Abstract
In this multicenter retrospective study, we studied the impact of the concurrent use of renin-angiotensin system inhibitors (RASi) on the outcomes of 229 metastatic renal cell carcinoma (mRCC) patients treated with immune-checkpoint inhibitors (ICI). The findings suggest that RASi could be repurposed to enhance outcomes with ICI in patients with mRCC, which may have a large global impact given their cost-efficacy.
Background:
Renin-angiotensin system inhibitors (RASi) have been shown to improve outcomes in studies of multiple malignancies by effects on the tumor microenvironment to enhance the immune repertoire and improve drug delivery. Repurposing RASi to treat metastatic renal cell carcinoma (mRCC) in combination with immune-checkpoint inhibitors (ICI) may improve survival coupled with tolerability and cost efficacy. We evaluated the impact of RASi on outcomes in mRCC patients receiving ICI.
Methods:
This multicenter, retrospective cohort study included mRCC patients treated with ICI with or without RASi. The patients from Dana-Farber Cancer Institute (DFCI) were used as a discovery cohort, and the patients from University of California San Diego (UCSD) were used for validation. Receipt of an ICI (PD1/L1 and/or CTLA-4 inhibitors) was required. RASi use was defined as receipt of a RASi at baseline and for a minimum of 30 days after ICI initiation. For both the discovery and validation cohorts, the primary outcome assessed was overall survival (OS) and the secondary endpoints were time-to-treatment failure (TTF), and objective response rate (ORR).
Results:
Overall, 229 patients who received an ICI were included: 100 patients from DFCI and 129 patients from UCSD. Concomitant RASi were administered in 30 patients (30%) in the DFCI cohort and 59 (45%) in the UCSD cohort. Median age at ICI initiation was 62.5 years in both cohorts. Median follow-up was 3.8 [IQR 3-5.3] years in the DFCI cohort, and 2.3 [IQR 1.4-3.6] years in the UCSD cohort. In the DFCI cohort, RASi was significantly associated with longer OS (adjusted-HR 0.35 [95% CI, 0.17-0.70], P = .003) and TTF (adjusted-HR 0.57 [0.36-0.92], P = .02). In the validation cohort, RASi was associated with TTF (adjusted HR, 0.60 [0.39-0.92], P = .02) and not statistically associated with OS (adjusted-HR 0.60 [0.34-1.06], P = .07). The propensity analysis, matching 83 patients from both cohorts receiving RASi while on ICI with 83 who did not, showed that RASi significantly improved OS (HR 0.59 [0.37-0.95], P = .03) and TTF (HR 0.60 [0.43-0.85], P = .0034).
Conclusions:
RASi was associated with improved OS and TTF in mRCC patients receiving ICI. This provides a rationale for prospective randomized studies combining ICI and RASi in mRCC patients.
Keywords: Angiotensin, Angiotensin receptor blockers, Angiotensin converting enzyme, Immunotherapy, PD1/L1 inhibitors, Kidnay cancer
Introduction
Immune-checkpoint inhibitors (ICI) have revolutionized the therapeutic landscape in patients with metastatic renal cell carcinoma (mRCC).1 However, the majority of patients do not respond. ICIs combined with VEGF inhibitors improve outcomes; however, therapy is associated with toxicities and cost.
The renin-angiotensin system (RAS) has been implicated in promoting tumor growth and progression in various cancers.2 RAS inhibitors (RASi), such as angiotensin-converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARB), are widely prescribed to treat of hypertension and cardiovascular disease. Interestingly, angiotensin inhibition has been demonstrated to activate the immune system and improve drug delivery due partly to the downregulation of transforming growth factor (TGF)-β, modification of the tumor microenvironment, resultant alleviation of hypoxia and increased perfusion of the tumor.3-7
The use of RASi has been associated with improved outcomes across malignancies.8-10 The addition of ARB to ICI improved ICI efficacy in mice with breast cancer by promoting T lymphocyte activity. However, limited studies have investigated the role of RASi in cancer patients treated with ICI and no data are available of the combination in mRCC.5,11,12
We hypothesized that concomitant RASi is associated with improved clinical outcomes in patients with mRCC receiving ICI and conducted a retrospective study to identify an association.
Materials and methods
Patients and data
We conducted a multicenter, retrospective cohort analysis of patients with mRCC treated with PD-1/L1 or CTLA-4 inhibitors, as monotherapy or in combination. Patients from Dana Farber Cancer Institute (DFCI) treated from 2015 to 2019 served as a discovery cohort. Patients from University of California San Diego (UCSD) treated between 2016 and 2019 served as a validation cohort.
Patients who received ≥2 doses of ICI (or combination of ICIs) were included. Patients with missing concomitant medication information or treated with ICI in combination with VEGF-targeted therapy or other non-ICI therapy were excluded. Baseline demographic, clinical and RASi use data were collected. RASi included ACEis and ARB. Patients within the RASi group were treated with a RASi at baseline and for a minimum of 30 days after ICI treatment initiation, for patients alive at day 30. The decision to start and the choice of RASi was at the discretion of the treating physician. This study was approved by the Institutional Review Board.
Study outcomes and statistical analysis
For both the discovery and validation cohorts, the primary outcome assessed was overall survival (OS) and the secondary endpoints were time-to-treatment failure (TTF), and objective response rate (ORR). For OS, patients who were alive were censored at the date of last follow-up. TTF was calculated from the start to the last date of ICI therapy or death. Patients who were alive and still receiving ICI were censored at the date of last follow-up. Response was assessed per Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 by investigators.
The distributions of OS and TTF were estimated with the Kaplan-Meier method along with 95% confidence intervals (95% CI), and their associations with RASi treatment, baseline hypertensive disease, beta-blocker treatment and any non-RASi antihypertensive were examined using a Cox proportional hazards model with histology (clear cell vs non-clear cell), IMDC category, number of prior lines of therapy and type of ICI treatment (single vs. combination) chosen as covariates; log-rank p-values and adjusted hazard ratios were reported. Association with response was assessed using a multivariable logistic regression model. All tests were two-tailed; statistical significance was defined as P < .05.
Propensity score matching was performed in the DFCI and UCSD cohorts using the R package “Matchlt”. Propensity scores were calculated using demographics (age at ICI start and sex), disease characteristics (clear-cell vs. non-clear cell histology, IMDC risk category) and treatment characteristics (number of prior lines of therapy, single vs. combination ICI treatment) and institution. Standardized differences-in-means were computed and plotted pre- and post-matching using the R package “cobalt”. Associations between RASi treatment and OS, TTF and ORR were assessed in the matched cohorts.
Results
Patients characteristics and treatment exposure
For the discovery cohort, 100 patients from DFCI met the inclusion criteria, of whom 30 (30%) received concomitant RASi (Table 1). The UCSD validation dataset included 129 patients, of whom 59 (45%) received concomitant RASi. Median age at treatment initiation was 62.5 years in both cohorts. Female patients constituted 30.0% (30/100) and 28.5% (38/129) of DFCI and UCSD cohorts, respectively. Most patients had clear cell histology (DFCI:89%; UCSD:76%). Median follow-up time was 3.8 [IQR 3-5.3] years in the DFCI cohort, and 2.3 [IQR 1.4-3.6] years in the UCSD cohort.
Table 1.
Summary of baseline characteristics of 229 mRCC patients treated with ICI with or without RASi.
| DFCI | UCSD | |||
|---|---|---|---|---|
| N (median) | % (range) | N (median) | % (range) | |
| Age at ICI start | 63 | 37-85 | 63 | 23-89 |
| Gender | ||||
| Female | 69 | 69.0 | 91 | 70.5 |
| Male | 31 | 31.0 | 38 | 29.5 |
| IMDC risk score | ||||
| Poor | 14 | 14.0 | 32 | 24.8 |
| Intermediate | 59 | 59.0 | 68 | 52.7 |
| Good | 24 | 24.0 | 29 | 22.5 |
| NA | 3 | 3.0 | 0 | 0.0 |
| Histology | ||||
| Clear cell | 89 | 89.0 | 84 | 65.1 |
| Non-clear cell | 11 | 11.0 | 45 | 34.9 |
| ICI type | ||||
| Single | 91 | 91.0 | 106 | 82.1 |
| Combination | 9 | 9.0 | 23 | 17.9 |
| Number of prior lines | ||||
| 1 | 15 | 15.0 | 51 | 39.5 |
| 2 | 46 | 46.0 | 50 | 38.8 |
| ≥3 | 39 | 39.0 | 28 | 21.7 |
| ACEi/ARB treatment | ||||
| ACEi | 23 | 23.0 | 36 | 27.9 |
| ARB | 7 | 7.0 | 24 | 18.6 |
| Neither | 70 | 70.0 | 69 | 53.5 |
Abbreviations: ACEi = angiotensin-converting enzyme inhibitors; ARB = angiotensin receptor blocker; DFCI = Dana-Farber Cancer Institute; ICI = immune-checkpoint inhibitors; UCSD = University of California San Diego.
Impact of RASi on clinical outcomes
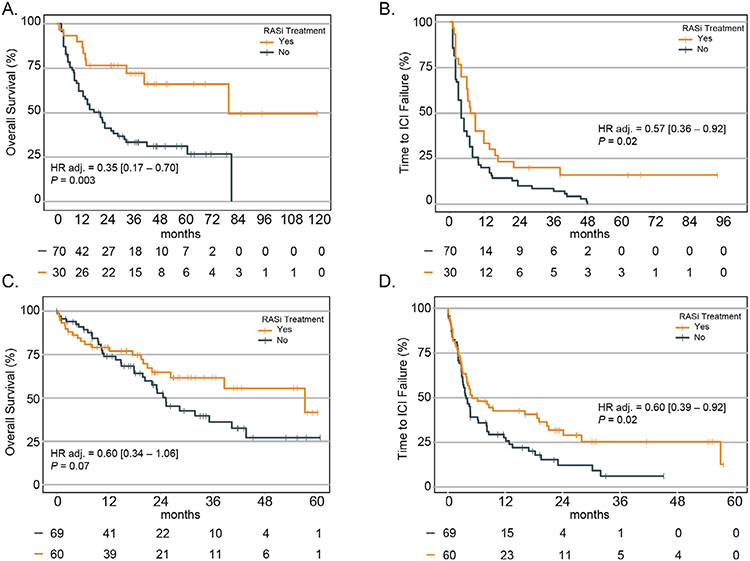
In the discovery cohort, RASi was significantly associated with longer OS (adjusted-HR 0.35 [95% CI, 0.17-0.70], P = .003; Figure 1A) and TTF (adjusted-HR 0.57 [0.36-0.92], P = .02; Figure 1B). In the validation cohort, RASi was associated with TTF (adjusted HR 0.60 [0.39-0.92], P = .02; Figure 1C) and was not statistically associated with OS (adjusted-HR 0.60 [0.34-1.06], P = .07; Figure 1D). RASi was not associated with ORR in either the DFCI (P = .15), or the UCSD (P = .95) cohorts. In addition, we did not find any association between hypertension at first dose of ICI and either OS (pDFCI = 0.68, pUCSD = 0 .75; Supplementary Table S3) or TTF (pDFCI = 0.60, pUCSD = 0.89; Supplementary Table S3). Similar findings were seen in patients with mRCC treated with beta-blockers (OS pDFCI = 0.49, pUCSD=0.18; TTF PDFCI = 0.68, pUCSD=0.11; Supplementary Table S4) or patients treated with any non-RASi antihypertensive (OS pDFCI = 0.28, PUCSD=0.29; TTF pDFCI = 0.46, pUCSD = 0.50; Supplementary Table S5).
Figure 1.
Survival and time-to-treatment failure in mRCC treated with ICI with or without RASi. (A) OS in the DFCI cohort. (B) TTF in the DFCI cohort. (C) OS in the UCSD cohort. (D) TTF in the UCSD cohort.
Propensity analysis
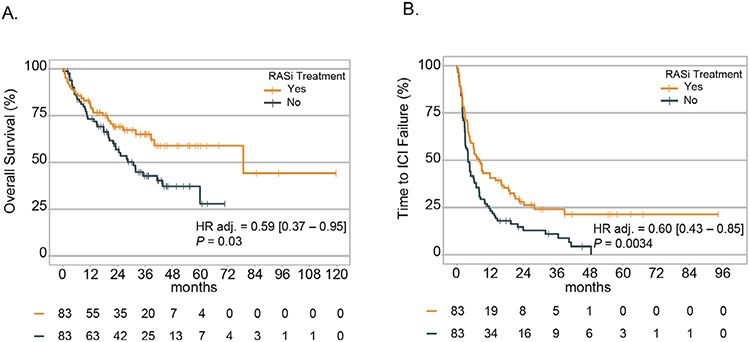
Eighty-three patients from both DFCI and UCSD who received RASi while on ICI were matched to 83 patients who had not received RASi (Supplementary Table S2; Supplementary Figs. S1 and S2). Patients on RASi had significantly longer OS (HR 0.59 [0.37-0.95], P = .03; Figure 2A) and TTF (HR 0.60 [0.43-0.85], P = .0034; Figure 2B), but no significant difference in ORR (22.8% vs. 13.3% P = .11).
Figure 2.
Overall survival (A) and time-to-treatment failure (B) in 166 propensity-matched mRCC patients.
Discussion
In this multicenter retrospective study, we assessed the association between RASi administered concomitantly with ICI and OS and TTF benefits in mRCC patients. A statistically significant association between RASi and OS was observed only in the discovery cohort, potentially due to modest sample size and shorter median follow-up. However, evidence from both cohorts and the propensity analysis supports an incremental benefit for RASi in combination with ICI.
Recently, there has been growing interest in drug repurposing for cancer therapy. RASi are widely used medications and are known for their favorable toxicity profile. Their efficacy in cancer treatment has been investigated and proven in preclinical models.9,13 Their effect on cancer-associated fibroblasts leads to remodeling of the immunosuppressive microenvironment. The angiotensin-II/angiotensin receptor axis regulates the activity of immunomodulatory cytokines (TGF-β, IL1-β, etc.) promoting the differentiation and recruitment of immunostimulatory CD8+ T-cells and NK cells.4 Perhaps more directly, RASi improve microvascular tumor perfusion by “relaxing” the ECM, which translates into efficacious drug delivery to tumor cells as well as improved ICI efficacy in mice with breast cancer.5,9
The additive effect of RASi on systemic treatment in cancer patients has been investigated in retrospective clinical studies.14 In mRCC, RASi improved survival in mRCC patients treated with VEGF inhibitors.15 In pancreatic cancer, losartan doubled R0 resection rate when combined with FOLFIRINOX and radiation therapy in a phase II trial.16 Based on these promising data, a randomized Phase II study in locally advanced pancreatic cancers (NCT03563248) is evaluating the efficacy of losartan, or the combination of losartan and nivolumab combined with chemo-radiotherapy followed by definitive local therapy. One retrospective study in non-small-cell lung carcinoma showed improved outcomes in those receiving PD-1/PD-L1 inhibitors combined with RASi.11,12
Limitations
The main limitation of this study is its retrospective nature and modest sample size, although we employed a multivariable analysis followed by propensity matching to overcomes confounding factors. Second, the patients included in the analysis are from 2 tertiary care centers. Although our analysis identified associations with OS and TTF, a statistically significant association with ORR was not observed, possibly because benefit from concurrent RASi is not captured by measuring tumor regressions. Third, we did not examine a differential impact of ACEi vs. ARB given the modest sample size. Fourth, tracking patient compliance to RASi treatment is suboptimal through retrospective chart review. Furthermore, given the reported benefit of RASi in VEGF-treated mRCC,15 it is challenging to claim a specific context associated benefit from RASi in the ICI setting. Also, we acknowledge that possible imbalance in subsequent receipt of VEGF TKI therapy might contribute to differences in OS, however receipt of VEGF TKIs post ICI will not impact ICI TTF. Finally, these findings cannot be extrapolated to patients receiving a combination of ICI and other targeted agents such as VEGF TKIs.
Conclusions
RASi administration in mRCC patients receiving ICI was associated with improved OS and TTF. Given the safety, low cost and global availability of RASi, prospective randomized studies may be justified to validate these findings and repurpose these agents in this context.
Supplementary Material
Clinical practice points.
The renin–angiotensin–aldosterone system regulates proliferation and angiogenesis, and the inhibition of angiotensin can improve drug delivery due to the downstream downregulation of transforming growth factor β.
Our retrospective multicenter study suggests that concurrent angiotensin blockade with ACEIs) or angiotensin receptor blockers (ARBs is associated with tumor regression and potentially improved survival in patients with mRCC receiving PD1/L1 inhibitors.
Further prospective validation of our data in a clinical trial examining this combination in mRCC may be justified to repurpose ACEIs or ARBs as anticancer therapy, especially in the context of these widely available, safe, and cost-effective agents.
Acknowledgments
We thank our patients for their contributions to research.
No funding or reagent from these organizations was used in this study. Rakesh Jain’s research is supported by NIH grants R35-CA197743, U01 -CA224348, R01-CA259253, R01-CA208205, R01-NS118929, National Foundation for Cancer Research, Ludwig Cancer Center at Harvard; Advanced Medical Research Foundation and Jane’s Trust Foundation
Abbreviations:
- ACEi
angiotensin-converting enzyme inhibitors
- aHTN
antihypertensive
- ARB
angiotensin receptor blockers
- CI
confidence interval
- CTLA-4
cytotoxic T-lymphocyte-associated antigen 4
- DFCI
Dana Farber Cancer institute
- ECM
extracellular matrix
- ECOG PS
Eastern Cooperative Oncology Group Performance Status
- HR
hazard ratio
- HTN
hypertension
- ICI
immune check-point inhibitors
- IMDC
international Metastatic RCC Database Consortium
- IQR
interquartile range
- MCC
Moores Cancer Center
- mRCC
metastatic renal cell carcinoma
- OR
odds ratio
- ORR
overall response rate
- OS
overall survival
- PD-1
programmed cell death protein 1
- PD-L1
programmed cell death ligand 1
- RAS
renin-angiotensin system
- RASi
renin-angiotensin system inhibitors
- TTF
time-to-treatment failure
- UCSD
University of California San Diego
Footnotes
Disclosures
Ziad Bakouny: Research support from Bristol-Myers Squibb and Genentech/imCORE. Honoraria from UpToDate. Toni K: Choueiri reports grants, personal fees, nonfinancial support, and other from BMS, Merck, Roche, Pfizer, EMD, Exelixis, Novartis, and AstraZeneca [advisory board, consultancy, honorarium, payments (personal and for institution)], and a patent for IO biomarkers pending, issued, and licensed to DFCI (related to IO). Rana R: McKay received research funding from Bayer, Pfizer, Tempus; serves on Advisory Board for AstraZeneca, Bayer, Bristol Myers Squibb, Calithera, Exelixis, Janssen, Merck, Novartis, Pfizer, Sanofi, Tempus; is a consultant for Dendreon, Vividion. Guru Sonpavde: Advisory Board: BMS, Genentech, EMD Serono, Merck, Sanofi, Seattle Genetics/Astellas, Astrazeneca, Exelixis, Janssen, Bicycle Therapeutics, Pfizer, Immunomedics/Gilead, Scholar Rock, G1 Therapeutics; Research Support to Institution: Sanofi, Astrazeneca, Immunomedics/Gilead, QED, Predicine, BMS; Steering committee of studies: BMS, Bavarian Nordic, Seattle Genetics, QED, G1 Therapeutics (all unpaid), and Astrazeneca, EMD Serono, Debiopharm (paid).; Data safety monitoring committee: Mereo; Travel costs: BMS (2019), Astrazeneca (2018); Writing/Editor fees: Uptodate, Editor of Elsevier Practice Update Bladder Cancer Center of Excellence; Speaking fees: Physicians Education Resource (PER), Onclive, Research to Practice, Medscape (all educational). Rakesh Jain has received an Honorarium from Amgen; Consultant fees from Elpis, SPARC, SynDevRx; Owns equity in Accurius, Enlight, SynDevRx; Board of Trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, Tekla Healthcare Opportunities Fund, Tekla World Healthcare Fund and received a Research Grant from Boehringer Ingelheim. The remaining authors have stated that they have no conflicts of interest.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board at Dana Farber Cancer Institute and University of California San Diego.
Consent for publication
All authors contributed to this study, read the final version of this manuscript and approved its publication.
Availability of data and materials
All data and materials relevant to this article are available to referees at submission and to readers promptly on request.
Meeting presentation
This article was presented as poster presentation during the 2021 ASCO-Genitourinary Cancers Symposium; Feb 17-19, 2021.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.10l6/j.clgc.2022.04.012.
References
- 1.Hofmann F, Hwang EC, Lam TB, et al. Targeted therapy for metastatic renal cell carcinoma. Cochrane Database Syst Rev. 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.George AJ, Thomas WG, Hannan RD. The renin-angiotensin system and cancer: old dog, new tricks. Nat Rev Cancer. 2010;10:745–759. [DOI] [PubMed] [Google Scholar]
- 3.Chauhan VP, Martin JD, Liu H, et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun. 2013;4:2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinter M, Jain RK. Targeting the renin-angiotensin system to improve cancer treatment: implications for immunotherapy. Set Transl Med. 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chauhan VP, Chen IX, Tong R, et al. Reprogramming the microenvironment with tumor-selective angiotensin blockers enhances cancer immunotherapy. Proc Natl Acad Set U S A. 2019;116:10674–10680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu H, Naxerova K, Pinter M, et al. Use of angiotensin system inhibitors is associated with immune activation and longer survival in nonmetastatic pancreatic ductal adenocarcinoma. Clin Cancer Res. 2017;23:5959–5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okwan-Duodu D, Landry J, Shen XZ, Diaz R. Angiotensin-converting enzyme and the tumor microenvironment: mechanisms beyond angiogenesis. Am J Physiol Regul Integr Comp Physiol. 2013;305:R205–R215. [DOI] [PubMed] [Google Scholar]
- 8.Wilop S, von Hobe S, Crysandt M, Osieka R, Jost E, Esser A. Impact of angiotensin I converting enzyme inhibitors and angiotensin II type 1 receptor blockers on survival in patients with advanced non-small-cell lung cancer undergoing first-line platinum-based chemotherapy. J Cancer Res Clin Oncol. 2009;135:1429–1435. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Y, Cao J, Melamed A, et al. Losartan treatment enhances chemotherapy efficacy and reduces ascites in ovarian cancer models by normalizing the tumor stroma. Proc Natl Acad Set U S A. 2019;116:2210–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakai Y, Isayama H, Ijichi H, et al. Inhibition of renin-angiotensin system affects prognosis of advanced pancreatic cancer receiving gemcitabine. Br J Cancer. 2010;103:1644–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tozuka T, Yanagitani N, Yoshida H, et al. Impact of renin-angiotensin system inhibitors on the efficacy of anti-PD-1/PD-L1 antibodies in NSCLC patients. Anticancer Res. 2021;4l:2093–2100. [DOI] [PubMed] [Google Scholar]
- 12.Jain RK, Skelton Iv WP, Pond GR, et al. Angiotensin blockade modulates the activity of PD1/L1 inhibitors in metastatic urothelial carcinoma. Clin Genitourin Cancer. 2021;19:540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coulson R, Liew SH, Connelly AA, et al. The angiotensin receptor blocker, Losartan, inhibits mammary tumor development and progression to invasive carcinoma. Oncotarget. 2017;8:18640–18656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinter M, Kwanten WJ, Jain RK. Renin-angiotensin system inhibitors to mitigate cancer treatment-related adverse events. Clin Cancer Res. 2018;24:3803–3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKay RR, Rodriguez GE, Lin X, et al. Angiotensin system inhibitors and survival outcomes in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2015;21:2471–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy JE, Wo JY, Ryan DP, et al. Total neoadjuvant therapy with FOLFIRINOX in combination with losartan followed by chemoradiotherapy for locally advanced pancreatic cancer: a phase 2 clinical trial. JAMA Oncol. 2019;5:1020–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.