Abstract
Across its clinical development program, ocrelizumab demonstrated efficacy in improving clinical outcomes in multiple sclerosis, including annualized relapse rates and confirmed disability progression. However, as with any new treatment, it was unclear how this efficacy would translate into real‐world clinical practice. The objective of this study was to systematically collate the published real‐world clinical effectiveness data for ocrelizumab in relapsing remitting multiple sclerosis and primary progressive multiple sclerosis. A search strategy was developed in MEDLINE and Embase to identify articles reporting real‐world evidence in people with relapsing remitting multiple sclerosis or primary progressive multiple sclerosis receiving treatment with ocrelizumab. The search focused on English language articles only but was not limited by the country in which the study was conducted or the time frame of the study. Additional manual searches of relevant websites were also performed. Fifty‐two studies were identified reporting relevant evidence. Real‐world effectiveness data for ocrelizumab were consistently favorable, with reductions in relapse rate and disease progression rates similar to those reported in the OPERA I/OPERA II and ORATORIO clinical trials, including in studies with more diverse patient populations not well represented in the pivotal trials. Although direct comparisons are confounded by lack of randomization of treatments, outcomes reported suggest that ocrelizumab has a similar or greater efficacy than other therapy options. Initial real‐world effectiveness data for ocrelizumab appear favorable and consistent with results reported in clinical trials, providing clinicians with an efficacious option to treat patients with multiple sclerosis.
Introduction
Multiple sclerosis (MS) is an immune‐mediated demyelinating neurodegenerative disease reported to affect 2.8 million people worldwide in 2020. 1 The International Advisory Committee on Clinical Trials of MS defined four MS phenotypes: clinically isolated syndrome (CIS), relapsing remitting MS (RRMS), primary progressive MS (PPMS), and secondary progressive MS (SPMS). 2
Current therapies have had a major impact on the management of RRMS through reduction or elimination of relapses. However, disease progression independent of relapses often continues and current medicines are less efficacious in people with PPMS or SPMS. 3 Ocrelizumab is a humanized monoclonal antibody, the mechanism of action for which is based on the selective depletion of CD20+ B‐cells. In two Phase III clinical trials of ocrelizumab versus interferon beta‐1a (OPERA I and OPERA II), ocrelizumab significantly lowered annualized relapse rate (ARR) compared to interferon beta‐1a in RRMS patients. 4 The proportion of patients at 12 and 24 weeks with confirmed disability progression (CDP) also was significantly lower for patients treated with ocrelizumab. The ORATORIO trial found ocrelizumab to be associated with lower rates of confirmed disability progression compared to placebo in patients with PPMS at 12 and 24 weeks. 5
Although randomized controlled trials (RCTs) are typically considered the most reliable source of evidence on new treatments, patients enrolled in RCTs are not necessarily representative of patients in real‐world clinical practice. Therefore, to have a fuller understanding of the impact of ocrelizumab, it is important to consider real‐world evidence alongside the results of RCTs. Here, we report results from a systematic literature review (SLR) to identify and summarize published literature reporting on the real‐world effectiveness of ocrelizumab in RRMS and PPMS.
Methods
A search strategy with no time period restriction was developed in MEDLINE and Embase to identify articles reporting real‐world evidence relating to clinical effectiveness and health‐related quality of life (HRQoL) in people with RRMS or PPMS receiving treatment with ocrelizumab. The search strategies broadly included terms to search for the health condition of interest (“relapsing remitting multiple sclerosis,” “RRMS,” “primary progressive multiple sclerosis,” and “PPMS”) and terms to search for the intervention of interest (“ocrelizumab” and “Ocrevus”). The search included English language articles only but did not limit by country in which the study was conducted or the time frame of the study. The search captured full peer‐reviewed articles and conference abstracts published before 22nd March 2022, when searches were run. The full search strategy is provided in the data supplement (Method S1).
Study selection was performed using Covidence software (www.covidence.org). Screening was performed by two independent reviewers against the Population, Intervention, Comparison, Outcomes, and Study (PICOS) scope for applicability to the research questions. 6 The PICOS is provided in the data supplement (Table S1). For studies where the applicability was not certain, a third reviewer (JLP) performed additional evaluation and a consensus decision was made through discussion between the reviewers. Where sufficient information was provided, included studies were checked for duplication of reported data (e.g., abstracts and full texts reporting the same data).
Results
In total, 52 relevant studies that were consistent with the scope were included (Fig. 1). A summary of included study characteristics are provided in the data supplement (Table S2). Full text articles were available for 23 (44%) of the studies, while only conference abstracts were available for the remainder. Studies varied widely by geographical region; 33 reported real‐world outcomes in Europe, 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 seven in the United States, 40 , 41 , 42 , 43 , 44 , 45 , 46 two in the Middle East, 47 , 48 three in various geographical regions, 49 , 50 , 51 one in South America, 52 and six did not report the country in which the study was conducted. 53 , 54 , 55 , 56 , 57 , 58 The most frequently reported study populations included combined populations of RRMS, PPMS, and SPMS patients for which data were not stratified by type of MS (n = 20). Where reported, mean/median age ranged from 35 to 62, mean/median disease duration ranged from 2.8 to 18.7 years, and mean expanded disability status scale (EDSS) ranged from 2 to 6.5 at baseline (Table S3).
Figure 1.
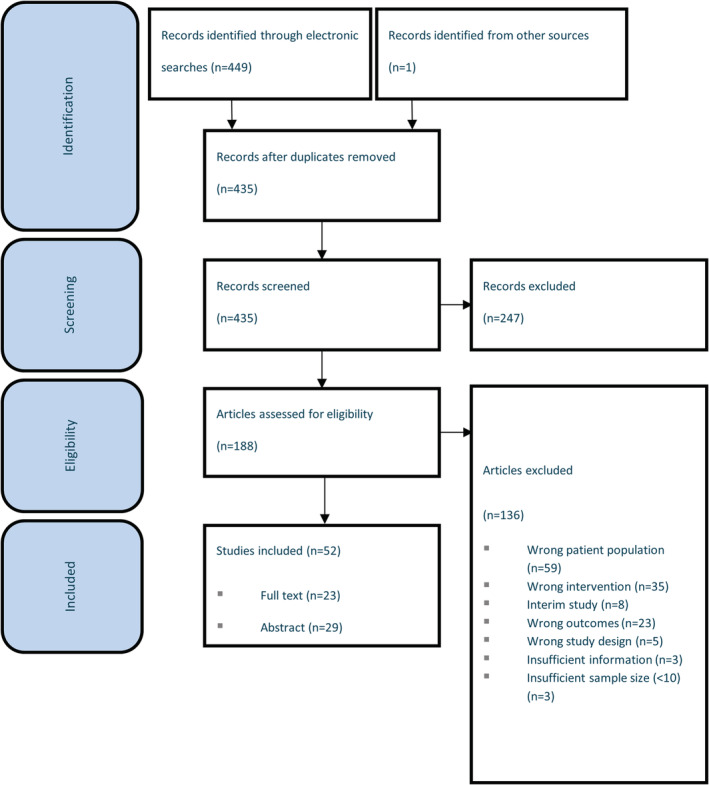
Preferred reporting items for systematic reviews and meta‐analyses (PRISMA) diagram.
Clinical relapse
The proportion of patients experiencing a clinical relapse following initiation of ocrelizumab treatment was low across the studies; all studies reported relapse in fewer than 20% of patients at follow‐up times ranging from 3 to 30 months, with the majority of studies reporting relapse in fewer than 10% of patients (Table S4). One study performed statistical analysis on relapse versus baseline and reported a statistically significant decrease in relapse activity in RRMS patients treated with ocrelizumab for a minimum of 12 months (patients with relapse in the 12 months prior to receiving ocrelizumab: 32 [62%], patients with relapse after at least 12 months of ocrelizumab [mean number of cycles received, 3.6]: 8 [15%], P = 0.01). 59 Two studies performed statistical analysis of relapse data between RRMS patients receiving different disease‐modifying therapies (DMTs) and both reported significantly fewer relapses in ocrelizumab‐treated patients. 8 , 39
Time to relapse
Six studies assessed time to first relapse in patients treated with ocrelizumab, with patient numbers ranging from 33 to 1104 8 , 18 , 28 , 34 , 39 , 51 , 58 (Table S5). Median time to first relapse following treatment initiation with ocrelizumab ranged from 52.5 days (411 RRMS patients switching to ocrelizumab following fingolimod treatment) 51 to 8.7 months (66 RRMS and PPMS patients switching to ocrelizumab following natalizumab treatment). 58 In the study reporting the shortest time to relapse, patients switching from fingolimod to ocrelizumab had relatively longer washout periods, possibly as a result of prescribers choosing to wait for lymphocyte counts to recover following fingolimod treatment before initiating ocrelizumab. This may account for the shorter time to relapse, as the study also reported that relapse incidence during washout was significantly higher in patients with longer washout periods, as was relapse incidence up to 1 year following ocrelizumab initiation. 51
Annualized relapse rate
Fourteen studies, with patient numbers ranging from 29 to 1104, assessed the change in ARR compared to baseline and all reported a numerical decrease in ARR following ocrelizumab initiation (Table S6). Three studies performed statistical analysis comparing follow‐up to baseline and all found the decrease in ARR to be statistically significant. 31 , 33 , 52 Two studies evaluated effects of ocrelizumab and other DMTs on ARR; ocrelizumab‐treated patients experienced significantly greater reductions in ARR compared with fingolimod‐treated patients (ocrelizumab: 0.12 vs fingolimod: 0.41, P = 0.04) 8 and had an estimated 51% lower risk of ARR compared with patients receiving cladribine (ExpB: 0.485, 95% CI: 0.264–0.893, P = 0.02). 39
Magnetic resonance imaging activity
Studies reporting magnetic resonance imaging (MRI) activity are summarized in the data supplement (Table S7). Statistical analysis of MRI activity compared with baseline was reported in two studies; Rojas et al reported a statistically significant decrease in the number of RRMS patients with T2 MRI activity following 12 months of ocrelizumab treatment (baseline: n = 41 [79%], 12 months: n = 18 [35%], P = 0.001) 52 and Sempere et al reported statistically significant decreases in the number of ocrelizumab‐treated patients with gadolinium‐enhancing lesions at 4–6 months (n = 1 [1%]) and 12 months (n = 0 [0%]) relative to baseline (n = 40 [57%], P < 0.001 for both). 31 Significantly fewer ocrelizumab‐treated patients had new T2 lesions 3–6 months following treatment initiation compared with fingolimod‐treated patients (ocrelizumab: n = 0 [0%], fingolimod: n = 5 [17%], P = 0.02) 8 and ocrelizumab patients also had a lower risk of MRI activity at 12 months compared with patients receiving cladribine (ExpB: 0.248, 95% CI: 0.065–0.948, P = 0.04). 39
Expanded disability status scale
Studies reporting EDSS data are summarized in the data supplement (Tables S8 and S9). Where reported, EDSS was assessed either by the treating neurologist or chart review. Five studies, in which patient numbers ranged from 35 to 110, performed statistical analysis of changes in absolute EDSS scores compared to baseline after receiving ocrelizumab; three reported no significant change at time points ranging from 6 months 33 , 56 to 1 year, 10 one reported significant improvement at 1 year, 21 and one reported progression in PPMS patients, but not RRMS patients, at a median follow‐up time of 2.09 years 19 (Table S8). One study assessed change in EDSS scores in different ethnicities exposed to ocrelizumab and found they remained stable across ethnic groups. 40 Based on change in EDSS score, CDP in RRMS patients was reported in 12 studies, with patient numbers ranging from 5 to 946 (Table S9): 11 studies reported CDP in fewer than 10% of patients (ranging from 0% to 9.5%) 8 , 13 , 17 , 23 , 28 , 30 , 31 , 39 , 47 , 51 , 52 and one study reported CDP in 20% of patients at a mean follow‐up of 5.6 months. 26 In PPMS patients, the rates of CDP were generally higher; the study by Sempere et al reported CDP in 5% (1/21) of patients with a mean follow‐up of 17 months 31 and the remaining six studies, in which patient numbers ranged from 16 to 48 and follow‐up ranged from 6 months to 2 years, reported CDP in 20.5%–37.5% of patients, 13 , 15 , 17 , 28 , 52 , 57 but the majority of patients in these studies experienced either improvement or no change in EDSS score. One study performed statistical analysis of changes in disability progression in RRMS and PPMS patients treated with ocrelizumab for a minimum of 12 months; compared to the proportion of patients experiencing EDSS progression in the year prior to receiving ocrelizumab (RRMS patients: n = 21 [40.4%], PPMS patients: n = 19 [65.5%]), a smaller proportion of patients progressed (RRMS patients: n = 2 [4%], PPMS patients: n = 9 [31%]), although no significant changes were reported in either patient group. 52
Evidence of disease activity
Four studies, with patient numbers ranging from 33 to 93, reported the proportion of patients with no evidence of disease activity (NEDA): >50% of patients treated with ocrelizumab for up to 2 years were classified as NEDA 13 , 17 , 31 , 47 (Table S10). Regarding evidence of disease activity (EDA), significantly fewer patients exhibited EDA when switched from natalizumab to ocrelizumab (n = 5 [15%]) compared with patients switched to fingolimod (n = 24 [56%], P < 0.001). 8
Health‐related quality of life
Three of the studies included presented HRQoL outcomes, with patient numbers ranging from 93 to 355 9 , 20 , 44 (Table S11). In the only study that carried out statistical tests of differences in HRQoL in ocrelizumab‐treated patients between baseline and subsequent time points, the Modified Fatigue Impact Scale (MFIS) suggested improvement with a mean change of −3.7 (p = 0.02) at 1 year. 44 No change in the Beck Depression Inventory‐II (BDI‐II) was observed. 44 In other studies, MFIS, BDI‐II, and the EuroQol‐5 Dimension Index (EQ‐5D) measure acquired at baseline and at 6 months remained stable, 9 while the Multiple Sclerosis Quality of Life‐54 (MSQoL‐54) measure was found to remain stable from baseline to 1 year. 20
Discussion
This SLR collates real‐world effectiveness evidence for ocrelizumab in RRMS and PPMS across 52 studies, summarizing data supporting the real‐world effectiveness of ocrelizumab for these indications.
Across most of the real‐world studies, baseline ARR and EDSS values and ARR and CDP outcomes following periods of ocrelizumab treatment were of similar magnitude to those reported by the pivotal OPERA I, 4 OPERA II, 4 and ORATORIO 5 trials (Figs. 2 and 3). Only the real‐world study by Rojas et al 52 reported an ARR numerically greater than that observed in the OPERA trials (0.23 vs 0.16) (Fig. 2). However, in this study, ARR values at baseline were slightly higher than in the trial and there was considerable uncertainty associated with the ARR estimate due to the small number of patients included and the relatively short follow‐up time (1 year). Similarly, the studies by Neo et al 26 and Fernandez‐Diaz et al 17 were the only ones reporting higher levels of CDP than in the RRMS trials and PPMS trials, respectively (Fig. 3). In both cases, these studies had higher baseline EDSS value than the trials and the other real‐world studies and the confidence intervals spanned the trial outcomes. These studies serve to illustrate how clinical outcomes in real‐world study populations can vary in relation to clinical trial cohorts.
Figure 2.
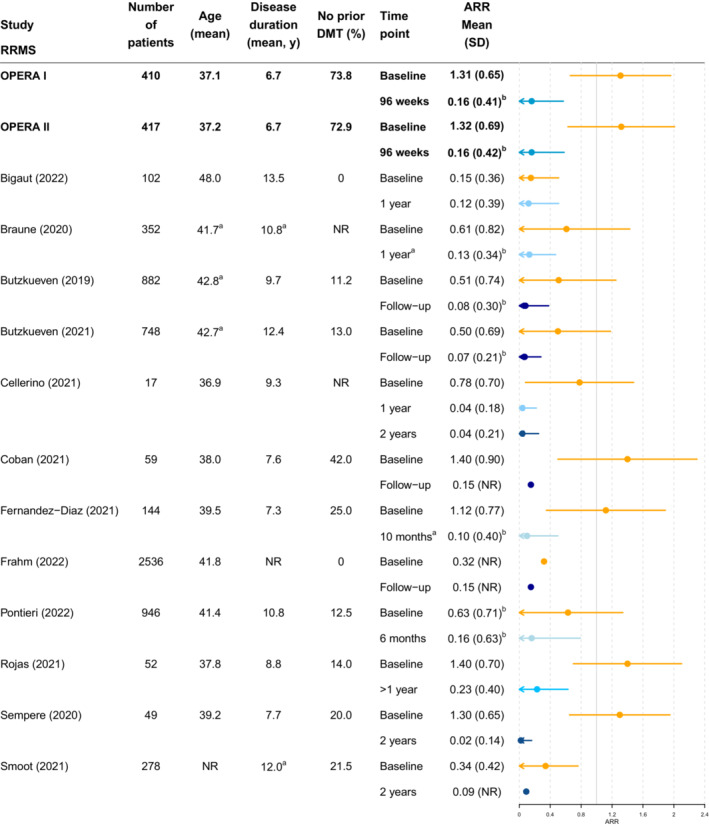
Annualized relapse rate in RRMS: clinical trials versus real‐world studies. Colors indicate comparable time points as indicated by the key. aMedians provided in figure as means not reported. bStandard deviations for ARR in OPERA I and II, Braune et al, 11 Butzkueven et al, 49 , 50 Fernandez‐Diaz et al, 17 and Pontieri et al 28 were back‐calculated from confidence intervals. ARR, annualized relapse rate; DMT, diseasemodifying therapy; EDSS, expanded disability status scale; NR, not reported; RRMS, relapsing remitting multiple sclerosis; SD, standard deviation.
Figure 3.
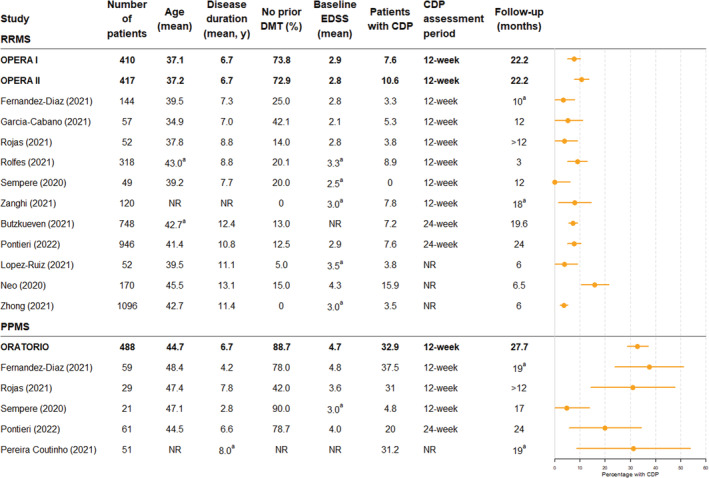
Percentage of patients with confirmed disability progression (CDP): clinical trials versus real‐world studies. Confidence intervals for confirmed disability progression percentages were calculated by assuming that the data follow a binomial distribution, except for Sempere et al, 31 in which the rule of three was used. aMedians provided in figure as means not reported. CDP, confirmed disability progression; DMT, disease‐modifying therapy; EDSS, expanded disability status scale; NR, not reported; PPMS, primary progressive multiple sclerosis; RRMS, relapsing remitting multiple sclerosis.
In the studies by Frahm et al 18 and Bigaut et al, 8 a low ARR at baseline relative to the trial and other real‐world studies was observed, which may be driven partly by the absence of treatment‐naïve patients in these studies. Notably, in the studies by Frahm et al and Bigaut et al, which capture patient groups that differ to those in the pivotal trials, ARR values following ocrelizumab were numerically lower than at baseline and of a similar magnitude to those in the other real‐world studies and the trials.
The study by Pereira‐Coutinho et al attempted to directly assess the applicability of ocrelizumab clinical trial data to a real‐world setting by splitting their retrospective cohort into a “control group” that mirrored the clinical trial cohorts (aged 18–55 years, baseline EDSS 3–6.5, symptom duration of less than 15 years for a baseline EDSS>5 or less than 10 years if baseline EDSS<5) and an “expanded group” which consisted of patients that did not meet these criteria. 57 The study found no significant differences in EDSS progression, time until EDSS progression, or secondary outcomes (timed‐25‐foot‐walk, 9‐hole‐peg test, and MRI activity) between the groups, although sample size was small (n = 16 in the control group and n = 35 in the expanded group). In general, the real‐world studies identified by this SLR included fewer treatment‐naïve patients, and several studies reported on study populations with a higher mean age, compared with the pivotal clinical trials (Figs. 2 and 3). Included studies also spanned various geographical regions and likely therefore included study populations of varying races and ethnicities, although this information was not specifically reported in the vast majority of studies. Despite the diversity of the real‐world study populations, effectiveness outcomes appear similar to the OPERA and ORATORIO trials. Three studies noted the similarity of clinical outcomes in real‐world settings with clinical trials in the discussion of their results, 40 , 48 , 52 although comparisons between real world and clinical trial populations should be treated with caution.
In addition to demonstrating favorable real‐world effectiveness that appear to be consistent with clinical trial data, this SLR highlights other important aspects of real‐world ocrelizumab use in RRMS and PPMS patients; three studies report stable or improving HRQoL following up to 1 year of ocrelizumab treatment, 9 , 20 , 44 five studies reported on the clinical effectiveness of switching to ocrelizumab from natalizumab, 8 , 25 , 36 , 39 , 58 and three further studies described the effective and safe transition to ocrelizumab from other DMTs, 16 , 18 , 27 highlighting ocrelizumab as a suitable option for previously treated patients that can mitigate the risk of disease reactivation.
Only six studies captured outcome data for both ocrelizumab and other treatments, which included rituximab, cladribine, fingolimod, dimethyl fumarate, natalizumab, and teriflunomide. 7 , 8 , 9 , 35 , 37 , 39 Two of the comparative studies reported significantly superior clinical outcomes in ocrelizumab‐treated patients; Bigaut et al reported significantly fewer relapses, a greater reduction in ARR, fewer patients with new T2 lesions, and fewer patients with EDA when treated with ocrelizumab compared with fingolimod 8 and Zanghi et al reported significantly fewer relapses compared to rituximab‐ and cladribine‐treated patients, decreased risk of ARR and MRI activity compared to cladribine‐treated patients only, and similar rates of infection and adverse events across all treatment groups. 39 However, all of the comparative studies are relatively small and may be impacted by bias, particularly due to residual confounding. For example, in the study by Bigaut et al, which compared DMTs following natalizumab cessation, time on natalizumab was longer in the ocrelizumab‐treated group and patient follow‐up times varied in the study by Zanghi et al. Rituximab‐treated patients also had a higher mean number of prior DMTs compared to ocrelizumab patients (1.7 vs 1.3) in the study by Zanghi et al, which may account for the better relapse outcome with ocrelizumab, although the difference in number of prior DMTs was not statistically significant. Other studies have reported varying results comparing rituximab with ocrelizumab, with some reporting lack of non‐inferiority of rituximab versus ocrelizumab 60 and others reporting no significant difference in clinical outcomes. 7 As a result, comparative effectiveness conclusions are difficult to confidently draw from these studies or from real‐world studies in general. In order to best support patients and healthcare professionals in selecting an optimum therapeutic approach in RRMS and PPMS, further work is needed to generate evidence on the comparative effectiveness of the available therapies in the form of prospective comparative cohort studies with matched pairs. 61 Any such work should seek to utilize best practices in the design and execution of real‐world studies.
Challenges encountered in synthesizing the literature include heterogeneity in study design, study populations and outcome definitions, lack of information available in conference abstracts, the lack of statistical tests for improvements in outcome, and the lack of studies comparing ocrelizumab with other DMTs. The limited information reported in some studies also precludes a thorough deduplication of studies, as such some studies capturing overlapping patient populations may have been included. While these issues render comparisons across studies challenging, the consistency of results observed is notable. In order to facilitate future syntheses of real‐world evidence on ocrelizumab, future studies should seek to use standardized outcome definitions and publish results in accordance with relevant reporting standards.
To conclude, this SLR demonstrates that initial real‐world effectiveness data for ocrelizumab appear favorable and similar to the results reported in the OPERA I/OPERA II and ORATORIO clinical trials, including in studies with relatively diverse patient populations across different geographical locations where baseline disease characteristics differed to the pivotal clinical trials. Although direct comparisons may be confounded by lack of randomization or patient matching, outcomes reported suggest that ocrelizumab has a similar or greater efficacy than other therapy options. However, the majority of studies identified reported outcomes based on ocrelizumab exposure times of 1 year or less. Therefore, further work will be required to monitor the long‐term impact of ocrelizumab in RRMS and PPMS patients as larger, comparative real‐world studies with longer follow‐up are published.
Author Contributions
Sreeram Ramagopalan, Cormac Sammon, and Donna Fountain PhD (employee of PHMR Ltd) were responsible for study conception and design. John L Petrie, Alexandra Ellicott MPH (employee of PHMR Ltd), and Emily Robertshaw MSc (employee of PHMR Ltd) performed screening of studies and data extraction. Emily Robertshaw also performed data visualization for Figures 2 and 3. John L Petrie and Cormac Sammon drafted the manuscript. All authors reviewed and edited the manuscript.
Conflict of Interest
Xavier Montalban has received speaking honoraria and travel expenses for participation in scientific meetings, has been a steering committee member of clinical trials or participated in advisory boards of clinical trials in the past years with Abbvie, Actelion, Alexion, Biogen, Bristol‐Myers Squibb/Celgene, EMD Serono, Genzyme, Hoffmann‐La Roche, Immunic, Janssen Pharmaceuticals, Medday, Merck, Mylan, Nervgen, Novartis, Sandoz, Sanofi‐Genzyme, Teva Pharmaceutical, TG Therapeutics, Excemed, MSIF, and NMSS. Paul Matthews has received consultancy fees from Novartis and Biogen. He has received honoraria or speakers' fees from Novartis and Biogen and has received research or educational funds from Biogen, Novartis, Merck, and Bristol Myers Squibb. Alex Simpson is an employee of F Hoffmann‐La Roche. John Petrie and Cormac Sammon are employees of PHMR Ltd, which received financial support from F Hoffmann‐La Roche for the work, including the development of the literature review and preparation of the manuscript. Sreeram Ramagopalan is an employee of F Hoffmann‐La Roche. Ente Ospedaliero Cantonale (employer) received compensation for Giulio Disanto: financial support for speaking, educational, or travel grants from Biogen Idec, Sanofi Genzyme, Roche, Merck, and Novartis. The submitted work is not related to these agreements. Jens Kuhle received speaker fees, research support, travel support, and/or served on advisory boards by Swiss MS Society, Swiss National Research Foundation (320030_189140/1), University of Basel, Progressive MS Alliance, Bayer, Biogen, Bristol Myers Squibb, Celgene, Merck, Novartis, Octave Bioscience, Roche, and Sanofi.
Supporting information
Table S1
Acknowledgements
This study was funded by F. Hoffmann‐La Roche. F. Hoffmann‐La Roche contributed to the study conception, study design, and editing of the manuscript.
Funding Statement
This work was funded by F. Hoffmann‐La Roche .
References
- 1. Multiple Sclerosis International Federation . Atlas of MS 3rd edition 2020.
- 2. Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hauser SL, Cree BAC. Treatment of multiple sclerosis: a review. Am J Med. 2020;133(12):1380‐90.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hauser SL, Bar‐Or A, Comi G, et al. Ocrelizumab versus interferon Beta‐1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221‐234. [DOI] [PubMed] [Google Scholar]
- 5. Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376(3):209‐220. [DOI] [PubMed] [Google Scholar]
- 6. Counsell C. Formulating questions and locating primary studies for inclusion in systematic reviews. Ann Intern Med. 1997;127(5):380‐387. [DOI] [PubMed] [Google Scholar]
- 7. Alcala C, Quintanilla‐Bordas C, Gascon F, et al. Effectiveness of rituximab vs. ocrelizumab for the treatment of primary progressive multiple sclerosis: a real‐world observational study. J Neurol. 2022;269:3676‐3681. [DOI] [PubMed] [Google Scholar]
- 8. Bigaut K, Kremer L, Fabacher T, et al. Ocrelizumab versus fingolimod after natalizumab cessation in multiple sclerosis: an observational study. J Neurol. 2022;269:3295‐3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bossart J, Kamm CP, Kaufmann M, et al. Real‐world disease‐modifying therapy usage in persons with relapsing‐remitting multiple sclerosis: cross‐sectional data from the swiss multiple sclerosis registry. Mult Scler Relat Disord. 2022;60:103706. [DOI] [PubMed] [Google Scholar]
- 10. Braune S, Bluemich S, Bruns C, et al. Real world experience with ocrelizumab in patients with primary progressive multiple sclerosis: insights from the German neuro trans data registry. Mult Scler J. 2021;27(2 Suppl):210‐211. [Google Scholar]
- 11. Braune S, Heer Y, Tozzi V, et al. Real‐world experience with ocrelizumab in the German neurotransdata registry. Mult Scler J. 2020;26(3 Suppl):69‐70. [Google Scholar]
- 12. Buttmann M, Meuth S, Weber M, et al. The effectiveness of ocrelizumab in real‐world patients with relapsing multiple sclerosis over 18 months‐interim analysis of the CONFIDENCE study. Mult Scler J. 2021;27(2 Suppl):684‐685. [Google Scholar]
- 13. Cellerino M, Boffa G, Lapucci C, et al. Predictors of ocrelizumab effectiveness in patients with multiple sclerosis. Neurotherapeutics. 2021;18(4):2579‐2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cellerino M, Mancuso E, Boffa G, et al. Effect of ocrelizumab treatment on retinal atrophy: preliminary results from a single‐center prospective observational study. Mult Scler J. 2021;27(2 Suppl):501‐502. [Google Scholar]
- 15. Daniels K, van der Nat PB, Frequin S, et al. Real‐world results of ocrelizumab treatment for primary progressive multiple sclerosis. Mult Scler Int. 2020;2020:5463451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ellwardt E, Rolfes L, Klein J, et al. Ocrelizumab initiation in patients with MS: a multicenter observational study. Neurol Neuroimmunol Neuroinflamm. 2020;7(4):e719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fernandez‐Diaz E, Perez‐Vicente JA, Villaverde‐Gonzalez R, et al. Real‐world experience of ocrelizumab in multiple sclerosis in a Spanish population. Ann Dent. 2021;8(2):385‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Frahm N, Fneish F, Ellenberger D, et al. Therapy switches in fingolimod‐treated patients with multiple sclerosis: long‐term experience from the German MS registry. Neurol Ther. 2022;11(1):319‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guerra T, Caputo F, Bollo L, Iaffaldano P, Paolicelli D, Trojano M. Effectiveness and safety of ocrelizumab in a real‐world setting: A single center experience from southern italy. Journal of the Neurological Sciences. Conference: World Congress of Neurology (WCN 2021); Rome Italy; 429 Supplement; 2021.
- 20. Impellizzeri M, Moiola L, Robotti M, et al. A 1 year follow‐up study of an italian cohort of primary progressive multiple sclerosis (PPMS) patients treated with ocrelizumab: changes of clinical, radiological, psychological and biological markers. Eur J Neurol. 2019;26(Suppl 1):662. [Google Scholar]
- 21. Lanzillo R, Carotenuto A, Moccia M, et al. Ocrelizumab treatment in multiple sclerosis: Prospective real world observational multi‐center study in Campania, Italy. Journal of the Neurological Sciences. Conference: World Congress of Neurology (WCN 2021); Rome Italy; 429 Supplement; 2021.
- 22. Lopez Ruiz R, Dotor Garcia‐Soto J, Hiraldo JDG, et al. Real world experience with ocrelizumab in patients with primary progressive multiple sclerosis. Mult Scler J. 2020;26(3 Suppl):301‐302. [Google Scholar]
- 23. Lopez Ruiz R, Eichau S, Guerra Hiraldo JD, Dotor Garcia‐Soto J, Ruiz De Arcos M, Ruiz‐Pena JL. Real world data on the use of ocrelizumab. Incidence of lymphopenia, B‐cell and immunoglobulins evolution. Mult Scler J. 2021;27(2 Suppl):631. [Google Scholar]
- 24. Magyari M, Pontieri L, Blinkenberg M, et al. Early experience with ocrelizumab in Denmark. A population‐based registry study. Mult Scler J. 2020;26(3 Suppl):530‐531. [Google Scholar]
- 25. Mancinelli CR, Scarpazza C, Cordioli C, et al. Switching to ocrelizumab in RRMS patients at risk of PML previously treated with extended interval dosing of natalizumab. Mult Scler. 2021;27(5):790‐794. [DOI] [PubMed] [Google Scholar]
- 26. Neo C, Singh‐Curry V, Nandoskar A, et al. Real‐world data on the use of ocrelizumab, among MS patients: B‐cell suppression and clinical outcomes. Mult Scler J. 2020;26(3 Suppl):185‐186. [Google Scholar]
- 27. Pasanisi MB, Vercellino M, Schillaci V, et al. Switching to ocrelizumab from other second line treatments in relapsing‐remitting multiple sclerosis: a single center cohort. Mult Scler J. 2020;26(3 Suppl):311‐312. [Google Scholar]
- 28. Pontieri L, Blinkenberg M, Bramow S, et al. Ocrelizumab treatment in multiple sclerosis: a Danish population‐based cohort study. Eur J Neurol. 2022;29(2):496‐504. [DOI] [PubMed] [Google Scholar]
- 29. Prockl V, Nickel FT, Utz KS, et al. Real world application of ocrelizumab in multiple sclerosis: single‐center experience of 128 patients. J Neurol Sci. 2020;415:116973. [DOI] [PubMed] [Google Scholar]
- 30. Rolfes L, Pawlitzki M, Pfeuffer S, et al. Ocrelizumab extended interval dosing in multiple sclerosis in times of COVID‐19. Neurol Neuroimmunol Neuroinflamm. 2021;8(5):e1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sempere AP, Berenguer‐Ruiz L, Borrego‐Soriano I, et al. Ocrelizumab in multiple sclerosis: a real‐world study from Spain. Front Neurol. 2020;11:592304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Signoriello E, Bonavita S, Di Pietro A, et al. BMI influences CD20 kinetics in multiple sclerosis patients treated with ocrelizumab. Mult Scler Relat Disord. 2020;43:102186. [DOI] [PubMed] [Google Scholar]
- 33. Signoriello E, Lus G, Bonavita S, et al. Switch from sequestering to anti‐CD20 depleting treatment: disease activity outcomes during wash‐out and in the first 6 months of ocrelizumab therapy. Mult Scler. 2022;28(1):93‐101. [DOI] [PubMed] [Google Scholar]
- 34. Toorop AA, van Lierop ZYGJ, Strijbis EMM, et al. The wearing‐off phenomenon of ocrelizumab in patients with multiple sclerosis. Mult Scler Relat Disord. 2022;57:103364. [DOI] [PubMed] [Google Scholar]
- 35. Treffts J, Van Kesteren Y, Thiel S, et al. Short term relapse risk after switching from natalizumab to ocrelizumab or cladribine‐An international cohort study. Neurology Conference: 73rd Annual Meeting of the American Academy of Neurology, AAN; 2021;96(15 Suppl 1).
- 36. Tsantes E, Curti E, Fiore A, Bazzurri V, Granella F. Ocrelizumab as exit strategy from natalizumab: results from a clinical series. Mult Scler J. 2020;26(3 Suppl):294. [Google Scholar]
- 37. Van Kesteren Y, Treffts J, Thiel S, et al. Short term relapse risk after switching from fingolimod to ocrelizumab orcladribine‐An international cohort study. Neurology Conference: 73rd Annual Meeting of the American Academy of Neurology, AAN; 2021;96(15 Suppl 1).
- 38. van Lierop ZYGJ, Toorop AA, van Ballegoij WJC, et al. Personalized B‐cell tailored dosing of ocrelizumab in patients with multiple sclerosis during the COVID‐19 pandemic. Mult Scler J. 2022;28(7):1121‐1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zanghi A, Gallo A, Avolio C, et al. Exit strategies in natalizumab‐treated RRMS at high risk of progressive multifocal leukoencephalopathy: a multicentre comparison study. Neurotherapeutics. 2021;18(2):1166‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Coban H, Germaine S, Dimaandal I, et al. Real‐world experience of ocrelizumab initiation in a diverse multiple sclerosis population. Mult Scler Relat Disord. 2021;53:103021. [DOI] [PubMed] [Google Scholar]
- 41. Fan J, Bilello M, Shinohara R, Bar‐Or A, Schindler M. Ocrelizumab infusion delays and radiologic relapse in multiple sclerosis. Mult Scler J. 2021;27(2 Suppl):691‐692. [Google Scholar]
- 42. Geils H, Stribling I, Katz J, Lathi E, Douglas E, Bouley A. Acapella: real‐world experience with ocrelizumab: an observational study evaluating safety in patients with relapsing and progressive ms year three. Mult Scler J. 2020;26(3 Suppl):256. [Google Scholar]
- 43. Smoot K, Chen C, Lucassen E, Stuchiner T, Grote L, Cohan S. Utilization, safety and tolerability of ocrelizumab: year 1 data from the Providence ocrelizumab registry. Mult Scler J. 2018;24(2 Suppl):288. [Google Scholar]
- 44. Smoot K, Chen C, Stuchiner T, Lucas L, Grote L, Cohan S. Clinical outcomes of patients with multiple sclerosis treated with ocrelizumab in a US community MS center: an observational study. BMJ Neurol. 2021;3(2):e000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vollmer B, Ijadi N, Declusin A, et al. Two‐year real‐world experience with ocrelizumab in the treatment of patients with multiple sclerosis. Mult Scler J. 2021;27(2 Suppl):595‐596. [Google Scholar]
- 46. Vollmer B, Nair K, Sillau S, Corboy J, Vollmer T, Alvarez E. Ocrelizumab real‐world safety and effectiveness in the one year treatment of multiple sclerosis compared to other disease modifying therapies. Mult Scler J. 2019;25(Suppl 2):747‐748. [Google Scholar]
- 47. Garcia‐Canibano B, Ouanes S, Ganesan GS, et al. Real‐world experience of ocrelizumab in multiple sclerosis in an Arab population. J Drug Assess. 2021;10(1):106‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yousuf W, Ganesan GS, Humos B, Baig T, Canibano B, Deleu D. Real‐world experience with Ocrelizumab in multiple sclerosis patients: two years follow up in Qatar. Neurology Conference: 73rd Annual Meeting of the American Academy of Neurology, AAN; 2021;96(15 Suppl 1).
- 49. Butzkueven H, Spelman T, Ozakbas S, et al. Real‐world experience with ocrelizumab in relapsing multiple sclerosis: insights from the MSOCR‐R cohort, an MS Base registry sub‐study. Mult Scler J. 2021;27(2 Suppl):104‐106. [Google Scholar]
- 50. Butzkueven H, Spelman T, Patti F, et al. Real‐world experience with ocrelizumab in the MSBase registry. Mult Scler J. 2019;25(Suppl 2):539‐540. [Google Scholar]
- 51. Zhong M, van der Walt A, Stankovich J, et al. Prediction of multiple sclerosis outcomes when switching to ocrelizumab. Mult Scler J. 2021;28:958‐969. [DOI] [PubMed] [Google Scholar]
- 52. Rojas JI, Patrucco L, Fruns M, et al. Real‐world experience of ocrelizumab in multiple sclerosis patients in Latin America. Arq Neuropsiquiatr. 2021;79(4):305‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Miscioscia A, Puthenparampil M, Pengo M, Miante S, Gallo P. Optical coherence tomography in primary progressive multiple sclerosis: INL thinning as a possible biomarker of response to ocrelizumab. Mult Scler J. 2021;27(2 Suppl):503. [Google Scholar]
- 54. Moss BP, Utigard E, Baldassari LE, Cohen JA, Ontaneda DD. Real‐world experience with ocrelizumab. Mult Scler J. 2019;25(Suppl 1):69‐70. [Google Scholar]
- 55. Novi G, Cellerino M, Nesi L, et al. Ocrelizumab in an expanded cohort of primary progressive MS patients: safety outcomes and clinical/ paraclinical follow‐up. Mult Scler J. 2018;24(2 Suppl):901‐902. [Google Scholar]
- 56. Ozakbas S, Ozcelik S, Kaya E, Ozdogar AT, Sagici O, Baba C. Early treatment response of ocrelizumab in persons with multiple sclerosis: six‐month results. Mult Scler J. 2021;27(2 Suppl):278. [Google Scholar]
- 57. Pereira Coutinho M, Leitao L, Ladeira F, et al. Ocrelizumab‐time to expand borders? Eur J Neurol. 2021;28(Suppl 1):916. [Google Scholar]
- 58. Smoot K, Gervasi‐Follmar T, Marginean H, Chen C, Zhang HJ, Repovic P. Evaluating the efficacy and safety of transitioning patients from natalizumab to ocrelizumab. Mult Scler J. 2021;27(2 Suppl):653. [DOI] [PubMed] [Google Scholar]
- 59. Rojas J, Patrucco L, Fruns M, et al. Real‐world experience of ocrelizumab in multiple sclerosis patients in Latin America. Mult Scler J. 2020;26(3 Suppl):548. [Google Scholar]
- 60. Roos I, Hughes S, Macdonell G. A non‐inferiority study of rituximab versus ocrelizumab in relapsing‐remitting multiple sclerosis. 2022 ECTRIMS Congress; October 26–28; Amsterdam, Netherlands; 2022.
- 61. Habs M. Prospective, comparative cohort studies and their contribution to the benefit assessments of therapeutic options: heart failure treatment with and without hawthorn special extract WS 1442. Forsch Komplementarmed Klass Naturheilkd. 2004;11(Suppl 1):36‐39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


