To the Editor: Extremes in birth weight are associated with adverse pregnancy outcomes and long-term risk of cardiometabolic disease. Fetal insulin has long been recognized as an important regulator of fetal growth, but the overall contribution of fetal insulin to birth weight in humans has not been quantified. Single-gene mutations resulting in absent fetal insulin secretion provide a unique opportunity to study the effects of fetal insulin on birth weight in humans. We sought to quantify the role of fetal insulin in fetal growth by studying birth weights in individuals without fetal insulin, either due to recessive loss-of-function mutations in the INS gene or pancreatic agenesis (Supplemental Methods and Supplemental Tables 1 and 2 for clinical details and genetics, respectively; supplemental material available online with this article; https://doi.org/10.1172/JCI165402DS1). We also investigated whether reduced insulin-mediated fetal growth affected postnatal growth once insulin was replaced. The study was approved by the Wales Research Ethics Committee (17/WA/0327). P values of less than 0.05 were considered statistically significant, and specific statistical tests are detailed in Figure 1.
In the absence of fetal insulin birth weight is halved in humans.
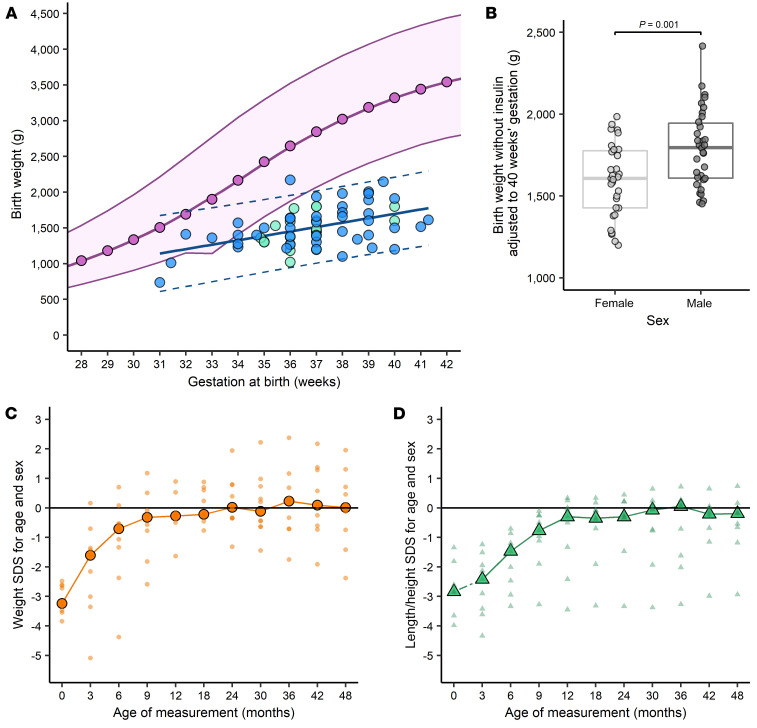
There was a substantial, global reduction in fetal growth in the absence of fetal insulin (Figure 1A and Supplemental Tables 1 and 3). Mean birth weight adjusted to 40 weeks’ gestation was 51% of normal birth weight (1,697 g [95% CI, 1,586–1,808 g] vs. 3,320 g [50th percentile at 40 weeks]) (1). Median birth length was greatly reduced (Supplemental Table 1) and 12% lower than normal (adjusted 43.7 cm [95% CI, 40.9–46.5 cm] vs. 49.6 cm at 40 weeks) (1).
Figure 1. The effect of absent fetal insulin on pre- and postnatal growth in humans.
(A) Birth weights in individuals without fetal insulin. Light green, INS (n = 21); blue, pancreatic agenesis (n = 43). Normal growth median, dark pink; the shaded pink area indicates ±2 SDs (INTERGROWTH-21st, refs. 1, 8). The relationship between birth weight without fetal insulin and gestational age is shown by the line-of-best fit (solid blue) and 95% prediction intervals (dashed blue) from a univariable linear regression model. (B) Adjusted birth weight (40 weeks) in the absence of insulin in female (light gray, n = 31) and male (dark gray, n = 33) individuals. Center line, median; box limits, IQR; whiskers, 1.5 × IQR. Unpaired, 2-tailed Student’s t test was used. (C and D) Growth in first 4 years following insulin treatment in individuals with a recessive INS mutation. (C) Weight (orange circles, n = 10) and (D) length/height (green triangles, n = 7 birth, n = 9 postnatal), with large symbols showing the median. Measurements were standardized (SDS) for sex and gestational age/age (9). Absent data points for individuals were approximated from available data using linear interpolation.
Female individuals without insulin were 196 g (95% CI, 80–312 g) lighter than male individuals, indicating that the sexual dimorphism in birth weight is accounted for by noninsulin-mediated fetal growth (Figure 1B).
Deficient insulin-mediated growth in utero is accompanied by rapid postnatal catch-up growth once insulin is replaced.
In utero growth restraint due to absent fetal insulin secretion did not persist postnatally. In individuals with loss-of-function INS mutations, after birth there was evidence of rapid, early catch-up growth of weight and length (Figure 1, C and D). Compared with birth size, there was a median gain of 2.97 SDS (IQR, 2.29 to 3.07 SDS) in weight and 2.11 SDS (IQR, –0.58 to 3.68 SDS) in length. Most recently available weight (n = 16) and height (n = 15) after the age of 2 years were within the normal ranges (Supplemental Table 4).
This study utilizing human monogenic disease as a model of absent fetal insulin has provided unique insights into the physiology of early growth.
Insulin-mediated fetal growth in humans contributes approximately 49% to birth weight at term, which is highest out of all species studied (2). Absent fetal insulin also reduced birth length, but its greater effect on weight confirms its main effects relate to fetal fat deposition. The high contribution of fetal insulin-mediated growth to birth weight could explain why, at birth, humans have a higher proportion of body fat compared with other species (3). This high proportion of fat at birth could confer a survival advantage, as lipids provide an efficient and vital fuel for the developing, large human brain (4). The relatively long length of gestation in humans could also result in a longer period of exposure to fetal insulin. We observed birth weight without fetal insulin to deviate further from the normal range as pregnancy progressed (Figure 1A), indicating that insulin-mediated growth becomes more important later in pregnancy.
A key regulator of fetal insulin-mediated growth is maternal glucose. Maternal glycemia has a substantial effect on birth weight (1 mmol/L higher maternal fasting glucose raises birth weight by 301 g; ref. 5). Similar to the situation in which insulin is replaced after birth following in utero deficiency, the effect of maternal glycemia is transient and rapidly lost in the first year of life, with catch-up and catch-down growth (6). In contrast, the lower birth weight in female individuals does not appear to have its origins in fetal insulin-mediated growth, and catch-up is not observed
Rapid catch-up growth once insulin is replaced in individuals with INS loss-of-function mutations is in marked contrast to the postnatal growth failure of those with severe insulin resistance secondary to biallelic mutations in the insulin receptor gene INSR (Donohue syndrome), despite similarly low birth weight (7). This is likely to reflect that, postnatally, it is not possible to correct the tissue insulin resistance in these individuals.
In conclusion, monogenic diseases resulting in absent fetal insulin have enabled us to answer fundamental questions about early growth in humans. We have used a monogenic human knockout of insulin to show that absence of fetal insulin reduces birth weight by approximately half and postnatally, there is rapid catch-up in weight and length. This establishes that insulin-mediated and noninsulin-mediated growth are equally important in humans. Whether other key modulators of fetal growth apart from maternal glucose act through fetal insulin is uncertain. In the future, all studies looking at fetal growth should determine whether insulin- or noninsulin-mediated growth are impacted, because the short- and long-term outcomes are likely to be different.
Supplementary Material
Acknowledgments
The authors are grateful to the participants and their families and acknowledge the genetic testing performed by Exeter Genomics Laboratory. This research was funded, in whole or in part, through grants from the Wellcome Trust to AEH (GW4 Clinical Academic Training PhD Fellowship, WT203918), RMF (Senior Research Fellowship, WT220390), SEF (Senior Research Fellowship, WT223187), and ATH and EDF (Collaborative Grant WT 224600) as well as from Diabetes UK to EDF (RD Lawrence Fellowship, 19/005971). ATH is employed as a core member of staff within the National Institute for Health Research (NIHR) Exeter Clinical Research Facility. This work was supported by the NIHR Exeter Biomedical Research Centre. The funders had no role in study design; data collection, analysis, or interpretation; or manuscript preparation. The views expressed are the authors’ and not necessarily those of any funder.
Version 1. 02/21/2023
In-Press Preview
Version 2. 03/15/2023
Electronic publication
Funding Statement
GW4-Clinical Academic Training PhD Fellowship to AEH
Senior Research Fellowships
RD Lawrence Fellowship to EDF
ATH is employed as a core member of staff within the National Institute for Health Research (NIHR) Exeter Clinical Research Facility.
This work was supported by the NIHR Exeter Biomedical Research Centre.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2023, Hughes et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Submitted: September 13, 2022; Accepted: February 2, 2023; Published: March 15, 2023.
Reference information: J Clin Invest. 2023;133(6):e165402.
Contributor Information
Alice E. Hughes, Email: a.e.hughes@exeter.ac.uk.
Elisa De Franco, Email: E.De-Franco@exeter.ac.uk.
Rachel M. Freathy, Email: r.freathy@exeter.ac.uk.
Sarah E. Flanagan, Email: s.flanagan@exeter.ac.uk.
Andrew T. Hattersley, Email: A.T.Hattersley@exeter.ac.uk.
References
- 1.Villar J, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384(9946):857–868. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 2.Fowden AL, Forhead AJ. Endocrine regulation of feto-placental growth. Horm Res. 2009;72(5):257–265. doi: 10.1159/000245927. [DOI] [PubMed] [Google Scholar]
- 3.Widdowson EM. Chemical composition of newly born mammals. Nature. 1950;166(4224):626–628. doi: 10.1038/166626a0. [DOI] [PubMed] [Google Scholar]
- 4.Cunnane SC, Crawford MA. Survival of the fattest: fat babies were the key to evolution of the large human brain. Comp Biochem Physiol A Mol Integr Physiol. 2003;136(1):17–26. doi: 10.1016/S1095-6433(03)00048-5. [DOI] [PubMed] [Google Scholar]
- 5.Knight B, et al. The Exeter Family Study of Childhood Health (EFSOCH): study protocol and methodology. Paediatr Perinat Epidemiol. 2006;20(2):172–179. doi: 10.1111/j.1365-3016.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 6.Knight B, et al. The impact of maternal glycemia and obesity on early postnatal growth in a nondiabetic Caucasian population. Diabetes Care. 2007;30(4):777–783. doi: 10.2337/dc06-1849. [DOI] [PubMed] [Google Scholar]
- 7.D’Ercole AJ, et al. Leprechaunism: studies of the relationship among hyperinsulinism, insulin resistance, and growth retardation. J Clin Endocrinol Metab. 1979;48(3):495–502. doi: 10.1210/jcem-48-3-495. [DOI] [PubMed] [Google Scholar]
- 8.Villar J, et al. INTERGROWTH-21st very preterm size at birth reference charts. Lancet. 2016;387(10021):844–845. doi: 10.1016/S0140-6736(16)00384-6. [DOI] [PubMed] [Google Scholar]
- 9.de Onis M. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatrica. 2006;95(s450):76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.