Abstract
Background and Purpose
Many patients with chronic pain report hypersensitivity not only to noxious stimuli, but also to other modalities including innocuous touch, sound, and light, possibly due to differences in the processing of these stimuli. The goal of this study was to characterize functional connectivity (FC) differences between subjects with temporomandibular disorders (TMD) and pain‐free controls during a visual functional magnetic resonance imaging (fMRI) task that included an unpleasant, strobing visual stimulus. We hypothesized the TMD cohort would exhibit maladaptations in brain networks consistent with multisensory hypersensitivities observed in TMD patients.
Methods
This pilot study included 16 subjects, 10 with TMD and 6 pain‐free controls. Clinical pain was characterized using self‐reported questionnaires. Visual task‐based fMRI data were collected on a 3T MR scanner and used to determine differences in FC via group independent component analysis.
Results
Compared to controls, subjects with TMD exhibited abnormally increased FC between the default mode network and lateral prefrontal areas involved in attention and executive function, and impaired FC between the frontoparietal network and higher order visual processing areas.
Conclusions
The results indicate maladaptation of brain functional networks, likely due to deficits in multisensory integration, default mode network function, and visual attention and engendered by chronic pain mechanisms.
Keywords: chronic pain, fMRI, MRI, TMD, visual processing
Using a visual task‐based paradigm combined with functional magnetic resonance imaging, we observed individuals with temporomandibular disorders (TMD) and chronic pain exhibited abnormally increased functional connectivity between the default mode network and lateral prefrontal areas involved in attention and executive function compared to pain‐free healthy controls (HC). Impaired functional connectivity in subjects with TMD was also observed between the frontoparietal network and higher order visual processing areas.
1. INTRODUCTION
Chronic pain is a major public health challenge, exacerbated to some extent by the lack of effective treatments. Research has identified peripheral and centralized, or nociplastic, contributions to chronic pain, including temporomandibular disorders (TMD) (Harper et al., 2016; Scholz, 2014). TMD has a lifetime prevalence of ∼10–12% (Manfredini et al., 2011) and is one of many chronic overlapping pain conditions including fibromyalgia and irritable bowel syndrome; however, much less is known about central pain processing in TMD compared to other conditions. Nociplastic pain mechanisms can include changes in brain structure, function, and metabolite concentrations (Eller‐Smith et al., 2018; Harfeldt et al., 2018; Harper et al., 2016). Additionally, demonstration of hypersensitivity to nonsomatosensory stimuli in chronic pain suggests the presence of generalized, central mechanisms of sensory amplification (Geisser et al., 2008; Harte et al., 2016; Hollins et al., 2009; Kmiecik et al., 2022).
The mechanisms underlying discomfort evoked by nonsomatosensory stimuli in TMD are unclear, despite reports of multisensory hypersensitivity (Greenspan et al., 2013; López‐Solà et al., 2017; Martenson et al., 2016; Phillips & Clauw, 2011; Schrepf et al., 2018; Ten Brink & Bultitude, 2022; Ten Brink et al., 2021). Differences in visual‐evoked functional connectivity (FC) and brain activation have been observed in other pain conditions (Harte et al., 2016; Cottam et al., 2018) and may explain the multisensory sensitivity in TMD (Shen et al., 2019). Visual stimulation is not an irritant for all TMD patients, but it is likely more unpleasant in a subset of individuals with nociplastic (as opposed to peripheral nociceptive) pain. Therefore, the goal of this pilot study was to identify differences in the brain networks of TMD patients, compared to pain‐free controls, during an unpleasant visual functional magnetic resonance imaging (fMRI) paradigm (i.e., a visual checkerboard stimulus) to uncover possible nociplastic pain‐associated mechanisms that would not be evoked by noxious stimulation of a painful site.
2. METHODS
2.1. Study design and subjects
This study was approved by the local Institutional Review Board. All subjects provided written informed consent prior to participation. Patients with painful and clinically diagnosed TMD were recruited from Oral and Maxillofacial Surgery/Hospital Dentistry. Pain‐free controls were recruited from the local community and were healthy without history of chronic pain. Exclusion criteria for both cohorts were severe physical impairments (e.g., bilateral amputation); medical conditions (e.g., autoimmune diseases, cancer); severe psychiatric illnesses; opioid, tobacco, or hormone use; or pregnancy. One subject was excluded due to challenges with data acquisition and poor image quality. The final cohort consisted of 10 subjects with TMD (n = 9 female), 18–49 years old (mean ± standard deviation [SD] = 32 ± 10 years), and 6 female pain‐free healthy controls (HC), 19–51 years old (31 ± 12 years). Subjects completed pain questionnaires followed by MRI acquired either the same or following day.
2.2. Clinical pain metrics and visual unpleasantness
The American College of Rheumatology's 2011 Preliminary Diagnostic Criteria for Fibromyalgia, which includes Symptom Severity and Widespread Pain Index (WPI) subscales, was administered (Wolfe et al., 2011), with higher scores (range: 0–31) (Wolfe et al., 2011) indicating likely nociplastic pain. The current TMD symptoms questionnaire from the TMD Research Diagnostic Criteria (RDC; 2002) was used to assess current face pain (Dworkin & LeResche, 1992). The short form of the Brief Pain Inventory (BPI) was used to assess pain severity and interference. The Pennebaker Inventory of Limbic Languidness (PILL) was used to measure somatic awareness or hypervigilance (Pennebaker, 1982). Immediately after the visual scan, participants were asked to rate the perceived unpleasantness of the visual stimulus on a scale from 0 to 100, where 0 means “not at all unpleasant” and 100 means “the most unpleasant sensation imaginable.” Mann–Whitney U tests were used to compare age, clinical pain metrics, and perceived visual unpleasantness between groups.
2.3. MR data acquisition
MR data were acquired on a 3T whole‐body MR scanner (Signa Discovery MR750, GE Healthcare, Chicago, IL) with a 32‐channel head coil. T1‐weighted images were acquired using a spoiled gradient recalled echo sequence (repetition time [TR] = 650 ms, echo time [TE] = 3.7 ms, flip angle = 8°, voxel size = 1.0 × 1.0 × 0.8 mm3, field of view [FOV] = 256 × 256 mm2, 166 slices). fMRI data were acquired using a multiband gradient echo pulse sequence (TR = 1200 ms, TE = 30 ms, flip angle = 70°, FOV = 210 × 210 mm2, 2.4 mm × 2.4 mm in‐plane resolution, 51–2.5 mm thick slices, multiband acceleration factor = 3). The visual paradigm included six cycles of 20 s “on” (8 Hz flashing checkerboard) and “off” (static crosshair) blocks (Figure 1). This same paradigm was used in Harte et al. (2016) to elicit unpleasantness and measure brain activity in fibromyalgia patients.
FIGURE 1.
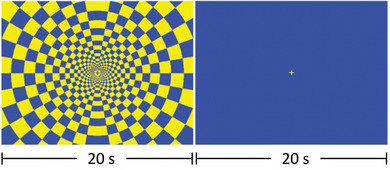
Visual paradigm used during fMRI. The visual task paradigm included six “on–off” cycles. The “on” block (left image) displayed an 8 Hz flashing checkered visual task for 20 s followed by an “off” block (right image) displaying a blue screen with a yellow crosshair in the center for 20 s.
2.4. MR data preprocessing
Functional data were preprocessed using SPM12 and employing well‐established pipelines (Anteraper et al., 2021; Glasser et al., 2013; Gopinath et al., 2015). The fMRI voxel time‐series data were temporally shifted to account for differences in slice acquisition times, and three‐dimensional volumes were registered to a base volume. These data were then corrected for physiological noise (respiratory and cardiac) with the well‐established RETROICOR (Glover et al., 2000) technique, and spatially normalized to the MNI‐152 template. Outlier identification was performed by flagging acquisitions with fMRI time‐series signal variation >5 SDs or frame‐to‐frame displacement >0.9 mm. First quartile, third quartile, and maximum motion across all subjects and scans were 0.17, 0.28, and 0.57 mm, respectively.
2.5. Group independent component analysis
The preprocessed fMRI data from all subjects were spatially smoothed with an isotropic Gaussian filter (full width at half maximum = 6 mm) for independent component analysis (ICA) analysis. Group ICA (GICA) was performed on temporally concatenated data from all subjects (TMD and HC) using the well‐established GIFT software (Calhoun et al., 2001). This ICA algorithm decomposes the concatenated fMRI time‐series data into group‐level independent components (ICs), which are composed of maps (component strength expressed as t‐scores), and their corresponding IC time‐courses. The ICA decomposition maximizes the independence between the spatial maps of different ICs, while not constraining the form of the time‐courses. Subject‐level ICs corresponding to the group ICs were obtained through the ICA back‐projection technique (Calhoun et al., 2001). The group‐level GICA spatial maps were visually examined for artifactual components representing draining veins, physiological noise, ventricular signal, or motion, using a well‐established approach (Allen et al., 2011; Cetin et al., 2014). The nonartifactual ICs constitute different brain functional networks. Voxel‐wise maps of group differences in FC to ICA‐derived brain functional networks were obtained with independent‐samples t‐tests on corresponding spatial maps. These group t‐test results were clustered, and the family‐wise error rate‐controlled significance (α) was computed for the given cluster detection threshold t‐score (p < .05) using Monte Carlo (MC) simulations of the process of image generation. Simulations were controlled for the estimated spatial autocorrelation function of regression residuals, intensity thresholding, masking, and cluster identification using the 3dClustSim program in AFNI (Cox, 2019; Gopinath et al., 2018).
3. RESULTS
Comparisons of clinical pain metrics and age are presented in Table 1. Current face pain, symptom severity, WPI, fibromyalgianess, PILL, and BPI were all significantly higher in the TMD cohort compared to HC (p ≤ .05). Despite perceived visual unpleasantness being somewhat higher in TMD patients (mean = 43.5) compared to HC (mean = 26.2), this difference was not statistically significant in our sample (Z(16) = −1.21, p = .23).
TABLE 1.
Group‐wise comparisons of clinical pain metrics between healthy pain‐free controls and subjects with temporomandibular disorders (TMD)
| Parameter a | Healthy controls (n = 6) | TMD subjects (n = 10) | U b | p c |
|---|---|---|---|---|
| Current face pain | 0.0 (0.0) a | 2.0 (1.3) | 6.0 | .005 |
| Symptom severity scale | 1.6 (1.1) | 5.2 (3.2) | 6.5 | .022 |
| Widespread Pain Index (WPI) | 0.5 (0.5) | 5.1 (3.9) | 1.5 | .002 |
| Fibromyalgianess | 2.2 (1.5) | 10.3 (6.9) | 2.0 | .005 |
| Pennebaker Inventory of Limbic Languidness (PILL) | 8.0 (5.2) | 16.1 (7.6) | 10.5 | .033 |
| Brief Pain Inventory (BPI) | 0.5 (1.2) | 4.8 (2.1) | 2.0 | .004 |
Values are reported as the mean (standard deviation).
Nonparametric Mann–Whitney U test statistic.
p‐values correspond to group‐wise comparisons.
Group ICA yielded 11 artifact‐free ICs including those belonging to the default mode network (DMN), left and right frontoparietal networks, frontostriatal network, and social cognition network (Table 2). The DMN IC exhibited significant (α < .05) abnormally increased FC to right ventrolateral and dorsolateral prefrontal cortices in subjects with TMD compared to HC (Figure 2; Table 3). The TMD cohort exhibited reduced DMN FC with left occipitotemporal/inferior temporal cortex (Table 3). Subjects with TMD showed reduced FC between left frontoparietal network (attention and executive function) and higher order visual processing regions, including the extrastriate body area and lateral occipital complex in the left hemisphere. Subjects with TMD also exhibited abnormally increased FC between the frontostriatal network and cerebellum, and decreased FC between frontostriatal network and supplementary motor area compared to HC. Additionally, the social cognition network exhibited abnormally increased FC to inferior frontal and middle frontal cortices in subjects with TMD compared to HC.
TABLE 2.
List of brain functional networks activated
| Default mode |
| Higher order visual processing |
| Right frontoparietal |
| Left frontoparietal |
| Somatosensory and pain processing |
| Sensorimotor |
| Frontostriatal |
| Visuospatial attention |
| Anterior visual |
| Posterior visual |
| Social cognition |
FIGURE 2.
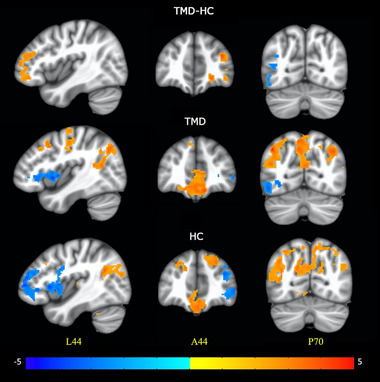
(Top) Significant (cluster‐level family‐wise error rate α < .05) differences in functional connectivity to the default mode network during the entire visual fMRI paradigm between subjects with temporomandibular disorders (TMD) and healthy control (HC) groups. One‐sample t‐test results for (middle) TMD and (bottom) HC groups. The color bar represents t‐test z‐scores. Slice locations are in MNI coordinates (L = left; A = anterior; P = posterior).
TABLE 3.
Areas exhibiting significant differences in functional connectivity (family‐wise error rate [FWER]‐corrected α < .05) to specific resting state networks (group independent components) during the visual functional magnetic resonance imaging paradigm between subjects with temporomandibular disorders (TMD) and healthy controls (HC)
| TMD vs. HC | Regions | Cluster peak z‐statistic | Cluster size (2 × 2 × 2 mm3 voxels) | Cluster detection threshold p‐values | Cluster‐level FWE rate α |
|---|---|---|---|---|---|
| Default mode network | |||||
| TMD > HC | Right hemisphere: dorsolateral and ventrolateral prefrontal cortex | 3.86 | 4688 | <.05 | <.05 |
| TMD < HC | Left occipitotemporal cortex | –3.26 | 4296 | <.05 | <.05 |
| Left frontoparietal network | |||||
| TMD > HC | Right posterior parietal cortex | 4.21 | 6532 | <.05 | <.05 |
| TMD < HC | Left extrastriate body area and lateral occipital complex | –4.22 | 5048 | <.05 | <.05 |
| Cognitive control network | |||||
| TMD > HC | Anterior cerebellar vermis | 3.83 | 3776 | <.05 | <.05 |
| TMD < HC | Supplementary motor area | –4.18 | 3928 | <.05 | <.05 |
| Social cognition | |||||
| TMD > HC | Right inferior frontal and middle frontal gyrus | 4.458 | 7632 | <.05 | <.05 |
Note: Regions were identified using brain atlases in AFNI. Cluster‐level α are obtained with the assumption the spatial autocorrelation function is Gaussian (see Section 2).
4. DISCUSSION
Sensory hypersensitivity is emerging as a key feature of nociplastic pain, which may drive clinical pain in many chronic overlapping pain conditions (Fitzcharles et al., 2021; Maixner et al., 2016). In this study, group ICA analysis of an unpleasant visual stimulus task during an fMRI paradigm revealed increased DMN FC to areas involved in attention, including dorsolateral and ventrolateral prefrontal cortices in TMD patients, possibly indicating increased attention to stimuli in TMD, consistent with prior work in chronic pain (Tracey & Bushnell, 2009; Villemure & Bushnell, 2009). Stronger activation of DMN regions associated with attention and salience was previously observed during a Stroop task in TMD compared to controls (Weissman‐Fogel et al., 2011). The DMN is typically suppressed during attention and executive functions, supporting the hypothesis that DMN‐associated dysfunction may relate to pain modulation. Indeed, DMN alterations have been associated with both chronic pain and pain‐evoked activity in healthy individuals (Alshelh et al., 2018). Resting‐state DMN alterations have been reported following gnathological treatment in TMD, suggesting that DMN‐associated changes may span rest, task‐evoked activation, and treatment response (Festa et al., 2021). The prefrontal cortex (PFC), in particular, is involved in executive function. Changes in grey matter, brain metabolites, and FC in the PFC have been reported in subjects with TMD as well as chronic back pain, myofascial pain, and fibromyalgia (Domin et al., 2021; Kucyi et al., 2014; Ong et al., 2019; Yin et al., 2020). Furthermore, transcranial direct current stimulation and repetitive transcranial magnetic stimulation targeting the dorsolateral PFC have shown some promise (Ong et al., 2019).
We also observed reduced FC in TMD compared to HC between the frontoparietal attention/executive function network and higher order visual processing areas, and between the cognitive control network and supplementary motor area. Motor dysfunction has been observed in TMD, including increased motor cortex activation (Weissman‐Fogel et al., 2011). Alterations in executive function and attention may be the result of pain hypervigilance or rumination (Broadbent et al., 2021; Michael & Burns, 2004). A recent review presents evidence for attentional bias to somatosensory stimuli in individuals with chronic pain (Broadbent et al., 2021), and pain rumination has been positively associated with FC in the medial PFC and other DMN regions in TMD (Kucyi et al., 2014). While more work is warranted, a growing body of literature suggests chronic pain is associated with widespread brain changes that may affect multisensory processing, attention, and executive function.
As a pilot study, we acknowledge limitations of sample size and primarily female participants. Future studies will expand these initial results to larger cohorts, additional sensory stimuli, and structural and metabolic changes to evaluate the central mechanisms associated with multisensory hypersensitivity in TMD. Larger studies will also enable the use of additional correlations with factors that could affect FC including clinical pain intensity and duration of TMD. We expect these and similar results from brain neuroimaging studies may be used to help differentiate pain mechanisms in TMD toward more personalized and tailored treatment.
CONFLICT OF INTEREST STATEMENT
Dr. Harte has received research support from Aptinyx and Arbor Medical Innovations, and consultation fees from Aptinyx, Memorial Slone Kettering Cancer Center, Indiana University, and University of North Carolina—Chapel Hill, not related to the present work.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/brb3.2916.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (NIH K99DE026810), Georgia Institute of Technology Computational Biology GRA, and the Emory University Department of Radiology and Imaging Sciences.
Harper, D. E. , Gopinath, K. , Smith, J. L. , Gregory, M. , Ichesco, E. , Aronovich, S. , Harris, R. E. , Harte, S. E. , Clauw, D. J. , & Fleischer, C. C. (2023). Characterization of visual processing in temporomandibular disorders using functional magnetic resonance imaging. Brain and Behavior, 13, e2916. 10.1002/brb3.2916
DATA AVAILABILITY STATEMENT
Data will be made available upon request after a data sharing agreement is executed.
REFERENCES
- Allen, E. A. , Erhardt, E. B. , Damaraju, E. , Gruner, W. , Segall, J. M. , Silva, R. F. , Havlicek, M. , Rachakonda, S. , Fries, J. , Kalyanam, R. , Michael, A. M. , Caprihan, A. , Turner, J. A. , Eichele, T. , Adelsheim, S. , Bryan, A. D. , Bustillo, J. , Clark, V. P. , Feldstein Ewing, S. W. , … Calhoun, V. D. (2011). A baseline for the multivariate comparison of resting‐state networks. Frontiers in Systems Neuroscience, 5, 2. 10.3389/fnsys.2011.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshelh, Z. , Marciszewski, K. K. , Akhter, R. , Di Pietro, F. , Mills, E. P. , Vickers, E. R. , Peck, C. C. , Murray, G. M. , & Henderson, L. A. (2018). Disruption of default mode network dynamics in acute and chronic pain states. NeuroImage: Clinical, 17, 222–231. 10.1016/j.nicl.2017.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anteraper, S. A. , Gopinath, K. , Hoch, M. J. , Waldrop‐Valverde, D. , Franklin, D. , Letendre, S. L. , Whitfield‐Gabrieli, S. , & Anderson, A. M. (2021). A comprehensive data‐driven analysis framework for detecting impairments in brain function networks with resting state fMRI in HIV‐infected individuals on cART. Journal of Neurovirology, 27, 239–248. 10.1007/s13365-021-00943-7 [DOI] [PubMed] [Google Scholar]
- Broadbent, P. , Liossi, C. , & Schoth, D. E. (2021). Attentional bias to somatosensory stimuli in chronic pain patients: A systematic review and meta‐analysis. Pain, 162, 332–352. 10.1097/j.pain.0000000000002040 [DOI] [PubMed] [Google Scholar]
- Calhoun, V. D. , Adali, T. , Pearlson, G. D. , & Pekar, J. J. (2001). A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapping, 14, 140–151. 10.1002/hbm.1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çetin, M. S. , Christensen, F. , Abbott, C. C. , Stephen, J. M. , Mayer, A. R. , Cañive, J. M. , Bustillo, J. R. , Pearlson, G. D. , & Calhoun, V. D. (2014). Thalamus and posterior temporal lobe show greater inter‐network connectivity at rest and across sensory paradigms in schizophrenia. Neuroimage, 97, 117–126. 10.1016/j.neuroimage.2014.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottam, W. J. , Iwabuchi, S. J. , Drabek, M. M. , Reckziegel, D. , & Auer, D. P. (2018). Altered connectivity of the right anterior insula drives the pain connectome changes in chronic knee osteoarthritis. Pain, 159, 929–938. 10.1097/j.pain.0000000000001209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, R. W. (2019). Equitable thresholding and clustering: A novel method for functional magnetic resonance imaging clustering in AFNI. Brain Connect, 9, 529–538. 10.1089/brain.2019.0666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domin, M. , Grimm, N. K. , Klepzig, K. , Schmidt, C. O. , Kordass, B. , & Lotze, M. (2021). Gray matter brain alterations in temporomandibular disorder tested in a population cohort and three clinical samples. The Journal of Pain, 22, 739–747. 10.1016/j.jpain.2021.01.003 [DOI] [PubMed] [Google Scholar]
- Dworkin, S. F. , & LeResche, L. (1992). Research diagnostic criteria for temporomandibular disorders: Review, criteria, examinations and specifications, critique. Journal of Craniomandibular Disorders, 6, 301–355. [PubMed] [Google Scholar]
- Eller‐Smith, O. C. , Nicol, A. L. , & Christianson, J. A. (2018). Potential mechanisms underlying centralized pain and emerging therapeutic interventions. Frontiers in Cellular Neuroscience, 12, 35. 10.3389/fncel.2018.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa, F. , Rotelli, C. , Scarano, A. , Navarra, R. , Caulo, M. , & Macrì, M. (2021). Functional magnetic resonance connectivity in patients with temporomadibular joint disorders. Frontiers in Neurology, 12, 629211. 10.3389/fneur.2021.629211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzcharles, M.‐A. , Cohen, S. P. , Clauw, D. J. , Littlejohn, G. , Usui, C. , & Häuser, W. (2021). Nociplastic pain: Towards an understanding of prevalent pain conditions. Lancet, 397, 2098–2110. 10.1016/S0140-6736(21)00392-5 [DOI] [PubMed] [Google Scholar]
- Geisser, M. E. , Glass, J. M. , Rajcevska, L. D. , Clauw, D. J. , Williams, D. A. , Kileny, P. R. , & Gracely, R. H. (2008). A psychophysical study of auditory and pressure sensitivity in patients with fibromyalgia and healthy controls. The Journal of Pain, 9, 417–422. 10.1016/j.jpain.2007.12.006 [DOI] [PubMed] [Google Scholar]
- Glasser, M. F. , Sotiropoulos, S. N. , Wilson, J. A. , Coalson, T. S. , Fischl, B. , Andersson, J. L. , Xu, J. , Jbabdi, S. , Webster, M. , Polimeni, J. R. , Van Essen, D. C. , & Jenkinson, M. (2013). The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage, 80, 105–124. 10.1016/j.neuroimage.2013.04.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover, G. H. , Li, T.‐Q. , & Ress, D. (2000). Image‐based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magnetic Resonance in Medicine, 44, 162–167. [DOI] [PubMed] [Google Scholar]
- Gopinath, K. , Krishnamurthy, V. , Cabanban, R. , & Crosson, B. A. (2015). Hubs of anticorrelation in high‐resolution resting‐state functional connectivity network architecture. Brain Connect, 5, 267–275. 10.1089/brain.2014.0323 [DOI] [PubMed] [Google Scholar]
- Gopinath, K. , Krishnamurthy, V. , & Sathian, K. (2018). Accounting for non‐gaussian sources of spatial correlation in parametric functional magnetic resonance imaging paradigms I: Revisiting cluster‐based inferences. Brain Connect, 8, 1–9. 10.1089/brain.2017.0521 [DOI] [PubMed] [Google Scholar]
- Greenspan, J. D. , Slade, G. D. , Bair, E. , Dubner, R. , Fillingim, R. B. , Ohrbach, R. , Knott, C. , Diatchenko, L. , Liu, Q. , & Maixner, W. (2013). Pain sensitivity and autonomic factors associated with development of TMD: The OPPERA prospective cohort study. The Journal of Pain, 14, T63.e6–T74.e6. 10.1016/j.jpain.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harfeldt, K. , Alexander, L. , Lam, J. , Månsson, S. , Westergren, H. , Svensson, P. , Sundgren, P. C. , & Alstergren, P. (2018). Spectroscopic differences in posterior insula in patients with chronic temporomandibular pain. Scandinavian Journal of Pain, 18, 351–361. 10.1515/sjpain-2017-0159 [DOI] [PubMed] [Google Scholar]
- Harper, D. E. , Schrepf, A. , & Clauw, D. J. (2016). Pain mechanisms and centralized pain in temporomandibular disorders. Journal of Dental Research, 95, 1102–1108. 10.1177/0022034516657070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte, S. E. , Ichesco, E. , Hampson, J. P. , Peltier, S. J. , Schmidt‐Wilcke, T. , Clauw, D. J. , & Harris, R. E. (2016). Pharmacologic attenuation of cross‐modal sensory augmentation within the chronic pain insula. Pain, 157, 1933–1945. 10.1097/j.pain.0000000000000593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollins, M. , Harper, D. , Gallagher, S. , Owings, E. W. , Lim, P. F. , Miller, V. , Siddiqi, M. Q. , & Maixner, W. (2009). Perceived intensity and unpleasantness of cutaneous and auditory stimuli: An evaluation of the generalized hypervigilance hypothesis. Pain, 141, 215–221. 10.1016/j.pain.2008.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmiecik, M. J. , Tu, F. F. , Silton, R. L. , Dillane, K. E. , Roth, G. E. , Harte, S. E. , & Hellman, K. M. (2022). Cortical mechanisms of visual hypersensitivity in women at risk for chronic pelvic pain. Pain, 163(6), 1035–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi, A. , Moayedi, M. , Weissman‐Fogel, I. , Goldberg, M. B. , Freeman, B. V. , Tenenbaum, H. C. , & Davis, K. D. (2014). Enhanced medial prefrontal‐default mode network functional connectivity in chronic pain and its association with pain rumination. Journal of Neuroscience, 34, 3969–3975. 10.1523/JNEUROSCI.5055-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López‐Solà, M. , Woo, C.‐W. , Pujol, J. , Deus, J. , Harrison, B. J. , Monfort, J. , & Wager, T. D. (2017). Towards a neurophysiological signature for fibromyalgia. Pain, 158, 34–47. 10.1097/j.pain.0000000000000707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maixner, W. , Fillingim, R. B. , Williams, D. A. , Smith, S. B. , & Slade, G. D. (2016). Overlapping chronic pain conditions: Implications for diagnosis and classification. The Journal of Pain, 17, T93–T107. 10.1016/j.jpain.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredini, D. , Guarda‐Nardini, L. , Winocur, E. , Piccotti, F. , Ahlberg, J. , & Lobbezoo, F. (2011). Research diagnostic criteria for temporomandibular disorders: A systematic review of axis I epidemiologic findings. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics, 112, 453–462. 10.1016/j.tripleo.2011.04.021 [DOI] [PubMed] [Google Scholar]
- Martenson, M. E. , Halawa, O. I. , Tonsfeldt, K. J. , Maxwell, C. A. , Hammack, N. , Mist, S. D. , Pennesi, M. E. , Bennett, R. M. , Mauer, K. M. , Jones, K. D. , & Heinricher, M. M. (2016). A possible neural mechanism for photosensitivity in chronic pain. Pain, 157, 868–878. 10.1097/j.pain.0000000000000450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael, E. S. , & Burns, J. W. (2004). Catastrophizing and pain sensitivity among chronic pain patients: Moderating effects of sensory and affect focus. Annals of Behavioral Medicine, 27, 185–194. 10.1207/s15324796abm2703_6 [DOI] [PubMed] [Google Scholar]
- Ong, W.‐Y. , Stohler, C. S. , & Herr, D. R. (2019). Role of the prefrontal cortex in pain processing. Molecular Neurobiology, 56, 1137–1166. 10.1007/s12035-018-1130-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennebaker, J. E. (1982). The psychology of physical symptoms. Springer‐Verlag. [Google Scholar]
- Phillips, K. , & Clauw, D. J. (2011). Central pain mechanisms in chronic pain states – Maybe it is all in their head. Best Practice & Research. Clinical Rheumatology, 25, 141–154. 10.1016/j.berh.2011.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz, J. (2014). Mechanisms of chronic pain. Mol Molecular Pain, 10, O15. 10.1186/1744-8069-10-S1-O15 [DOI] [Google Scholar]
- Schrepf, A. , Williams, D. A. , Gallop, R. , Naliboff, B. D. , Basu, N. , Kaplan, C. , Harper, D. E. , Landis, J. R. , Clemens, J. Q. , Strachan, E. , Griffith, J. W. , Afari, N. , Hassett, A. , Pontari, M. A. , Clauw, D. J. , & Harte, S. E. (2018). Sensory sensitivity and symptom severity represent unique dimensions of chronic pain: A MAPP Research Network study. Pain, 159, 2002–2011. 10.1097/j.pain.0000000000001299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, W. , Tu, Y. , Gollub, R. L. , Ortiz, A. , Napadow, V. , Yu, S. , Wilson, G. , Park, J. , Lang, C. , Jung, M. , Gerber, J. , Mawla, I. , Chan, S. ‐T. , Wasan, A. D. , Edwards, R. R. , Kaptchuk, T. , Li, S. , Rosen, B. , & Kong, J. (2019). Visual network alterations in brain functional connectivity in chronic low back pain: A resting state functional connectivity and machine learning study. NeuroImage: Clinical, 22, 101775. 10.1016/j.nicl.2019.101775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Brink, A. F. , & Bultitude, J. H. (2022). Visual sensitivity in complex regional pain syndrome and fibromyalgia: An online study. Perception, 51(3), 187–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Brink, A. F. , Proulx, M. J. , & Bultitude, J. H. (2021). Validation of the Leiden Visual Sensitivity Scale and Visual Discomfort Scale in chronic pain conditions. Perception, 50, 399–417. 10.1177/03010066211005327 [DOI] [PubMed] [Google Scholar]
- Tracey, I. , & Bushnell, M. C. (2009). How neuroimaging studies have challenged us to rethink: Is chronic pain a disease? The Journal of Pain, 10, 1113–1120. 10.1016/j.jpain.2009.09.001 [DOI] [PubMed] [Google Scholar]
- Villemure, C. , & Bushnell, M. C. (2009). Mood influences supraspinal pain processing separately from attention. Journal of Neuroscience, 29, 705–715. 10.1523/JNEUROSCI.3822-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman‐Fogel, I. , Moayedi, M. , Tenenbaum, H. C. , Goldberg, M. B. , Freeman, B. V. , & Davis, K. D. (2011). Abnormal cortical activity in patients with temporomandibular disorder evoked by cognitive and emotional tasks. Pain, 152, 384–396. 10.1016/j.pain.2010.10.046 [DOI] [PubMed] [Google Scholar]
- Wolfe, F. , Clauw, D. J. , Fitzcharles, M.‐A. , Goldenberg, D. L. , Häuser, W. , Katz, R. S. , Mease, P. , Russell, A. S. , Russell, I. J. , & Winfield, J. B. (2011). Fibromyalgia criteria and severity scales for clinical and epidemiological studies: A modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. Journal of Rheumatology, 38, 1113–1122. 10.3899/jrheum.100594 [DOI] [PubMed] [Google Scholar]
- Yin, Y. , He, S. , Xu, J. , You, W. , Li, Q. , Long, J. , Luo, L. , Kemp, G. J. , Sweeney, J. A. , Li, F. , Chen, S. , & Gong, Q. (2020). The neuro‐pathophysiology of temporomandibular disorders‐related pain: A systematic review of structural and functional MRI studies. The Journal of Headache and Pain, 21, 78. 10.1186/s10194-020-01131-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon request after a data sharing agreement is executed.


