a gonococcal vaccine. Currently, we do not understand how N. gonorrhoeae expressing opacity (Opa) proteins survive neutrophil defenses and are recovered viable from infected patients.
Here, we investigated how soluble elements of gonorrhea infection, present in human serum, contribute to N. gonorrhoeae survival from neutrophils. We found that the serum component C4b-binding protein (C4BP) protects N. gonorrhoeae from neutrophil killing and suppresses neutrophil activation. C4BP limited neutrophil phagocytosis of N. gonorrhoeae that expressed Opa proteins that bound to neutrophil receptors of the CEACAM family. This work provides novel insight into the interplay between the noncellular and cellular aspects of the innate immune response to N. gonorrhoeae.
Introduction
Neisseria gonorrhoeae (Gc), an obligate human pathogen and cause of the sexually transmitted infection gonorrhea, is an urgent public health threat that causes an estimated 86.9 million cases worldwide each year [1,2]. Infection with Gc fails to elicit an effective host immune response, there is no protective immune memory to infection, a vaccine is not available, and resistance to antibiotics is increasing [3,4]. There is only one remaining class of antibiotics that are recommended for treatment of gonorrhea, the cephalosporins, but strains resistant to ceftriaxone and cefixime have emerged, increasing the likelihood of untreatable gonorrhea [5,6].
Gc infects human mucosal surfaces, including the female cervix and male urethra. At these sites, Gc stimulates a robust inflammatory response characterized by an abundant recruitment of neutrophils [7]. Although neutrophils produce and secrete antimicrobial components including proteases, cationic peptides, and reactive oxygen species, Gc is not cleared by the local neutrophil influx. Our group and others have identified several ways in which Gc resists killing by primary human neutrophils, including resistance to the antimicrobial proteins and reactive oxygen species that are made by activated neutrophils, limiting phagocytosis and phagosome maturation, and degradation of neutrophil extracellular traps [8]. If neutrophilic inflammation is not resolved, it can cause irreversible tissue damage, leading to pelvic inflammatory disease and infertility [7].
Neutrophils associate with and internalize Gc through opsonic and non-opsonic means. The primary opsonins are antibodies and complement components, which are recognized by Fc receptors and complement receptors, respectively. Engagement of these receptors leads to internalization of the opsonized bacteria [9]. The primary form of non-opsonic phagocytosis by neutrophils is through the interaction between the outer membrane opacity-associated (Opa) proteins and human carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) [10,11]. Isolates of Gc encode ≥ 9 distinct Opa proteins, and each opa gene is phase variable, generating extensive diversity in Opa expression within a bacterial population [12]. Most Opa proteins bind one or more human CEACAMs. Expression of Opa proteins confers an advantage to the bacteria during cervical colonization by promoting interaction with the epithelial-expressed CEACAMs 1 and 5 [13]. In contrast, Opa protein engagement of CEACAM-1 and CEACAM-3 [10,14–17] on human neutrophils stimulates production of reactive oxygen species (ROS) [18], efficient bacterial binding and phagocytosis, and bacterial killing [19]. Curiously, Opa-expressing (Opa+) Gc predominate in neutrophil-rich exudates from individuals with gonorrhea [20–22]. It is an open question in the field why there is this discrepancy between in vivo and ex vivo observations with human neutrophils.
Inflammatory secretions are replete with human serum components as a result of serum transudate into the cervical lumen, which occurs under homeostatic conditions, and serum leakage due to breaching of the epithelial barrier, which occurs during the inflammatory conditions of Gc infection [7]. One prominent serum component is the fluid-phase complement inhibitor C4b-binding protein (C4BP) (reviewed in [23]). The C4BP that primarily circulates in human blood is a 570 kDa multimer: 7 alpha (α) subunits (75 kDa, composed of 8 complement control protein (CCP) domains) and 1 beta (β) subunit (45 kDa, composed of 3 CCP domains). The C-terminal domains of the α and β chains mediate multimerization, and form a high affinity complex with the anticoagulation factor Protein S (PS) (7α1βPS). A 7α form of C4BP, which lacks a β chain and PS, is also present in the bloodstream, and increases in relative abundance compared to the 7α1βPS form of C4BP during conditions of inflammation. C4BP is present in human serum at an estimated concentration of 200 μg/mL [24]. It inhibits complement activity in the classical and mannose-binding lectin pathways, where CCP1 of the α chain binds complement component C4b to prevent assembly of the classical C3 convertase. C4BP also accelerates the decay of the C3 convertase by acting as a cofactor for Factor I, a serum protease that cleaves C3b and C4b. The inhibition of complement activation by C4BP prevents excessive inflammation and consequent tissue damage [25].
C4BP binds to the surface of most strains of Gc and to other pathogenic bacteria including Streptococcus pyogenes, Moraxella catarrhalis, Escherichia coli strain K1, Borrelia recurrentis, Haemophilus influenzae, Yersinia pestis, Bordetella pertussis, and Neisseria meningitidis, through which it confers resistance to serum bactericidal activity in Gram-negatives and decreases complement-mediated phagocytosis in Gram-positives [26]. The primary target of C4BP on Gc is the PorB porin; CCP1 of C4BP binds alleles of both PorB1a and PorB1b genotypes [27]. Of 190 recently isolated clinical strains of Gc, 89.7% of PorB1a isolates and 19.3% of PorB1b isolates bound human C4BP [28]. Commonly used lab strains FA1090 (genotype PorB1b) and 1291 (genotype PorB1b) bind C4BP [27]. Though the binding sites on C4BP for Gc porin and C4b overlap on CCP1 of the α chain, C4BP on the surface of Gc retains its cofactor activity in the degradation of C4b by Factor I [27]. Gc does not bind C4BP of other species with the exception of chimpanzee, a species in which successful experimental gonorrhea infection has been accomplished; this specificity for human C4BP may account in part for humans being the only natural host for Gc [29]. Roles of C4BP in the pathogenesis of Gc beyond inhibiting complement-mediated lysis have not been reported.
In this study, we made the unexpected observation that incubation of Opa+ Gc of strain FA1090 with normal human serum enhances its resistance to killing by primary human neutrophils and suppresses the neutrophil oxidative burst, independently of complement. We directly linked this phenomenon to a novel complement-independent function for C4BP. Using a derivative of FA1090 that constitutively expresses only the CEACAM-1 and -3 binding OpaD protein (OpaD+ Gc) [14], we found that binding of C4BP by Gc significantly enhances its survival from primary human neutrophils and suppresses neutrophil production of reactive oxygen species. Conversely, serum depleted of C4BP was unable to suppress the neutrophil oxidative burst and did not increase Gc survival after neutrophil challenge. C4BP addition reduced the association and phagocytosis of OpaD+ Gc by neutrophils. These outcomes all required binding of C4BP to the Gc surface, as shown using mutants of both C4BP and PorB1b that abrogate their interaction, and extended to variants of Gc expressing other Opa proteins. We propose that binding of C4BP enables Gc to avoid multiple facets of human innate immunity, by resisting neutrophil phagocytic killing as well as its canonical role in limiting complement-mediated lysis.
Results
Incubation of Neisseria gonorrhoeae with normal human serum limits neutrophil anti-gonococcal activity
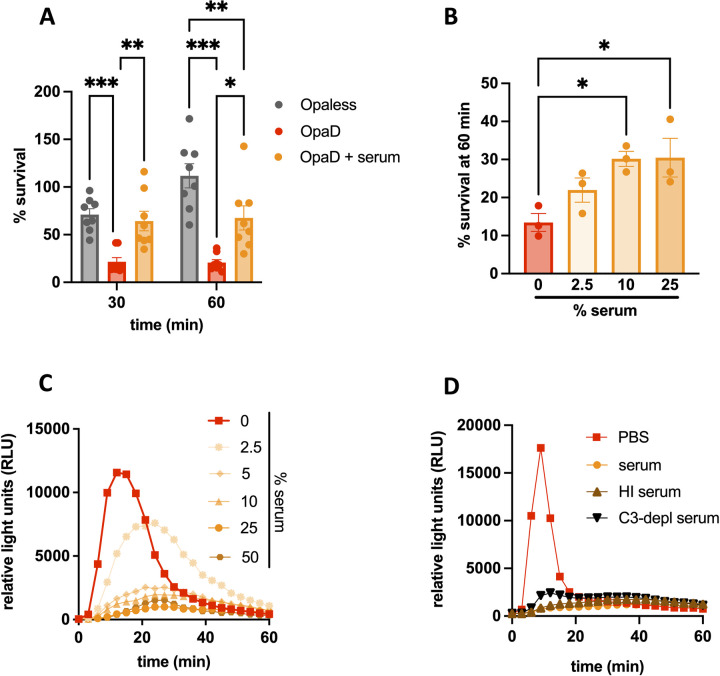
To investigate the effect of serum on Gc-neutrophil interactions, OpaD+ Gc was incubated in pooled normal human serum, then exposed to adherent, IL-8 treated primary human neutrophils, and CFU were enumerated from cell lysates over time. We expected that serum would decrease survival of OpaD+ Gc by neutrophils via complement-mediated opsonophagocytosis. Instead, preincubation of the bacteria with serum significantly increased the number of OpaD+ Gc recovered from neutrophils, and in fact was equivalent to recovery of Opaless Gc at 30 minutes post-infection (Fig 1A). The effect of serum on OpaD+ Gc was concentration-dependent, with OpaD+ Gc incubated in 10% and 25% serum surviving significantly better than bacteria without serum at 60 minutes (Fig 1B). Serum had no significant effect on survival of OpaD+ Gc in the absence of neutrophils, reflecting the serum-resistant nature of the FA1090 genetic background (S1 Fig).
Fig 1. Incubation with normal human serum decreases neutrophil anti-gonococcal activity.
(A) Adherent, IL-8-treated primary human neutrophils were exposed to Opaless Gc, OpaD+ Gc, or OpaD+ Gc that were preincubated in 25% normal human serum from healthy subjects (UVA). CFU were enumerated from neutrophil lysates at 0, 30, and 60 minutes (min) post infection, and bacterial survival is presented as the mean ± SEM of CFU at the indicated time point relative to the mean CFU for the same condition at 0 minutes for 8 independent experiments. Two-way ANOVA with Sidak’s post-hoc comparisons were used to compare each condition within each time point. In (B), OpaD+ Gc was mixed with the indicated percentage of normal human serum (UVA), and survival from neutrophils at 60 minutes was performed as in (A). One-way ANOVA followed by Tukey’s post-hoc comparisons was used to compare each condition to PBS alone (0% serum). *p<0.05, **p<0.01,***p<0.001. (C) OpaD+ Gc alone (PBS) or incubated in the indicated percentage of normal human serum (UVA) was exposed to neutrophils in suspension at an MOI of 100 in the presence of luminol. ROS production was measured over the course of 60 minutes (min) as the relative light units (RLU) generated by luminol-dependent chemiluminescence. (D) ROS production was measured as in (C) from OpaD+ Gc incubated in serum (UVA), complement component 3-depleted serum (“C3-depl serum”), or heat-inactivated serum (“HI serum” (56°C, 30 minutes)). (C-D) Results are one representative of 3 independent experiments.
We next examined how incubation with serum affects neutrophil functionality, using generation of reactive oxygen species (ROS) via luminol-dependent chemiluminescence as a readout. Preincubation of OpaD+ Gc with serum suppressed the resulting neutrophil ROS response in a concentration-dependent manner, with serum concentrations of 10% and higher abrogating ROS production (Fig 1C). The suppressive effect of serum on neutrophil ROS production was replicated with a different isogenic derivative of Opaless Gc that constitutively expresses the Opa60 protein, which also binds to CEACAMs 1 and 3 [19,30], as well as Gc of strain 1291 [27] expressing undefined Opa proteins (S2 Fig).
Given that complement is a major opsonic activity in serum, we tested whether the effects observed with serum on Opa+ Gc were complement-dependent. Heat-inactivated serum behaved identically to untreated serum in OpaD+ Gc-mediated suppression of neutrophil ROS (Fig 1D). Complement component C3-depleted human serum also suppressed the ability of OpaD+ Gc to elicit neutrophil ROS (Fig 1D).
Together these results show that the presence of human serum unexpectedly increases the survival of Opa+ Gc from primary neutrophils and suppresses neutrophil activation, in a complement-independent manner.
Identification of C4BP as the suppressive serum component
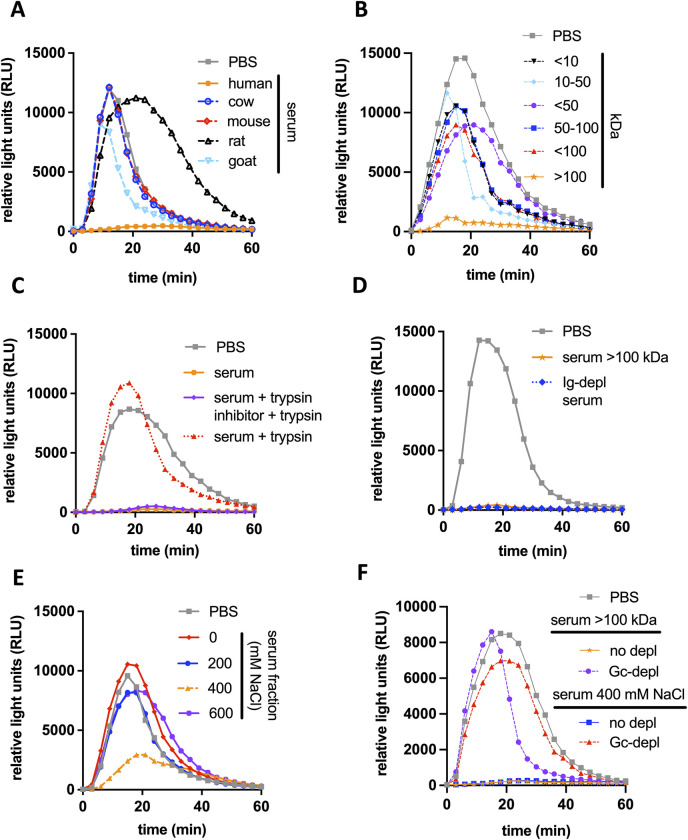
We characterized the component in serum that is responsible for suppressing neutrophil anti-gonococcal activity, using the reduction in ROS production as a readout. The suppressive activity was present in human serum but not serum from goat, mouse, rat, or cow (Fig 2A). The suppressive component remained in the retentate of a 100 kDa molecular weight cutoff centrifugal filter device (Fig 2B) and was sensitive to trypsinization (Fig 2C), but the component was not immunoglobulin, as shown using IgG/IgA/IgM-depleted human serum (Fig 2D). Notably, the > 100 kDa retentate lost its ability to suppress ROS when it was preincubated with Gc, the bacteria pelleted, and the supernatant incubated with a new culture of OpaD+ Gc (“Gc-depleted” fraction) (Fig 2F). This observation enabled us to take an unbiased biochemical approach to identify the suppressive component from human serum, by following the reduction in neutrophil ROS production by OpaD+ Gc.
Fig 2. Characteristics of the serum component that suppresses Opa+ Gc induced neutrophil ROS.
(A-F) OpaD+ Gc were exposed to primary human neutrophils and ROS production was measured as in Fig 1C. Before addition to neutrophils, OpaD+ Gc was incubated with serum from the indicated species (final concentration, 25%) (A); the indicated molecular weight fraction of human serum (B); human serum that was intact (solid orange; UVA), treated with trypsin (dotted red), or treated sequentially with trypsin inhibitor then trypsin (purple) (C); the ≥ 100 kD human serum fraction from (B) (orange), or serum depleted of immunoglobulins G, M, and A (“Ig-depl serum”, dotted blue) (D); human serum fractions generated by anion exchange chromatography via elution with the indicated molarity of NaCl (E); or the indicated suppressive serum fraction from B and E that was pre-incubated with Gc (“Gc-depl”) or not (“no-depl”) (F). Incubation of OpaD+ Gc with PBS+ was used as a positive control for neutrophil ROS production in all conditions (grey).
To this end, pooled normal human serum was fractionated using anion exchange chromatography. Only the 400 mM NaCl eluate retained neutrophil suppressive activity (Fig 2E) and was depletable by preincubation with Gc (Fig 2F). The intact and Gc-depleted 400 mM fractions were trypsinized and analyzed by mass spectrometry (see Methods and S1 and S2 Files). The most abundant peptide in the intact 400 mM fraction that was absent from the Gc-depleted fraction was C4BP α. Two proteins that complex with C4BP α in serum—C4BP β and Protein S [31]–were also present in the intact 400 mM fraction and absent (in the case of C4BP β) or markedly reduced (in the case of Protein S) in the Gc-depleted fraction.
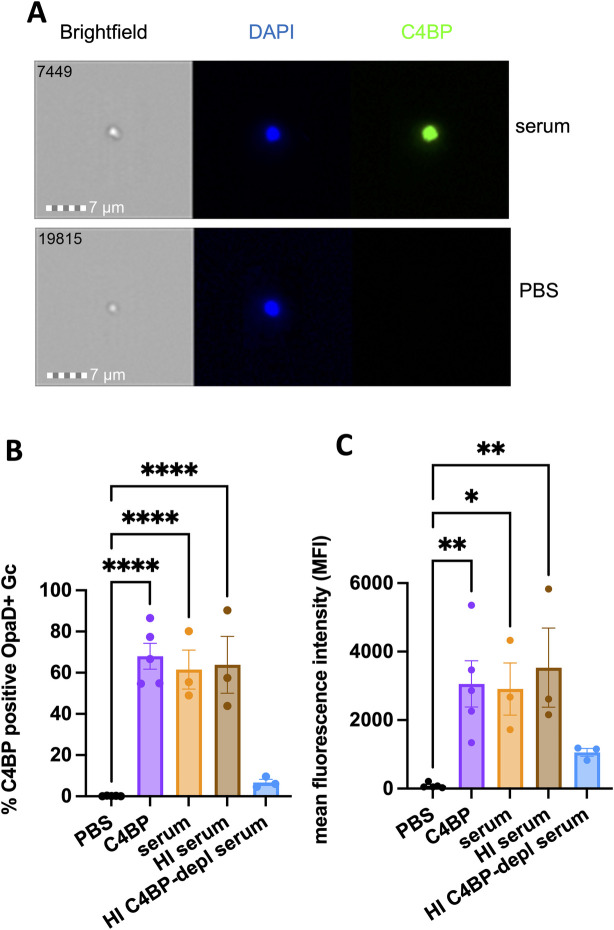
By imaging flow cytometry, C4BP was detectable on the surface of OpaD+ Gc that was incubated with intact or heat-inactivated human serum, or purified C4BP at 50 μg/mL (approximating the concentration of C4BP in 25% serum) (Fig 3A–3C). No C4BP reactivity on OpaD+ Gc was detected using 25% serum that was C4BP-depleted (Fig 3B and 3C). C4BP-depleted serum had heat-sensitive bactericidal activity against OpaD+ Gc, as expected for bacteria of strain FA1090 [29] (S1 Fig). Going forward, C4BP-depleted serum and matched replete serum were heat-inactivated when mixed with Gc, such that any effects on Gc interactions with neutrophils were independent of complement-mediated lysis.
Fig 3. C4BP binds to OpaD+ Gc.
OpaD+ Gc was incubated with 25% normal human serum (orange; UVA), heat-inactivated (HI) serum (brown), heat-inactivated C4BP-depleted (depl) serum (blue), or purified C4BP (50 μg/ml; purple) for 20 minutes at 37°C. Gc was fixed and stained with DAPI for intact bacteria (blue) and for C4BP using anti-C4BP antibody followed by Alexa Fluor 488-coupled goat anti-rabbit IgG (green), and analyzed by imaging flow cytometry. (A) Representative images (image numbers 7449 and 19815) of a single serum-incubated (top) and PBS-incubated (bottom) bacterium. (B) is the percent C4BP-positive bacteria and (C) is the mean intensity of AF488 fluorescence of the total bacterial population. In B-C, results are the mean ± SEM of 3 independent experiments. Statistical analyses were performed using one-way ANOVA with Tukey’s post-hoc comparisons to PBS. *p<0.05, **p<0.01,****p<0.0001.
Together, these results led us to hypothesize that C4BP is the component of serum responsible for suppressing neutrophil anti-gonococcal activity and enhancing Gc survival from neutrophils.
Binding of C4BP to Neisseria gonorrhoeae limits neutrophil anti-gonococcal activity
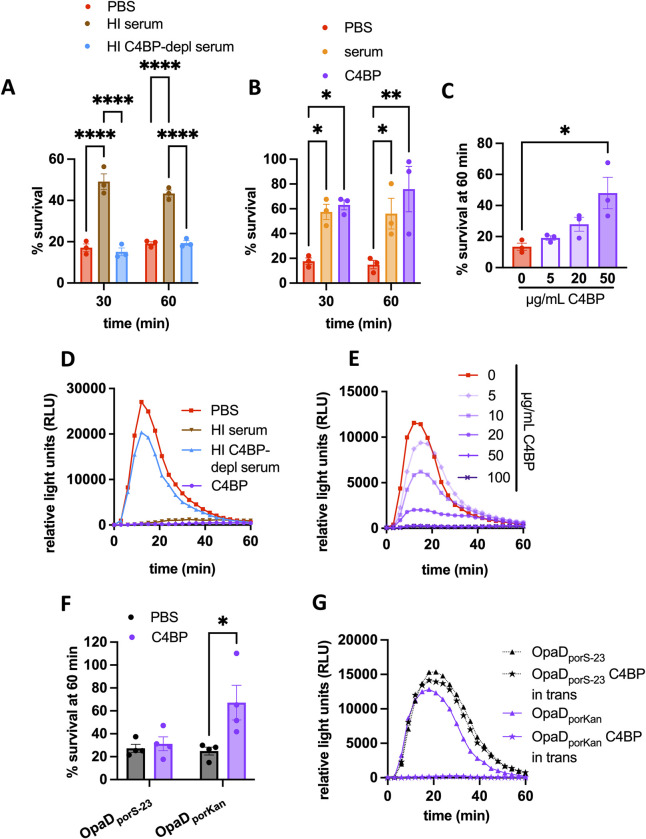
We used heat-inactivated C4BP-depleted serum, purified C4BP, and C4BP-replete serum to test the contribution of C4BP in modulating Gc interactions with neutrophils. Serum that was heat inactivated retained the ability to enhance the survival of opsonized OpaD+ Gc that was exposed to neutrophils (see brown bars, Fig 4A and orange bars, Fig 4B). OpaD+ Gc that was incubated with C4BP-depleted serum survived just as poorly after exposure to human neutrophils as OpaD+ bacteria alone, and both survived significantly less well than bacteria incubated with C4BP-replete serum (Fig 4A). Conversely, OpaD+ Gc that was incubated in purified C4BP survived similarly to Gc incubated with C4BP-replete serum, and both survived significantly better than untreated OpaD+ bacteria (Fig 4B). As with normal human serum (Fig 1B), incubation with purified C4BP enhanced the survival of OpaD+ Gc from neutrophils in a concentration-dependent manner (Fig 4C). C4BP recapitulated the serum-mediated suppression of OpaD+ Gc-induced neutrophil ROS (Fig 4D) in a concentration-dependent manner (Fig 4E). Conversely, incubation of OpaD+ Gc in C4BP-depleted serum induced ROS release from neutrophils similarly to untreated OpaD+ Gc (Fig 4D). As with OpaD+ Gc, incubation with C4BP significantly increased the survival of Opa60+ Gc from neutrophils (S3A Fig), and neutrophil ROS elicited by Opa60+ Gc was suppressed by incubation with C4BP (S3B Fig).
Fig 4. C4BP is necessary and sufficient for serum-mediated suppression of neutrophil anti-gonococcal activity.
(A-C) Adherent, IL-8-treated neutrophils were exposed to OpaD+ Gc incubated in PBS+ (red) or the following (all at 25% final concentration) as in Fig 1A: (A) heat inactivated serum that was C4BP-replete (brown; Lund University) or C4BP-depleted (depl, blue); (B) C4BP-replete serum (orange; Lund Universtiy) or 50 μg/mL purified C4BP (purple); (C) C4BP at the indicated concentrations. In (A-B), two-way ANOVA with Sidak’s post-hoc test was used to compare each condition within each time point for 3 independent experiments; (C) used one-way ANOVA followed by Tukey’s post-hoc comparisons to compare each condition to PBS alone (0 μg/mL C4BP). (D-E) Neutrophil ROS production was measured as in Fig 1C in response to OpaD+ Gc that was incubated (D) in PBS, heat-inactivated C4BP-replete serum (brown; Lund University), heat inactivated C4BP-depleted serum (blue), or purified C4BP at 50 μg/mL (purple); or (E) in the indicated concentration of C4BP. (F) Neutrophils were exposed to OpaDporS-23 Gc or OpaDporKan Gc, with or without C4BP addition to the infection milieu (50 μg/mL), and bacterial survival after 60 minutes was measured as in (A). Results are the mean ± SEM of 3 independent experiments; two-way ANOVA with Sidak’s post-hoc comparisons were used to compare each condition. *p<0.05, **p<0.01,****p<0.0001. (G) OpaD+ Gc (grey solid lines), OpaDporS-23 Gc (black dotted lines), and OpaDporKan Gc (purple solid lines) alone (PBS; triangles), incubated with C4BP (open circles), or added to wells containing C4BP at a final concentration of 50 ug/mL (C4BP “in trans”; stars) were exposed to neutrophils, and ROS production was measured as in (E). Results in (D, E, and G) are one representative of 3 independent experiments.
To investigate the structural requirements of C4BP for suppression of neutrophil anti-gonococcal activity, we utilized 3 different forms of C4BP (S4A Fig): 7α1βPS (predominant form in plasma), 7α1β (no protein S), and 7α (plasma purified, no β chain or protein S). Each was recognized with anti-C4BP α antibody (S4B Fig) and was able to bind to the surface of OpaD+ Gc (S4C Fig). When mixed with OpaD+ Gc, all three forms of C4BP enhanced bacterial survival from neutrophils (S4D Fig) and suppressed neutrophil ROS (S4G Fig), when compared with the effect of C4BP-depleted serum. Thus, the α chain multimer, which is made in inflammatory conditions, is sufficient to enhance OpaD+ Gc survival from neutrophils and suppress neutrophil ROS.
To directly test whether binding to Gc was necessary for the suppressive activity of C4BP on neutrophils, we took two complementary approaches. First, we used two forms of recombinant C4BP that have been reported [27,32], and which we validated, to be incapable of binding to Gc (S4A and S4C Fig): 7α D15N/K24E, which contains two human-to-rhesus mutations in α chain CCP1 that abrogate PorB binding [32], and 1α, which is a monomer due to a deletion of the C-terminal linker and shows decreased binding to ligands due to loss of avidity [27]. 7α D15N/K24E and 1α did not enhance OpaD+ Gc survival from neutrophils (S4E and S4F Fig) or suppress neutrophil ROS release (S4G Fig). Second, we engineered a strain of OpaD+ Gc with a mutant porB gene (see Methods for mutated residues) that was reported to not bind C4BP. We confirmed that the resulting mutant, OpaDporS-23, was unable to bind C4BP by imaging flow cytometry, while the isogenic control, OpaDporKan, bound C4BP similarly to OpaD+ Gc (S5A and S5B Fig). These strains allowed us to ask if C4BP affected neutrophil response to Gc under conditions where it did not directly bind the bacterial surface. To this end, we mixed OpaDporS-23 or OpaDporKan Gc, purified multimeric C4BP, and neutrophils together simultaneously without washing away the C4BP, a condition termed “in trans”. Addition of C4BP in trans did not rescue the survival of OpaDporS-23 from neutrophils (Fig 4F) and did not block the production of neutrophil ROS (Fig 4G), suggesting that unbound C4BP in solution could not recapitulate the effect of C4BP on Gc-neutrophil interactions. However, C4BP added in trans did enhance the survival of OpaDporKan Gc from neutrophils (Fig 4F), and blunted the neutrophil oxidative burst that was stimulated by OpaDporKan Gc, similar to its effect on OpaD+ Gc (Fig 4G). These results confirmed that the suppression of neutrophil antigonococcal activity by C4BP required its binding to Gc, and was not due to an unexpected, direct interaction of C4BP with neutrophils.
Taken together, these results show that C4BP is necessary and sufficient for serum-mediated enhanced survival of Opa+ Gc from neutrophils and for suppressing the oxidative burst that is elicited by Opa+ Gc, in a manner that is dependent on binding of C4BP to the bacterial surface.
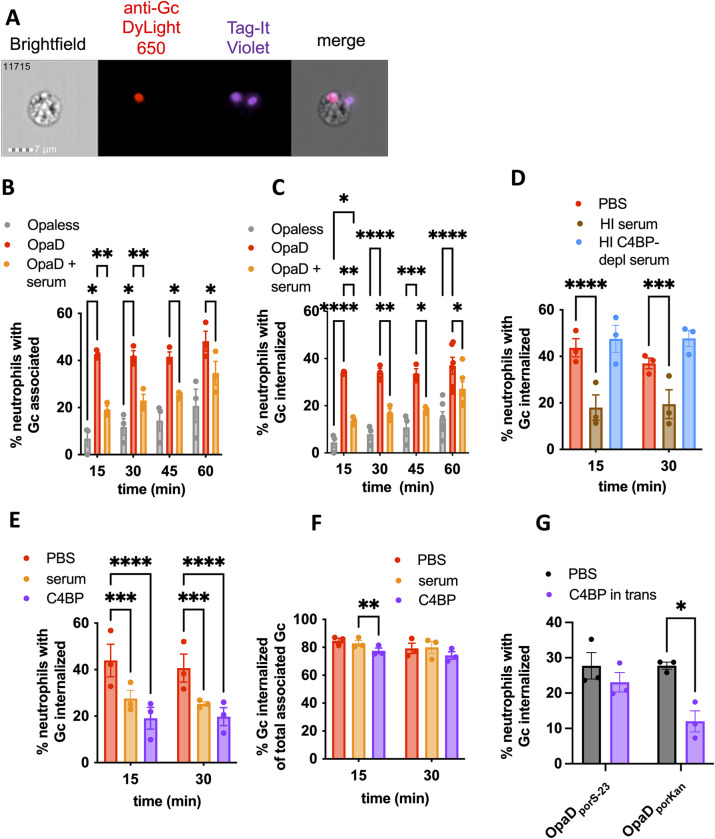
C4BP significantly decreases neutrophils’ association with and internalization of OpaD+ Neisseria gonorrhoeae
We recently reported that the main driver of Gc susceptibility to killing by neutrophils is the efficiency and extent of phagocytosis [19], in keeping with the finding that Gc internalized by neutrophils has significantly poorer survival than Gc that remains extracellular [15]. Moreover, interaction of Gc with surface-expressed receptors such as CEACAMs can activate neutrophils, leading to release of ROS [18]. For these reasons, we hypothesized that C4BP in serum enhances survival of Gc and suppresses ROS release from neutrophils by blocking bacterial binding and phagocytosis. To test this hypothesis, we measured the effect of C4BP on Gc association with and internalization by neutrophils over 1 hour by imaging flow cytometry (Fig 5A) [33]. In keeping with previous reports [14,15,19], OpaD expression promoted the rapid association and internalization of Gc with neutrophils (Fig 5B and 5C), which was significantly reduced when OpaD+ Gc was mixed with normal human serum (Fig 5B and 5C). This effect was C4BP-dependent, since OpaD+ Gc that was incubated in C4BP-depleted serum was indistinguishable from untreated bacteria in neutrophil association (S6A Fig) and internalization (Fig 5D), and both were significantly less than Gc incubated in C4BP-replete serum. Conversely, incubation of OpaD+ Gc with C4BP significantly decreased the percentage of neutrophils with associated (S6B Fig) and internalized (Fig 5E) OpaD+ Gc compared with untreated bacteria. The effect of serum and C4BP on internalization was predominantly driven by a reduction in bacterial interaction with the neutrophil surface, since incubation of Gc with serum or C4BP, compared with PBS as a control, did not affect the percentage of neutrophil-associated bacteria that were phagocytosed at 30 minutes (Fig 5F). Gc binding to C4BP was required for these effects, since the addition of C4BP in trans had no effect on neutrophil association with (S6C Fig) or internalization of (Fig 5G) OpaDporS-23 Gc. Thus, C4BP is necessary and sufficient for the serum-mediated decrease in association and internalization of OpaD+ Gc by neutrophils.
Fig 5. C4BP is necessary and sufficient for serum-mediated decrease in neutrophil internalization of OpaD+ Gc.
Gc were labeled with Tag-IT Violet (TIV), treated as indicated in the following graphs, and incubated with adherent, IL-8-treated primary human neutrophils. At the indicated times, cells were fixed and stained with DyLight 650 (DL650)-labeled anti-Gc antibody without permeabilization to recognize extracellular bacteria. Neutrophils were analyzed via imaging flow cytometry. (A) Data from 12,000 neutrophils with untreated OpaD+ Gc at 60 min were collected. One of these cells, number 11715, is displayed as a representative, with images captured from channels for (left to right) phase contrast, intracellular bacteria (red), total associated bacteria (purple), and merge. (B-C) Neutrophils were exposed to Opaless Gc (gray), OpaD+ Gc (red), or OpaD+ Gc incubated in C4BP-replete serum (25%, orange; Lund University). (B) reports the percentage of single, focused, intact neutrophils with ≥1 cell-associated bacterium (TIV+); (C) reports the percentage of neutrophils with ≥1 phagocytosed bacterium (TIV+ DL650−). (D) Neutrophils were exposed to OpaD+ Gc in PBS (red), or heat-inactivated serum that was intact (brown; Lund University) or C4BP-depleted (blue, both at 25% final), and the percentage of phagocytosed bacteria was calculated as in (C). (E-F) Neutrophils were exposed to OpaD+ Gc in PBS (red), serum (25%, orange; Lund University), or purified C4BP (50 μg/ml, purple). In (E), the percentage of phagocytosed bacteria was calculated as in (C). In (F), the percentage of cell-associated Gc that are internalized was calculated. Results are the mean ± SEM from ≥3 independent experiments. Statistical analyses were performed by two-way ANOVA followed by Sidak’s multiple comparisons. (G) OpaDporS-23 Gc and OpaDporKan Gc were incubated with neutrophils alone (grey bars) or with 50 μg/mL C4BP added to the media immediately prior to infection (“in trans”) for 30 minutes. The percentage of neutrophils with ≥ 1 phagocytosed bacterium was calculated as in (C) from 3 independent experiments. Statistical analyses were performed by two-way ANOVA followed by Sidak’s multiple comparisons. *p<0.05, **p<0.01,***p<0.001, ****p<0.0001.
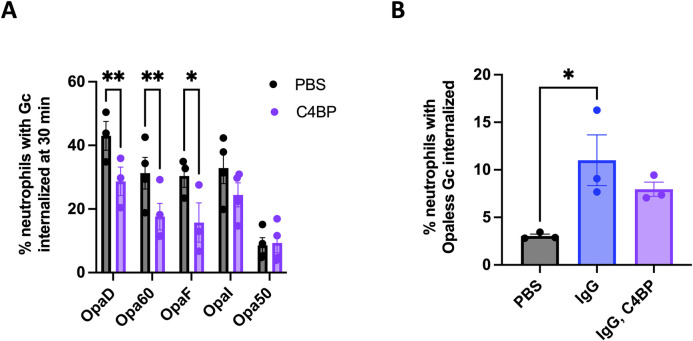
To evaluate the effect of C4BP on the different routes of phagocytosis of Gc by neutrophils, we made use of a panel of single-Opa-expressing Gc that have different receptor-binding capacities, and their Opaless parent [19]. All isolates bound C4BP similarly (S7A–S7D Fig). As we found for OpaD+ Gc, incubation of Opa60+ Gc (CEACAM-1 and CEACAM-3 binding) and OpaF+ Gc (CEACAM-1 binding) with C4BP significantly decreased bacterial association with and internalization by neutrophils (Figs 6A and S8A). In contrast, C4BP binding to Opa50+ Gc (binds heparan sulfate proteoglycans (HSPGs) and not CEACAMs [16]) and OpaI+ Gc (CEACAMs 1 and 3 as well as HSPGs [19,34]) had no effect on interactions with neutrophils (Figs 6A and S8A). The lack of effect of C4BP on Opa50+ Gc held true after 60 minutes of infection, where more neutrophils had associated and internalized the bacteria (S8B and S8C Fig). Moreover, neutrophils’ association with and internalization of Opaless Gc, which does not express Opa proteins and does not bind CEACAMs or HSPGs, was similarly unaffected by incubation with C4BP at 60 minutes (S8B and S8C Fig). To test if C4BP could block Gc-neutrophil interactions that are promoted by phagocytic receptors other than CEACAM family members, we used a Gc-specific IgG to drive phagocytosis of Opaless Gc through neutrophil Fc receptors. As expected, opsonization with IgG significantly increased the percentage of neutrophils with associated and internalized Opaless Gc, compared to unopsonized bacteria (blue vs. gray bars, Figs 6B and S8D). However, adding C4BP had no effect on the ability of neutrophils to associate with or internalize IgG-opsonized Gc (purple bars, Figs 6B and S8D). Addition of IgG did not affect C4BP binding, and vice versa (S8E Fig). Together, these results suggest that the antimicrobial, antiphagocytic effect of C4BP on Gc is predominantly exerted on CEACAM-binding bacteria, and does not extend to Gc that are recognized by neutrophils by other means.
Fig 6. Binding of C4BP prevents internalization of CEACAM-binding Gc by neutrophils.
(A) The indicated variants of Gc were incubated with C4BP (purple), or PBS (grey). The percentage of neutrophils with≥ 1 phagocytosed bacterium was calculated as in Fig 5C. Data represent the mean ± SEM of ≥3 independent experiments. Statistical analyses were performed by two-way ANOVA followed by Sidak’s multiple comparisons. (B) Opaless Gc was incubated with C4BP (50 ug/mL), opsonized in rabbit anti-Gc IgG (80 μg/mL, 20 minutes, 37°C), sequentially incubated with IgG then C4BP, or left untreated. Unopsonized Gc and IgG-opsonized Opaless Gc bound similar amounts of C4BP (S8E Fig). The percentage of neutrophils with ≥ 1 phagocytosed bacterium was calculated as in (A). Statistical analyses were performed by one-way ANOVA followed by Tukey’s multiple comparisons. Opaless Gc incubated with C4BP behaved similarly to Opaless Gc (not shown). *p<0.05, **p<0.01.
Discussion
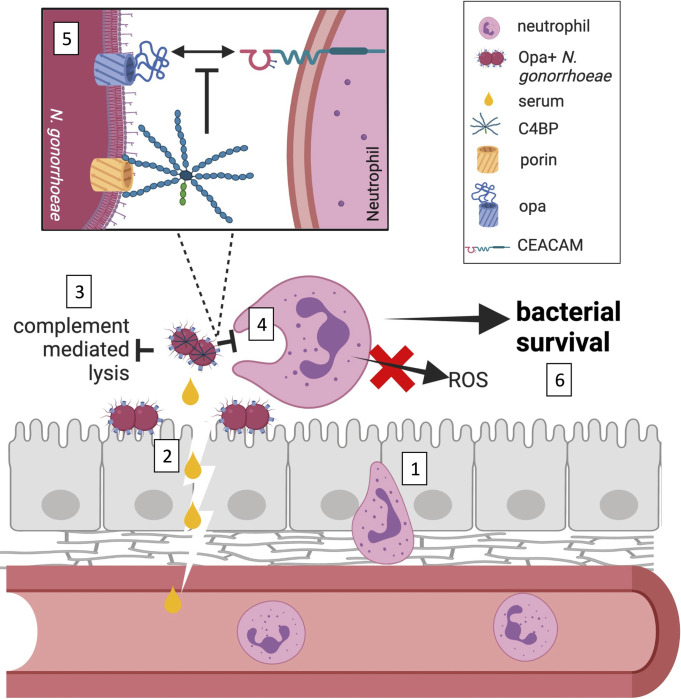
Our work uncovers an unexpected complement-independent role for C4BP in Gc pathogenesis: enhancing resistance to neutrophil clearance. Through an unbiased biochemical screen, C4BP multimer was identified as the complement-independent serum component that enhanced survival of Opa+ Gc from neutrophils and suppressed neutrophil activation, as read out by ROS production. The effects of C4BP were ascribed to inhibition of bacterial binding and phagocytosis by neutrophils. Use of both bacterial mutants and C4BP variants demonstrated that the effect of C4BP required binding to the bacterial surface. These effects were limited to Gc expressing Opa proteins that bind to some CEACAMs. Binding of C4BP may provide one explanation for why Opa+ Gc predominate in infected patient exudates, in spite of the potential for Opa proteins to activate neutrophils and promote bacterial killing in vitro in the absence of serum and C4BP. Our findings add novelty to an extensive literature demonstrating that many pathogens recruit C4BP to limit complement-mediated lysis, by showcasing that C4BP can also act in a complement-independent manner to thwart phagocytic killing (Fig 7).
Fig 7. C4BP in gonococcal pathogenesis.
Neutrophils are recruited to mucosal sites of Neisseria gonorrhoeae infection by extravasating from the blood stream and crossing the epithelia paracellularly (1). Inflammatory conditions of the infection as well as serum transudate bring N. gonorrhoeae into contact with C4b-binding protein (C4BP) (2). The ability of N. gonorrhoeae to bind to C4BP to inhibit complement-mediated lysis has been extensively documented (3). This work shows that C4BP that is bound to the surface of N. gonorrhoeae decreases phagocytic uptake by neutrophils and suppresses neutrophil reactive oxygen species (ROS) production, in a complement-independent manner (4). C4BP binding decreases the interaction of CEACAM-binding, opacity protein (Opa)-expressing N. gonorrhoeae with neutrophils, but not bacteria that are opsonized with IgG or that express Opa proteins that engage other ligands (5). As a consequence, C4BP enhances survival of N. gonorrhoeae from neutrophils, a novel function for this canonical complement inhibitor (6). Figure generated using Biorender (https://biorender.com/).
Gc is expected to encounter C4BP in three conditions during infection. The first is when encountering serum transudate during cervical infection. The lytic activity of cervical fluid is reported to be the equivalent of 11% serum; thus complement components such as C4BP are anticipated to be present in the female genital tract at concentrations that are approximately 11% of their concentrations in serum [35]. We detected C4BP in human serum, and lower but measurable concentrations of C4BP in human mucosal fluids, including seminal plasma, vaginal fluid, menstrual blood, saliva, and tears by mass spectrometry (S1 Table), implying Gc could be exposed to C4BP at the earliest stages of human infection at other sites as well. Even at C4BP concentrations well below what is found in serum, Gc concentrates C4BP on its surface, as shown here by the ability of the bacteria to deplete C4BP from fractionated human serum. The second situation where Gc would encounter C4BP is in conditions of inflammation in acute gonorrhea, where serum leakage occurs due to mucosal damage and breaches from the influx of neutrophils. Inflammatory conditions also increase circulating levels of the 7α form of C4BP [25,36]. Interestingly, C4BP 7α has been found to reduce innate immune inflammation in the context of systemic lupus erythematosus [37], another non-canonical role for C4BP. If 7α has similar anti-inflammatory properties in gonorrhea, future studies could investigate how different forms of C4BP are generated and function in the context of human disease. In the third scenario, Gc would encounter C4BP in the bloodstream during a disseminated gonococcal infection. Future studies could utilize transgenic animal models to demonstrate the effect of human C4BP on infection outcome in each of these scenarios.
The ability of Gc to survive exposure to neutrophils correlates with its resistance to phagocytosis [19]. We found here that incubation with C4BP reduces neutrophil association with and internalization of Opa+ Gc, which can explain how C4BP limits killing of Gc by neutrophils in this infection model. However, C4BP may also enhance Gc resistance to neutrophils by inhibiting signaling pathways that lead to release of antimicrobial products. This is in line with our finding that C4BP incubation suppressed the ability of Opa+ Gc to elicit neutrophil ROS, even though ROS does not contribute to neutrophil anti-gonococcal activity [15,38]. Neutrophil NADPH oxidase activation requires a series of cytoplasmic phosphorylation events, Rac GTPase activation, and granule release, all of which contribute to the overall activation state of neutrophils [18]. Moreover, release of anti-gonococcal proteases from neutrophil primary granules requires signaling via nonreceptor tyrosine kinases like Src and Syk [39,40]. How C4BP would prevent these intracellular signaling pathways is unclear. The primary target for C4BP on Gc is the PorB porin, and while the PorB.1A subtype porin is a ligand for the neutrophil receptor Gp96, the PorB.1B subtype, found on strain FA1090 used in this study, is not known to be a ligand for any neutrophil receptors [41]. However, it is possible that FA1090 PorB.1B could signal through Gp96 or an unappreciated neutrophil receptor, and C4BP could modulate its signaling. Alternatively, purified gonococcal porin suppresses the neutrophil oxidative burst and degranulation, and is able to translocate into the membranes of human cells [42–44]. This raises the possibility that porin or C4BP delivery to neutrophils could affect intracellular neutrophil signaling. In either situation, the ability of C4BP to block proinflammatory, antimicrobial signaling pathways in neutrophils would be expected to work in concert with C4BP-mediated prevention of phagocytosis to enhance Gc survival from neutrophils.
The inhibitory effect of C4BP on bacterial binding and phagocytosis by neutrophils was limited to Opa-CEACAM interactions on the neutrophils, and did not affect phagocytosis of HSPG-binding Opa+ Gc or IgG-opsonized Gc. Our data suggest that C4BP is not interfering with Opa-CEACAM binding by occluding the binding interface, as shown with a bacterial N-CEACAM precipitation assay (S9A and S9B Fig). Alternatively, C4BP could bind to a target on the neutrophil to send signals that prevent phagocytosis, but such a target has not been described. While protein S can bind to phosphatidylserine residues on apoptotic cells [45], multimeric C4BP lacking protein S still inhibited Gc phagocytosis and ROS production, suggesting this mode of interaction is not at play. Also, addition of C4BP to the porBS-23 non-binding mutant Gc did not suppress neutrophil functions, suggesting that soluble C4BP does not directly engage neutrophils to account for these effects.
It is noteworthy that serum incubation had the most pronounced effect on Gc-neutrophil association at early time points and was minimized over time. There are two nonexclusive explanations for this observation. One, if C4BP effects on nonopsonic phagocytosis are predominantly by steric hindrance, interaction between Gc and neutrophils could be expected to “catch up” over time. Two, release of neutrophil proteases could degrade C4BP on the surface of Gc. This could occur for Gc bound to neutrophils, if the proteases are released extracellularly, or phagocytosed bacteria, if released into the phagosome. Interestingly, an intracellular role for C3 in activating antibacterial autophagy for cytoinvasive bacteria has been described [46]. It is intriguing to consider the possibility that C4BP on the surface of phagocytosed Gc could help protect the bacteria from intraphagosomal killing, potentially helping to explain how viable, intact Opa+ Gc are found inside neutrophils recovered from infected individuals [47,48].
This work has implications for the development of new antimicrobials and vaccines for gonorrhea, which are urgently needed for global public health. The Blom and Ram groups are developing therapeutics for Gc that make use of bacterial-binding C4BP CCPs that are fused to IgG or IgM, which recruit complement components to the bacterial surface and elicit serum bactericidal activity [28]. It is intriguing to consider how the C4BP effects on neutrophils reported here would affect the function of these therapeutics as treatments for gonorrhea. While IgG-fused C4BP might facilitate bacterial phagocytosis, the multimeric IgM fusion could block interaction with neutrophils rather than stimulate it, even with complement deposition to drive opsonphagocytosis. Moreover, in light of efforts to develop a gonococcal vaccine, it would be crucial that a vaccine candidate can still bind Gc and recruit complement components in the presence of C4BP. If opsonophagocytosis contributes to vaccine-mediated protection, a vaccine candidate would need to overcome not only the effect of C4BP on limiting complement-dependent phagocytosis, but also the effect of C4BP on nonopsonic Opa-driven interactions between Gc and neutrophils. Since many gonorrhea vaccine studies use mouse sera, and mouse C4BP does not bind to Gc, these results highlight the need to address how C4BP, as well as other complement-limiting factors like factor H and lipooligosaccharide sialylation, would affect the efficacy of a vaccine candidate.
To our knowledge, C4BP has never been reported to enhance the survival of pathogenic bacteria in a complement-independent manner. In fact, the only report to date of C4BP affecting host-pathogen interactions in a complement-independent manner is a report from Varghese and colleagues showing that C4BP restricts entry of Influenza A subtype H1N1 virus into lung epithelial cells [49]. It is intriguing to consider that the many pathogenic bacteria that recruit C4BP to their surface may do so to prevent complement-dependent and -independent neutrophil responses. This may be especially important for bacteria like Group A streptococci that, like the pathogenic Neisseria, stimulate a potent pyogenic response [50]. The protection afforded to Opa+ Gc by C4BP binding could allow Gc to express Opa proteins for the benefit of tight adherence to epithelial cells during infection, while decreasing the Opa-induced activation of neutrophils that would occur during the peak of inflammation. The complement-independent role for C4BP reported here shifts our understanding of the relationship between the cellular and noncellular components of the innate immune system, and how pathogens like Gc can exploit these factors to evade immune clearance for successful infection.
Methods
Ethics statement
All experiments with human neutrophils were conducted in accordance with a protocol approved by the UVA Institutional Review Board for Health Sciences Research (IRB HSR #13909). Formal written consent was obtained from each participant.
Biological fluids and proteins
Normal human serum preparation
Venous blood from 10 healthy human subjects, who were consented under a protocol approved by the University of Virginia Institutional Review Board for Health Sciences Research (IRB-HSR #13909), was collected into serum vacutainer blood collection tubes (BD 366430). After incubation at 37°C for 30 minutes, the serum supernatant was collected, pooled, diluted to 50% with PBS with 100 mg/L CaCl2 and 100 mg/L MgCl2 (“PBS+”; Gibco 14040133), and passed through a 0.2 μm filter. Serum was aliquoted and stored at -20°C and thawed on ice. Aliquots of this single serum pool were used in this manuscript. This serum source appears in figure legends with label “UVA” to differentiate it from the C4BP-replete serum from Lund University (see C4BP-depleted serum and matched C4BP-replete serum).
C4BP-depleted serum and matched C4BP-replete serum
C4BP was depleted from pooled healthy human serum as previously described [51] by passing fresh serum through a column coupled with MK104, a mouse mAb directed against CCP1 of the α-chain of C4BP, with C1q addback (80 μg/mL). Serum was aliquoted, stored at -80°C, and thawed on ice. C4BP-replete serum (i.e. undepleted serum from the same donor pool) was used for comparison and appears in figure legends with label “Lund University” to differentiate it from the UVA normal human serum preparation (see Normal human serum preparation). Serum was collected with written consent under ethical permit from Lund University (2017–582). C4BP depletion was verified via western blot and mass spectrometry (S1 Table).
Modified sera
IgG/M/A-depleted serum (Sigma-Aldrich) was reconstituted from a lyophilized powder and diluted to 25% in PBS+. Complement C3-depleted serum was sourced from Complement Technologies (A314).
To create fractions of serum, pooled normal human serum (UVA) was separated using size-exclusion centrifugal filter units (Amicon). For the <10 kDa fraction, the flow-through of a 10 kDa molecular weight cut off (MWCO) filter was collected. For the >100 kDa fraction, the retentate of a 100 kDa MWCO filter was collected and brought up to original volume with PBS+, and the flow-through collected as the <100 kDa fraction. The <50 kDa fraction was collected from the flow-through of a 50 kDa MWCO filter. For the 10–50 kDa fraction, the retentate of a 10 kDa MWCO filter was subsequently added to a 50 kDa MWCO filter, and the flow-through was collected. For the 50–100 kDa fraction, the retentate of a 50 kDa MWCO column was subsequently added to a 100 kDa MWCO filter, and the flow-through was collected. Serum fractions were sterilized by passage through a 0.2 μM filter and stored at -20° C.
For digestion, pooled normal human serum, diluted to 25% in PBS+, was incubated with 1.5 mg/mL trypsin (Sigma T6763) for 2 hours at 37°C. Serum was placed on ice, soybean trypsin inhibitor (3 mg/mL) (Gibco 17075–029) was added, and the serum was used immediately after preparation.
Other animal sera
Normal rat serum (Enco Scientific Services 13551), fetal bovine serum (HyClone SH30071.03, Lot AC10223670), and normal goat serum (Gibco, 16210–064) were commercially sourced. Fresh normal mouse serum was a gift from the lab of Jonathan Kipnis (formerly of UVA).
Non-serum human biological fluids
The following human biological fluids were sourced from Lee Biosolutions, Inc: Vaginal fluid (991-10-P-1), seminal plasma (991-04-SPP-1), menstrual blood (991-15-P), tears (991-12-P), and saliva (991-05-P-PreC). All were stored at -20°C, thawed on ice, and centrifuged to remove insoluble material prior to use.
Purified and recombinant C4BP
Human serum-purified C4BP was sourced from Complement Technologies, Inc (A109). It was stored at -80°C, thawed on ice, and diluted in PBS+ to the desired concentration for experimentation.
C4BP 7α1βPS that was used in experiments with C4BP-depleted and -replete human serum was purified from human plasma by affinity chromatography using a MK104 antibody column as previously described [52]. C4BP 7α1β was purified from plasma via a standard method that uses barium chloride precipitation followed by anion exchange chromatography and gel filtration. Protein S was then removed after incubation with ethylene glycol, which breaks the hydrophobic bond between C4BP and protein S, followed by affinity chromatography on heparin-Sepharose [53,54]. C4BP 7α, the species lacking the β chain, was purified from the supernatant of the barium chloride precipitation [53] using affinity chromatography with MK104. Recombinant forms of C4BP were expressed in human embryonic kidney (HEK) 293 cells. Recombinant multimeric C4BP 7α was purified via MK104 column as previously described [32]. Recombinant C4BP 1α, the monomeric form of C4BP, has a deletion of the C-terminal linker that is required for multimerization, and was purified as previously described [27]. Recombinant C4BP 7α D15N/K24E, in which CCP1 has two human-to-rhesus mutations in CCP1 that abrogate binding to Gc, was purified as previously described [32].
Bacterial strains
This study used piliated FA1090 Gc that is deleted for all opa genes (Opaless [14]) and containing constitutively expressed versions of OpaD [14], Opa60 [55], OpaI [19], or Opa50 [55], which have a mutated signal sequence that does not phase-vary. Predominantly OpaF+ Gc was selected phenotypically based on colony opacity from strain ΔopaBEGK [14]. Expression of OpaF was verified by Western blot using an OpaF-specific antibody (a gift from Marcia Hobbs, UNC) [21]. Opa+ Gc from strain background 1291 was selected visually by colony opacity.
For all assays, Gc was grown overnight on gonococcal medium base (GCB, Difco) with Kellogg’s supplements I and II [56] at 37°C with 5% CO2. Piliated colonies were selected the next morning and grown for 8 hours on GCB, then grown in gonococcal base medium liquid (GCBL) culture with Kellogg’s supplements I and II overnight with rotation with two back dilutions the next day as described previously [57]. Piliated Gc were enriched by using sedimented bacteria for the final back dilution.
OpaDporS-23 was generated by transforming OpaD+ Gc with genomic DNA from FA1090 S-23 (from Hank Seifert, Northwestern University). FA1090 S-23 contains 5 residues (amino acids 254–259) mutated to alanine in loop 6 of porB (Y254A, G255A, M257A, S258A, G259A), with a kanamycin resistance cassette inserted downstream [58]. Transformants were selected on GCB containing 50 μg/mL kanamycin. porB was amplified from transformants by PCR using PorI (GGCGAATTCCGGCCTGCTTAAATTTCTTA) and PorII (GCGAAGCTTATTAGAATTTGTGGCGCAG), using conditions described in [59], and sent for commercial Sanger sequencing using the same primers. Isolates with the mutated porB were verified to not bind C4BP by flow cytometry (see Detection of C4BP on bacteria). OpaDporKan is isogenic to OpaDporS-23 but retains the parental FA1090 porB.
Neutrophil purification
Neutrophils were purified from venous blood of healthy human subjects who signed informed consent in accordance with UVA IRB-HSR protocol #13909, using dextran sedimentation followed by a Ficoll (Cytiva) gradient and erythrocyte lysis, as described previously [57,60]. Neutrophils were resuspended in 1x PBS (no calcium or magnesium) (Gibco 14190–144) containing 0.1% glucose, stored on ice, and used within 2 hours of purification.
Serum/C4BP incubation
Serum or C4BP was diluted in PBS+ to the desired final concentration. Gc was suspended in serum or C4BP, at a final volume of 250 μL for 10^8 Gc, for 20 minutes at 37°C. Bacteria were pelleted, the supernatant was discarded, and the pellet was washed in PBS+. No free serum or C4BP was present in experiments unless otherwise noted.
Serum bactericidal activity assay
OpaD+ Gc (10^8) was mixed (see Serum/C4BP incubation) for 20 minutes in the indicated percentage of serum or C4BP-depleted serum, with or without heat inactivation at 56°C for 30 minutes. Bacteria were pelleted, washed, diluted, and plated on GCB agar. Colony forming units (CFU) were enumerated after overnight growth.
Mass spectrometry
Identification of C4BP
Pooled normal human serum (UVA, see Normal human serum preparation) was fractionated using strong anion exchange chromatography with a HiTrap Q HP column (Cytiva). Fractions eluted with increasing concentrations of salt were tested for ROS suppressive activity (see Neutrophil Assays below). The suppressive fraction (400 mM NaCl) and activity-depleted fraction (400 mM NaCl preincubated with 5x10^8 OpaD+ Gc for 30 minutes at 37°C) were analyzed by mass spectrometry (MS) and tandem mass spectrometry (MS/MS) at the Biomolecular Analysis Core Facility at the University of Virginia. Samples were reduced with 10 mM DTT in 0.1 M ammonium bicarbonate followed by alkylation with 50 mM iodoacetamide in 0.1 M ammonium bicarbonate (both room temperature for 0.5 h). The samples were then digested overnight at 37°C with 0.1 μg trypsin in 50 mM ammonium bicarbonate. The samples were acidified with acetic acid to stop digestion and then purified using magnetic beads. The solution was evaporated for MS analysis.
The LC-MS system consisted of a Thermo Q Exactive HF mass spectrometer system with an Easy Spray ion source connected to a Thermo 3 μm C18 Easy Spray column (through pre-column). The extract (1 μg) was injected and the peptides eluted from the column by an acetonitrile/0.1 M acetic acid gradient at a flow rate of 0.3 μL/minute over 1.0 hours. The nanospray ion source was operated at 1.9 kV. The digest was analyzed using the rapid switching capability of the instrument acquiring a full scan mass spectrum to determine peptide molecular weights followed by product ion spectra (10 HCD) to determine amino acid sequence in sequential scans. This mode of analysis produces approximately 25000 MS/MS spectra of ions ranging in abundance over several orders of magnitude.
The data were analyzed by database searching using the Sequest search algorithm [61] against Uniprot Human. Scaffold Viewer software (Proteome Software, Inc) was used to visualize and compare the relative abundance of peptides in each fraction (S1 File). Peptides with only one spectral count in the 400 mM fraction were removed to decrease the false discovery rate, and the remaining peptides were ordered from highest to lowest fold change (400 mM fraction vs activity-depleted 400 mM fraction) (S2 File).
Detection of C4BP in secretions
We performed an untargeted data-dependent analysis of tryptic peptides of purified C4BP to identify target peptides and transitions for parallel reaction monitoring (PRM) analysis along with literature and PeptideAtlas (http://www.peptideatlas.org/) searches. Peptide LNNGEITQHR from C4BPα was selected, and a C-terminal isotope-labeled (“heavy” peptide) version was synthesized (Thermo Fisher Scientific).
Samples from distinct fluids were prepared for mass spectrometry analysis as follows. Proteins from 100 μL of each sample were precipitated by adding 1 mL of cold Methanol/Acetone (9:1) and incubated at -80°C. Samples were centrifuged and washed twice with 1 mL of cold methanol. Protein pellets were reconstituted in 100 mM ammonium bicarbonate and total protein was measured using BCA. 10 μg of total protein of each sample were reduced with 10 mM DTT followed by alkylation with 50 mM IAA both at room temperature for 30 min. Trypsin digestion was performed using 1:25 enzyme to protein ration at 37 C for 16 h. Samples were acidified using acetic acid, peptides were purified using C-18 ZipTips and dried under vacuum. Each sample was reconstituted in 10 μL of formic acid containing 1 fmol/μL of the isotope-labeled peptide.
Peptide mixtures were analyzed on Thermo Orbitrap Exploris 480 system coupled to an EASY- nLC 1200 system. 1 μL of each sample was automatically injected into a Thermo 3 μm C18 Easy Spray column (through pre-column) and peptides eluted from the column by acetonitrile/0.1% formic acid gradient at a flow rate of 300 μL/min over 68 minutes. The nanospray ion source was operated at 1.5 kV and the mass spectrometer was operated on positive mode acquiring targeted MS2 scans using Orbitrap resolution at 60,000, AGC target 300, isolation window of 1.2 m/z, and optimized collision energy for HCD of 20.
For quantification, the total peak areas of each peptide were used to obtain light to heavy ratio (L:H). Since the spiked-in heavy concentration is known, the L:H ratios were used to calculate the concentration of the peptide corresponding to the endogenous C4BP in each sample.
Western blotting
Proteins or Gc were boiled for 5 minutes in reducing sample buffer containing 60 mM Tris pH 6.8, 25% glycerol, 0.4% SDS, 5% β-mercaptoethanol, and 0.1% bromophenol blue. Proteins or lysates were resolved on a 4–20% gradient SDS-polyacrylamide gel (Criterion TGX 5671094, Bio-Rad), transferred in Towbin [62] buffer to nitrocellulose membrane, and the membrane was incubated in 5% BSA in Tris buffered saline with 0.1% Tween for 1 hour (blocking buffer). C4BP was detected using rabbit anti-C4BPA antibody (Novus Biologicals 88262) followed by goat anti-Rabbit H+L Alexa Fluor 680 cross adsorbed secondary antibody (Invitrogen A21109), and Opa or porin (loading controls) were detected with mouse anti-Opa 4B12 and mouse anti-porin H5.2 (gift from Marcia Hobbs) respectively followed by goat anti-Mouse IgG (H+L) Dylight 800 cross adsorbed secondary antibody (Invitrogen SA5-10176). All antibodies were diluted in blocking buffer. Bands were visualized via LI-COR Odyssey.
Detection of C4BP on bacteria
Gc (10^8) was pelleted, washed, and incubated with 50 μg/mL C4BP diluted in PBS+ for 20 minutes at 37°C. Gc were pelleted, washed, and resuspended in 5 μg/mL rabbit anti-C4BPA antibody (Novus Biologicals 88262), diluted in RPMI + 10% FBS, for 30 minutes at 37°C. Gc were pelleted and resuspended in 5 μg/mL goat anti-mouse AF488 (Invitrogen, Carlsbad, CA) diluted in RPMI + 10% FBS for 30 minutes at 37°C. The samples were fixed in 2% PFA with 5 μg/mL 4′,6-diamidino-2-phenylindole (DAPI) for visualization of the bacteria. Samples were analyzed by imaging flow cytometry using ImagestreamX Mk II with INSPIRE software (Luminex Corporation) at the Flow Cytometry Core Facility at the University of Virginia, and data were analyzed using IDEAS software.
N-CEACAM binding to Gc
Binding of recombinant N-termini of CEACAM1 or CEACAM3 to Gc, with and without incubation with C4BP, was measured by imaging flow cytometry, based on fluorescence of mouse anti-GST antibody p1A12 (Biolegend) as in [30].
Neutrophil assays
Gc survival from neutrophils
Gc survival from primary human neutrophils was measured as described previously [57]. In brief, 10^6 adherent, IL-8 treated neutrophils were synchronously exposed to 10^6 Gc (MOI 1) by centrifugation in RPMI containing 10% fetal bovine serum. After incubation at 37°C with 5% CO2 for the indicated times, neutrophils were lysed with 1% saponin and lysates were serially diluted and plated on GCB. Colony forming units (CFU) were enumerated after overnight growth, and survival was calculated as a percentage relative to the CFU enumerated at the 0 minute time point.
ROS measurement
ROS release from neutrophils was measured as described previously [18] by suspending neutrophils in Morse’s Defined Medium (MDM) [63] with luminol in a white-bottomed 96 well plate (Falcon 353296). Bacteria were added to neutrophils at an MOI of 100. Luminescence was measured every 3 minutes over 1 hour using a VICTOR3 Wallac luminometer (Perkin-Elmer).
Neutrophil association with and internalization of Gc by imaging flow cytometry
Following the protocol outlined in [33], 2x10^6 adherent, IL-8 treated neutrophils were synchronously infected with 2x10^6 Tag-It Violet stained Gc by centrifugation (MOI 1). After incubation for the indicated times at 37°C with 5% CO2, paraformaldehyde was added (final concentration 2%) and cells were lifted with a cell scraper (Corning 353085). Cells were washed in PBS and blocked in 10% normal goat serum. Extracellular associated bacteria identified by reaction with rabbit anti-Gc antibody (Meridian B65111R) that was conjugated in-house with 1 μg/mL Dylight 650 (Thermo Scientific) according to manufacturer’s recommendations. Results are expressed as the percentage of neutrophils with at least 1 associated (bound and/or internalized; Tag-IT Violet+ Dylight 650+) Gc and the percentage of neutrophils with at least 1 internalized (Tag-IT Violet+ Dylight 650-) Gc.
Statistics
Survival, binding, association, and internalization results are displayed as the mean ± SEM for n≥ 3 biological replicates, conducted on different days with different bacterial cultures and subjects’ neutrophils. Each dot on the bar graphs represents a single, independent biological replicate. Statistical comparisons were performed using one-way ANOVA followed by Tukey’s multiple comparisons post-hoc test, two-way ANOVA followed by Sidak’s multiple comparisons post-hoc test, or paired Student’s t-test as appropriate, using Prism GraphPad. For data sets analyzed by t-test or two-way ANOVA, data from the same biological replicate are paired, to account for inter-subject neutrophil variation. ROS results are presented as one representative graph of n≥3 biological replicates, which cannot be averaged because of day-to-day differences in the magnitude of luminescence emitted.
Supporting information
OpaD+ Gc (10^8 CFU/ml) was resuspended in the indicated percent of normal human serum (Lund University) or C4BP-depleted (depl) serum, with or without heat-inactivation (HI), for 20 minutes at 37°C. CFU were then enumerated from washed bacterial suspensions. Two-way ANOVA with Sidak’s post-hoc comparisons was used to compare across conditions at matched serum concentrations. Data represent the mean ± SEM of 3 independent experiments. **p<0.01.
(TIF)
Strain FA1090 Gc constitutively expressing Opa60 (Opa60+) and an undefined Opa+ isolate of strain 1291 Gc were incubated with normal human serum (UVA) or left untreated, then exposed to primary human neutrophils. ROS production was measured as in Fig 1C. Results are one representative of 3 independent experiments.
(TIF)
(A) Survival of Opa60+ Gc that was preincubated with purified C4BP (blue) or PBS (grey) was measured after 60 minutes of exposure to neutrophils as in Fig 1A. Results are the mean ± SEM of 4 independent experiments and were compared using a one-way ANOVA with Tukey’s post-hoc comparisons. *p<0.05. (B) Opa60+ Gc (blue circles) or OpaD+ Gc (grey squares) were incubated with C4BP (solid lines) or PBS (dotted lines), and neutrophil ROS production in response to each was measured as in Fig 1C. Results are one representative of 3 independent experiments.
(TIF)
(A) Schematic and description for each C4BP species used in (B-G). (B) The indicated form of C4BP or sera (Lund University) were separated by SDS-PAGE and subjected to Western blot for C4BP (rabbit anti-C4BPA IgG, Novus). (C) OpaD+ Gc was incubated in heat-inactivated C4BP-depleted serum, alone or with each indicated form of C4BP added back. Bacterial lysates were resolved on a 4–20% gradient gel C4BP in each lysate was detected as in (B). Anti-Opa 4B12 mAb served as a loading control. Blots were developed by LI-COR Odyssey. (D-F) OpaD+ Gc was incubated in 25% heat-inactivated C4BP-depleted serum alone (“(-)”) or to which the indicated species as defined in (A) were added back (50 μg/mL). Bacterial survival after 60 minutes of exposure to neutrophils was measured as in Fig 1A, for 3–4 independent experiments. One-way ANOVA followed by Tukey’s post-hoc comparisons was used to compare each condition to C4BP-depleted serum alone. (G) OpaD+ Gc was incubated with the indicated species of C4BP added to heat-inactivated C4BP-depleted serum as in (F). Neutrophil ROS production in response to each condition was measured as in Fig 1C. Results are one representative of 3 independent experiments. *p<0.05, **p<0.01,****p<0.0001.
(TIF)
OpaD+ Gc, OpaDporS-23 Gc, and OpaDporKan Gc were incubated with C4BP (50 μg/ml) or left untreated (PBS). C4BP bound to the surface of the bacteria was measured by imaging flow cytometry as in Fig 3B and 3C. (B), percent C4BP-positive bacteria; (C), mean intensity of AF488 fluorescence of the total bacterial population. Results are the mean ± SEM from 3 independent experiments. Statistics are performed using two-way ANOVA with Sidak’s post-hoc comparisons to OpaDporS-23 Gc incubated with C4BP. **p<0.01,***p<0.001, ****p<0.0001.
(TIF)
(A-B) OpaD+ Gc was incubated in PBS (red), C4BP-replete serum (Lund University) that was not heated (orange) or was heat inactivated (brown), C4BP-depleted heat-inactivated serum (blue), or purified C4BP (purple). Gc was then exposed to neutrophils, and the percentage of neutrophils with ≥1 associated bacterium was measured as in Fig 5B. Statistics were performed by two-way ANOVA followed by Sidak’s multiple comparisons for data from 3 independent experiments. Results in (A) and (B) are from the same infected cell population as shown in Fig 5D and 5E, respectively, for phagocytosed Gc. (C) OpaDporS-23 Gc and OpaDporKan Gc were incubated with neutrophils, with (purple) or without (grey) C4BP added to the medium immediately prior to infection (“in trans”). The percentage of neutrophils with ≥1 associated bacterium was measured as in Fig 5B. Statistics were performed by two-way ANOVA followed by Sidak’s multiple comparisons for data from 3 independent experiments. *p<0.05, ***p<0.001, ****p<0.0001. Results in (C) are from the same infected cell population as in Fig 5G for phagocytosed Gc.
(TIF)
The indicated variants or mutants of Gc were incubated in C4BP or PBS, then processed for imaging flow cytometry as in Fig 3. OpaD+ Gc and OpaDporS-23 Gc are positive and negative controls for C4BP binding, respectively. (A,C) Percentage of C4BP-positive bacteria; (B,D) mean intensity of C4BP (AF488) fluorescence of the total bacterial population. Statistical analyses in (A) and (B) are performed by two-way ANOVA with Sidak’s post-hoc comparisons to OpaDporS-23 Gc incubated with C4BP. Statistics in (C) and (D) are performed by two-way ANOVA with Sidak’s post-hoc comparisons to untreated Gc. Results are the mean ± SEM of 3 independent experiments. **p<0.01,***p<0.001, ****p<0.0001.
(TIF)
(A-C) The indicated variants of Gc were incubated in C4BP (50 μg/ml, purple) or PBS (grey) as in Fig 6A. The bacteria were incubated with neutrophils for 30 minutes (A) or 1 hour (B,C), then processed for imaging flow cytometry as in Fig 5. (A,C) report the percentage of neutrophils with ≥1 associated Gc as in Fig 5B, and (B) reports the percentage of neutrophils with ≥ 1 phagocytosed Gc as in Fig 5C. Results are the mean ± SEM from ≥3 independent experiments. Statistical analyses were performed by two-way ANOVA followed by Sidak’s multiple comparisons. Data in (A) are from the same infected cell population as in Fig 6A for phagocytosed Gc. (D-E) Opaless Gc was incubated with C4BP, opsonized in rabbit anti-Gc IgG, sequentially incubated with IgG then C4BP, or left untreated as in Fig 6B. (D) The bacteria were incubated with neutrophils for 30 minutes, then processed for imaging flow cytometry as in A. Statistics were performed by one-way ANOVA followed by Tukey’s multiple comparisons. Data in (D) are from the same infected cell population as in Fig 6B for phagocytosed Gc. (E) C4BP in bacterial lysates was detected as in S4 Fig, except anti-porin H5.2 antibody served as the loading control. *p<0.05, **p<0.01,***p<0.001.
(TIF)
(A-B) OpaD+ Gc was incubated with C4BP (purple) or in PBS (gray), washed, and mixed with GST-tagged recombinant N-terminal domains of CEACAM-1 (CCM 1) or CEACAM-3 (CCM 3), or in buffer (no CCM). N-CEACAM precipitation with Gc was detected with an anti-GST antibody followed by Alexa Fluor 488-coupled goat anti-mouse IgG, and analyzed by imaging flow cytometry. (A) Percent CEACAM-positive bacteria; (B) mean intensity of CEACAM (AF488) fluorescence of the total in focus, singlet bacterial population. Results are the mean ± SEM from 3 independent experiments. Statistics were performed by two-way ANOVA followed by Sidak’s multiple comparisons.
(TIF)
(TIF)
Scaffold Viewer, a free software available online (https://support.proteomesoftware.com/hc/en-us/articles/360058295552-Free-Scaffold-Viewer), is required to view this file.
(SF3)
(XLS)
Acknowledgments
We thank Louise Ball, Samuel Clark, and Christopher Baiocco, who performed preliminary experiments that informed this project. We thank Mike Solga of the UVA Flow Cytometry Core Facility (RRID: SCR_017829) and Nick Sherman, PhD of the UVA Biomolecular Analysis Facility for their advice. We thank Hank Seifert, Marcia Hobbs, Jonathan Kipnis, and Igor Smirnov for reagents and strains. We thank Sanjay Ram for strains and thoughtful discussions of the work.
Data Availability
Data for the results presented in this manuscript are deposited in the Open Science Framework repository: https://osf.io/6smfu/?view_only=f870019813d04cd38b6f89a9421f3788. Raw imaging flow cytometry data are uploaded to the MyFlowCyt repository as follows: Phagocytosis and association of bacteria with neutrophils: https://flowrepository.org/id/RvFrI1RnnCQSpSV7q5tLgTflvbNzVfC8Edq5I9pyOU8BOPRNuxMrLinIOVpmKGZ2 Detecting C4BP on the bacterial surface: https://flowrepository.org/id/RvFrDE8iJvnxTCQOq0MD65s5vgpRts4oRz1oo7CkGKOdphuE37bfO9VQ1VpmXXpJ Bacterial binding of N-CEACAM: https://flowrepository.org/id/RvFrBUyaxitfZHTiomYyk7gif26T9cxgdshJ4z0cSNkMvr8aAuD4HKRrxEvASHDF.
Funding Statement
This work was supported by NIH R01AI097312 and R21AI157539 (AKC). LMW was supported in part by NIH T32 AI007046 and the University of Virginia School of Medicine Wagner Fellowship (https://med.virginia.edu/bims/whats-new-in-bims/wagner-fellowship/). AMB was supported by Swedish Research Council (2018-02392). MBD was supported in part by NIH F32GM136076. JCR was supported in part by NIH T32 GM007267 and NIH T32 AI005432. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Rowley J, Vander Hoorn S, Korenromp E, Low N, Unemo M, Abu-Raddad LJ, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019. Aug 1;97(8):548–562. doi: 10.2471/BLT.18.228486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirkcaldy RD, Weston E, Segurado AC, Hughes G. Epidemiology of Gonorrhea: A Global Perspective. Sex Health. 2019;16: 401–411. doi: 10.1071/SH19061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Feinen B, Russell MW. New Concepts in Immunity to Neisseria Gonorrhoeae: Innate Responses and Suppression of Adaptive Immunity Favor the Pathogen, Not the Host. Front Microbiol. 2011;2: 52. doi: 10.3389/fmicb.2011.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rice PA, Shafer WM, Ram S, Jerse AE. Neisseria gonorrhoeae: Drug Resistance, Mouse Models, and Vaccine Development. Annu Rev Microbiol. 2017;71: 665–686. doi: 10.1146/annurev-micro-090816-093530 [DOI] [PubMed] [Google Scholar]
- 5.Bodie M, Gale-Rowe M, Alexandre S, Auguste U, Tomas K, Martin I. Addressing the rising rates of gonorrhea and drug-resistant gonorrhea: There is no time like the present. Can Commun Dis Rep. 2019;45: 54–62. doi: 10.14745/ccdr.v45i23a02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unemo M, Shafer WM. Antimicrobial Resistance in Neisseria gonorrhoeae in the 21st Century: Past, Evolution, and Future. Clin Microbiol Rev. 2014;27: 587–613. doi: 10.1128/CMR.00010-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens JS, Criss AK. Pathogenesis of Neisseria gonorrhoeae in the female reproductive tract: neutrophilic host response, sustained infection, and clinical sequelae. Curr Opin Hematol. 2018;25: 13–21. doi: 10.1097/MOH.0000000000000394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmer A, Criss AK. Gonococcal defenses against antimicrobial activities of neutrophils. Trends Microbiol. 2018;26: 1022–1034. doi: 10.1016/j.tim.2018.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vandendriessche S, Cambier S, Proost P, Marques PE. Complement Receptors and Their Role in Leukocyte Recruitment and Phagocytosis. Front Cell Dev Biol. 2021;9: 624025. doi: 10.3389/fcell.2021.624025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Virji M, Evans D, Hadfield A, Grunert F, Teixeira AM, Watt SM. Critical determinants of host receptor targeting by Neisseria meningitidis and Neisseria gonorrhoeae: identification of Opa adhesiotopes on the N-domain of CD66 molecules. Mol Microbiol. 1999;34: 538–551. doi: 10.1046/j.1365-2958.1999.01620.x [DOI] [PubMed] [Google Scholar]
- 11.Popp A, Dehio C, Grunert F, Meyer TF, Gray-Owen SD. Molecular analysis of neisserial Opa protein interactions with the CEA family of receptors: identification of determinants contributing to the differential specificities of binding. Cell Microbiol. 1999;1: 169–181. doi: 10.1046/j.1462-5822.1999.00017.x [DOI] [PubMed] [Google Scholar]
- 12.Murphy GL, Connell TD, Barritt DS, Koomey M, Cannon JG. Phase variation of gonococcal protein II: regulation of gene expression by slipped-strand mispairing of a repetitive DNA sequence. Cell. 1989;56: 539–547. doi: 10.1016/0092-8674(89)90577-1 [DOI] [PubMed] [Google Scholar]
- 13.Yu Q, Wang L-C, Benigno SD, Gray-Owen SD, Stein DC, Song W. Neisseria gonorrhoeae infects the heterogeneous epithelia of the human cervix using distinct mechanisms. PLOS Pathog. 2019;15: e1008136. doi: 10.1371/journal.ppat.1008136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ball LM, Criss AK. Constitutively Opa-Expressing and Opa-Deficient Neisseria gonorrhoeae Strains Differentially Stimulate and Survive Exposure to Human Neutrophils. J Bacteriol. 2013;195: 2982–2990. doi: 10.1128/JB.00171-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson MB, Ball LM, Daily KP, Martin JN, Columbus L, Criss AK. Opa+ Neisseria gonorrhoeae Exhibits Reduced Survival in Human Neutrophils Via Src Family Kinase-Mediated Bacterial Trafficking Into Mature Phagolysosomes. Cell Microbiol. 2015;17: 648–665. doi: 10.1111/cmi.12389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray-Owen SD, Dehio C, Haude A, Grunert F, Meyer TF. CD66 carcinoembryonic antigens mediate interactions between Opa-expressing Neisseria gonorrhoeae and human polymorphonuclear phagocytes. EMBO J. 1997;16: 3435–3445. doi: 10.1093/emboj/16.12.3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen T, Grunert F, Medina-Marino A, Gotschlich EC. Several Carcinoembryonic Antigens (CD66) Serve as Receptors for Gonococcal Opacity Proteins. J Exp Med. 1997;185: 1557–1564. doi: 10.1084/jem.185.9.1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smirnov A, Daily KP, Criss AK. Assembly of NADPH oxidase in human neutrophils is modulated by the opacity-associated protein expression state of Neisseria gonorrhoeae. Infect Immun. 2013/12/18 ed. 2014;82: 1036–44. doi: 10.1128/IAI.00881-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alcott AM, Werner LM, Baiocco CM, Belcher Dufrisne M, Columbus L, Criss AK. Variable Expression of Opa Proteins by Neisseria gonorrhoeae Influences Bacterial Association and Phagocytic Killing by Human Neutrophils. J Bacteriol. 204: e00035–22. doi: 10.1128/jb.00035-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.James JF, Swanson J. Studies on gonococcus infection. XIII. Occurrence of color/opacity colonial variants in clinical cultures. Infect Immun. 1978;19: 332–340. doi: 10.1128/iai.19.1.332-340.1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jerse AE, Cohen MS, Drown PM, Whicker LG, Isbey SF, Seifert HS, et al. Multiple gonococcal opacity proteins are expressed during experimental urethral infection in the male. J Exp Med. 1994;179: 911–920. doi: 10.1084/jem.179.3.911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swanson J, Barrera O, Sola J, Boslego J. Expression of outer membrane protein II by gonococci in experimental gonorrhea. J Exp Med. 1988/12/01 ed. 1988;168: 2121–9. doi: 10.1084/jem.168.6.2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blom AM, Villoutreix BO, Dahlbäck B. Complement inhibitor C4b-binding protein-friend or foe in the innate immune system? Mol Immunol. 2004;40: 1333–1346. doi: 10.1016/j.molimm.2003.12.002 [DOI] [PubMed] [Google Scholar]
- 24.Marcovina SM, Zoppo A, Viganó-D’Angelo S, Di Cola G, D’Angelo A. Determination of serum levels of complement component C4b-binding protein: influence of age and inflammation. Int J Clin Lab Res. 1991;21: 171–175. doi: 10.1007/BF02591638 [DOI] [PubMed] [Google Scholar]
- 25.Ermert D, Blom AM. C4b-binding protein: The good, the bad and the deadly. Novel functions of an old friend. Immunol Lett. 2016;169: 82–92. doi: 10.1016/j.imlet.2015.11.014 [DOI] [PubMed] [Google Scholar]
- 26.Blom AM, Ram S. Contribution of interactions between complement inhibitor C4b-binding protein and pathogens to their ability to establish infection with particular emphasis on Neisseria gonorrhoeae. Vaccine. 2008;26 Suppl 8: I49–55. doi: 10.1016/j.vaccine.2008.11.049 [DOI] [PubMed] [Google Scholar]
- 27.Ram S, Cullinane M, Blom AM, Gulati S, McQuillen DP, Monks BG, et al. Binding of C4b-binding protein to porin: a molecular mechanism of serum resistance of Neisseria gonorrhoeae. J Exp Med. 2001/02/07 ed. 2001;193: 281–95. doi: 10.1084/jem.193.3.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bettoni S, Shaughnessy J, Maziarz K, Ermert D, Gulati S, Zheng B, et al. C4BP-IgM protein as a therapeutic approach to treat Neisseria gonorrhoeae infections. JCI Insight. 2019;4. doi: 10.1172/jci.insight.131886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ngampasutadol J, Ram S, Blom AM, Jarva H, Jerse AE, Lien E, et al. Human C4b-binding protein selectively interacts with Neisseria gonorrhoeae and results in species-specific infection. Proc Natl Acad Sci U S A. 2005;102: 17142–17147. doi: 10.1073/pnas.0506471102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Werner LM, Palmer A, Smirnov A, Dufrisne MB, Columbus L, Criss AK. Imaging Flow Cytometry Analysis of CEACAM Binding to Opa-Expressing Neisseria gonorrhoeae. Cytom Part J Int Soc Anal Cytol. 2020;97: 1081–1089. doi: 10.1002/cyto.a.24037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dahlbäck B. Protein S and C4b-binding protein: components involved in the regulation of the protein C anticoagulant system. Thromb Haemost. 1991;66: 49–61. [PubMed] [Google Scholar]
- 32.Jarva H, Ngampasutadol J, Ram S, Rice PA, Villoutreix BO, Blom AM. Molecular Characterization of the Interaction between Porins of Neisseria gonorrhoeae and C4b-Binding Protein. J Immunol. 2007;179: 540–547. doi: 10.4049/jimmunol.179.1.540 [DOI] [PubMed] [Google Scholar]
- 33.Smirnov A, Solga MD, Lannigan J, Criss AK. Using Imaging Flow Cytometry to Quantify Neutrophil Phagocytosis. Methods Mol Biol Clifton NJ. 2020;2087: 127–140. doi: 10.1007/978-1-0716-0154-9_10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cole JG, Fulcher NB, Jerse AE. Opacity Proteins Increase Neisseria gonorrhoeae Fitness in the Female Genital Tract Due to a Factor under Ovarian Control. Infect Immun. 2010;78: 1629–1641. doi: 10.1128/IAI.00996-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Price RJ, Boettcher B. The Presence of Complement in Human Cervical Mucus and its Possible Relevance to Infertility in Women with Complement-Dependent Sperm-Immobilizing Antibodies. Fertil Steril. 1979;32: 61–66. doi: 10.1016/s0015-0282(16)44117-8 [DOI] [PubMed] [Google Scholar]
- 36.Frutos PG de Alim RIM, Härdig Y, Zoller B, Dahlbâck B. Differential Regulation of α and β Chains of C4b-Binding Protein During Acute-Phase Response Resulting in Stable Plasma Levels of Free Anticoagulant Protein S. Blood. 1994;84: 815–822. doi: 10.1182/blood.V84.3.815.815 [DOI] [PubMed] [Google Scholar]
- 37.Serrano I, Luque A, Mitjavila F, Blom AM, Rodríguez de Córdoba S, Vega MC, et al. The Hidden Side of Complement Regulator C4BP: Dissection and Evaluation of Its Immunomodulatory Activity. Front Immunol. 2022;13: 883743. doi: 10.3389/fimmu.2022.883743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seib KL, Wu H-J, Kidd SP, Apicella MA, Jennings MP, McEwan AG. Defenses against Oxidative Stress in Neisseria gonorrhoeae: a System Tailored for a Challenging Environment. Microbiol Mol Biol Rev. 2006;70: 344–361. doi: 10.1128/MMBR.00044-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lacy P. Mechanisms of Degranulation in Neutrophils. Allergy Asthma Clin Immunol Off J Can Soc Allergy Clin Immunol. 2006;2: 98–108. doi: 10.1186/1710-1492-2-3-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarantis H, Gray-Owen SD. The specific innate immune receptor CEACAM3 triggers neutrophil bactericidal activities via a Syk kinase-dependent pathway. Cell Microbiol. 2007;9: 2167–2180. doi: 10.1111/j.1462-5822.2007.00947.x [DOI] [PubMed] [Google Scholar]
- 41.Rechner C, Kühlewein C, Müller A, Schild H, Rudel T. Host Glycoprotein Gp96 and Scavenger Receptor SREC Interact with PorB of Disseminating Neisseria gonorrhoeae in an Epithelial Invasion Pathway. Cell Host Microbe. 2007;2: 393–403. doi: 10.1016/j.chom.2007.11.002 [DOI] [PubMed] [Google Scholar]
- 42.Haines KA, Yeh L, Blake MS, Cristello P, Korchak H, Weissmann G. Protein I, a translocatable ion channel from Neisseria gonorrhoeae, selectively inhibits exocytosis from human neutrophils without inhibiting O2- generation. J Biol Chem. 1988;263: 945–951. doi: 10.1016/S0021-9258(19)35444-4 [DOI] [PubMed] [Google Scholar]
- 43.Lynch EC, Blake MS, Gotschlich EC, Mauro A. Studies of Porins: Spontaneously Transferred from Whole Cells and Reconstituted from Purified Proteins of Neisseria gonorrhoeae and Neisseria meningitidis. Biophys J. 1984;45: 104–107. doi: 10.1016/S0006-3495(84)84127-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lorenzen DR, Günther D, Pandit J, Rudel T, Brandt E, Meyer TF. Neisseria gonorrhoeae Porin Modifies the Oxidative Burst of Human Professional Phagocytes. Infect Immun. 2000;68: 6215–6222. doi: 10.1128/IAI.68.11.6215-6222.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Webb JH, Blom AM, Dahlbäck B. The binding of protein S and the protein S-C4BP complex to neutrophils is apoptosis dependent. Blood Coagul Fibrinolysis Int J Haemost Thromb. 2003;14: 355–359. doi: 10.1097/00001721-200306000-00006 [DOI] [PubMed] [Google Scholar]
- 46.Sorbara MT, Foerster EG, Tsalikis J, Abdel-Nour M, Mangiapane J, Sirluck-Schroeder I, et al. Complement C3 Drives Autophagy-Dependent Restriction of Cyto-invasive Bacteria. Cell Host Microbe. 2018;23: 644–652.e5. doi: 10.1016/j.chom.2018.04.008 [DOI] [PubMed] [Google Scholar]
- 47.Wiesner PJ, Thompson SE. Gonococcal diseases. Dis Mon. 1980;26: 1–44. doi: 10.1016/s0011-5029(80)80002-2 [DOI] [PubMed] [Google Scholar]
- 48.Johnson MB, Criss AK. Resistance of Neisseria gonorrhoeae to neutrophils. Front Microbiol. 2011;2: 77. doi: 10.3389/fmicb.2011.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Varghese PM, Murugaiah V, Beirag N, Temperton N, Khan HA, Alrokayan SH, et al. C4b Binding Protein Acts as an Innate Immune Effector Against Influenza A Virus. Front Immunol. 2020;11: 585361. doi: 10.3389/fimmu.2020.585361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jenkins HT, Mark L, Ball G, Persson J, Lindahl G, Uhrin D, et al. Human C4b-binding Protein, Structural Basis for Interaction with Streptococcal M Protein, a Major Bacterial Virulence Factor. J Biol Chem. 2006;281: 3690–3697. doi: 10.1074/jbc.M511563200 [DOI] [PubMed] [Google Scholar]
- 51.Kask L, Trouw LA, Dahlbäck B, Blom AM. The C4b-binding protein-protein S complex inhibits the phagocytosis of apoptotic cells. J Biol Chem. 2004;279: 23869–23873. doi: 10.1074/jbc.C400159200 [DOI] [PubMed] [Google Scholar]
- 52.Dahlbäck B. Purification of human C4b-binding protein and formation of its complex with vitamin K-dependent protein S. Biochem J. 1983;209: 847–856. doi: 10.1042/bj2090847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mohlin FC, Blom AM. Purification and functional characterization of C4b-binding protein (C4BP). Methods Mol Biol Clifton NJ. 2014;1100: 169–176. doi: 10.1007/978-1-62703-724-2_14 [DOI] [PubMed] [Google Scholar]
- 54.Webb JH, Blom AM, Dahlbäck B. Vitamin K-Dependent Protein S Localizing Complement Regulator C4b-Binding Protein to the Surface of Apoptotic Cells. J Immunol. 2002;169: 2580–2586. doi: 10.4049/jimmunol.169.5.2580 [DOI] [PubMed] [Google Scholar]
- 55.Martin JN, Ball LM, Solomon TL, Dewald AH, Criss AK, Columbus L. Neisserial Opa protein–CEACAM interactions: competition for receptors as a means for bacterial invasion and pathogenesis. Biochemistry. 2016;55: 4286–4294. doi: 10.1021/acs.biochem.6b00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kellogg DS, Peacock WL, Deacon WE, Brown L, Pirkle CI. NEISSERIA GONORRHOEAE I. J Bacteriol. 1963;85: 1274–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ragland SA, Criss AK. Protocols to interrogate the interactions between Neisseria gonorrhoeae and primary human neutrophils. Methods Mol Biol Clifton NJ. 2019;1997: 319–345. doi: 10.1007/978-1-4939-9496-0_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen A, Seifert HS. Structure-Function Studies of the Neisseria gonorrhoeae Major Outer Membrane Porin. Infect Immun. 2013;81: 4383–4391. doi: 10.1128/IAI.00367-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hobbs MM, Alcorn TM, Davis RH, Fischer W, Thomas JC, Martin I, et al. Molecular Typing of Neisseria gonorrhoeae Causing Repeated Infections: Evolution of Porin during Passage within a Community. J Infect Dis. 1999;179: 371–381. doi: 10.1086/314608 [DOI] [PubMed] [Google Scholar]
- 60.Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Investig Suppl. 1968;97: 77–89. [PubMed] [Google Scholar]
- 61.Eng JK, McCormack AL, Yates JR. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom. 1994;5: 976–989. doi: 10.1016/1044-0305(94)80016-2 [DOI] [PubMed] [Google Scholar]
- 62.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci. 1979;76: 4350–4354. doi: 10.1073/pnas.76.9.4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morse SA, Bartenstein L. Purine metabolism in Neisseria gonorrhoeae: the requirement for hypoxanthine. Can J Microbiol. 1980;26: 13–20. doi: 10.1139/m80-003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
OpaD+ Gc (10^8 CFU/ml) was resuspended in the indicated percent of normal human serum (Lund University) or C4BP-depleted (depl) serum, with or without heat-inactivation (HI), for 20 minutes at 37°C. CFU were then enumerated from washed bacterial suspensions. Two-way ANOVA with Sidak’s post-hoc comparisons was used to compare across conditions at matched serum concentrations. Data represent the mean ± SEM of 3 independent experiments. **p<0.01.
(TIF)
Strain FA1090 Gc constitutively expressing Opa60 (Opa60+) and an undefined Opa+ isolate of strain 1291 Gc were incubated with normal human serum (UVA) or left untreated, then exposed to primary human neutrophils. ROS production was measured as in Fig 1C. Results are one representative of 3 independent experiments.
(TIF)
(A) Survival of Opa60+ Gc that was preincubated with purified C4BP (blue) or PBS (grey) was measured after 60 minutes of exposure to neutrophils as in Fig 1A. Results are the mean ± SEM of 4 independent experiments and were compared using a one-way ANOVA with Tukey’s post-hoc comparisons. *p<0.05. (B) Opa60+ Gc (blue circles) or OpaD+ Gc (grey squares) were incubated with C4BP (solid lines) or PBS (dotted lines), and neutrophil ROS production in response to each was measured as in Fig 1C. Results are one representative of 3 independent experiments.
(TIF)
(A) Schematic and description for each C4BP species used in (B-G). (B) The indicated form of C4BP or sera (Lund University) were separated by SDS-PAGE and subjected to Western blot for C4BP (rabbit anti-C4BPA IgG, Novus). (C) OpaD+ Gc was incubated in heat-inactivated C4BP-depleted serum, alone or with each indicated form of C4BP added back. Bacterial lysates were resolved on a 4–20% gradient gel C4BP in each lysate was detected as in (B). Anti-Opa 4B12 mAb served as a loading control. Blots were developed by LI-COR Odyssey. (D-F) OpaD+ Gc was incubated in 25% heat-inactivated C4BP-depleted serum alone (“(-)”) or to which the indicated species as defined in (A) were added back (50 μg/mL). Bacterial survival after 60 minutes of exposure to neutrophils was measured as in Fig 1A, for 3–4 independent experiments. One-way ANOVA followed by Tukey’s post-hoc comparisons was used to compare each condition to C4BP-depleted serum alone. (G) OpaD+ Gc was incubated with the indicated species of C4BP added to heat-inactivated C4BP-depleted serum as in (F). Neutrophil ROS production in response to each condition was measured as in Fig 1C. Results are one representative of 3 independent experiments. *p<0.05, **p<0.01,****p<0.0001.
(TIF)
OpaD+ Gc, OpaDporS-23 Gc, and OpaDporKan Gc were incubated with C4BP (50 μg/ml) or left untreated (PBS). C4BP bound to the surface of the bacteria was measured by imaging flow cytometry as in Fig 3B and 3C. (B), percent C4BP-positive bacteria; (C), mean intensity of AF488 fluorescence of the total bacterial population. Results are the mean ± SEM from 3 independent experiments. Statistics are performed using two-way ANOVA with Sidak’s post-hoc comparisons to OpaDporS-23 Gc incubated with C4BP. **p<0.01,***p<0.001, ****p<0.0001.
(TIF)
(A-B) OpaD+ Gc was incubated in PBS (red), C4BP-replete serum (Lund University) that was not heated (orange) or was heat inactivated (brown), C4BP-depleted heat-inactivated serum (blue), or purified C4BP (purple). Gc was then exposed to neutrophils, and the percentage of neutrophils with ≥1 associated bacterium was measured as in Fig 5B. Statistics were performed by two-way ANOVA followed by Sidak’s multiple comparisons for data from 3 independent experiments. Results in (A) and (B) are from the same infected cell population as shown in Fig 5D and 5E, respectively, for phagocytosed Gc. (C) OpaDporS-23 Gc and OpaDporKan Gc were incubated with neutrophils, with (purple) or without (grey) C4BP added to the medium immediately prior to infection (“in trans”). The percentage of neutrophils with ≥1 associated bacterium was measured as in Fig 5B. Statistics were performed by two-way ANOVA followed by Sidak’s multiple comparisons for data from 3 independent experiments. *p<0.05, ***p<0.001, ****p<0.0001. Results in (C) are from the same infected cell population as in Fig 5G for phagocytosed Gc.
(TIF)
The indicated variants or mutants of Gc were incubated in C4BP or PBS, then processed for imaging flow cytometry as in Fig 3. OpaD+ Gc and OpaDporS-23 Gc are positive and negative controls for C4BP binding, respectively. (A,C) Percentage of C4BP-positive bacteria; (B,D) mean intensity of C4BP (AF488) fluorescence of the total bacterial population. Statistical analyses in (A) and (B) are performed by two-way ANOVA with Sidak’s post-hoc comparisons to OpaDporS-23 Gc incubated with C4BP. Statistics in (C) and (D) are performed by two-way ANOVA with Sidak’s post-hoc comparisons to untreated Gc. Results are the mean ± SEM of 3 independent experiments. **p<0.01,***p<0.001, ****p<0.0001.
(TIF)
(A-C) The indicated variants of Gc were incubated in C4BP (50 μg/ml, purple) or PBS (grey) as in Fig 6A. The bacteria were incubated with neutrophils for 30 minutes (A) or 1 hour (B,C), then processed for imaging flow cytometry as in Fig 5. (A,C) report the percentage of neutrophils with ≥1 associated Gc as in Fig 5B, and (B) reports the percentage of neutrophils with ≥ 1 phagocytosed Gc as in Fig 5C. Results are the mean ± SEM from ≥3 independent experiments. Statistical analyses were performed by two-way ANOVA followed by Sidak’s multiple comparisons. Data in (A) are from the same infected cell population as in Fig 6A for phagocytosed Gc. (D-E) Opaless Gc was incubated with C4BP, opsonized in rabbit anti-Gc IgG, sequentially incubated with IgG then C4BP, or left untreated as in Fig 6B. (D) The bacteria were incubated with neutrophils for 30 minutes, then processed for imaging flow cytometry as in A. Statistics were performed by one-way ANOVA followed by Tukey’s multiple comparisons. Data in (D) are from the same infected cell population as in Fig 6B for phagocytosed Gc. (E) C4BP in bacterial lysates was detected as in S4 Fig, except anti-porin H5.2 antibody served as the loading control. *p<0.05, **p<0.01,***p<0.001.
(TIF)
(A-B) OpaD+ Gc was incubated with C4BP (purple) or in PBS (gray), washed, and mixed with GST-tagged recombinant N-terminal domains of CEACAM-1 (CCM 1) or CEACAM-3 (CCM 3), or in buffer (no CCM). N-CEACAM precipitation with Gc was detected with an anti-GST antibody followed by Alexa Fluor 488-coupled goat anti-mouse IgG, and analyzed by imaging flow cytometry. (A) Percent CEACAM-positive bacteria; (B) mean intensity of CEACAM (AF488) fluorescence of the total in focus, singlet bacterial population. Results are the mean ± SEM from 3 independent experiments. Statistics were performed by two-way ANOVA followed by Sidak’s multiple comparisons.
(TIF)
(TIF)
Scaffold Viewer, a free software available online (https://support.proteomesoftware.com/hc/en-us/articles/360058295552-Free-Scaffold-Viewer), is required to view this file.
(SF3)
(XLS)
Data Availability Statement
Data for the results presented in this manuscript are deposited in the Open Science Framework repository: https://osf.io/6smfu/?view_only=f870019813d04cd38b6f89a9421f3788. Raw imaging flow cytometry data are uploaded to the MyFlowCyt repository as follows: Phagocytosis and association of bacteria with neutrophils: https://flowrepository.org/id/RvFrI1RnnCQSpSV7q5tLgTflvbNzVfC8Edq5I9pyOU8BOPRNuxMrLinIOVpmKGZ2 Detecting C4BP on the bacterial surface: https://flowrepository.org/id/RvFrDE8iJvnxTCQOq0MD65s5vgpRts4oRz1oo7CkGKOdphuE37bfO9VQ1VpmXXpJ Bacterial binding of N-CEACAM: https://flowrepository.org/id/RvFrBUyaxitfZHTiomYyk7gif26T9cxgdshJ4z0cSNkMvr8aAuD4HKRrxEvASHDF.