Abstract
Mycobacterium tuberculosis, the causative agent of tuberculosis (TB), infects primarily macrophages, causing them to differentiate into lipid-laden foamy macrophages that are a primary source of tissue destruction in patients with TB. In this issue of the JCI, Bedard et al. demonstrate that 1-tuberculosinyladenosine, a virulence factor produced by M. tuberculosis, caused lysosomal dysfunction associated with lipid storage in the phagolysosome of macrophages in a manner that mimicked lysosomal storage diseases. This work sheds light on how M. tuberculosis manipulates host lipid metabolism for its survival and opens avenues toward host-directed therapy against TB.
Foamy macrophages are a hallmark of tuberculous granulomas
Mycobacterium tuberculosis, the etiological agent of tuberculosis (TB), has plagued mankind for millennia and remains one of the deadliest pathogens today, as evidenced by the 10.6 million cases and 1.6 million deaths in 2021. M. tuberculosis is an intracellular pathogen that resides primarily within macrophages. M. tuberculosis–infected macrophages differentiate into lipid-laden foamy macrophages and aggregate together with other immune cells to form granulomas. Although granulomas generally serve as the physical and immunological barrier to bacterial growth and spreading (1, 2), M. tuberculosis has evolved to survive nutritional, hypoxic, and immunological constraints imposed by this environment. Furthermore, M. tuberculosis–infected foamy macrophages represent the primary source of caseous necrosis that causes tissue destruction and pulmonary cavitation in patients with TB (2–4). Accumulating evidence supports a model whereby M. tuberculosis induces reprogramming of host lipid metabolism to promote the accumulation of lipid droplets within the infected macrophages and uses these lipids as the primary carbon source for survival within the TB granuloma (4–10).
1-Tuberculosinyladenosine is a virulence factor of M. tuberculosis
Although macrophages are professional phagocytes equipped with a range of microbicidal weapons for killing intracellular microbes, M. tuberculosis has developed strategies to sidestep these defenses (1). In particular, the TB bacillus produces specific virulence factors that allow it to inhibit phagosome-lysosome fusion and autophagy, resist acidification of the phagolysosome, and disrupt the phagosome membrane to escape into the cytosol. In 2014, D. Branch Moody and collaborators performed a comparative lipidomic screen of M. tuberculosis and Calmette-Guérin bacillus (BCG), an attenuated Mycobacterium bovis strain used as a TB vaccine, and identified a M. tuberculosis–specific lipid they named 1-tubercolosinyladenosine (1-TbAd) (11). Since that initial discovery, 1-TbAd has been shown to be one of the most abundantly produced lipids in virulent M. tuberculosis strains, including clinical isolates (12, 13). Further studies have identified the 1-TbAd biosynthetic genes (Rv3377c and Rv3378c) and provided evidence that these genes appeared early in the evolution of the M. tuberculosis and were likely acquired by a horizontal gene transfer (11, 12, 14). Interestingly, a decade before the 1-TbAd discovery, genetic screening showed that Rv3377c and Rv3378c prevent phagolysosome acidification in macrophages and promote intracellular survival of M. tuberculosis (15). Consistent with these findings, Moody and colleagues demonstrated that 1-TbAd accumulates in acidic compartments, raising their pH, and causes lysosomes to swell in human macrophages (13). Importantly, these effects of 1-TbAd, especially phagolysosome swelling, could be recapitulated in human macrophages infected with wild-type M. tuberculosis but not with a 1-TbAd–deficient (Rv3378c-KO) mutant (13). Collectively, the results from these studies suggested that 1-TbAd is a M. tuberculosis–produced lipidic virulence factor that protects M. tuberculosis from macrophage-mediated intracellular killing. However, the link and exact mechanism governing these effects remain incompletely understood. In particular, when studying the mode of action of 1-TbAd, Moody and coworkers observed the formation of electron-dense inclusion bodies in swollen phagolysosomes of macrophages stimulated by 1-TbAd or infected by M. tuberculosis; however, they did not identify their nature.
1-TbAd causes lysosomal dysfunction and lysosomal lipid storage
In this issue of the JCI, Bedard et al. (16) shine a light on these processes. By using correlative light and electron microscopy (CLEM), Bedard et al. demonstrate that the particular inclusions within swollen lysosomes of human macrophages were of a lipid nature, suggesting that 1-TbAd induced lipid storage in these cells (Figure 1). This finding was confirmed by immunofluorescence microscopy using BODIPY staining. A two-hour pulse of 1-TbAd was sufficient to trigger durable lipid accumulation in macrophages over several days. 1-TbAd–induced lipid accumulation in human macrophages was clearly related to lysosome remodeling, since this phenotype could be recapitulated with chloroquine, a classical lysosomotropic drug, but not with N6-tubercolosinyladenosine (N6-TbAd), a natural isomer of 1-TbAd with no lysosomotropism or antiacid properties (12, 13). To obtain a comprehensive picture of the lipids that accumulated in macrophages treated with 1-TbAd, Bedard et al. (16) performed lipidomic analysis and found that 1-TbAd increased the pools of cholesteryl esters and triacylglycerol, which are the main lipids that accumulate in M. tuberculosis–induced foamy macrophages (2–4). 1-TbAd also enhanced the amounts of monoalkyl-diacylglycerol, β-glucosylceramide, and lactosylceramide, which are lysosomal hydrolase substrates known to be stored in specific lysosomal storage disorders, including Wolman and Gaucher diseases.
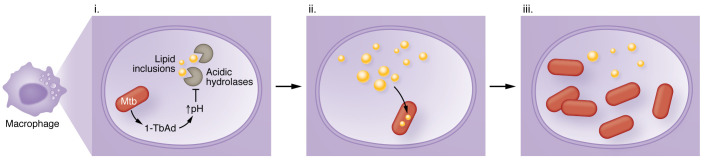
Figure 1. 1-TbAd produced by M. tuberculosis induces lysosomal lipid storage.
(i) M. tuberculosis (Mtb) produces 1-TbAd, which raises the vacuolar pH and inhibits acidic hydrolases, including lipases, inside the phagolysosome. (ii) Consequently, lipids, including cholesteryl esters and triglycerides, accumulate in the vacuole, mimicking lysosomal storage diseases. (iii) Under conditions of restricted lipid access, M. tuberculosis can use these lipids as a carbon source to promote its intracellular growth.
On the basis of these results, Bedard et al. (16) hypothesized that by raising lysosomal pH, 1-TbAd inhibits the activity of intralysosomal acid hydrolases, resulting in the accumulation of lipids in this compartment. To address this hypothesis, the authors used enzyme-activated fluorogenic probes and showed that 1-TbAd strongly inhibited the acid-dependent glycosidase and protease activity within intact macrophages. Lysosomes play a fundamental role in the autophagic process by fusing with autophagosomes and degrading their contents. Consequently, the autophagic flux can be strongly disrupted as a consequence of any lysosomal dysfunction, as observed in lysosomal storage diseases or when using lysosomotropic drugs such as chloroquine. In agreement, Bedard et al. found that 1-TbAd, but not N6-TbAd, induced the accumulation of autophagosomes in macrophages, most likely by impairing autophagosome-lysosome fusion. Next, Bedard et al. asked whether direct effects of 1-TbAd on lysosomal lipid storage could also be observed in macrophages infected with 1-TbAd–producing M. tuberculosis strains. Electron microscopy (EM) analyses combined with immunogold staining for CD63, a lysosomal marker, confirmed, that infection with wild-type M. tuberculosis, but not with the Rv3378c-KO strain, which does not produce 1-TbAd, induced swelling of macrophage phagolysosomes. In addition, immunofluorescence microscopy revealed a substantial induction of lipid inclusions (indicated by Nile red staining) at day four after infection with wild-type and complemented bacteria, compared with the 1-TbAd–deficient mutant. Lipid inclusions in M. tuberculosis–infected macrophages were clearly localized to phagolysosomal (i.e., LAMP1+) compartments, as revealed by CLEM (16).
Since some lipid inclusions were observed in the phagolysosomes that contained bacteria, Bedard et al. then wondered whether M. tuberculosis could use these lipids, in particular cholesterol, as a carbon source to grow inside these cells. To answer this question, they measured intracellular M. tuberculosis growth in the presence of all-trans-retinoic acid (ATRA), which limits M. tuberculosis growth by restricting access to cholesterol (8), alone or in combination with 1-TbAd. Remarkably, treatment with 1-TbAd, but not N6-TbAd, abrogated ATRA-mediated restriction of M. tuberculosis growth in macrophages in a dose-dependent manner, suggesting that 1-TbAd–induced lipid storage in phagolysosomes provides nutrients to M. tuberculosis to support bacterial growth. Finally, Bedard et al. sought to assess whether the lysosomal failure of macrophages triggered by 1-TbAd could be reversed using pharmacological regulators of lysosomal activity. In particular, they focused on an agonist (C8) of transient receptor potential mucolipin channel 1 (TRPML1), a lysosomal Ca2+ release channel that prevents lysosomal dyshomeostasis (16–20). As hypothesized, treatment of 1-TbAd–stimulated macrophages with C8 decreased the size of swollen lysosomes by more than two-fold, dramatically reduced the number of lipid inclusions, and restored glycolipid catabolism (16).
Conclusion and perspectives
Bedard et al. (16) demonstrate that 1-TbAd produced by M. tuberculosis caused lysosomal failure and accumulation of lipids in macrophages, mimicking lysosomal storage diseases. Furthermore, their data suggest that M. tuberculosis used lipids stored in lysosomes to support its growth inside macrophages, especially under conditions of limited lipid access. Finally, they show that 1-TbAd–induced, lysosome-dependent lipid accumulation could be counteracted by the use of a drug that restores lysosomal function.
These findings raise several questions and open several perspectives. First, 1-TbAd–induced lysosomal lipid storage could only be observed in human macrophages primed with both granulocyte-macrophage colony-stimulating factor (GM-CSF) and M-CSF (indicating M1 macrophages), but not, or only slightly, in M-CSF–polarized human macrophages (suggesting M2 macrophages) or mouse alveolar macrophages. In contrast, 1-TbAd swelled lysosomes in all these macrophage subtypes. The reason why 1-TbAd induces lipid accumulation specifically in M1 macrophages remains to be clarified. However, this apparent discrepancy could be linked to cell-specific differences in lipid handling and metabolism, since alveolar macrophages and M2 macrophages are committed to fatty acid oxidation, whereas M1 macrophages rely mainly on glycolysis for energy generation (21, 22). Second, foamy macrophages observed during M. tuberculosis infection have often been reported to be filled with lipid droplets, a phenotype not observed in this study (16). Thus, it is yet to be determined whether the unconventional, so-called “foamy” macrophages induced by 1-TbAd can be found in the host during TB infection. Nonetheless, the results of Bedard et al. (16) provide a possible explanation for some earlier observations, such as that intracellular M. tuberculosis can readily acquire lipids from macrophages, even when macrophages do not form lipid droplets (23). Finally, Bedard et al. (16) provide evidence that the use of specific lysosome-targeting drugs can reverse 1-TbAd–induced lysosomal dysfunction. Although future studies are needed to confirm these results in M. tuberculosis–infected macrophages and in vivo, this study provides initial evidence that lysosomes may be an attractive therapeutic target for the development of host-directed therapies against TB.
Acknowledgments
The authors did not receive specific funding for this work. The Neyrolles laboratory is supported by the Centre National de la Recherche Scientifique (CNRS); the University of Toulouse–University Toulouse III Paul Sabatier; the Fondation pour la Recherche Médicale; the Fondation Bettencourt Schueller; the Agence Nationale de la Recherche; and MSDAVENIR.
Version 1. 03/15/2023
Electronic publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2023, Rombouts et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: J Clin Invest. 2023;133(6):e168366. https://doi.org/10.1172/JCI168366.
See the related article at A terpene nucleoside from M. tuberculosis induces lysosomal lipid storage in foamy macrophages.
Contributor Information
Yoann Rombouts, Email: Yoann.Rombouts@ipbs.fr.
Olivier Neyrolles, Email: olivier.neyrolles@ipbs.fr.
References
- 1.Cambier CJ, Falkow S, Ramakrishnan L. Host evasion and exploitation schemes of Mycobacterium tuberculosis. Cell. 2014;159(7):1497–1509. doi: 10.1016/j.cell.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 2.Russell DG, et al. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat Immunol. 2009;10(9):943–948. doi: 10.1038/ni.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim M, et al. Caseation of human tuberculosis granulomas correlates with elevated host lipid metabolism. EMBO Mol Med. 2010;2(7):258–274. doi: 10.1002/emmm.201000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guerrini V, et al. Storage lipid studies in tuberculosis reveal that foam cell biogenesis is disease-specific. PLoS Pathog. 2018;14(8):e1007223. doi: 10.1371/journal.ppat.1007223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peyron P, et al. Foamy macrophages from tuberculous patients’ granulomas constitute a nutrient-rich reservoir for M. tuberculosis persistence. PLoS Pathog. 2008;4(11):e1000204. doi: 10.1371/journal.ppat.1000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muñoz-Elías EJ, McKinney JD. Mycobacterium tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nat Med. 2005;11(6):638–644. doi: 10.1038/nm1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pandey AK, Sassetti CM. Mycobacterial persistence requires the utilization of host cholesterol. Proc Natl Acad Sci U S A. 2008;105(11):4376–4380. doi: 10.1073/pnas.0711159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babunovic GH, et al. CRISPR Interference reveals that all-trans-retinoic acid promotes macrophage control of Mycobacterium tuberculosis by limiting bacterial access to cholesterol and propionyl coenzyme A. mBio. 2022;13(1):e03683–21. doi: 10.1128/mbio.03683-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandenburg J, et al. WNT6/ACC2-induced storage of triacylglycerols in macrophages is exploited by Mycobacterium tuberculosis. J Clin Invest. 2021;131(16):e141833. doi: 10.1172/JCI141833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenwood DJ, et al. Subcellular antibiotic visualization reveals a dynamic drug reservoir in infected macrophages. Science. 2019;364(6447):1279–1282. doi: 10.1126/science.aat9689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Layre E, et al. Molecular profiling of Mycobacterium tuberculosis identifies tuberculosinyl nucleoside products of the virulence-associated enzyme Rv3378c. Proc Natl Acad Sci U S A. 2014;111(8):2978–2983. doi: 10.1073/pnas.1315883111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young DC, et al. In vivo biosynthesis of terpene nucleosides provides unique chemical markers of Mycobacterium tuberculosis infection. Chem Biol. 2015;22(4):516–526. doi: 10.1016/j.chembiol.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buter J, et al. Mycobacterium tuberculosis releases an antacid that remodels phagosomes. Nat Chem Biol. 2019;15(9):889–899. doi: 10.1038/s41589-019-0336-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becq J, et al. Contribution of horizontally acquired genomic islands to the evolution of the tubercle bacilli. Mol Biol Evol. 2007;24(8):1861–1871. doi: 10.1093/molbev/msm111. [DOI] [PubMed] [Google Scholar]
- 15.Pethe K, et al. Isolation of Mycobacterium tuberculosis mutants defective in the arrest of phagosome maturation. Proc Natl Acad Sci U S A. 2004;101(37):13642–13647. doi: 10.1073/pnas.0401657101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bedard M, et al. A terpene nucleoside from M. tuberculosis induces lysosomal lipid storage in foamy macrophages. J Clin Invest. 2023;133(6):e161964. doi: 10.1172/JCI161944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, et al. A molecular mechanism to regulate lysosome motility for lysosome positioning and tubulation. Nat Cell Biol. 2016;18(4):404–417. doi: 10.1038/ncb3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medina DL, et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol. 2015;17(3):288–299. doi: 10.1038/ncb3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bae M, et al. Activation of TRPML1 clears intraneuronal Aβ in preclinical models of HIV infection. J Neurosci. 2014;34(34):11485–11503. doi: 10.1523/JNEUROSCI.0210-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodwin JM, et al. GABARAP sequesters the FLCN-FNIP tumor suppressor complex to couple autophagy with lysosomal biogenesis. Sci Adv. 2021;7(40):eabj2485. doi: 10.1126/sciadv.abj2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang L, et al. Growth of Mycobacterium tuberculosis in vivo segregates with host macrophage metabolism and ontogeny. J Exp Med. 2018;215(4):1135–1152. doi: 10.1084/jem.20172020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wculek SK, et al. Oxidative phosphorylation selectively orchestrates tissue macrophage homeostasis. Immunity. doi: 10.1016/j.immuni.2023.01.011. [published online February 3, 2023]. [DOI] [PubMed] [Google Scholar]
- 23.Knight M, et al. Lipid droplet formation in Mycobacterium tuberculosis infected macrophages requires IFN-γ/HIF-1α signaling and supports host defense. PLoS Pathog. 2018;14(1):e1006874. doi: 10.1371/journal.ppat.1006874. [DOI] [PMC free article] [PubMed] [Google Scholar]