Abstract
Objectives
This study aimed to investigate the safety of the inactivated COVID-19 vaccines in early pregnant women in view of their adverse-effect profile and associated maternal-fetal complications, as well as to evaluate their immunogenicity.
Methods
In this prospective observational cohort study, 232 women in their first trimester or those in the periconception period who inadvertently received two doses of inactivated COVID-19 vaccine between January 21, 2021, and January 14, 2022 were analyzed. Meanwhile, 735 unvaccinated early pregnancy women were also included in the study at a case-to-control ratio of 1:3.
Results
The vaccination group did not have an increased miscarriage rate compared with that of the control group (P = 0.918). Furthermore, the birth defect rates in the vaccine group and control group were 0.83% and 1.0%, respectively. Vaccination did not increase the risk of small for gestational age, gestational diabetes mellitus, preterm, or hypertensive disorders of pregnancy (P >0.01). Within 12 weeks after the second dose, the inactivated vaccine effectively produced neutralizing antibody (NAb) against SARS-CoV-2. The NAb levels in the paired umbilical cord serum and maternal serum samples during delivery were negative in both groups. The T-cell subset remained within the normal range in both groups.
Conclusion
Therefore, our study proves that inactivated COVID-19 vaccines are safe for mothers and fetuses and also effective in producing NAb against SARS-CoV-2.
Keywords: Inactivated COVID-19 vaccine, Pregnancy complications, First trimester, Neutralizing antibody, Cord blood
Introduction
With the urgent need to combat COVID-19 in China, the safety, immunogenicity, and efficacy data of the two authorized inactivated vaccines (CoronaVac from Sinovac Life Sciences, Beijing, China, and BBIBP-CorV from Beijing Institute of Biological Products, Beijing, China) are promising [1], [2], [3]. However, they are not comprehensively investigated among pregnant women. Pregnancy may pose an increased risk of severe COVID-19 [4]. It is a state in which immune cells involved in antibody production may respond differently and thus negatively affect vaccine efficacy [5]. Vaccine efficacy and safety for mothers and their fetuses have emerged as primary concerns [6]. Therefore, the Japan Society of Obstetrics and Gynecology and the Japanese Society of Infectious Diseases in Obstetrics and Gynecology recommended that vaccines should be avoided during the first trimester to prevent any harm to fetal organ development [7]. Nevertheless, recent studies have revealed that mRNA vaccination has promising effects on antibody development among pregnant women by crossing the placenta and protecting the fetus [8], [9], [10]. On this basis, the American College of Obstetricians and Gynecologists proposed that pregnant subjects should also be vaccinated. One case report suggested that the levels of SARS-CoV-2 immunoglobulin (Ig)G antibodies were remarkable within the cord blood of the neonate following two doses of inactivated COVID-19 vaccination during pregnancy [11]. However, the average duration of human pregnancy is about 9 months. Studies are yet needed to elucidate whether inactivated COVID-19 vaccination in pregnancy can produce adequate responses that can ensure immunity during the whole gestational period and protect the fetus by crossing the placenta. A previous study also suggested that antibody levels rapidly decreased after individuals received an inactivated vaccine 12 weeks after the second dose [12]. To provide evidence-based suggestions, this study concentrated on early pregnancy associated with high fetal malformation and genetic mutation frequencies, safety and efficacy of inactivated COVID-19 vaccines among pregnant women, and the potential antibody transfer via the placenta.
Subjects and methods
Ethics statement
The only human materials used in this study were serum samples collected from early pregnancy women for public health purposes. Written informed consent for the use of clinical samples was obtained from some participants involved in this study. This study was approved by the Ethics Review Committee of the Shanghai First Maternity and Infant Hospital, Tongji University School of Medicine (approval number: KS21348), and the procedures were performed in accordance with approved guidelines.
Study design and subjects
This prospective cohort study was conducted from July 01, 2021, to May 30, 2022, at Shanghai First Maternity and Infant Hospital, Shanghai, China.
Women were considered eligible under the following criteria: (i) pregnant, (ii) with natural conception, (iii) given a two-dose regimen of inactivated vaccine in periconception (30 days before the last menstrual period through 14 days after) or in the first trimester, (iv) not infected with SARS-CoV or SARS-CoV-2, and (v) without chronic illness. The vaccinated and unvaccinated early pregnancy women were matched at a ratio of 1: 3. Demographic data, medical history, medications, COVID-19 vaccination timing, inactivated COVID-19 vaccine type, and side effects were provided by all participants. Most participants were required to provide a blood sample for lymphocyte subset analysis or neutralizing antibody (NAb) detection. Each of the pregnant women in the present study was followed up in the obstetrics outpatient clinic at Shanghai First Maternity and Infant Hospital, and most of them delivered in this hospital. Gestational age records of live-born infants and stillbirths were based on ultrasonography, whereas gestational age records of abortive outcomes were based on either ultrasonography or the 1st day of the last menstrual period. In addition, birth defects listed in the National Stocktaking Report on Birth Defects Prevention were also examined.
These unfavorable birth outcomes included preterm birth, small for gestational age (SGA), gestational diabetes mellitus (GDM), and hypertensive disorders of pregnancy (PIH), and they were chosen because they are common and important markers of maternal health. Weight for gestational age percentiles was assigned on the basis of reference values derived by Oken et al. [13], with a cutoff of less than the 10th percentile used to classify birth as SGA. Preterm birth was defined as birth before the 37th gestational week, as indicated in medical records. GDM was diagnosed according to the guidelines of the International Association of Diabetes and Pregnancy Study Groups [14]. PIH was diagnosed as recommended by the International Society for the Study of Hypertension in Pregnancy [15]. Miscarriage was defined as the loss of pregnancy before the 28th gestational week. Stillbirth was defined as fetal loss after 28 weeks of gestation. The research protocol was approved by the Ethics Committee of Shanghai First Maternity and Infant Hospital. Informed consent for data collection was signed by the patients. Maternal blood and paired cord blood were harvested upon delivery to test for NAb.
Sampling and processing
Blood samples were obtained from some participants when they first visited the Shanghai First Maternity and Infant Hospital after they confirmed their pregnancy. Some blood specimens were not collected because of refusal by the pregnant women. Paired cord and maternal blood samples were collected upon delivery from the vaccination and control groups. Blood samples were harvested through venipuncture (or from the umbilical vein following the delivery of cord blood) into serum separator tubes and centrifuged at 3500 × g for 5 minutes at 20°C. Sera were aliquoted into cryogenic vials and stored at -20°C.
Microneutralization test
A microneutralization test was performed through microtitration, and cytopathic effects (CPEs) were observed to determine the serum-neutralizing titer. After heat inactivation, the serum was diluted and incubated with a live virus (strain WH01, 100 lg CCID50/well) at 37°C for 2 hours. Vero cells (105 cells/ml) were added and incubated at 37°C and 5% CO2 for 7 days. Then, CPE was monitored to determine the serum NAb titer. NAb geometric mean titers (GMTs) were also determined. NAb positivity was defined as an antibody titer of ≥4.
Lymphocyte subsets
Immunocyte subsets were separated under standard instructions. Briefly, peripheral blood mononuclear cells were separated through lymphocyte separation (Ficoll-Paque PREMIUM; GE Healthcare), added with antibodies against cluster of differentiation (CD)3, CD20, and CD16 (BD558639; BD, USA), and incubated at ambient temperature in dark for 30 minutes. Reagents for red blood cell lysis were sequentially added. In addition, membranes were permeabilized. Then, cells were rinsed with phosphate buffer solution thrice, resuspended in phosphate buffer solution, and examined through flow cytometry (FCM, BD, USA). Each experiment was performed in line with specific protocols.
Statistical analysis
The features and pregnancy outcomes of the subjects were summarized through frequency statistics. Fisher's exact test or chi-square test was employed to compare intergroup differences in categorical variables. Analysis of variance was conducted to compare GMTs among the groups. Multivariable logistic regression was performed for obstetric outcomes to obtain adjusted risk ratios (RRs) with 95% confidence intervals (CIs). Potential confounding variables, including nullipara, were incorporated into the regression equation.
Results
Participant characteristics and pregnancy outcomes
Table 1 presents the baseline characteristics and pregnancy outcomes of participants excluding those of losing follow-up or artificial abortion in early pregnancy. The two groups had no significant differences in age, body mass index, previous miscarriage, twin pregnancy, or uterine anatomy. The difference in the percentage of nullipara was statistically significant between the two groups (P = 0.003; 72.12% vs 82.29%).
Table 1.
Baseline characteristics and pregnancy outcomes in participants excluding those of losing follow-up or artificial abortion in early pregnancy.
| Characteristic | Vaccinated group (165) | % | No-vaccination group (717) | % | P-value |
|---|---|---|---|---|---|
| Age, mean (SD),y | |||||
| <35 | 137/165 | 594/717 | |||
| ≥35 | 28/165 | 16.97 | 123/717 | 17.15 | 0.955 |
| Body mass index (kg/m2) | |||||
| Normal (<24 kg/m2) | 139/165 | 613/717 | |||
| Overweight (≥24 kg/m2) | 26/165 | 15.76 | 104/717 | 14.50 | 0.682 |
| Previous miscarriage | 21/165 | 12.73 | 122/717 | 17.02 | 0.178 |
| Twin pregnancy | 1/165 | 0.61 | 3/717 | 0.42 | 0.746 |
| Uterine anatomy | 1/165 | 0.61 | 3/717 | 0.42 | 0.746 |
| Nulliparous | 119/165 | 72.12 | 590/717 | 82.29 | 0.003 |
| Pregnancy loss | |||||
| Miscarriage | 20/165 | 12.12 | 89/717 | 12.41 | 0.918 |
| Stillbirth | 1/119 | 0 | |||
| Neonatal death | 0 | 0 | |||
| Birth defect | 1/120 | 0.83 | 5/514 | 1.00 | 0.887 |
| Congenital anomalies | 1 | 2 | |||
| Chromosome abnormality | 0 | 3 |
The denominator of the birth defect rate in the vaccination group included live-born infants. A total of 119 women in the study group delivered their babies, including one twin, during the study period. One case of congenital anomaly, i.e., atrial septal defect, was recorded in the vaccinated group, and the baby was still alive.
The denominator of the birth defect rate in the nonvaccinated group included live-born infants, and 511 women delivered their babies, including three twins, during the study period. Three cases of chromosomal abnormalities in the nonvaccinated group included two cases of trisomy 21 and one case of Noonan syndrome, which were manually terminated. Two cases of congenital malformations in the nonvaccinated group included one case of loss of the corpus callosum and one case of a solid mass in the right abdomen of the fetus, which were also manually terminated.
The denominator of stillbirth in the vaccination group was the number of live birth.
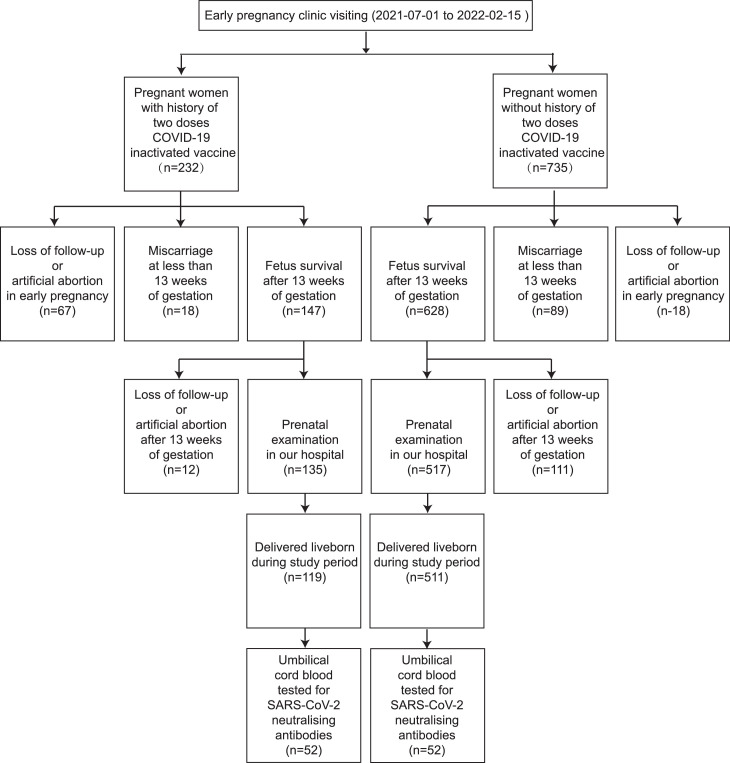
All pregnancies with abortive outcomes or live births from July 01, 2021, to May 30, 2022, were identified. During the study period, 119 cases in the vaccination group and 511 cases in the control group were delivered. A total of 232 pregnant women were included in the vaccination group; apart from 67 cases of loss to follow-up or artificial abortion in early pregnancy, 18 cases of miscarriage were recorded in less than 13 weeks of gestation, indicating that the early miscarriage rate was 10.91% (18/165). The same method was employed to calculate the early miscarriage rate of the control group (12.41%; 89/717; Figure 1 ). Two cases of late miscarriage in the vaccination group occurred on weeks 20 and 22 of gestation, respectively. One stillbirth case in the vaccination group was reported on weeks 35 + 4 of gestation. No case of late miscarriage or stillbirth was found in the control group. The miscarriage rates were 12.12% and 12.41% in the vaccination and control groups, respectively (P = 0.918), while the birth defect rates were 0.83% and 1.0% (P = 0.887). One case of a major birth defect, i.e., a cardiac defect, was found in the vaccination group. Three cases of chromosomal abnormalities, including two cases of trisomy 21 and one case of Noonan syndrome, were reported in the control group. Two cases of congenital malformations were detected in the control group, containing one case of loss of the corpus callosum and one case of a solid mass in the right abdomen of the fetus.
Figure 1.
Flowchart showing enrolment of study participants.
After adjusting for nullipara in the vaccination and control groups, our study revealed that exposure to inactivated COVID-19 vaccines was not related to preterm birth, PIH, GDM, or SGA (Table 2 ). The preterm birth rates were 5.9% and 5.3% in the vaccination and control groups, respectively (P = 0.07, adjusted RR [aRR] 1.00, 95% CI = 0.44-2.29), the incidence rates of PIH were 4.2% and 6.3% (P = 0.74, aRR 0.67, 95% CI = 0.27-1.69), the SGA rates were 2.5% and 3.1% (P = 0.12, aRR 0.81, 95% CI = 0.34-2.73), and the GDM rates were 16% and 12.7% (P = 0.88, aRR 1.23, 95% CI = 0.77-1.98) in the vaccination and control groups, respectively.
Table 2.
Rate and risks of complications of vaccine group and no-vaccine group.
| No-vaccine group (n=511) | Rate per 100 deliveries | Vaccine group (n = 119) | Rate per 100 deliveries | Crude risk ratio (95% confidence interval) | Adjusted risk ratio (95% confidence interval) | P-value | X2 | |
|---|---|---|---|---|---|---|---|---|
| Preterm | 27 | 5.3% | 7 | 5.9% | 1.11 (0.50-2.50) | 1.00 (0.44-2.29) | 0.07 | 0.80 |
| Pregnancy-induced hypertension syndrome | 32 | 6.3% | 5 | 4.2% | 0.67 (0.27-1.69) | 0.67 (0.27-1.69) | 0.74 | 0.39 |
| Small-size for gestational age | 16 | 3.1% | 3 | 2.5% | 0.81 (0.24-2.72) | 0.81 (0.34-2.73) | 0.12 | 0.73 |
| Gestational diabetes mellitus | 65 | 12.7% | 19 | 16.0% | 1.26 (0.78-2.01) | 1.23 (0.77-1.98) | 0.88 | 0.35 |
Vaccination characteristics and vaccine side effects
Among the 133 participants vaccinated with CoronaVac, 100 had the first dose during the periconception period, and 33 received theirs in the first trimester (Table 3 ). Among the 99 participants vaccinated with BBIBP-CorV, 73 were given the first dose during the periconception period, and 26 received their shot during early pregnancy. CoronaVac and BBIBP-CorV elicited similar adverse reactions. Notably, fatigue, reaction, and pain at the injection site were common side effects. Vaccination-related fever was reported in 3.4% of the subjects.
Table 3.
Sampling timepoints and adverse reaction profiles of inactivated COVID-19 vaccine.
| Vaccination Characteristic | Corona Vac (n = 133) | BBIBP-CorV (n = 99) | P-value |
|---|---|---|---|
| Pregnancy status | / | ||
| Periconception | 100 | 73 | |
| Pregnant at time of vaccination | 33 | 26 | |
| Gestational age at second vaccine dose | / | ||
| <2 weeks | 100 | 73 | |
| 2-6 weeks | 31 | 25 | |
| 6-12 weeks | 2 | 1 | |
| Timepoints for blood collection after second dose | / | ||
| ≤12 weeks | 53 | 39 | |
| 12-24 weeks | 22 | 18 | |
| Seroconversion rate | |||
| Time from COVID-19 vaccination at the second dose to blood sampling ≤12 weeks | 62.3% (33/53) | 41% (16/39) | <0.0001 |
| Time from COVID-19 vaccination at the second dose to blood sampling at 12-24 weeks | 45.5% (10/22) | 0% (0/18) | 10.91 |
| The geometric mean titer of neutralizing antibodies | |||
| Time from COVID-19 vaccination at the second dose to blood sampling ≤12 weeks | 5.63 | 3.16 | 0.02 |
| Time from COVID-19 vaccination at the second dose to blood sampling at 12-24 weeks | 3.09 | 2.09 | 0.07 |
| Side effects of vaccine | |||
| Injection site soreness | 9.8% (13/133) | 10.1% (10/99) | 0.93 |
| Injection site reaction or rash | 12% (16/133) | 13.1% (13/99) | 0.80 |
| Headache | 3.8% (5/133) | 4.0% (4/99) | 0.91 |
| Fatigue | 8.3% (11/133) | 8.1% (8/99) | 0.96 |
| Fever or chills | 3.8% (5/133) | 3% (3/99) | 0.76 |
| Allergic reaction | 0.8% (1/133) | 0 | 0.75 |
Maternal vaccine response and antibody transfer
All participants in the control group were seronegative (with NAb titers < 1:4). In the Sinovac group, the NAb-positive rates were 62.3% and 45.5% for 53 blood samples collected within 12 weeks of the second dose and for 22 samples taken between 12 and 24 weeks after the second dose, respectively. The NAb-positive rates of BBIBP-CorV were 41% and 0% for 39 blood samples collected within 12 weeks following the second dose and for 18 samples taken between 12 and 24 weeks following the second dose, respectively. The NAb GMTs were 5.63 in the Sinovac group in <12 weeks after the second dose and 3.09 in the group between 12 and 24 weeks after the second dose. The GMTs of NAb were 3.16 in the BBIBP-CorV group at <12 weeks after the second dose and 2.09 in the group between 12 and 24 weeks after the second dose. The GMT and NAb-positive rates in the Sinovac and BBIBP-CorV groups decreased from the group within 12 weeks after the second dose to the group between 12 and 24 weeks after the second dose. The NAb-positive rate was zero in the maternal blood and paired cord blood upon delivery in both groups.
Lymphocyte subsets results
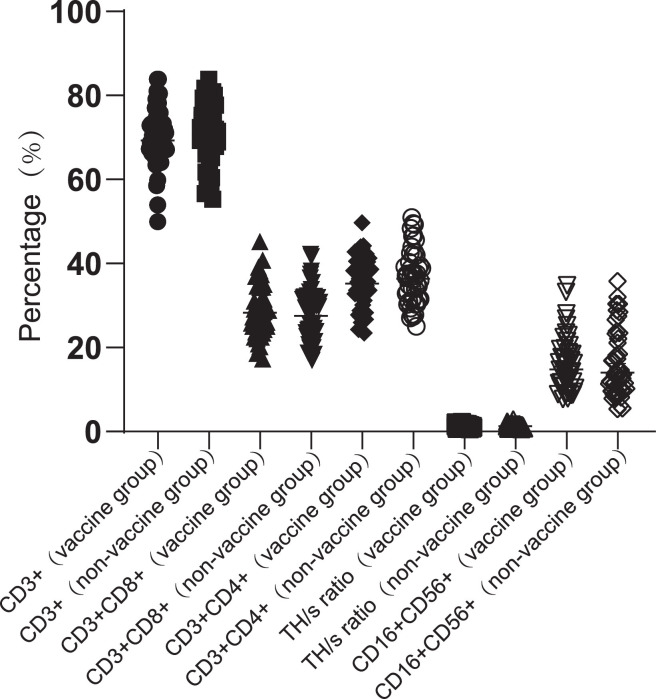
The vaccine and control groups had no significant differences in the percentages of lymphocyte expression, including CD4+ T cells, CD8+ T cells, CD56+ T cells, and the TH/s ratio (Figure 2 ). The T-cell subset remained within the normal range in both groups.
Figure 2.
Lymphocyte subsets in vaccine group and non-vaccine group.
CD, cluster of differentiation; TH, T helper.
Discussion
In this study, severe side effects associated with vaccination were not observed in any of the included pregnant women. Most of the common side effects occurred within 2-3 days and recovered or improved immediately, including itching (P = 0.93; 9.8% vs 10.1%), inoculation site swelling or rash (P = 0.8; 12% vs 13.1%), and mild fatigue (P = 0.96; 8.3% vs 8.1%), with no significant differences between BBIBP-CorV and CoronaVac.
Our study suggested that inoculation with inactivated COVID-19 vaccines during early pregnancy did not increase the unfavorable obstetric or birth outcome rate. The vaccination group did not have an increased miscarriage rate compared with that of the control group (P = 0.918; 12.12% vs 12.41%). The major birth defect rates were 0.83% and 1.0% in the vaccination and control groups, respectively (P = 0.887). The preterm, SGA, GDM, and PIH rates in the vaccination group were similar to those in the control group. Therefore, these findings support the acceptability and reliability of COVID-19 vaccines among pregnant women who are unwilling to be vaccinated. Nevertheless, information about the safety of inactivated COVID-19 vaccines during pregnancy is still limited. To date, only two reports have described pregnant women receiving inactivated COVID-19 vaccines during late pregnancy [11,16]. Pregnant women have no adverse effects and develop good antibody responses. Theoretically, the strong activation of the maternal immune system during early pregnancy may influence embryo implantation and fetal acceptance by destroying anti-fetal immune tolerance. Subsequently, it may cause pregnancy loss, restricted fetal/placental development, or preeclampsia occurrence [17]. High natural killer cell proportion and cytotoxicity are unfavorable factors predicting poor fertility outcomes. The shift of CD4+ cells toward T helper (Th)1 polarization in the peripheral blood is also related to reproductive failure [18]. However, our lymphocyte subset data indicated no significant differences in the natural killer cell percentage or the Th1/Th2 ratio between the vaccination and control groups. Consequently, consistent with our preliminary data, the result indicated favorable obstetric outcomes following vaccination, with similar rates of pregnancy complications to those in the control group.
The antibody response rate of CoronaVac is higher than 95% [19], and clinical trials have reported that inactivated vaccines can significantly reduce new coronavirus infections [20], [21], [22]. BBIBP-CorV is highly effective in the prevention of infection, hospitalization, and death caused by COVID-19 [23]. Their efficacy in protecting against severe COVID-19 and mortality is similar to that of mRNA vaccines.
In this study, the inactivated vaccine-induced humoral immunity in 132 subjects. In the Sinovac group, the NAb-positive rates were 62.3% and 45.5% in blood samples taken within 12 weeks of the second dose and in samples taken between 12 and 24 weeks after the second dose, respectively. The NAb-positive rates of the BBIBP-CorV group were 41% and 0% in blood samples taken within 12 weeks following the second dose and in samples taken between 12 and 24 weeks following the second dose. Based on our results, the GMT and NAb-positive rates of both inactivated vaccines decreased from the group within 12 weeks after the second dose to the group between 12 and 24 weeks after the second dose. This finding was consistent with the conclusion of one study reporting a rapid decline in antibody levels 12 weeks after the second dose of inactivated vaccine [12]. The antibody content is low within 6 months following two doses of CoronaVac, and the NAb detection rate is 20.4% (95% CI = 12.8-30.1) among adults aged 18-60 years [24]. Because of the reduced immunity following two doses of inactivated COVID-19 vaccines and the decreased efficacy, pregnant women should be vaccinated during the second or third trimester or offered the third or booster doses 12 weeks after they receive two doses of inactivated COVID-19 vaccines to guarantee the efficient transplacental transfer of NAb. Boosting with heterologous inactivated vaccine promotes immunity compared with that of boosting with homologous vaccines, thereby promoting their protective effects [24].
SARS-CoV-2 IgG antibodies can be detected in the cord blood of neonates following two doses of SARS-CoV-2 inactivated vaccine (CoronaVac) inoculation administered during the third trimester of pregnancy [11]. Passive anti-SARS-CoV-2 immunity occurs in one neonate via immunoprophylaxis in a pregnant woman receiving CoronaVac during late pregnancy [25]. At 24 hours post-delivery, blood was sampled from the neonate for analysis through enzyme immunoassays, revealing anti-SARS-CoV-2 antibodies [16]. Effective transplacental transfer of SARS-CoV-2 IgG antibodies occurs in most pregnant women with seropositivity following natural infection [26]. NAb existing within the neonatal circulation can be an additional vaccination benefit that protects against COVID-19 in fetuses and neonates. Maternal vaccination can protect mothers, their fetuses, and even neonates. However, our study suggested that the positive rate and GMTs of NAb decreased from the second dose vaccination and might even disappear during delivery. No NAb was detected in umbilical cord blood. As a result, the best vaccination time during pregnancy must be determined to optimize the balance between maternal/fetal safety and vaccine effectiveness.
Given the low incidence of obstetric complications, drawing reliable conclusions from our study on obstetric/neonatal complications could be difficult because of the limited number of participants that delivered. Expanding the sample size of our study was also difficult after the vaccine window was closed. Because most fertile women have been fully vaccinated within a few months, subsequent natural conception occurs after vaccination. At the beginning of the study, because of the lack of data on vaccine safety for pregnancy, pregnant women inadvertently vaccinated in early pregnancy chose to terminate the pregnancy; consequently, fewer participants delivered during our study process.
By far, this study covered the largest scale to evaluate whether inactivated COVID-19 vaccine was safe and immunogenic among early pregnancy women, although the sample size was still insufficient. Nonvaccinated early pregnancy women were enrolled as controls to demonstrate vaccine-associated obstetric outcomes. This study also compared different T-cell subset proportions and found that the difference was not significant between the groups.
In conclusion, inactivated COVID-19 vaccines can be considered safe for mothers & fetuses and effective in producing NAb against COVID-19. Because of the reduced immunity following two doses of COVID-19 vaccine inoculation, pregnant women lose their antibodies upon delivery, and no NAb is found in cord blood samples from the vaccination group. Thus, boosting with heterologous inactivated vaccines may be needed to strengthen and guarantee immunity during the entire pregnancy and consequently protect the fetus through the placental crossing.
Declaration of competing interest
The authors have no competing interests to declare.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
This study was approved by the Ethics Review Committee of the Shanghai First Maternity and Infant Hospital, Tongji University School of Medicine (approval number: KS21348), and the procedures were performed in accordance with approved guidelines.
Acknowledgments
We thank all the patients and doctors who participated in this study. Thanks to Dongmei Yan and Shuangli Zhu of National Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention for their support in laboratory work. Our thanks should go to Dr. Yong Zhang, Prof. of National Institute for Viral Disease Control and Prevention, CDC China, for his help in this paper. Written informed consent has been obtained from the patient(s) to publish this paper.
Author contributions
Study design: Yan Ma and Yiying Huang. Data collection: Yan Ma, Zhenli Shan and Yicun Gu. Data analysis: Yan Ma and Zhenli Shan. Writing: Yan Ma and Yiying Huang.
References
- 1.Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21:39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han B, Song Y, Li C, Yang W, Ma Q, Jiang Z, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy children and adolescents: a double-blind, randomised, controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:1645–1653. doi: 10.1016/S1473-3099(21)00319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim CNH, Hutcheon J, van Schalkwyk J, Marquette G. Maternal outcome of pregnant women admitted to intensive care units for coronavirus disease 2019. Am J Obstet Gynecol. 2020;223:773–774. doi: 10.1016/j.ajog.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saeed Z, Greer O, Shah NM. Is the host viral response and the immunogenicity of vaccines altered in pregnancy? Antibodies (Basel) 2020;9:38. doi: 10.3390/antib9030038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goncu Ayhan S, Oluklu D, Atalay A, Menekse Beser D, Tanacan A, Moraloglu Tekin O, et al. COVID-19 vaccine acceptance in pregnant women. Int J Gynaecol Obstet. 2021;154:291–296. doi: 10.1002/ijgo.13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayakawa S, Komine-Aizawa S, Takada K, Kimura T, Yamada H. Anti-SARS-CoV-2 vaccination strategy for pregnant women in Japan. J Obstet Gynaecol Res. 2021;47:1958–1964. doi: 10.1111/jog.14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill L, Jones CW. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies in neonatal cord blood after vaccination in pregnancy. Obstet Gynecol. 2021;137:894–896. doi: 10.1097/AOG.0000000000004367. [DOI] [PubMed] [Google Scholar]
- 9.Zdanowski W, Waśniewski T. Evaluation of SARS-CoV-2 spike protein antibody titers in cord blood after COVID-19 vaccination during pregnancy in polish healthcare workers: preliminary results. Vaccines (Basel) 2021;9:675. doi: 10.3390/vaccines9060675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mithal LB, Otero S, Shanes ED, Goldstein JA, Miller ES. Cord blood antibodies following maternal coronavirus disease 2019 vaccination during pregnancy. Am J Obstet Gynecol. 2021;225:192–194. doi: 10.1016/j.ajog.2021.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soysal A, Bilazer C, Gönüllü E, Barın E, Çivilibal M. Cord blood antibody following maternal SARS-CoV-2 inactive vaccine (CoronaVac) administration during the pregnancy. Hum Vaccin Immunother. 2021;17:3484–3486. doi: 10.1080/21645515.2021.1947099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zee JST, Lai KTW, Ho MKS, Leung ACP, Fung LH, Luk WP, et al. Serological response to mRNA and inactivated COVID-19 vaccine in healthcare workers in Hong Kong: decline in antibodies 12 weeks after two doses. Hong Kong Med J. 2021;27:380–383. doi: 10.12809/hkmj219744. [DOI] [PubMed] [Google Scholar]
- 13.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International Association of Diabetes and Pregnancy Study Groups Consensus Panel. Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72:24–43. doi: 10.1161/HYPERTENSIONAHA.117.10803. [DOI] [PubMed] [Google Scholar]
- 16.Menegali BT, Schuelter-Trevisol F, Barbosa AN, Izidoro TM, Feurschuette OHM, Marcon CEM, et al. Vertical transmission of maternal COVID-19 antibodies after CoronaVac vaccine: a case report. Rev Soc Bras Med Trop. 2021;54:e0385. doi: 10.1590/0037-8682-0385-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saito S, Nakashima A, Shima T, Ito M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol. 2010;63:601–610. doi: 10.1111/j.1600-0897.2010.00852.x. [DOI] [PubMed] [Google Scholar]
- 18.Kwak-Kim JY, Chung-Bang HS, Ng SC, Ntrivalas EI, Mangubat CP, Beaman KD, et al. Increased T helper 1 cytokine responses by circulating T cells are present in women with recurrent pregnancy losses and in infertile women with multiple implantation failures after IVF. Hum Reprod. 2003;18:767–773. doi: 10.1093/humrep/deg156. [DOI] [PubMed] [Google Scholar]
- 19.Zee JST, Lai KTW, Ho MKS, Leung ACP, Chan QWL, Ma ESK, et al. Serological response to mRNA and inactivated COVID-19 vaccine in healthcare workers in Hong Kong: preliminary results. Hong Kong Med J. 2021;27:312–313. doi: 10.12809/hkmj219605. [DOI] [PubMed] [Google Scholar]
- 20.Al Kaabi N, Zhang Y, Xia S, Yang Y, Al Qahtani MM, Abdulrazzaq N, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA. 2021;326:35–45. doi: 10.1001/jama.2021.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398:213–222. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jara A, Undurraga EA, González C, Paredes F, Fontecilla T, Jara G, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385:875–884. doi: 10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nadeem I, Ul Munamm SA, Ur Rasool M, Fatimah M, Abu Bakar M, Rana ZK, et al. Safety and efficacy of Sinopharm vaccine (BBIBP-CorV) in elderly population of Faisalabad district of Pakistan. Postgrad Med J. 2022 doi: 10.1136/postgradmedj-2022-141649. [DOI] [PubMed] [Google Scholar]
- 24.Costa Clemens SA, Weckx L, Clemens R, Almeida Mendes AV, Ramos Souza A, Silveira MBV, et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study. Lancet. 2022;399:521–529. doi: 10.1016/S0140-6736(22)00094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paixao ES, Wong KLM, Alves FJO, de Araújo Oliveira V, Cerqueira-Silva T, Júnior JB, et al. CoronaVac vaccine is effective in preventing symptomatic and severe COVID-19 in pregnant women in Brazil: a test-negative case-control study. BMC Med. 2022;20:146. doi: 10.1186/s12916-022-02353-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flannery DD, Gouma S, Dhudasia MB, Mukhopadhyay S, Pfeifer MR, Woodford EC, et al. Assessment of maternal and neonatal cord blood SARS-CoV-2 antibodies and placental transfer ratios. JAMA Pediatr. 2021;175:594–600. doi: 10.1001/jamapediatrics.2021.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]