Artificial intelligence (AI) involves using data and algorithms to perform activities normally achieved through human intelligence. AI and its key component machine learning contextualize data and enhance decision making to transform how we operate, discover, and develop drugs. Transforming clinical pharmacology (CP) as AI‐augmented CP (AI/CP) requires an ecosystem including digitized data collection, standardized processes, complementary technologies, and an ethical framework. This commentary aims to highlight the future perspectives of AI/CP in drug development.
DIGITAL AND DATA CONSIDERATIONS
For decades, clinical pharmacologists have embraced the mathematical representation of physiology and explored modeling options to derive relationships between drug and temporal changes in pharmacokinetics (PK) and pharmacodynamics. Now, as an evolution toward defining better therapies, we strive toward more digitization. Digitized drug interaction databases, for example, consisted of curated qualitative and quantitative data related to various extrinsic and intrinsic factors, including comedications, excipients, food products, organ impairment, and genetics that can affect human systemic drug exposure. Besides, digital biomarkers (measured by means of digital devices such as portables, wearables, implantable) provide new and faster data in real time, giving clinicians a better understanding of how medication impacts the disease and its interaction with an individual's overall health. With recent advancements in collecting electronic health records and processing patient genomics data, digital twins and virtual populations are becoming achievable. With the development of natural language processing–like techniques, artificial intelligence (AI) models could use physician notes and laboratory books as data for predictive modeling. With the development of the Internet of Things (network of devices work together seamlessly connecting medical devices and databases), it is now possible to collect more electronic data than ever using wearable devices. The availability of curated databases, real‐world evidence databases, patient‐centric sampling, and futuristic wearable data would provide the foundation for AI/clinical pharmacology (CP) to develop and deliver life‐changing medicine for patients.
OPPORTUNITIES IN CP
Our expectation for AI‐augmented CP is to enable accurate predictions, drive making unbiased decisions, and provide efficient CP systems (Figure 1) to deliver the core part of the guidance to the prescriber (e.g., labels, summaries of product characteristics). In this perspective we focus on the intersection of AI and the field of clinical pharmacology with a focus on the potential impact of AI in these aspects: dose recommendations, drug interaction, variability in PK and patient stratification/selection.
FIGURE 1.
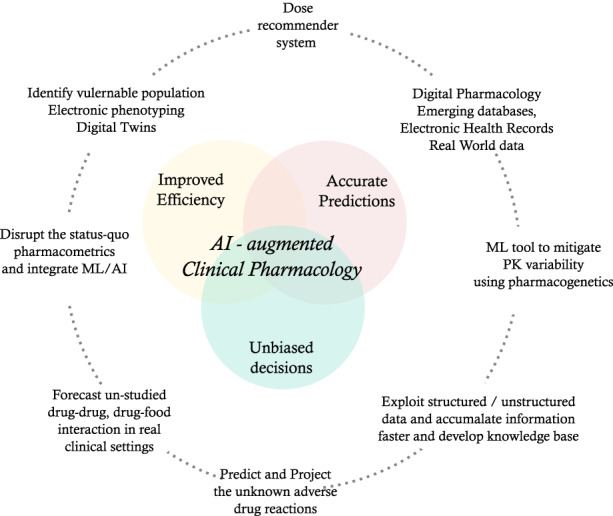
Potential application of artificial intelligence and machine learning in clinical pharmacology. AI, artificial intelligence; ML, machine learning; PK, pharmacokinetics
DOSE RECOMMENDATIONS
A dose‐recommender system based on AI/machine learning (ML), which integrates data across domains including but not limited to multiple safety and efficacy measures, electronic records about current health status, information about the disease and previous treatment history, and patient‐reported outcomes, would provide tailored dosing options for patients, enhancing efficacy and minimizing adverse events. Currently, reinforcement learning–based algorithms showed potential for dose predictions and dose modifications during treatment by precision dosing for oncology patients. 1 The Dose–Response Network 2 uses deep‐learning (DL) approaches that can estimate individual patient outcomes at different intervals of dose–response curves. The ability of AI to recommend doses in counterfactual conditions is questionable. However, generative adversarial networks, with their ability to learn from current data and expand the learning to the unknown dose–response surface, could revolutionize individualized dose–response curve predictions. 2
DRUG INTERACTIONS AND ADVERSE REACTIONS
CP‐based impact on prescribing information depends predominantly on studies evaluating drug–drug interactions (DDIs), drug–food interaction, bioavailability, and PK changes in special populations. However, this information is limited compared to the potential drug interactions in clinical practice and real‐world settings. AI/CP can expand beyond the patient population evaluated in clinical studies. Innovative algorithms based on knowledge graphs (KGs) showed the potential to predict unknown adverse drug reactions, 3 DDIs, and drug–food interactions. 4 Bougiatiotis et al. 5 demonstrated the utility of biomedical literature KGs and link prediction models to assess the DDIs in Alzheimer's disease and lung cancer. With the help of the KG framework, existing physiologically based PK (PBPK) expertise and data sources (see Digital and Data Considerations), clinical pharmacologists can project potential and dangerous DDIs due to simultaneous administration of multiple drugs. This provides opportunities to include both known and unknown (potential) DDIs in patient information leaflets.
VARIABILITY AND PATIENT STRATIFICATION/SELECTION
In clinical practice, therapeutic drug monitoring offers dose recommendations for drugs with relatively high variability in PK and a narrow therapeutic index. AI/ML has demonstrated better dose recommendations for propofol and remifentanil with less error in predicting bispectral index during anesthesia than traditional modeling methods. 6 AI/ML approaches can now recognize patterns by identifying complex and nonlinear relationships and the influence of intrinsic and extrinsic factors on the variability of PK in different subpopulations. We envision that integrating ML capability with population‐based approaches would help further explain PK variability and offer options to modify dose in subgroups of patients.
US Food and Drug Administration guidance 7 on clinical trial enhancement strategies suggest including patients with a high chance of showing a disease‐related end point (prognostic indicators) and patients who are likely to respond to the treatment (predictive indicators). DL methods can handle the array of data (liquid biopsy, pathology imaging, computerized tomography scans, and extensive omics data) and understand/recognize patterns with the prognostic/predictive potential. Reduced population heterogeneity includes choosing patients with baseline measurements of a disease or a biomarker characterizing the disease in a narrow range. In contrast, excluding patients whose disease or symptoms improve spontaneously or whose measurements are highly variable would help to increase study power, reduce costs, and bring new medicine to patients faster. Decreasing variability often uses a process known as electronic phenotyping, which focuses on reducing population heterogeneity. Electronic phenotyping 8 requires mining large databases of electronic health records and accounting for heterogeneity between patient records and data types. Applying AI technologies, especially ML and DL, to electronic phenotyping processes can accelerate the identification of eligible patients for clinical trials.
PHARMACOMETRICS AND AI
Efforts to automate pharmacometric modeling and the development of neural network/neural ordinary differential equation–based predictive modeling and novel algorithm‐based clinical trial designs (Table 1) lay the foundation for the future of model‐based drug development (MBDD). We postulate that exploiting AI methods extracting information from unstructured data (e.g., imaging/electronic records) would enhance current approaches for MBDD by improving personalized projections and decision making across clinical trials. The potential to hybridize ML and pharmacological models helps ML to perform well in limited data scenarios and conversely the ML models help with improving misspecification of pharmacological models (Table 1). Meta‐analyses of clinical and observational studies aggregate meaningful inferences supporting drug development, but these analyses are hugely time‐consuming. However, with the combination of AI and human intelligence, Michelson et al. 9 performed a rapid meta‐analysis to generate insights indicating ocular toxicity as a side effect of hydroxychloroquine in a much shorter period (<30 min) than traditional meta‐analysis. Similarly, unsupervised ML assisted with the automated screening and study selection process for meta‐analysis. 10 Efficient and rapid ML‐based literature analysis could help with a well‐informed comparative analysis in early clinical trials. A predictive modeling ecosystem including nonlinear mixed‐effect models, mechanistic models, PBPK, quantitative systems pharmacology, AI/ML algorithms, structured/unstructured data, and ML‐assisted meta‐analysis would drive advancements in MBDD. Overall, a synergism in terms of efficiency and developing accurate predictive models are expected while integrating pharmacometrics and ML.
TABLE 1.
Summary of examples featuring artificial intelligence and clinical pharmacology
| Description | Clinical pharmacology feature | Reference |
|---|---|---|
| PK/PD modeling | ||
| Latent hybridization model integrating expert PK/PD models with hospital observation data through neural ODEs | Informing clinical decisions with expert pharmacological models; potential for improving the PK/PD model based on clinical observation variables | Qian Z, Zame WR, Fleuren LM, Elbers P, van der Schaar M. Integrating expert ODEs into neural ODEs: pharmacology and disease progression. 2021. doi:10.48550/ARXIV.2106.02875 |
| An automated tool to distil closed‐form ODEs from observed trajectories | Discovering an interpretable set of differential equations that corresponds to the PK/PD model of a drug | Qian Z, Kacprzyk K, van der Schaar M. D‐CODE: discovering closed‐form ODEs from observed trajectories. Presented at: International Conference on Learning Representations, 2022. https://openreview.net/forum?id=wENMvIsxNN [accessed 04 Oct 2022] |
| Nonpharmacometric models to predict longitudinal changes in tumor size | Predict changes in tumor trajectory and optimize the treatment options | Talianu A, Johnson M. Long short‐term memory recurrent neural networks to predict longitudinal changes in tumor size. Presented at: Population Approach Group Europe 29. 2021. Abstract 9815. https://www.page‐meeting.org/?abstract=9815 [accessed 2–3 Sep 2021] |
| Algorithm exploring different dosing regimens for cancer treatment | Potential for personalized dosing regimen balancing safety and efficacy | Sotto Mayor T, Irurzun Arana I, Johnson M. Developing a reinforcement learning algorithm to determine an optimal dosing regimen for cancer treatment. Presented at: Population Approach Group Europe 30. 2022. Abstract 10151. https://www.page‐meeting.org/?abstract=10151 [accessed 28 Jun 2022] |
| Apply machine‐learning algorithms to develop population PK models | High potential for automated PK model selection | Sale M, Ismail M, Wang F, et al. Comparison of robustness and efficiency of four machine learning algorithms for identification of optimal population pharmacokinetic models. Presented at: Population Approach Group Europe 30. 2022. Abstract 10053. https://www.page‐meeting.org/?abstract=10053 [accessed 28 Jun 2022] |
| Clinical trials | ||
| A Bayesian framework for finding the maximum tolerated dose for drug combinations in the presence of safety constraints | Better clinical designs for testing most optimal dose combinations while having a constrained number of patients in a safe, informative, and efficient way | Lee H‐S, Shen C, Zame WR, Lee J‐W, van der Schaar M. SDF‐Bayes: cautious optimism in safe dose‐finding clinical trials with drug combinations and heterogeneous patient groups. Presented at: Proceedings of the 24th International Conference on Artificial Intelligence and Statistics (AISTATS) 2021; April 13–15, 2021; San Diego, CA. |
| Safe efficacy exploration dose allocation: a model for maximizing the cumulative efficacies while satisfying the toxicity constraints with high probability | State‐of‐the‐art clinical designs that find the optimal dose at phase I with a higher success rate and fewer patients while preserving the validity of the study | Shen C, Wang Z, Villar S, Van Der Schaar M. Learning for dose allocation in adaptive clinical trials with safety constraints. In: Daumé H III, Singh A, eds. Proceedings of the 37th International Conference on Machine Learning. Vol. 119. Omnipress. 2020:8730–8740. |
| Contextual constrained clinical trial algorithm for dose finding under a multitude of budget and safety constraints | Learning both about toxicity and efficacy at phase I while reducing costs; balancing the trade‐off between learning and treatment | Lee H‐S, Shen C, Jordon J, van der Schaar M. Contextual constrained learning for dose‐finding clinical trials. 2020. doi:10.48550/ARXIV.2001.02463 |
| Other applications | ||
| A state space model for disease progression that can build on the entire patient history as opposed to the current state only | Disease progression could be used for finding better drug administration regimens depending on the stage of disease | Alaa AM, van der Schaar M. Attentive state‐space modeling of disease progression. In: Wallach H, et al., eds. Advances in Neural Information Processing Systems (NEROIPS). Vol. 32. Curran Associates, Inc.; 2019:1–11 |
| A machine‐learning method for partitioning patients into subgroups with uncertainty quantification | Ability to identify patient subpopulations that would benefit from a drug faster and make trials more successful and efficient | Lee H‐S, Zhang Y, Zame W, Shen C, Lee J‐W, van der Schaar M. Robust recursive partitioning for heterogeneous treatment effects with uncertainty quantification. In: Larochelle H, Ranzato M, Hadsell R, Balcan MF, Lin H, eds. Advances in Neural Information Processing Systems (NEROIPS). Vol. 33. Curran Associates, Inc.; 2020:2282–2292 |
Abbreviations: ODE, ordinary differential equation; PD, pharmacodynamic; PK, pharmacokinetic.
PITFALLS
Causality and bias
In general, ML approaches use inductive reasoning, and the inferences are correlative, not causal. Furthermore, true causal‐based understanding comes from applying deductive reasoning and the application of a scientific method. In health care, it is vital to understand the root cause of any changes (physiological, pharmacological) causally related to an underlying disease. In the absence of such knowledge, we may make poor medical decisions. Although AI in health care has promise, algorithms are trained on big and extensive datasets with high variability and imbalance, resulting in algorithmic bias. Several other avenues of data accumulation can import bias into the algorithm, including but not limited to differences in infrastructure used for data collection (e.g., wearable device data) and the quality of training provided for patients and practitioners for data collection.
Data privacy and ethical concerns
Regarding data privacy, much progress has been made with the influential “General Data Protection Regulation” compliance efforts. Most healthcare companies have more awareness of data privacy and structural components in terms of infrastructure and governance boards for data use. However, applying these tools in a clinical setting requires robust information technology platforms for commercial technology giants. To protect patient privacy and control the utility of data by third parties, strong data privacy regulation (across the globe) is required. Implementing AI tools in clinical practice requires further ethical considerations regarding data privacy and patient consent. Recommendations and guidance for using AI in clinical practice are scarce or nonexistent; from a patient perspective, patient consent for data usage and awareness of using AI for their life/health decisions needs well‐formulated moral guidance.
SUMMARY
Rapid growth in digitization is the foundation for implementing AI in CP. With the transformation in data and digital systems, AI could help improve dose recommendations that are the core CP deliverable and improve the efficiency of pharmacometrics. Overall, the patient can benefit by expanding the CP section of the label (providing expected DDI and unknown adverse reactions). However, a lack of causal inference and ethical data‐sharing issues must be addressed for a successful and thriving AI in CP.
FUNDING INFORMATION
No funding was received for this work.
CONFLICT OF INTEREST
Martin Johnson, Alex Phipps, Dave Boulton, and Megan Gibbs are AstraZeneca employees and shareholders. Megan Gibbs is on the editorial board of the journal Clinical Pharmacology and Therapeutics. Mishal Patel previously worked for AstraZeneca and has nothing to disclose. Mihaela van der Schaar has nothing to disclose.
Johnson M, Patel M, Phipps A, van der Schaar M, Boulton D, Gibbs M. The potential and pitfalls of artificial intelligence in clinical pharmacology. CPT Pharmacometrics Syst Pharmacol. 2023;12:279‐284. doi: 10.1002/psp4.12902
REFERENCES
- 1. Yauney G, Shah P. Reinforcement learning with action‐derived rewards for chemotherapy and clinical trial dosing regimen selection. Machine Learning for Healthcare Conference. PMLR; 2018:161‐226. [Google Scholar]
- 2. Bica I, Alaa AM, Lambert C, van der Schaar M. From real‐world patient data to individualized treatment effects using machine learning: current and future methods to address underlying challenges. Clin Pharmacol Ther. 2021;109:87‐100. [DOI] [PubMed] [Google Scholar]
- 3. Bean DM, Wu H, Iqbal E, et al. Knowledge graph prediction of unknown adverse drug reactions and validation in electronic health records. Sci Rep. 2017;7:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ryu JY, Kim HU, Lee SY. Deep learning improves prediction of drug–drug and drug–food interactions. Proc Natl Acad Sci U S A. 2018;115:E4304‐E4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bougiatiotis K, Aisopos F, Nentidis A, Krithara A, Paliouras G. Drug–drug interaction prediction on a biomedical literature knowledge graph. In: AIME, ed. Artificial Intelligence in Medicine. Lecture Notes in Computer Science. Vol 12299. AIME, Springer; 2020. [Google Scholar]
- 6. Lee H‐C, Ryu H‐G, Chung E‐J, Jung C‐W. Prediction of bispectral index during target‐controlled infusion of propofol and remifentanil: a deep learning approach. Anesthesiology. 2018;128:492‐501. [DOI] [PubMed] [Google Scholar]
- 7. U.S. Food and Drug Administration . Enrichment Strategies for Clinical Trials to Support Approval of Human Drugs and Biological Products – Guidance for Industry. U.S. Food and Drug Administration; 2019. Available from: https://www.fda.gov/media/121320/download [accessed 04 Oct 2022] [Google Scholar]
- 8. Banda JM, Seneviratne M, Hernandez‐Boussard T, Shah NH. Advances in electronic phenotyping: from rule‐based definitions to machine learning models. Annu Rev Biomed Data Sci. 2018;1:53‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Michelson M, Chow T, Martin NA, Ross M, Tee Qiao Ying A, Minton S. Artificial intelligence for rapid meta‐analysis: case study on ocular toxicity of hydroxychloroquine. J Med Internet Res. 2020;22:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xiong Z, Liu T, Tse G, et al. A machine learning aided systematic review and meta‐analysis of the relative risk of atrial fibrillation in patients with diabetes mellitus. Front Physiol. 2018;9:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]


