Abstract
Talaromycosis (penicilliosis) is an invasive mycosis endemic in tropical and subtropical Asia. Talaromycosis primarily affects individuals with advanced HIV disease and other immunocompromised conditions and disproportionally affects people in low and middle-income countries, particularly agricultural workers in rural areas during their most economically-productive years. Approximately 17,300 talaromycosis cases and 4,900 deaths occur annually. Talaromycosis is highly associated with the tropical monsoon season, where flooding and cyclones can exacerbate the poverty-inducing potential of the disease. Talaromycosis can present as localised or disseminated disease, the latter causing cutaneous lesions that are disfiguring and stigmatising. Despite a mortality of up to one in three diagnosed cases, talaromycosis has received little attention and investment from regional and global funders, policy-makers, researchers, and industry. Diagnostic and treatment modalities remain extremely limited. This paper is a global call for talaromycosis to be recognised as a Neglected Tropical Disease to alleviate its impact on vulnerable populations.
Introduction
In 2005, the World Health Organization (WHO) established a list of Neglected Tropical Diseases (NTDs). The list included a diverse group of largely chronic, parasitic, tropical infections that disproportionately impact people living in poverty.1 Fungal diseases were not included on the NTD list until 2016 when the WHO expanded the list to include mycetoma, chromoblastomycosis, and an undefined category of ‘other deep mycoses’. In January 2021 the WHO released the 2021–2030 road map for NTDs, a visionary framework that emphasises impact measurement, cross-cutting programming, and country-driven policy.2 The 2021–2030 NTD road map has, for the first time, named paracoccidioidomycosis and sporotrichosis among the ‘deep mycoses’. The editors of PLOS Neglected Tropical Diseases (PLOS NTD) have also increasingly recognised neglected fungal diseases, adding histoplasmosis and cryptococcosis ‘on the cusp’ of the PLOS NTD list in 2020.3
Talaromycosis is an invasive fungal infection with a high mortality, killing up to one in three diagnosed individuals.4,5 As with other NTDs, talaromycosis disproportionally affects people living in poverty in the tropical and subtropical zones of Asia. It is not currently recognised as an NTD despite being quintessentially so. Talaromycosis satisfies all NTD criteria identified by the WHO, PLOS NTD, and the Food and Drug Administration (FDA), and warrants recognition by these global entities. Talaromycosis has been neglected by local, regional, and international clinicians, researchers, funders, and public health organisations; this multi-level neglect is the main barrier to reducing its significant morbidity and mortality. This paper brings together physicians and scientists working in talaromycosis endemic areas, policy-makers, and fungal experts to elevate the profile of this neglected mycosis. We present a global viewpoint on why talaromycosis should be considered an NTD by the WHO, PLOS NTD, and the Tropical Disease Priority Review Voucher Programme of the FDA.
Talaromycosis is a tropical infectious disease with high morbidity and mortality
Talaromycosis (formerly penicilliosis) is caused by the thermally dimorphic fungus Talaromyces marneffei (Tm) that is endemic in the tropical and subtropical regions of Asia (Appendix Figure 1). Tm has a reservoir in wild bamboo rats living in the highlands of endemic regions and in the soil associated with the bamboo rats. Human infection is presumed to occur via inhalation of Tm spores from the environment.6 The HIV pandemic has led to a rapid rise in global incidence, particularly in the hyperendemic areas of Southeast Asia (Thailand, Vietnam, Myanmar), East Asia (southern China, Hong Kong, Taiwan), and north-eastern India6. Although prevalence in the general population is unknown, talaromycosis has a pooled prevalence of 3·6% among people living with HIV, ranging between 0·1% to 19·6%, depending on the geographic region and country (Appendix Table 1).7 A total of 288,000 cases have been reported in 33 countries to the end of 2018, with an estimated 17,300 cases (95% confidence interval [CI]: 9,900 – 23,700) and 4,900 deaths (95% CI: 2,500 – 7,300) a year.8 The highest reported incidence of talaromycosis is in China, Thailand, and Vietnam, where it is the third most common opportunistic infection and a leading cause of HIV-associated death, surpassing tuberculosis and cryptococcal meningitis.4,5
Most individuals diagnosed with talaromycosis are immunocompromised, but apparently healthy individuals can develop talaromycosis, albeit rarely.9 Disease can be localised to the upper or lower respiratory tract,10–12 bones, joints, and intestinal tract, or disseminated to multiple organ systems.13 Advanced HIV disease (defined by the WHO as a CD4 cell count <200 cells/m3) is a major risk factor for talaromycosis. Patients with advanced HIV disease commonly present with disseminated disease involving the lungs, liver, spleen, gastrointestinal tract, blood stream, skin, and bone marrow.4,5,13 Talaromycosis is not limited to people living with HIV. It is increasingly diagnosed in people with other immunosuppressing conditions, including the primary immunodeficiency condition due to interferon-gamma autoantibodies, auto-immune diseases, malignancy, and solid organ and bone marrow transplantations.13,14 Non-HIV-infected individuals are less likely to have skin lesions (44% vs. 71%)5,14 and positive blood cultures (47% vs. 77%).15 As a result, diagnosis is delayed (180 days vs. 45 days),16 and mortality is higher (29% vs. 21%) compared to HIV-infected individuals.15 Similar to some other endemic mycoses, primary pulmonary talaromycosis has been described in apparently healthy individuals.9,17 The diverse manifestations of primary pulmonary infections include tracheal and endobronchial lesions that can cause airway collapse (Appendix Figure 2a),18 alveolar consolidation, cavitary lung disease, solitary or multiple nodules, mediastinal lymphadenopathy, and pleural effusion (Appendix Figure 2b–c).19 Individuals with underlying structural lung disease, such as chronic obstructive pulmonary disease, lung malignancy, and cavitation associated with tuberculosis or sarcoidosis, are at risk for pulmonary infection.9,17,20 These cases suggest that talaromycosis may be a more common cause of pneumonia in endemic areas than currently recognised.
Talaromycosis is a tropical infectious disease trapped in a cycle of poverty, stigma, and neglect
The WHO has four criteria for inclusion on the NTD list (Appendix Table 2). Talaromycosis fulfils all four criteria as detailed below.
1). Talaromycosis causes stigma, morbidity and mortality among impoverished people
Several predisposing factors of talaromycosis are inextricably linked with poverty. The endemic region of talaromycosis consists almost entirely of low and middle income countries (LMIC) (Appendix Figure 1), with most countries falling into the lower middle income category.21 Many people in Tm hyperendemic regions live in poor, rural areas. For example, 63% of the Vietnamese population and 67% of southern China’s Guangxi province live rurally.22,23 Although the soil-burrowing bamboo rats are the enzootic reservoir of Tm, human infection is neither linked to bamboo rat exposure nor consumption. Rather, infection is linked to occupational exposure to crops or livestock, and travel to highland farming areas.6,24,25 Farmers have a 70% to 90% greater odds of developing talaromycosis than non-farmers,24,25 and residence in or any travel of more than three days duration to highland communities is associated with a three-fold greater odds of disease.25 Talaromycosis disproportionally affects rural communities and agricultural workers due to prolonged exposure to soil in the endemic regions.24 The disease predominantly affects young people during their peak years of economic productivity,4,5,13 burdening the families of primary wage-earners in rural communities. These factors, combined with the high cost of treatment (average USD $1,300, approximately one-year salary for an average farmer in Vietnam), exacerbates the poverty inducing potential of talaromycosis.
Talaromycosis is characterised by disfiguring cutaneous lesions that predominate on the face and extremities (Appendix Figure 3). They are a feature of disseminated infection which prompts hospital admission and epitomise the visually stigmatising nature of the disease. The pathogen, Talaromyces marneffei, and the disease, talaromycosis, are difficult to pronounce, further hampering efforts to bring the disease on to national health agendas in endemic countries. Although talaromycosis has been associated with HIV, it has not benefited from the overall decline in HIV incidence in Asia nor from funding through HIV programmes. In a recent estimate of the global burden of talaromycosis, the incidence is projected to increase by 35% by 2025.8 This is driven by the persistent or rising incidence of advanced HIV disease among people newly diagnosed with HIV in Indonesia, Thailand, Vietnam, the Philippines, and China.26 Despite improved access to antiretroviral therapy across Asia, talaromycosis mortality remains unchanged,4 suggesting diagnostic delay and access to HIV services remain significant barriers to survival.
2). Talaromycosis primarily occurs in tropical or subtropical regions of Asia
The endemicity of talaromycosis includes most countries in the tropical and subtropical zones of Asia (Appendix Figure 1). The tropical ecosystems in Southeast Asia are among the most vulnerable areas in the world to climate change due to a large low-income population, an economy dependent on natural resources and agriculture, and the threat of climate-related disasters including floods and typhoons.27 Talaromycosis is highly associated with the tropical monsoon weather with disease incidence increasing by 30% to 73% during the rainy months in Thailand, Vietnam, and southern China.5,28 This remarkable seasonality is driven by high humidity,29 which likely promotes infection through poor air quality and expansion of the fungal reservoir in the environment. The monsoon season brings intense, prolonged rainfall and floods, and the warmer oceans feed tropical cyclones which threaten agriculture production and food supply. These climate factors exacerbate the impact of talaromycosis on the rural poor and are likely to increase with global warming.30
3). Research support for talaromycosis has been insufficient to determine optimum diagnosis and treatment
Diagnosis.
Current culture-based diagnosis is slow and insensitive. Blood culture takes up to 14 days for identification, only detects disease in its advanced stage, and misses up to 50% of infections.4,5,15 Although a presumptive diagnosis can be made based on typical microscopic findings on a skin smear, skin lesions are absent in 30% to 60% of patients.15,28 Talaromycosis mortality doubles from 24% to 50% when the diagnosis is delayed and reaches 100% when the diagnosis is missed.31 Non-culture diagnostic approaches are urgently needed to disrupt the cycle of delayed or misdiagnosis, disseminated disease, and high mortality.
There are few translational scientists and companies working on developing novel diagnostics for talaromycosis. Two promising monoclonal antibody (mAb)-based antigen detection enzyme immunoassays (EIAs) are currently in development. The mAb-4D1 EIA and its immunochromatographic platform developed in Thailand show high sensitivity and specificity in small studies.32,33 The mAb-Mp1p EIA developed in Hong Kong has been studied more extensively and was shown to be superior to blood culture in sensitivity (86% versus 73%) and had a specificity of 98%. Sensitivity was higher in urine than in plasma and was the highest when testing plasma and urine in combination.34 The Mp1p EIA is currently being evaluated in a multi-centre prospective study as a rapid diagnostic and screening tool for talaromycosis (ClinicalTrials.gov: NCT04033120). A commercial version of the Mp1p EIA was approved for clinical use in China in October 2019, and a Mp1p point-of-care lateral flow antigen (LFA) test is being developed through industry-public partnership with support from the National Institute of Health in the U.S. The WHO 2021–2030 NTD road map has identified diagnostics as one of four priority areas, and established a Diagnostics Technical Advisory Group (DTAG) to centralise diagnostic advances and drive progress within the field in a coordinated manner.2 Talaromycosis stands to benefit from this initiative if included on the NTD list. DTAG coordination can advance non-culture diagnostics for talaromycosis and has the best chance of saving lives, by effectively facilitating industry and research partnership to rapidly validate and commercialise these antigen detection assays for clinical use.
Treatment.
Amphotericin B and itraconazole have been the mainstay of treatment for talaromycosis. In 2017, a multi-centre randomised controlled trial found induction therapy with amphotericin B achieved more rapid fungal clearance in blood and reduced six-month mortality from 21% to 11% compared to itraconazole.35 Despite this large mortality benefit, many patients in Vietnam, where the trial was conducted, and in other countries in Asia still struggle to gain access to amphotericin B due to its high cost and difficulties with procurement and distribution. Even where there is access, LMICs in Asia are still using the deoxycholate amphotericin B formulations that have not been used in high income countries (HICs) for two decades. The less toxic liposomal amphotericin B formulation (AmBisome™) is still not available in most of Asia despite coming off patent protection in the U.S. in 2016.36 Therapeutic options for patient in LMICs who cannot tolerate amphotericin B are largely absent. The role of newer triazole compounds that are widely available in developed countries (voriconazole, posaconazole, isavuconazole) and the role of novel antifungal compounds in development in the treatment of talaromycosis have never been systematically studied in animals or in humans. It is also unknown whether combination therapy with amphotericin B plus flucytosine, shown to be more efficacious than amphotericin B alone for treatment of cryptococcosis,37 is also more efficacious for talaromycosis. No controlled studies have been conducted for treatment of non-HIV-infected people, and the duration of consolidation/maintenance antifungal therapy is unknown. For an infectious disease that has an on-treatment mortality of 30%, research to improve treatment is imperative. This will require substantial investment from both the global scientific community and industry. Inclusion on the NTD list would raise the profile of talaromycosis globally and would increase access to less toxic formulations of amphotericin B and newer antifungal drugs used routinely in HICs. This can be achieved collectively through the Drugs for Neglected Diseases initiative and the WHO Model Lists of Essential Medicines.38
4). Control of talaromycosis is feasible with known public health strategies
Primary prophylaxis with itraconazole has been shown to reduce the incidence of talaromycosis and other invasive fungal infections in patients with advanced HIV disease.39 However, this blanket approach to disease prevention has not been widely adopted due to concerns of toxicity, drug-drug interactions, drug resistance, and cost. In cryptococcosis, a more targeted approach of antigen screening and pre-emptive fluconazole therapy prevents cryptococcal meningitis, reduces mortality,40 is highly cost effective,41 and is being implemented in HIV programmes across the world.42,43 A similar diagnostic-driven approach is likely to be an effective strategy to control talaromycosis, as Tm antigenaemia has been shown to precede development of culture-confirmed talaromycosis by up to 16 weeks,44,45 and antigenaemia is associated with 12-month mortality.46 The Tm LFA assays in development would allow for testing at the point of care in the community and enable a screen-and-treat strategy to reduce disease burden at a population level.
While antigen screening is an effective approach in patients with advanced HIV disease, pathogen-based detection is still limited by delayed clinical presentatio.. Host-based diagnostics such as an antibody test or interferon-gamma release assay would enable identification of latent infections in people undergoing immunosuppressive therapy, chemotherapy, and organ transplantations, and would allow for pre-emptive therapy to interrupt disease development. Host-based assays would permit new knowledge of disease exposure, latent infection, and population burden. Seroprevalence data could advance our understanding of pathogen ecology and the environmental reservoir of Tm. This understanding could inform strategies that control Tm at its source and could delineate geographic risk regions to effectively guide resource allocation for diagnosis, treatment, and prevention strategies.
The WHO 2021–2030 NTD road map is focused on integration of NTD prevention and control strategies within national healthcare systems with the support of domestic financing.2 Currently there is no provision for talaromycosis funding within national healthcare policies in the endemic regions. Sustainable national health resource allocation for talaromycosis will not occur without an endorsement from global health entities. Talaromycosis and other neglected mycoses stand to greatly benefit from being included on the NTD road map focusing on health system strengthening and national cross-cutting approaches to tackle the root causes of poverty and access to care for rural populations.47
Conclusions
Talaromycosis meets all criteria to be included in the WHO NTD list and shares many features of other infectious diseases associated with poverty currently recognised by the WHO, PLOS NTD, and the FDA. The significant challenges in diagnosis and treatment of talaromycosis represent enormous opportunities to make an impact on the disease at both the individual and population level. Recognition of talaromycosis as an NTD by global public health organisations, funders, and other stakeholders will demonstrate the commitment and provide the necessary impetus to improve the control and prevention of this deadly infectious disease.
Acknowledgements
This work was supported by a NIAID-funded Duke Center for AIDS Research (CFAR) (5P30AI064518 to S.N. and T.L.) and a NIAID research project grant (R01AI143409 to T.L.).
Declaration of Interests
Nelesh P Govender reports grants from National Institutes of Health, the Centers for Disease Control and Prevention, the CDC Foundation, the Bill and Melinda Gates Foundation, the UK Medical Research Council, and from NHLS Research Trust, outside the submitted work. Jasper Fuk-Woo Chan and Kwok-Yung Yuen have an issued patent on antibodies targeting Talaromyces marneffei Mp1p proteins and their methods of use. John Perfect reports grants from Merck, Astellas, Pfizer, Amplyx, and Minnetronix, and he reports membership on advisory boards or consulting roles for Merck, Amplyx, Minnetronix, F2G, Scynexis, Ampili, and Matinas, outside the submitted work. Thuy Le reports research funding from Gilead Sciences, outside the submitted work. All other authors declare no competing interests.
Appendix
FIGURE 1:
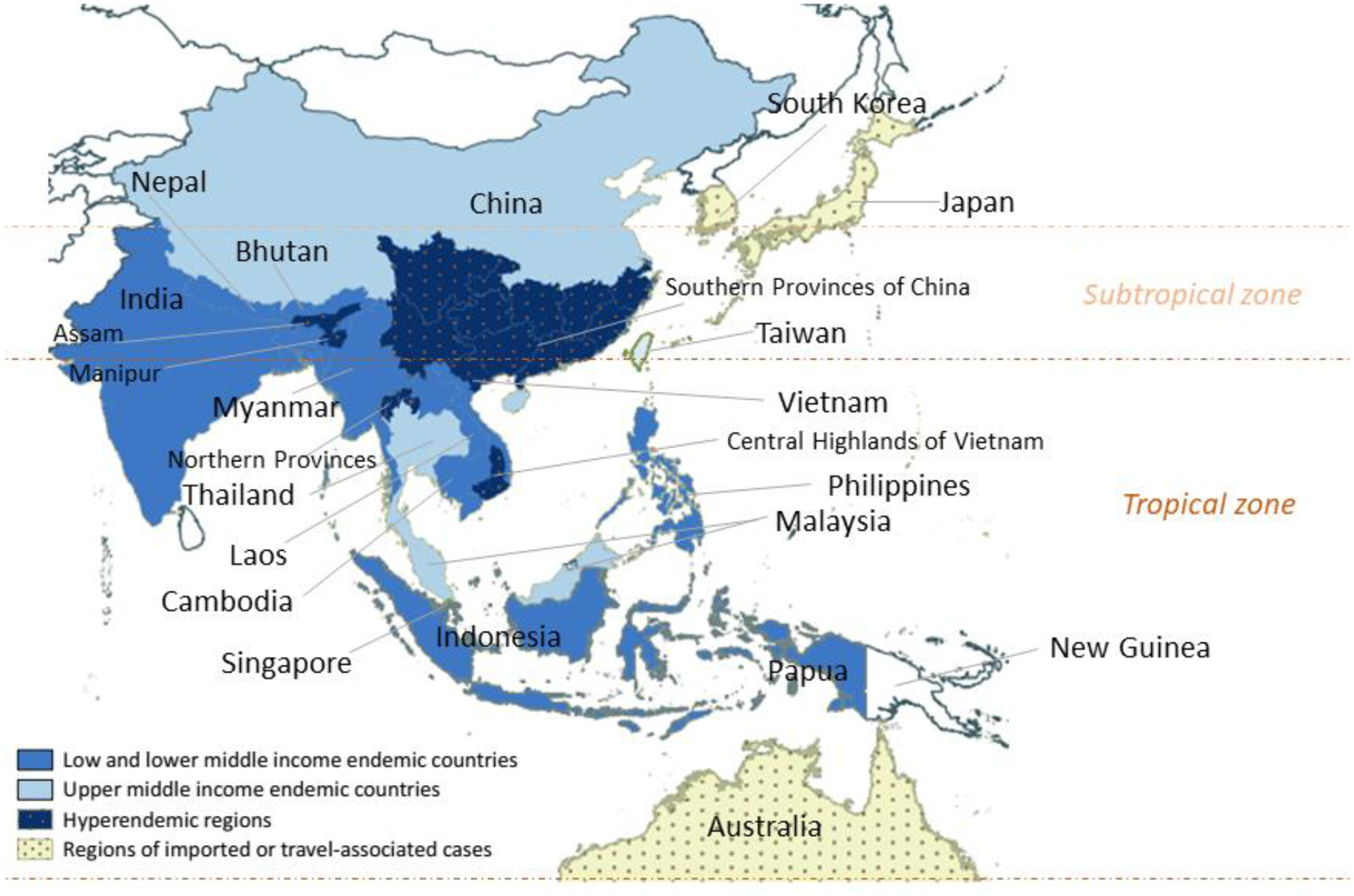
Geographic distribution of talaromycosis according to income status and climate zones
FIGURE 2.
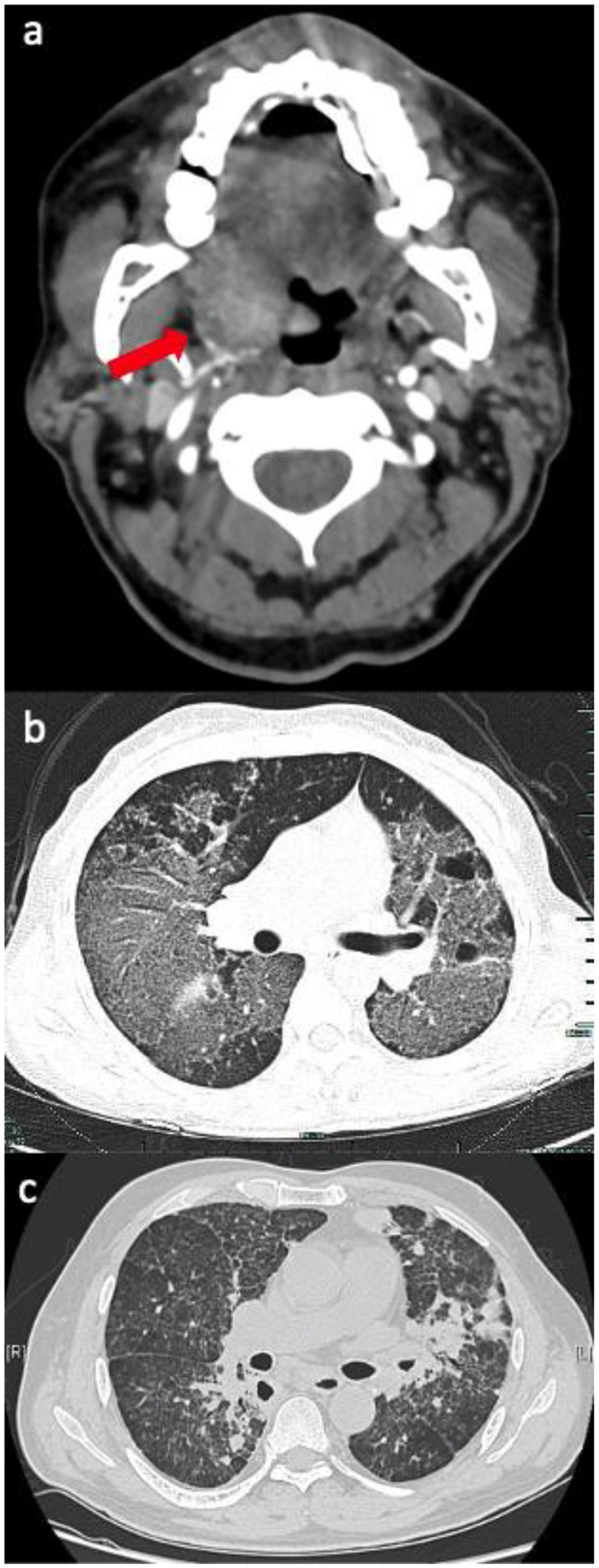
Upper and lower respiratory tract manifestations of talaromycosis.
a) Computed tomography (CT) Angiogram of the neck demonstrating an ill-defined mass along the right lateral aspect of the hypopharynx involving the base of the tongue, right lingual tonsil, and right vallecula extending along the right palatine tonsil and into the pharyngeal space, in a 63 year-old man with HIV,1 b) Axial CT Chest demonstrating multiple disseminated ground glass opacities and bullae in a 34 year old immunocompromised female with a STAT3 mutation,2 c) Chest CT with interstitial infiltrates and nodules in a 57-year-old non-HIV-infected man with a history of prolonged steroid use.3
FIGURE 3:
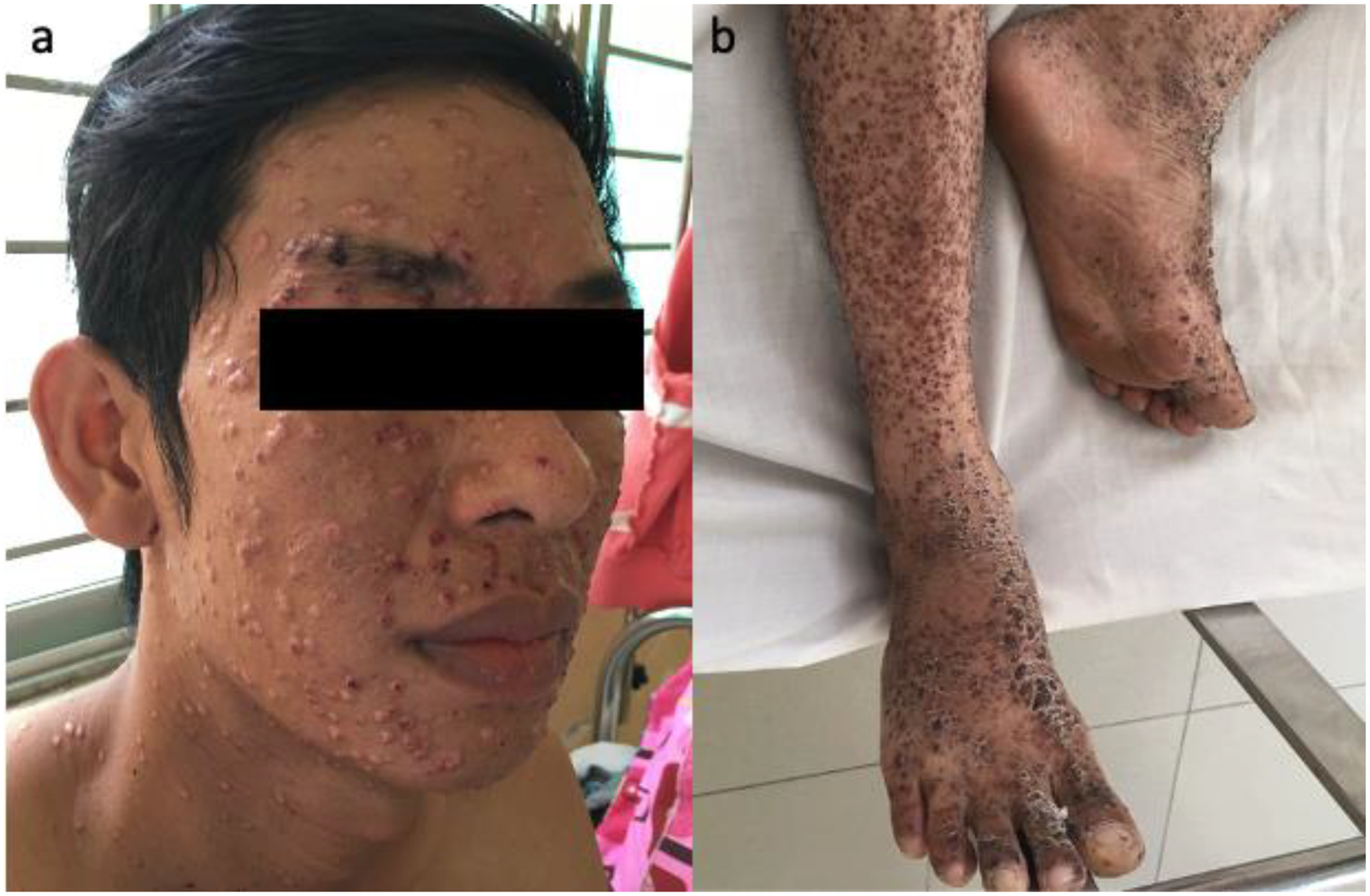
Disfiguring skin lesions in talaromycosis.
a) Typical disfiguring central-umbilicated skin lesions on the face of a patient with advanced HIV and disseminated talaromycosis in Vietnam, b) Talaromycosis cutaneous lesions in the lower extremities in another patient with HIV in Vietnam.
Table 1:
Talaromycosis prevalence and mortality among people living with HIV/AIDS in endemic countries
| Prevalence | Mortality | |
|---|---|---|
| China | 16.1%4 3.3% (95% CI 1.8–5.8)5 16%6 |
17.5%4 14%6 |
| Vietnam | 6.4% (95% CI 4.4–9.5)5 0.23/100,000 population7 4.4% (range, 3.4% - 5.4%)8 4.9%9 |
6.3% (range, 5%−8.3%)8 33.3%9 12.6%10 |
| Thailand | 3.9% (95% CI 1.8–8.3)5 | 20.7% (HIV)5 |
| Malaysia | 2.1% (95% CI 0.7 – 6.6)5 | 4.8%, case report data 11,12 |
| Taiwan | 1.1% (95%CI: 0.5–2.8)5 0.6%13 |
50%, case report data14 |
| India | 3.2% (95% CI 0.3–32.6)5 77 reported cases, most in Manipur state15 |
6.5%15 |
Table 2.
WHO criteria for classifying a condition as a neglected tropical disease (NTD)16
Disease conditions that
|
REFERENCES
- 1.World Health Organization. Report of the global partners’ meeting on neglected tropical diseases : 2007 - a turning point : Geneva, Swizerland, 17-18 April 2007. Geneva: World Health Organization; 2007. [Google Scholar]
- 2.World Health Organization. Ending the neglect to attain the sustainable development goals: a sustainability framework for action against neglected tropical diseases 2021–2030. Geneva: World Health Organization; 2021. [Google Scholar]
- 3.Hotez PJ, Aksoy S, Brindley PJ, Kamhawi S. What constitutes a neglected tropical disease? PLoS Negl Trop Dis 2020; 14(1): e0008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang J, Meng S, Huang S, et al. Effects of Talaromyces marneffei infection on mortality of HIV/AIDS patients in southern China: a retrospective cohort study. Clin Microbiol Infect 2019; 25(2): 233–41. [DOI] [PubMed] [Google Scholar]
- 5.Le T, Wolbers M, Chi NH, et al. Epidemiology, seasonality, and predictors of outcome of AIDS-associated Penicillium marneffei infection in Ho Chi Minh City, Viet Nam. Clin Infect Dis 2011; 52(7): 945–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanittanakom N, Cooper CR Jr., Fisher MC, Sirisanthana T. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin Microbiol Rev 2006; 19(1): 95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qin Y, Huang X, Chen H, et al. Burden of Talaromyces marneffei infection in people living with HIV/AIDS in Asia during ART era: a systematic review and meta-analysis. BMC Infect Dis 2020; 20(1): 551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ning C W W X B, Thanh NT, Ye Li, Liang H, Le T. The global distribution, drivers, and burden of talaromycosis 1964–2018. Conference of Retrovirus and Opportunistic Infections; 2020; Boston, MA, USA; 2020. [Google Scholar]
- 9.Yu X, Cai X, Xu X, et al. Fungemia caused by Penicillium marneffei in an immunocompetent patient with COPD: A unique case report. Medicine (Baltimore) 2018; 97(3): e9658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li HR, Xu NL, Lin M, et al. Diffuse interstitial and multiple cavitary lung lesions due to Talaromyces marneffei infection in a non-HIV patient. New Microbes New Infect 2015; 8: 14–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh A, Atallah S, Al-Shyoukh A, DaCunha M, Mizusawa M. Localized Talaromyces marneffei infection presenting as a tonsillar mass mimicking malignancy. IDCases 2020; 21: e00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang W, Ye J, Qiu C, et al. Rapid and precise diagnosis of T. marneffei pulmonary infection in a HIV-negative patient with autosomal-dominant STAT3 mutation: a case report. Ther Adv Respir Dis 2020; 14: 1753466620929225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao C, Xi L, Chaturvedi V. Talaromycosis (Penicilliosis) Due to Talaromyces (Penicillium) marneffei: Insights into the Clinical Trends of a Major Fungal Disease 60 Years After the Discovery of the Pathogen. Mycopathologia 2019; 184(6): 709–20. [DOI] [PubMed] [Google Scholar]
- 14.Chan JF, Lau SK, Yuen KY, Woo PC. Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerg Microbes Infect 2016; 5(3): e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawila R, Chaiwarith R, Supparatpinyo K. Clinical and laboratory characteristics of penicilliosis marneffei among patients with and without HIV infection in Northern Thailand: a retrospective study. BMC Infect Dis 2013; 13: 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang JQ, Yang ML, Zhong XN, et al. A comparative analysis of the clinical and laboratory characteristics in disseminated penicilliosis marneffei in patients with and without human immunodeficiency virus infection. Chinese Journal of Tuberculosis and Respiratory Diseases 2008; 31(10): 740–6. [PubMed] [Google Scholar]
- 17.Wang PH, Wang HC, Liao CH. Disseminated Penicillium marneffei mimicking paradoxical response and relapse in a non-HIV patient with pulmonary tuberculosis. J Chin Med Assoc 2015; 78(4): 258–60. [DOI] [PubMed] [Google Scholar]
- 18.Joosten SA, Hannan L, Heroit G, Boerner E, Irving L. Penicillium marneffei presenting as an obstructing endobronchial lesion in an immunocompetent host. Eur Respir J 2012; 39(6): 1540–3. [DOI] [PubMed] [Google Scholar]
- 19.Qiu Y, Zhang JQ, Pan ML, Zeng W, Tang SD, Tan CM. Determinants of prognosis in Talaromyces marneffei infections with respiratory system lesions. Chin Med J (Engl) 2019; 132(16): 1909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin F, Qiu Y, Zeng W, Liang Y, Zhang J. Talaromyces marneffei infection in a lung cancer patient: a rare case report. BMC Infect Dis 2019; 19(1): 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The World Bank. World Bank Country and Lending Groups. 2021. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519 (accessed 11th January 2021).
- 22.The World Bank. The World Bank Open Data. 2019. https://data.worldbank.org/indicator/SP.RUR.TOTL.ZS (accessed January 19 2021).
- 23.Zhao Y-j, Lu Y. Mapping determinants of rural poverty in Guangxi — a less developed region of China. Journal of Mountain Science 2020; 17(7): 1749–62. [Google Scholar]
- 24.Chariyalertsak S, Sirisanthana T, Supparatpinyo K, Praparattanapan J, Nelson KE. Case-control study of risk factors for Penicillium marneffei infection in human immunodeficiency virus-infected patients in northern Thailand. Clin Infect Dis 1997; 24(6): 1080–6. [DOI] [PubMed] [Google Scholar]
- 25.Le T J B, Cuc NTK, Thanh NT, Lam PS, Khuong PT, Bich DT, Thompson C, Wertheim H, Farrar J, Chau NVV, Day J, Shikuma C, Wolbers M, Thwaites G. The exposure and geospatial risk factors for AIDS-associated penicilliosis in Vietnam. Conference on Retroviruses and Opportunistic Infections. Seattle, WA, USA; 2015. [Google Scholar]
- 26.UNAIDS. UNAIDS Data 2020, 2020.
- 27.National Intelligence Council. Southeast Asia and Pacific Islands: The Impact of Climate Change to 2030: National Intelligence Council, 2009.
- 28.Ying RS, Le T, Cai WP, et al. Clinical epidemiology and outcome of HIV-associated talaromycosis in Guangdong, China, during 2011–2017. HIV Med 2020; 21(11): 729–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bulterys PL, Le T, Quang VM, Nelson KE, Lloyd-Smith JO. Environmental predictors and incubation period of AIDS-associated penicillium marneffei infection in Ho Chi Minh City, Vietnam. Clin Infect Dis 2013; 56(9): 1273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myers N Environmental refugees: a growing phenomenon of the 21st century. Philos Trans R Soc Lond B Biol Sci 2002; 357(1420): 609–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu Y, Zhang J, Li X, et al. Penicillium marneffei infection: an emerging disease in mainland China. Mycopathologia 2013; 175(1–2): 57–67. [DOI] [PubMed] [Google Scholar]
- 32.Prakit K, Nosanchuk JD, Pruksaphon K, Vanittanakom N, Youngchim S. A novel inhibition ELISA for the detection and monitoring of Penicillium marneffei antigen in human serum. Eur J Clin Microbiol Infect Dis 2016; 35(4): 647–56. [DOI] [PubMed] [Google Scholar]
- 33.Pruksaphon K, Intaramat A, Simsiriwong P, et al. An inexpensive point-of-care immunochromatographic test for Talaromyces marneffei infection based on the yeast phase specific monoclonal antibody 4D1 and Galanthus nivalis agglutinin. PLoS Negl Trop Dis 2021; 15(5): e0009058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thu NTM, Chan JFW, Ly VT, et al. Superiority of a novel Mp1p antigen detection enzyme immunoassay compared to standard BACTEC blood culture in the diagnosis of talaromycosis. Clin Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le T, Kinh NV, Cuc NTK, et al. A Trial of Itraconazole or Amphotericin B for HIV-Associated Talaromycosis. N Engl J Med 2017; 376(24): 2329–40. [DOI] [PubMed] [Google Scholar]
- 36.Kneale M, Bartholomew JS, Davies E, Denning DW. Global access to antifungal therapy and its variable cost. J Antimicrob Chemother 2016; 71(12): 3599–606. [DOI] [PubMed] [Google Scholar]
- 37.Day JN, Chau TTH, Wolbers M, et al. Combination antifungal therapy for cryptococcal meningitis. N Engl J Med 2013; 368(14): 1291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laing R, Waning B, Gray A, Ford N, t Hoen E. 25 years of the WHO essential medicines lists: progress and challenges. Lancet 2003; 361(9370): 1723–9. [DOI] [PubMed] [Google Scholar]
- 39.Chariyalertsak S, Supparatpinyo K, Sirisanthana T, Nelson KE. A controlled trial of itraconazole as primary prophylaxis for systemic fungal infections in patients with advanced human immunodeficiency virus infection in Thailand. Clin Infect Dis 2002; 34(2): 277–84. [DOI] [PubMed] [Google Scholar]
- 40.Mfinanga S, Chanda D, Kivuyo SL, et al. Cryptococcal meningitis screening and community-based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: an open-label, randomised controlled trial. Lancet 2015; 385(9983): 2173–82. [DOI] [PubMed] [Google Scholar]
- 41.Jarvis JN, Harrison TS, Lawn SD, Meintjes G, Wood R, Cleary S. Cost effectiveness of cryptococcal antigen screening as a strategy to prevent HIV-associated cryptococcal meningitis in South Africa. PLoS One 2013; 8(7): e69288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Longley N, Jarvis JN, Meintjes G, et al. Cryptococcal Antigen Screening in Patients Initiating ART in South Africa: A Prospective Cohort Study. Clin Infect Dis 2016; 62(5): 581–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tenforde MW, Wake R, Leeme T, Jarvis JN. HIV-Associated Cryptococcal Meningitis: Bridging the Gap Between Developed and Resource-Limited Settings. Curr Clin Microbiol Rep 2016; 3: 92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ly VT, Thanh NT, Thu NTM, et al. Occult Talaromyces marneffei Infection Unveiled by the Novel Mp1p Antigen Detection Assay. Open Forum Infect Dis 2020; 7(11): ofaa502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ly VT TN, Thanh NT, Tung NLN, Chan J, Woo P, Chau NVV, Nga CN, Yuen KY, Le T. Superior Accuracy of the Mp1p Antigen Assay Over Cultures in Diagnosing Talaromycosis. Conference of Retroviruses and Opportunistic Infections. Boston, MA, USA; 2020. [Google Scholar]
- 46.Thu NT, Dat Vu Quoc, Chan Jasper F., Ha Hien T., Nguyen Dung T., Ho Anh T., Woo Patrick C., Yuen Kwok-Yung, Lyss Sheryl, Bateganya Moses, Nguyen Kinh V., Le Thuy. Asymptomatic Talaromyces marneffei antigenemia and mortality in advanced HIV disease. Conference on Retroviruses and Opportunistic Infections. Seattle; 2019. [Google Scholar]
- 47.Espinal M, Kruk ME, Mohamed MCM, Wainwright E. Considerations for a sustainability framework for neglected tropical diseases programming. Trans R Soc Trop Med Hyg 2021; 115(2): 176–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Appendix References
- 1.Singh A, Atallah S, Al-Shyoukh A, DaCunha M, Mizusawa M. Localized Talaromyces marneffei infection presenting as a tonsillar mass mimicking malignancy. IDCases 2020; 21: e00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang W, Ye J, Qiu C, et al. Rapid and precise diagnosis of T. marneffei pulmonary infection in a HIV-negative patient with autosomal-dominant STAT3 mutation: a case report. Ther Adv Respir Dis 2020; 14: 1753466620929225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li HR, Xu NL, Lin M, et al. Diffuse interstitial and multiple cavitary lung lesions due to Talaromyces marneffei infection in a non-HIV patient. New Microbes New Infect 2015; 8: 14–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang J, Meng S, Huang S, et al. Effects of Talaromyces marneffei infection on mortality of HIV/AIDS patients in southern China: a retrospective cohort study. Clin Microbiol Infect 2019; 25(2): 233–41. [DOI] [PubMed] [Google Scholar]
- 5.Qin Y, Huang X, Chen H, et al. Burden of Talaromyces marneffei infection in people living with HIV/AIDS in Asia during ART era: a systematic review and meta-analysis. BMC Infect Dis 2020; 20(1): 551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ying RS, Le T, Cai WP, et al. Clinical epidemiology and outcome of HIV-associated talaromycosis in Guangdong, China, during 2011–2017. HIV Med 2020; 21(11): 729–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beardsley J, Denning DW, Chau NV, Yen NT, Crump JA, Day JN. Estimating the burden of fungal disease in Vietnam. Mycoses 2015; 58 Suppl 5(Suppl Suppl 5): 101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le T, Wolbers M, Chi NH, et al. Epidemiology, seasonality, and predictors of outcome of AIDS-associated Penicillium marneffei infection in Ho Chi Minh City, Viet Nam. Clin Infect Dis 2011; 52(7): 945–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Son VT, Khue PM, Strobel M. Penicilliosis and AIDS in Haiphong, Vietnam: evolution and predictive factors of death. Med Mal Infect 2014; 44(11–12): 495–501. [DOI] [PubMed] [Google Scholar]
- 10.Larsson M, Nguyen LH, Wertheim HF, et al. Clinical characteristics and outcome of Penicillium marneffei infection among HIV-infected patients in northern Vietnam. AIDS Res Ther 2012; 9(1): 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nor-Hayati S, Sahlawati M, Suresh-Kumar C, Lee KC. A retrospective review on successful management of Penicillium marneffei infections in patients with advanced HIV in Hospital Sungai Buloh. Med J Malaysia 2012; 67(1): 66–70. [PubMed] [Google Scholar]
- 12.Rokiah I, Ng KP, Soo-Hoo TS. Penicillium marneffei infection in an AIDS patient--a first case report from Malaysia. Med J Malaysia 1995; 50(1): 101–4. [PubMed] [Google Scholar]
- 13.Yen YF, Chen M, Jen I, et al. Association of HIV and Opportunistic Infections With Incident Stroke: A Nationwide Population-Based Cohort Study in Taiwan. J Acquir Immune Defic Syndr 2017; 74(2): 117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hung CC, Hsueh PR, Chen MY, Hsiao CH, Chang SC, Luh KT. Invasive infection caused by Penicillium marneffei: an emerging pathogen in Taiwan. Clin Infect Dis 1998; 26(1): 202–3. [DOI] [PubMed] [Google Scholar]
- 15.Sethuraman N, Thirunarayan MA, Gopalakrishnan R, Rudramurthy S, Ramasubramanian V, Parameswaran A. Talaromyces marneffei Outside Endemic Areas in India: an Emerging Infection with Atypical Clinical Presentations and Review of Published Reports from India. Mycopathologia 2020. [DOI] [PubMed] [Google Scholar]
- 16.The WHO Strategic and Technical Advisory Group for Neglected Tropical Disease (WHO STAG). Recommendations for the adoption of additional diseases as neglected tropical diseases: The World Health Organization, 2017. [Google Scholar]


