Abstract
Using immunofluorescence microscopy and a fusion of a cold shock protein (CSP), CspB, to green fluorescent protein (GFP), we showed that in growing cells Bacillus subtilis CSPs specifically localize to cytosolic regions surrounding the nucleoid. The subcellular localization of CSPs is influenced by the structure of the nucleoid. Decondensed chromosomes in smc mutant cells reduced the sizes of the regions in which CSPs localized, while cold shock-induced chromosome compaction was accompanied by an expansion of the space in which CSPs were present. As a control, histone-like protein HBsu localized to the nucleoids, while β-galactosidase and GFP were detectable throughout the cell. After inhibition of translation, CspB-GFP was still present around the nucleoids in a manner similar to that in cold-shocked cells. However, in stationary-phase cells and after inhibition of transcription, CspB was distributed throughout the cell, indicating that specific localization of CspB depends on active transcription and is not due to simple exclusion from the nucleoid. Furthermore, we observed that nucleoids are more condensed and frequently abnormal in cspB cspC and cspB cspD double-mutant cells. This suggests that the function of CSPs affects chromosome structure, probably through coupling of transcription to translation, which is thought to decondense nucleoids. In addition, we found that cspB cspD and cspB cspC double mutants are defective in sporulation, with a block at or before stage 0. Interestingly, CspB and CspC are depleted from the forespore compartment but not from the mother cell. In toto, our findings suggest that CSPs localize to zones of newly synthesized RNA, coupling transcription with initiation of translation.
Cold shock proteins (CSPs) constitute a widespread and highly conserved protein family in bacteria, and multiple copies of CSPs are often present (10, 25). Haemophilus influenzae contains a single csp gene, while Bacillus subtilis contains three csp genes and Escherichia coli contains nine. The sequence identity for CSPs and the cold shock domain that has been identified in a variety of eukaryotic RNA-binding proteins is remarkably high, suggesting that there is a conserved function for CSPs and the cold shock domain (11).
Synthesis of all CSPs (CspB, CspC, CspD) in B. subtilis is strongly induced following cold shock and entry into the stationary phase (for CspB and CspC), while CSPs are also present at 37°C (6, 9). Cold shock induction occurs mainly at the posttranscriptional level, while transcriptional activation of csp genes is only modest (6, 19, 31). Deletion of one or two csp genes leads to an increase in synthesis of the other CSP(s) and influences the synthesis of many other cytosolic proteins (6). cspB cspD and cspB cspC double mutants show growth impairment at 15°C as well as at 37°C. These findings suggest that CSPs can complement each other and cross-regulate their synthesis. The presence of at least one CSP is necessary for viability of B. subtilis at low and optimal growth temperatures, and depletion of CSPs leads to compromised and deregulated protein synthesis (6). CSPs consist of a five-stranded β-barrel with highly conserved RNA-binding epitopes (RNP motifs) on adjacent β-strands. Mutational analysis has shown that RNP motifs are essential for binding to single-stranded DNA (ssDNA) and to RNA (28). CSPs bind cooperatively to single-stranded nucleic acids, and their affinity for ssDNA is somewhat greater than their affinity for RNA (6, 17). Longer RNA molecules are bound only after heat denaturation, suggesting that CSPs bind to linear single-stranded nucleic acids instead of highly structured nucleic acids. Interestingly, B. subtilis CSPs are highly susceptible to proteolysis in vitro, while they are rather stable in vivo. Based on the finding that binding to substrate nucleic acids strongly protects CSPs in vitro, CSPs appear to be heavily associated with ssDNA and/or RNA in vivo (27). It has been suggested that CSPs may act as transcription activators of other cold-induced genes or as RNA chaperones during initiation of translation.
According to recent reports (1, 23), transcription in bacteria seems to occur mostly on the centrally located nucleoid that contains the bulk of the DNA, while translation appears to take place mainly at cytosolic sites surrounding the nucleoid. It has been proposed that the coupling of transcription and translation to insertion of proteins into membranes and transport across the cytosolic membrane pulls DNA towards the membrane, thereby decondensing chromosomes, which otherwise form a more compacted structure (e.g., in the stationary phase) (32). We have proposed a model in which CSPs cooperatively bind to nascent transcripts and prevent formation of secondary structures in RNA that would impair initiation of translation (6). As the affinity of CSPs for ssDNA and RNA is moderate, ribosomes could displace CSPs from RNA and initiate translation on a linear template. Thus, CSPs would strongly affect the coupling of transcription to translation in bacteria. This model was recently supported by biochemical data obtained by Hanna and Liu (13), who reported a strong association of E. coli CspE with several short transcripts after in vivo cross-linking.
In this study, we investigated the subcellular localization of B. subtilis CSPs. We found that all three CSPs are present in cytosolic spaces surrounding the central nucleoid. So far, a similar localization has been reported only for a ribosomal protein in B. subtilis (23), suggesting that CSPs function in the same cellular location as ribosomes rather than on the nucleoid. However, inhibition of transcription eliminated specific localization of CspB, indicating that CspB binds to RNA during transcription. We also found that CSPs influence the structure of the nucleoid, which supports the hypothesis that these molecules function in coupling transcription and translation.
MATERIALS AND METHODS
Media and growth conditions.
E. coli XL1-Blue {recA endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10(Tetr)]; Stratagene} was grown in Luria-Bertani (LB) rich medium supplemented with 50 μg of ampicillin per ml when it was appropriate. Bacillus strains were grown in 2xYT rich medium or DSM sporulation medium. For expression of green fluorescent protein (GFP) in B. subtilis MW1 (see below), Spizizen's minimal medium (14) supplemented with 0.2% (wt/vol) fructose instead of glucose, 50 μg of tryptophan per ml, and 50 μg of phenylalanine per ml was used. Xylose was added to a final concentration of 0.5% to activate the Pxyl promoter controlling transcription of gfp. Isopropyl-β-d-thiogalactopyranoside (IPTG) (final concentration, 1 mM) was added to the growth medium of B. subtilis MW4 (see below) and 64BCDbt (6). For inhibition studies, rifampin, chloramphenicol, kanamycin, or spectinomycin was added to a final concentration of 200 μg/ml. Cells were incubated for 30 min before image acquisition. S750 minimal medium (16) was used for growth for microscopy.
Plasmid construction.
By using primers 5′cspB-OrfUp (5′-AGAGAGCTCAGAAAATTTGAAGAAACCAC-3′) and 3′cspB-gfp (5′-AAACCCGGGACGCTTCTTTAGTAACGT-3′) and primers 3′cspB-gfp-Trm3′Orf (5′-GAAAAGCTTAAGCATAAATTGATATGAAAAACT-3′) and 3′cspB-OrfDown (5′-GGACTCGAGAAATCAGAGATCATTTAAA-3′), a cspB-containing upstream fragment that did not include the cspB stop codon (5′ fragment, 1,958 bp) and a fragment downstream of the cspB gene (3′ fragment) were amplified from B. subtilis JH642 chromosomal DNA by PCR. The 5′ fragment was cloned into pCW8 (30) by using SacI and SmaI, resulting in plasmid pMW_BGFP-1. Next, the 3′ fragment was cloned into pMW_BGFP-1 by using HindIII and XhoI. The resulting plasmid, pMW_BGFP-2, was linearized by HindIII digestion and ligated with a 1,660-bp HindIII fragment obtained from pDG647 (Bacillus Genetic Stock Center, Columbus, Ohio) containing an erythromycin resistance cassette, which yielded pMW_BGFP. The integrity of this plasmid was verified by sequencing the entire cspB gene, including its 5′ untranslated leader region and promoter region, as well as the junction between cspB and gfp, using an ABI Prism 310 genetic analyzer. The linker sequence between CspB and GFP is SRAAGIRLEK, and the entire fusion protein comprises 317 amino acids (molecular weight, 35,600; pI 5.50). By introducing SpeI and BamHI restriction sites, a DNA fragment carrying the entire gfp gene was amplified from pCW8 (30) with primers 5′gfp-Express-pX (5′-ATAAACTAGTAGGAGGTAGAAAAAATGAGTAAAGGAGAAG-3′) and 3′gfp-Express-pX (5′-CCTAGGATCCATGCTATTTGTATAGTTCATCCAT-3′), and it was subsequently cloned into the SpeI and BamHI sites of the B. subtilis amyE integration vector pX (20). Plasmid pMW_gfp was tested for the presence of gfp by digestion analysis and by PCR.
Bacterial strains.
For construction of B. subtilis MW1 (amyE::xylR-Pxyl-gfp-cat), plasmid pMW_gfp was linearized with ScaI and transformed into B. subtilis JH642 (pheA1 trpC2), with selection for resistance to 10 μg of chloramphenicol per ml. Double-crossover integration was verified by performing a starch assay with Lugol's solution (Merck, Darmstadt, Germany) on LB medium plates containing 1% starch. Transformation of B. subtilis JH642 and 64C (6) with ScaI- and XhoI-linearized plasmid pMW_BGFP and selection for resistance to 1 μg of erythromycin per ml and 25 μg of lincomycin per ml resulted in B. subtilis MW2 (cspB-gfp) and MW3 (cspB-gfp cspC::kan), respectively. To construct MW4 (cspB::cat cspC::kan cspD::cat pDGcspB), B. subtilis 64BCDbt (6) was transformed with ScaI-linearized pX (20). Transformants were selected on LB medium plates containing 30 μg of chloramphenicol per ml. In contrast to parental strain 64BCDbt, strain MW4 did not contain the spectinomycin resistance cassette in the cat-disrupted cspB gene locus, as determined by PCR analysis of the cspB gene locus (Table 1), but instead contained two cat genes. For construction of MW5 (cspC::kan cspD::cat cspB-gfp pDGcspB), chromosomal DNA prepared from MW3 was used for transformation of MW4 to obtain MW5 after selection on LB medium plates containing 1 μg of erythromycin per ml and 25 μg of lincomycin per ml. In contrast to 64BCDbt, growth of MW5 was not affected by depletion of IPTG. As determined by PCR, continued growth of MW5 without selection for phleomycin resistance resulted in loss of pDGcspB after approximately 2 weeks, in contrast to growth of 64BCDbt, which did not lose this plasmid.
TABLE 1.
B. subtilis strains
| Strain | Genotype | Reference |
|---|---|---|
| 64BCDbt | cspB::spc cspC::kan cspD::cat pDGcspB | 6 |
| JH642 | pheA1 trpC2 | 31 |
| PK9C8 | hbs-gfp Pspac-hbs | 21 |
| MW1 | amyE::xylR-Pxyl-gfp-cat | This study |
| MW2 | cspB-gfp | This study |
| MW3 | cspB-gfp cspC::kan | This study |
| MW4 | cspB::cat cspC::kan cspD::cat pDGcspB | This study |
| MW5 | cspC::kan cspD::cat cspB-gfp pDGcspB | This study |
Immunofluorescence microscopy.
Immunofluorescence microscopy was performed as described by Pogliano et al. (26). Anti-CspB, anti-CspC, and anti-CspD sera were used at concentrations of 1:2,500 to 1:10,000. 4′,6′-Diamidino-2-phenylindole (DAPI) was used at concentrations of 200 to 500 ng/ml; FM4-64 was used at a concentration of 1 nM.
Image acquisition.
Fluorescence microscopy was performed with an Olympus AX70 microscope by using Xenon epifluorescence. Cells were grown in S750 medium (16) and were mounted on agarose pads containing growth medium on object slides. Images of live cells or images of fixed cells were taken with the same exposure time. For stationary-phase cells, culture supernatant was used for the agarose pads. Images were acquired with a digital charge-coupled device camera, and three-dimensional image reconstitution was generated with the Metamorph program.
RESULTS
Intracellular localization of CSPs in growing cells.
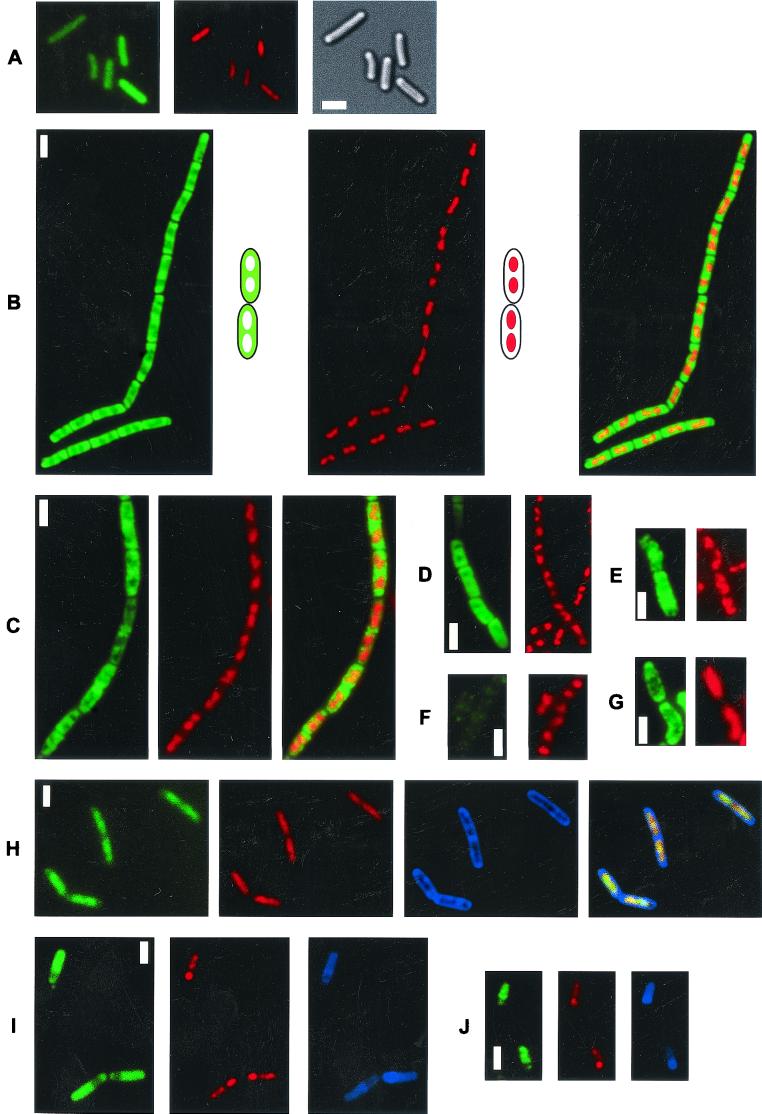
To study the subcellular localization of the major CSP, CspB, in B. subtilis, we constructed a fusion of CspB to GFP. The fusion was integrated by double crossover into the cspB locus of strain JH642 so that the cspB-gfp fusion was under the same control as wild-type cspB. Western blot analysis verified that the fusion was produced, replacing wild-type CspB. To investigate whether the fusion was active, it was necessary to combine the cspB-gfp fusion with cspD and cspC deletion mutants, since csp single mutants had no detectable phenotype (6). A strain carrying cspB-gfp and having deletions of cspC and cspD is viable, whereas cells having deletions of all csp genes can survive only when they carry a plasmid encoding cspB in trans (6). Therefore, the CspB-GFP fusion was able to complement CspB function, at least in part (see below). Fluorescence microscopy of the resulting strain, strain MW2 (cspB-gfp), showed that CspB-GFP localized to the cytosol, as expected. However, the pattern of localization was different from that of GFP, which was present throughout the cell (Fig. 1A). CspB-GFP was observed only in cytosolic regions surrounding the nucleoids; most occurred at the cell poles, while nucleoids were devoid of CspB or had a drastically lower concentration of CspB (Fig. 1B). An overlay of GFP fluorescence with DNA stain (Fig. 1B, green-blue panel) revealed little overlay (yellow) of CspB and the DNA stain. To obtain better resolution of the location of the GFP fusion, three-dimensional images were reconstructed from Z sections taken through MW2 cells (data not shown). These analyses showed that central spaces in the cells were mostly devoid of CspB-GFP and that CspB was present not only at the cell poles but also between nucleoids and the membranes along the long axis of the cells. To determine if this pattern of localization is also valid for wild-type CSPs, we performed immunofluorescence microscopy with antisera raised against all B. subtilis CSPs (CspB, CspC, and CspD) (6). As shown in Fig. 1C, CspB localized around nucleoids indistinguishable from the CspB-GFP fusion protein; this was also observed for CspC and CspD (data not shown), while background staining was observed in a cspB deletion strain (Fig. 1F) (the same results were obtained with cspC or cspD mutant cells when the corresponding sera were used [data not shown]). In contrast, β-galactosidase was observed throughout the cells (Fig. 1E), without pronounced exclusion from spaces occupied by nucleoids. As a further control, we used HBsu-GFP, which localized entirely to nucleoids (Fig. 1H) (note that the overlay of GFP and blue DNA staining resulted in a yellowish color), as previously reported (21). Thus, HBsu and CSPs localize to two distinct subcellular regions in B. subtilis.
FIG. 1.
Fluorescence microscopy of exponentially growing B. subtilis cells. Green, GFP; red, DAPI DNA stain; blue, membrane stain; monochrome, Nomarski differential interference contrast. (A) Live cells (MW1) expressing GFP at 25°C. (B) Live cells (MW2) expressing CspB-GFP at 25°C. (C) Immunofluorescence with anti-CspB serum and fixed wild-type cells (JH642) growing at 37°C. (D) Immunofluorescence with anti-CspB serum and wild-type cells 2 h after cold shock from 37 to 15°C. (E) Immunofluorescence with anti-β-galactosidase serum and wild-type cells growing at 37°C. (F) Immunofluorescence with anti-CspB serum and cspB deletion strain 64B growing at 37°C. (G) Immunofluorescence with anti-CspB serum and smc mutant cells growing at 23°C. (H) Live cells (PK9C8) expressing HBsu-GFP at 25°C. (I) Live cells (MW2) expressing CspB-GFP 4 h after induction of sporulation at 25°C. (J) Immunofluoresence with anti-CspB antiserum and fixed wild-type cells 3 h after induction of sporulation at 37°C.
The fluorescence intensity was greater in a cspC deletion mutant carrying cspB-gfp than in wild-type cells and greatest in cells with a deletion of cspC and cspD (data not shown). This observation is in accordance with the previous finding that deletion of csp genes leads to higher levels of the remaining CSPs (6).
Nucleoid structure influences the localization of CSPs.
After a shift from 37 to 15°C during the mid-exponential phase in rich medium, B. subtilis cells continue to grow, albeit at a very reduced rate, while the level of CSPs increases about twofold (10). Following cold shock, nucleoids in B. subtilis cells condensed significantly, as shown in Fig. 1D (Fig. 1C and D, compare the red stains), although the cell size and cell shape remained similar to the cell size and cell shape of cells growing at 37°C. Thus, as in cells in the stationary phase and after a block in translation (see below), cold shock leads to chromosome compaction, probably due to a decrease in the transcriptional and translational capacity of the cell. Immunofluorescence microscopy revealed that CSPs still localized around nucleoids and that the zones of localization increased concomitant with nucleoid compaction (Fig. 1D). Conversely, in smc mutant cells growing exponentially at a permissive temperature (23°C) in rich medium, which have a DNA-to-protein ratio similar to that of wild-type cells but strongly decondensed chromosomes (4, 8, 24), the cellular spaces occupied by CSPs decreased, and CSPs were detectable only in small spaces close to the cell membrane (Fig. 1G). Thus, in growing cells, CSP localization depends on the state of compaction of the DNA.
Entry into the stationary phase and inhibition of transcription but not inhibition of translation affect localization of CSPs.
CSPs may localize to zones surrounding the nucleoids simply because of exclusion from the nucleoids, or alternatively, they may localize to zones of active transcription and/or translation. CSPs have been shown to bind to ssDNA, as well as to RNA, via a positively charged RNA-binding epitope (28), so there is no repulsion from DNA per se due to their overall negative charge. We therefore analyzed the localization of CSPs in stationary-phase cells, in which chromosomes were more compact (Fig. 2C) than they were in growing cells (Fig. 2A). Nucleoid compaction during the stationary phase was also observed in cells expressing HBsu-GFP (Fig. 2E) and thus is likely to represent the true situation in vivo instead of a fixation artifact. Soon after entry into the stationary phase, CspB-GFP was observed throughout cells (Fig. 2C), in contrast to the pronounced localization around nucleoids in growing cells (Fig. 2A). Similarly, using immunofluorescence, we found that CspB and CspC localized throughout the cytosol in the stationary phase (data not shown).
FIG. 2.
Fluorescence microscopy of live B. subtilis cells grown at 25°C. (A) Exponentially growing cells expressing CspB-GFP (MW2). (B) MW2 cells treated with chloramphenicol for 30 min. (C) MW2 cells 3 h after entry into the stationary phase. (D) MW2 cells treated with rifampin for 30 min. (E) Cells expressing HBsu-GFP 3 h after entry into the stationary phase. The white lines indicate septa between cells. Bars = 2 μm. Note that immunofluorescence using anti-CspB antibodies with fixed wild-type cells confirmed the delocalization of CspB upon inhibition of transcription (D) (data not shown).
In order to analyze whether active transcription or translation or both are required for specific localization of CspB, exponentially growing cells were treated with inhibitors of transcription or translation. In contrast to growing cells (Fig. 2A), CspB-GFP was found throughout the cells after addition of rifampin (Fig. 2D), while CspB remained localized around nucleoids after addition of chloramphenicol (Fig. 2B), spectinomycin, or kanamycin (data not shown) in a manner similar to that in cold-shocked cells (Fig. 1D). To confirm relocalization of CspB upon inhibition of transcription, we performed immunofluorescence microscopy using CspB antiserum with wild-type cells and found that CspB localized throughout the cells after addition of rifampin (data not shown). We have no straightforward explanation for our finding that nucleoids decondensed after rifampin treatment, while treatment with translation inhibitors induced nucleoid compaction. In control experiments, we did not observe a noticeable effect on CspB-GFP localization or on nucleoid structure after addition of ethanol and methanol at concentrations used for the chloramphenicol and rifampin experiments, respectively (data not shown). Additional control experiments in which Western blotting was used showed that the CspB-GFP fusion is stable during the stationary phase and after addition of rifampin, eliminating the possibility that delocalized fluorescence might have been due to a GFP-containing proteolysis product of the fusion. These findings show that inhibition of transcription eliminates specific localization of CspB.
Absence of CSPs leads to a defect in nucleoid structure and in chromosome segregation.
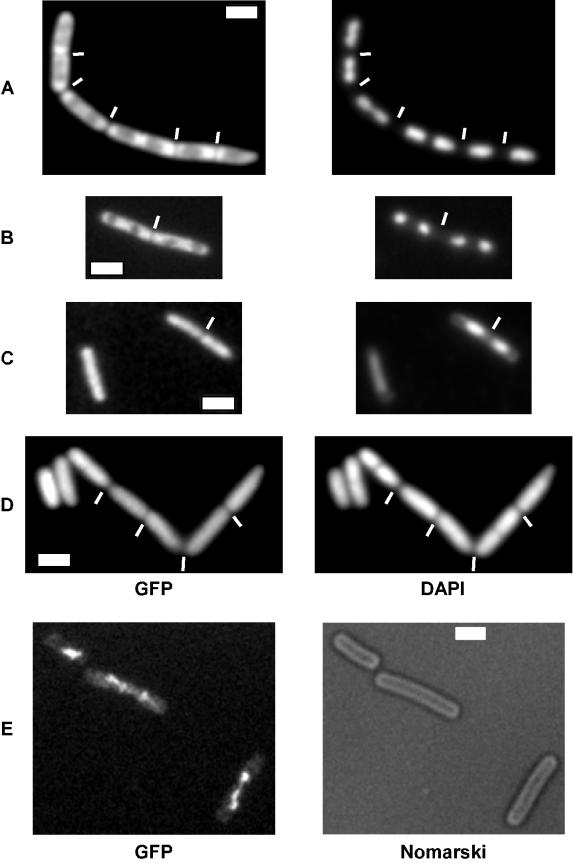
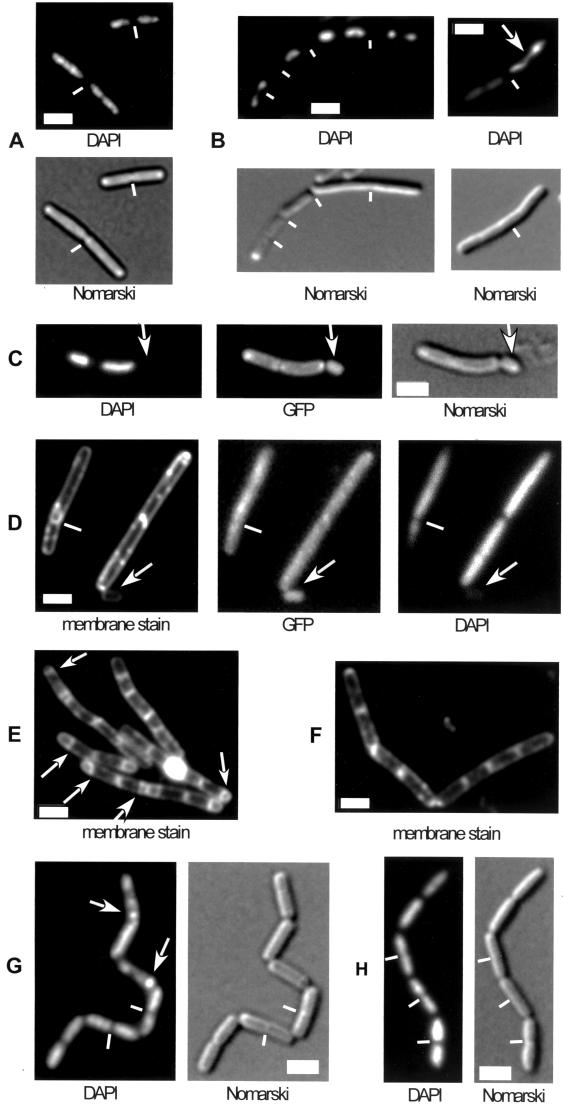
Two CSPs have been identified as high-copy-number suppressors of chromosome decondensation defects in E. coli cells (15, 34). We therefore analyzed nucleoid morphology in csp deletion strains during exponential growth. Figure 3 shows nucleoid staining in typical fields of wild-type cells (Fig. 3A) and cspB cspD double-mutant cells (Fig. 3B) grown in rich medium at 37°C. In the mutant strain, nucleoids were generally more condensed (Fig. 3B) and frequently had irregular shapes (this was observed in 5 to 15% of the cells). Similar nucleoid defects were found in cspB cspC double-mutant cells (data not shown) and in cspC mutant cells containing cspB-GFP (indicating that the fusion does not fully complement the wild-type protein). Occasionally, small cells devoid of DNA were detected (0.1 to 0.5% of the cells) adjacent to DNA-free zones in larger mutant cells (Fig. 3C), showing that a defect in CSP function leads to minicell formation. We speculate that in order to function optimally, CSPs need to be small proteins, a requirement which is compromised through the GFP fusion. However, we cannot rule out the possibility that the fusion has an influence on proper folding of CspB. In contrast, depletion of HBsu from cells that constitutively expressed HBsu-GFP and contained wild-type hbsu under an inducible promoter led to chromosome decondensation (Fig. 3D), as previously reported (21), as well as to generation of 5 to 10% anucleate cells 2 h after removal of the inducer (when cells were still growing, as judged from the increase in optical density). Note that anucleate cells are generally normal cell size and are generated by guillotining of DNA entrapped by a prematurely closing septum (Fig. 3D, white lines). These findings show that CSPs influence the structure of the nucleoid, although they localize to zones surrounding the chromosomes. Additionally, CSPs influence the position of cell division, probably in an indirect way. In contrast, HBsu has a direct impact on chromosome condensation and segregation, analogous to the impact of the SMC condensation factor.
FIG. 3.
Fluorescence microscopy of live B. subtilis cells growing in rich medium. DAPI, DNA stain; Nomarski, outline of cells; membrane stain, vital stain FM4-64. (A) Wild-type cells. (B) Two fields of 64BD cells (cspB::spc cspD::cat) grown at 37°C. The arrow indicates an abnormal nucleoid structure. (C) Strain MW3 (cspB-gfp cspC::kan) growing at 25°C. The arrow indicates a minicell devoid of DNA. (D) Cells depleted of HBsu (see text) for 3 h. The arrow indicates an anucleate cell. The white line indicates a septum dissecting a nucleoid. (E) Wild-type cells 3 h after the onset of sporulation at 37°C. The arrows indicate polar septa. (F) 64BD cells (cspB::spc cspD::cat) 3 h after the onset of sporulation. (G) Wild-type cells 2 h after the onset of sporulation. The arrows indicate condensed nucleoids in forespore compartments that have proceeded to stage III (engulfment) of sporulation. The white lines indicate septa. (H) Cells of strain 64BD (cspB::spc cspD::cat) 2 h after the onset of sporulation. The white lines indicate septa. Note that nucleoids are more decondensed and extended in cells entering sporulation (G and H) than in growing cells (A and B).
Sporulation defect in csp double mutants.
B. subtilis has the unique ability to undergo sporulation in response to adverse environmental conditions. This differentiation process involves sequential activation of sporulation genes and synthesis of the corresponding gene products. CSPs have previously been shown to affect protein synthesis in growing cells, so we investigated a possible role of CSPs during sporulation. All csp single-mutant strains formed heat-resistant spores comparable to those of wild-type cells (sporulation efficiency, 70 to 80%). In contrast, cspB cspD mutant cells were strongly impaired in terms of sporulation, producing only 0.05% spores. Cells with a deletion of cspB and cspC are defective in adaptation to the stationary phase; such mutant cells lyse after cessation of growth (6). Likewise, only 0.002% viable colonies remained 24 h after the beginning of sporulation in cspB cspC mutant cells, compared to wild-type cells. However, the sporulation frequency was 0.03% (compared to viable cells), revealing that the csp double mutants did not sporulate. A cspC deletion strain expressing cspB-gfp formed 0.5 to 1% spores, as well as a number of viable cells comparable to the number formed by the parental strain, showing that a CspB-GFP fusion can substantially but not completely complement the function of CspB during sporulation.
In order to analyze at which stage the block in sporulation occurs, cspB cspC and cspB cspD mutant cells were stained with FM4-64 membrane stain several hours after entry into sporulation. In contrast to the parental strain, in which polar septation started 2 h after initiation of sporulation (Fig. 3E), no polar septa were detectable in the csp double mutants for up to 48 h after entry into sporulation (Fig. 3F). Thus, deletion of cspD or cspC in addition to cspB leads to a defect at stage 0 of sporulation. Recent data suggest that formation of the polar septum during sporulation depends not only on Spo0A activity but also on the migration of replication origins to the extreme cell poles, a process termed axial filament formation (7). To test whether csp mutant cells that have more compacted nucleoids during growth are defective in formation of axial filaments, we investigated nucleoid structure at the onset of sporulation. DAPI staining of live cells at the transition to the stationary phase in sporulation medium showed that nucleoids extended to the cell poles (and more or less filled the whole cell) in wild-type cells (Fig. 3G). In cspB cspC and cspB cspD mutant cells, nucleoids also extended to the poles in a majority of the cells (Fig. 3H). Thus, csp mutant cells are not obviously affected in axial filament formation.
Localization of CSPs in sporulating cells.
Cells expressing CspB-GFP were used to study the localization of CspB during sporulation. Although CspB-GFP was found to generally localize throughout the cell at the onset of sporulation (data not shown), comparable to what happens in cells entering the stationary phase (Fig. 2C), the amount of fusion protein decreased considerably within the forespore compartment soon after polar septation (Fig. 1I). Likewise, using immunofluorescence as a control, we observed depletion of CspB and CspC from the forespores in wild-type sporangia (Fig. 1J). CspD appeared to be present in the forespore compartment at least until engulfment (data not shown); however, in the absence of an available GFP fusion construct, this finding remains preliminary.
DISCUSSION
The results of this study establish that there is a connection among specific subcellular localization of B. subtilis CSPs, active transcription, and nucleoid structure. In growing cells, CSPs localize in cytosolic spaces surrounding the nucleoids, in contrast to histone-like protein HBsu, which localizes exclusively in the nucleoid, as shown previously (21). B. subtilis CSPs remained at locations surrounding nucleoids in cells with more condensed nucleoids, such as cells after cold shock, and also in cells with decondensed chromosomes, such as smc mutant cells. According to recent reports, ribosomal proteins localize in a manner similar to CSPs, while RNA polymerase subunits and the main sigma factor localize mainly in nucleoids (1, 23). In control experiments, we found that GFP and β-galactosidase localize throughout the cell, providing evidence that non-nucleoid-associated proteins are not generally excluded from nucleoids.
CSPs have been shown to affect the synthesis of other cytosolic proteins at 37°C and after cold shock (5, 6, 18, 22, 33). Two main modes of activation or repression by CSPs have been proposed. Due to their binding to ssDNA, it has been proposed that CSPs may facilitate open complex formation by RNA polymerase through stabilization of ssDNA during transcription initiation (3). In an alternative model, it was suggested that CSPs function as RNA chaperones (17) by binding to newly synthesized RNA and by keeping RNA in a linear form (6). This is a prerequisite for translation initiation, which depends on a linear RNA template in bacteria (12). Although our data do not exclude a possible function of CSPs in transcription or in replication initiation, the pattern of localization suggests that the main function of CSPs takes place at sites of translation.
Recently, E. coli CSPs were shown to act as antiterminators in transcription of a cold stress-induced operon (2). This finding indicates that coupling of transcription and translation may take place at the interface between the nucleoid and surrounding spaces containing ribosomes. Consequently, CSPs could act mainly on translation initiation within translation areas and could act as antiterminators at the interface between nucleoid and cytosol. In support of this model, we found that in the absence of transcription and during the stationary phase, CSPs localize throughout the cytoplasm, while inhibition of translation retains the specific localization of CSPs. We interpret these findings as an association of CSPs with mRNA, which is preserved upon a block in translation but is eliminated when there is a decrease in RNA synthesis. Our data show that CSPs change their pattern of localization depending on cellular activity, which has not been reported previously for bacterial proteins with respect to transcription and translation.
Intriguingly, although CSPs do not localize to the nucleoid like condensation and compaction factors such as HBsu and SMC, they affect the structure of the nucleoid. In contrast to depletion of HBsu (21) or deletion of smc (4, 8), each of which leads to chromosome decondensation, a cspB cspD double mutant had more compact nucleoids with frequently abnormal structure than wild-type cells. This finding suggests that through their function, CSPs are involved in decondensing chromosomes in growing cells. Chromosome condensation occurs during the stationary phase, during a block in translation, and after cold shock, as shown in this study. Woldringh et al. (32) proposed a model in which simultaneous insertion into and transport of proteins across membranes and coupled transcription-translation that takes place in bacteria provide expansion forces for chromosome decondensation in growing cells. Our findings are consistent with this model, since a decrease in cellular CSP levels compromises protein synthesis (6) and thereby probably affects chromosome decondensation. The data which we obtained also support the hypothesis that CSPs are important mediators in the coupling of transcription and translation. Moreover, the effect of CSPs on nucleoid morphology discovered in this study is a first clue to why two CSPs in E. coli were found to be high-copy-number suppressors of a mutation in condensation factor MukB (34) and confer resistance to chromosome decondensation through camphor (15).
We also found that CSPs are important for the developmental process of sporulation in B. subtilis. CSP double mutants were strongly impaired in terms of initiation of sporulation, at a stage before polar septation. In light of a defect in protein synthesis in csp mutants during growth (6), it appears likely that absence of CSPs leads to deregulation of protein levels at the onset of sporulation. Interestingly, we found that CspB and CspC were depleted from the forespore compartment but not from the mother cell. The significance of this observation is not clear; however, degradation of CSPs from the forespore may indicate a generally higher rate of proteolysis within the forespore. This may be relevant in compartmentalized proteolysis during sporulation, which, for example, could also lead to degradation of sigma factor E, which is depleted from the forespore (26).
Changes in localization of CSPs during growth, sporulation, and the stationary phase provide evidence not only that membrane and DNA-binding proteins switch their subcellular locations (29) but also that the position of cytosolic, non-DNA-binding proteins is more dynamic in bacteria than anticipated.
ACKNOWLEDGMENTS
Immunofluorescence microscopy experiments were performed primarily in the laboratory of Richard Losick (Cambridge, Mass.).
This study was supported by the Deutsche Forschungsgemeinschaft (Emmie Noether Program and SFB 395).
ADDENDUM IN PROOF
It was recently shown that CSPs colocalize with ribosomes in B. subtilis and that localization of ribosomes to sites surrounding nucleoids also depends on active transcription (J. Mascarenhas, M. H. Weber, and P. L. Graumann, EMBO Rep. 2:685–689, 2001).
REFERENCES
- 1.Azam T A, Hiraga S, Ishihama A. Two types of localization of the DNA-binding proteins within the Escherichia coli nucleoid. Genes Cells. 2000;5:613–626. doi: 10.1046/j.1365-2443.2000.00350.x. [DOI] [PubMed] [Google Scholar]
- 2.Bae W, Xia B, Inouye M, Severinov K. Escherichia coli CspA-family RNA chaperones are transcription antiterminators. Proc Natl Acad Sci USA. 2000;97:7784–7789. doi: 10.1073/pnas.97.14.7784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandi A, Pon C L, Gualerzi C O. Interaction of the main cold shock protein CS 7.4 (CspA) of Escherichia coli with the promoter region of hns. Biochimie. 1994;76:1090–1098. doi: 10.1016/0300-9084(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 4.Britton R A, Lin D C-H, Grossman A D. Characterization of a procaryotic SMC protein involved in chromosome partitioning. Genes Dev. 1998;12:1254–1259. doi: 10.1101/gad.12.9.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graumann P, Marahiel M A. Effects of heterologous expression of CspB from Bacillus subtilis on gene expression in Escherichia coli. Mol Gen Genet. 1997;253:745–752. doi: 10.1007/s004380050379. [DOI] [PubMed] [Google Scholar]
- 6.Graumann P, Wendrich T M, Weber M H W, Schröder K, Marahiel M A. A family of cold shock proteins in Bacillus subtilis is essential for cellular growth and for efficient protein synthesis at optimal and low temperatures. Mol Microbiol. 1997;25:741–756. doi: 10.1046/j.1365-2958.1997.5121878.x. [DOI] [PubMed] [Google Scholar]
- 7.Graumann P L, Losick R. Coupling of asymmetric division to polar placement of replication origin regions in Bacillus subtilis. J Bacteriol. 2001;183:4052–4060. doi: 10.1128/JB.183.13.4052-4060.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graumann P L, Losick R, Strunnikov A V. Subcellular localization of Bacillus subtilis SMC, a protein involved in chromosome condensation and segregation. J Bacteriol. 1998;180:5749–5755. doi: 10.1128/jb.180.21.5749-5755.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graumann P L, Marahiel M A. Cold shock proteins CspB and CspC are major stationary-phase-induced proteins in Bacillus subtilis. Arch Microbiol. 1999;171:135–138. doi: 10.1007/s002030050690. [DOI] [PubMed] [Google Scholar]
- 10.Graumann P L, Marahiel M A. Cold shock response in Bacillus subtilis. J Mol Microbiol Biotechnol. 1999;1:203–209. [PubMed] [Google Scholar]
- 11.Graumann P L, Marahiel M A. A superfamily of proteins that contain the cold-shock domain. Trends Biochem Sci. 1998;23:286–290. doi: 10.1016/s0968-0004(98)01255-9. [DOI] [PubMed] [Google Scholar]
- 12.Gualerzi C O, Pon C L. Initiation of mRNA translation in procaryotes. Biochemistry. 1990;29:5881–5889. doi: 10.1021/bi00477a001. [DOI] [PubMed] [Google Scholar]
- 13.Hanna M M, Liu K. Nascent RNA in transcription complexes interacts with CspE, a small protein in E. coli implicated in chromatin condensation. J Mol Biol. 1998;282:227–239. doi: 10.1006/jmbi.1998.2005. [DOI] [PubMed] [Google Scholar]
- 14.Harwood C R, Cutting S M. Molecular biological methods for Bacillus. New York, N.Y: Wiley; 1990. [Google Scholar]
- 15.Hu K H, Liu E, Dean K, Gingras M, DeGraff W, Trun N J. Overproduction of three genes leads to camphor resistance and chromosome condensation in Escherichia coli. Genetics. 1996;143:1521–1532. doi: 10.1093/genetics/143.4.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaacks K J, Healy J, Losick R, Grossman A D. Identification and characterization of genes controlled by the sporulation regulatory gene spo0H in Bacillus subtilis. J Bacteriol. 1989;171:4121–4129. doi: 10.1128/jb.171.8.4121-4129.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang W, Hou Y, Inouye M. CspA, the major cold-shock protein of Escherichia coli, is an RNA chaperone. J Biol Chem. 1997;272:196–202. doi: 10.1074/jbc.272.1.196. [DOI] [PubMed] [Google Scholar]
- 18.Jones P G, Krah R, Tafuri S, Wolffe A P. DNA gyrase, CS7.4, and the cold shock response in Escherichia coli. J Bacteriol. 1992;174:5798–5802. doi: 10.1128/jb.174.18.5798-5802.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaan T, Jurgen B, Schweder T. Regulation of the expression of the cold shock proteins CspB and CspC in Bacillus subtilis. Mol Gen Genet. 1999;262:351–354. doi: 10.1007/s004380051093. [DOI] [PubMed] [Google Scholar]
- 20.Kim L, Mogk A, Schumann W. A xylose-inducible Bacillus subtilis integration vector and its application. Gene. 1996;181:71–76. doi: 10.1016/s0378-1119(96)00466-0. [DOI] [PubMed] [Google Scholar]
- 21.Köhler P, Marahiel M A. Association of the histone-like protein HBsu with the nucleoid of Bacillus subtilis. J Bacteriol. 1997;179:2060–2064. doi: 10.1128/jb.179.6.2060-2064.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.La Taena A, Brandi A, Falconi M, Spurio R, Pon C, Gualerzi C O. Identification of a cold shock transcriptional enhancer of the Escherichia coli gene encoding nucleoid protein H-NS. Proc Natl Acad Sci USA. 1991;88:10907–10911. doi: 10.1073/pnas.88.23.10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis P J, Thaker S D, Errington J. Compartmentalization of transcription and translation in Bacillus subtilis. EMBO J. 2000;19:710–718. doi: 10.1093/emboj/19.4.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moriya S, Tsujikawa E, Hassan A K M, Asai K, Kodama T, Osagawara N. A Bacillus subtilis gene encoding protein homologous to eukaryotic SMC motor protein is necessary for chromosome partition. Mol Microbiol. 1998;29:179–187. doi: 10.1046/j.1365-2958.1998.00919.x. [DOI] [PubMed] [Google Scholar]
- 25.Phadtare S, Alsina J, Inouye M. Cold-shock response and cold-shock proteins. Curr Opin Microbiol. 1999;2:175–180. doi: 10.1016/S1369-5274(99)80031-9. [DOI] [PubMed] [Google Scholar]
- 26.Pogliano K, Hofmeister A E, Losick R. Disappearance of the sigma E transcription factor from the forespore and the SpoIIE phosphatase from the mother cell contributes to establishment of cell-specific gene expression during sporulation in Bacillus subtilis. J Bacteriol. 1997;179:3331–3341. doi: 10.1128/jb.179.10.3331-3341.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schindler T, Graumann P L, Perl D, Ma S, Schmid F X, Marahiel M A. The family of cold shock proteins of Bacillus subtilis. Stability and dynamics in vitro and in vivo. J Biol Chem. 1999;274:3407–3413. doi: 10.1074/jbc.274.6.3407. [DOI] [PubMed] [Google Scholar]
- 28.Schröder K, Graumann P, Schnuchel A, Holak T A, Marahiel M A. Mutational analysis of the putative nucleic acid-binding surface of the cold-shock domain, CspB, revealed an essential role of aromatic and basic residues in binding of single-stranded DNA containing the Y-box motif. Mol Microbiol. 1995;16:699–708. doi: 10.1111/j.1365-2958.1995.tb02431.x. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro L, Losick R. Dynamic spatial regulation in the bacterial cell. Cell. 2000;100:89–98. doi: 10.1016/s0092-8674(00)81686-4. [DOI] [PubMed] [Google Scholar]
- 30.Webb C D, Decatur A, Teleman A, Losick R. Use of green fluorescent protein for visualization of cell-specific gene expression and subcellular protein localization during sporulation in Bacillus subtilis. J Bacteriol. 1995;177:5906–5911. doi: 10.1128/jb.177.20.5906-5911.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willimsky G, Bang H, Fischer G, Marahiel M A. Characterization of cspB, a Bacillus subtilis inducible cold shock gene affecting viability at low temperatures. J Bacteriol. 1992;174:6326–6335. doi: 10.1128/jb.174.20.6326-6335.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woldringh C L, Jensen P R, Westerhoff H V. Structure and partitioning of bacterial DNA: determined by a balance of compaction and expansion forces? FEMS Microbiol Lett. 1995;131:235–242. doi: 10.1111/j.1574-6968.1995.tb07782.x. [DOI] [PubMed] [Google Scholar]
- 33.Wouters J A, Mailhes M, Rombouts F M, de Vos W M, Kuipers O P, Abee T. Physiological and regulatory effects of controlled overproduction of five cold shock proteins of Lactococcus lactis MG1363. Appl Environ Microbiol. 2000;66:3756–3763. doi: 10.1128/aem.66.9.3756-3763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamanaka K, Mitani T, Ogura T, Niki H, Hiraga S. Cloning, sequencing and characterization of multicopy suppressors of a mukB mutation in Escherichia coli. Mol Microbiol. 1994;13:301–312. doi: 10.1111/j.1365-2958.1994.tb00424.x. [DOI] [PubMed] [Google Scholar]