Abstract
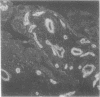
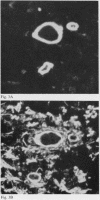
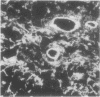
Laminin is a high molecular weight basement membrane structural glycoprotein. In rheumatoid arthritis and other arthropathies immunoreactive laminin was prominent in synovial blood vessel basement membranes and acted as a marker for them. It codistributed with collagen type IV. Immunohistological reactivity to laminin showed extensive vascular proliferation in rheumatoid arthritis together with basement membrane reduplication, which was confirmed ultrastructurally. Parallel histological studies showed vascular proliferation was predominantly in the subintimal rheumatoid synovium, where it was related to connective tissue proliferation but not to the inflammatory cell infiltrate. Vascular proliferation was also seen in relation to connective tissue changes in biopsies from cases of haemophilic arthritis, osteoarthritis, and meniscal tears. We suggest connective tissue activation is non-specific reaction associated with vascular proliferation. This involves laminin and other structural proteins. It occurs in rheumatoid arthritis and other arthropathies but is distinct from inflammatory cell infiltration.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boselli J. M., Macarak E. J., Clark C. C., Brownell A. G., Martinez-Hernandez A. Fibronectin: its relationship to basement membranes. I. Light microscopic studies. Coll Relat Res. 1981 Sep;1(5):391–404. doi: 10.1016/s0174-173x(81)80024-6. [DOI] [PubMed] [Google Scholar]
- Foidart J. M., Bere E. W., Jr, Yaar M., Rennard S. I., Gullino M., Martin G. R., Katz S. I. Distribution and immunoelectron microscopic localization of laminin, a noncollagenous basement membrane glycoprotein. Lab Invest. 1980 Mar;42(3):336–342. [PubMed] [Google Scholar]
- Gay S., Gay R. E., Miller E. F. The collagens of the joint. Arthritis Rheum. 1980 Aug;23(8):937–941. doi: 10.1002/art.1780230810. [DOI] [PubMed] [Google Scholar]
- Gordon H., Sweets H. H. A Simple Method for the Silver Impregnation of Reticulum. Am J Pathol. 1936 Jul;12(4):545–552.1. [PMC free article] [PubMed] [Google Scholar]
- HIROHATA K., KOBAYASHI I. FINE STRUCTURES OF THE SYNOVIAL TISSUES IN RHEUMATOID ARTHRITIS. Kobe J Med Sci. 1964 Dec;10:195–225. [PubMed] [Google Scholar]
- Hahn E., Wick G., Pencev D., Timpl R. Distribution of basement membrane proteins in normal and fibrotic human liver: collagen type IV, laminin, and fibronectin. Gut. 1980 Jan;21(1):63–71. doi: 10.1136/gut.21.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENDRUM A. C., FRASER D. S., SLIDDERS W., HENDERSON R. Studies on the character and staining of fibrin. J Clin Pathol. 1962 Sep;15:401–413. doi: 10.1136/jcp.15.5.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie G. W., Leblond C. P., Martin G. R. Light microscopic immunolocalization of type IV collagen, laminin, heparan sulfate proteoglycan, and fibronectin in the basement membranes of a variety of rat organs. Am J Anat. 1983 May;167(1):71–82. doi: 10.1002/aja.1001670107. [DOI] [PubMed] [Google Scholar]
- Miettinen M., Foidart J. M., Ekblom P. Immunohistochemical demonstration of laminin, the major glycoprotein of basement membranes, as an aid in the diagnosis of soft tissue tumors. Am J Clin Pathol. 1983 Mar;79(3):306–311. doi: 10.1093/ajcp/79.3.306. [DOI] [PubMed] [Google Scholar]
- Miller P. J. An elastin stain. Med Lab Technol. 1971 Apr;28(2):148–149. [PubMed] [Google Scholar]
- Rohde H., Wick G., Timpl R. Immunochemical characterization of the basement membrane glycoprotein laminin. Eur J Biochem. 1979 Dec;102(1):195–201. doi: 10.1111/j.1432-1033.1979.tb06280.x. [DOI] [PubMed] [Google Scholar]
- Rothschild B. M., Masi A. T. Pathogenesis of rheumatoid arthritis: a vascular hypothesis. Semin Arthritis Rheum. 1982 Aug;12(1):11–31. doi: 10.1016/0049-0172(82)90020-8. [DOI] [PubMed] [Google Scholar]
- Scott D. L., Bedford P. A., Walton K. W. The preparation of plasma fibronectin antigen and antiserum. J Immunol Methods. 1981;43(1):29–33. doi: 10.1016/0022-1759(81)90033-8. [DOI] [PubMed] [Google Scholar]
- Scott D. L., Delamere J. P., Walton K. W. The distribution of fibronectin in the pannus in rheumatoid arthritis. Br J Exp Pathol. 1981 Aug;62(4):362–368. [PMC free article] [PubMed] [Google Scholar]
- Soren A., Klein W., Huth F. The synovial changes in post-traumatic synovitis and osteoarthritis. Rheumatol Rehabil. 1978 Feb;17(1):38–45. doi: 10.1093/rheumatology/17.1.38. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Unsworth D. J., Scott D. L., Almond T. J., Beard H. K., Holborow E. J., Walton K. W. Studies on reticulin. I: Serological and immunohistological investigations of the occurrence of collagen type III, fibronectin and the non-collagenous glycoprotein of Pras and Glynn in reticulin. Br J Exp Pathol. 1982 Apr;63(2):154–166. [PMC free article] [PubMed] [Google Scholar]