Abstract
Objectives
Recombination related to coinfection is a huge driving force in determining the virus genetic variability, particularly in conditions of partial immune control, leading to prolonged infection. Here, we characterized a distinctive mutational pattern, highly suggestive of Delta-Omicron double infection, in a lymphoma patient.
Methods
The specimen was characterized through a combined approach, analyzing the results of deep sequencing in primary sample, viral culture, and plaque assay.
Results
Bioinformatic analysis on the sequences deriving from the primary sample supports the hypothesis of a double viral population within the host. Plaque assay on viral culture led to the isolation of a recombinant strain deriving from Delta and Omicron lineages, named XS, which virtually replaced its parent lineages within a single viral propagation.
Conclusion
It is impossible to establish whether the recombination event happened within the host or in vitro; however, it is important to monitor co-infections, especially in the exceptional intrahost environment of patients who are immunocompromised, as strong driving forces of viral evolution.
Keywords: SARS-CoV-2 variants, Whole genome sequencing, Recombinant XS, Plaque assay, Co-infection
Background
Since late 2020, SARS-CoV-2 has clearly demonstrated its capacity to generate new variants, marked by the emergence of sets of mutations that impact virus characteristics, including transmissibility and antigenicity.
SARS-CoV-2 evolution is an intricate process related to the different mechanisms on the molecular, organism, and population scale. The development of point mutations has played a big role in the emergence of new variants [1]; on the other hand, the recombination between closely related genotypes occurs readily due to the high sequence identity and may result in the emergence of new strains [2]. Coronaviruses have an intrinsically high intratypic recombination rate (approximately 25%) across the genome. To allow for homologous recombination, coinfection of genetically different viruses must occur in the same host cell. The crossover sites may occur anywhere, but the selection pressure can lead them to cluster in certain hotspots [3,4].
Favorable conditions for coinfection—and subsequent recombination—spike in periods of coexistence of two major lineages. The most recent one in our geographic area happened between October 11, 2021 and March 27, 2022, when Omicron succeeded Delta as the predominant lineage but the two variants co-circulated for a time. Coinfections have been reported multiple times [5], [6], [7], more recently involving Delta and Omicron [8,9]. Delta-Omicron recombinants have also been reported [10], [11], [12], [13].
Recombinant viruses were initially identified only through bioinformatic tools, but they have now been isolated in culture as well, which allows the investigation of their epidemic potential. Most often, this has been done on patients who were presumably infected with a recombinant strain to begin with [10,12,13]. However, Burel et al. were able to monitor a coinfection between B.1.160 and Alpha for 14 months, until its evolution in a recombinant strain, and culture it [14].
The origin of variants is still a matter of speculation. Several hypotheses take zoonotic origin, selective pressure during treatment with antiviral drugs, monoclonal antibodies, or convalescent plasma into consideration and a few studies point to the significance of the intrahost environment of patients who are immunocompromised to explain the evolution of immune escape variants [15,16]. Individuals who are immunocompromised are more likely to be long carriers, which increases the likelihood of subsequent coinfection and recombination events.
Because homologous recombination related to coinfection and conditions of partial immune control are strong driving forces of viral evolution, it is very important to monitor such instances. Here, we describe the composite approach we used to accurately characterize a peculiar SARS-CoV-2 sequence, suggestive of a double viral population, combining bioinformatic tools and plaque assay on viral culture.
The case
A male patient, aged 47 years, diagnosed with stage IVa nodular sclerosing non-Hodgkin lymphoma and diabetes, was admitted to the hospital on January 14, 2022 due to severe respiratory distress. The point-of-care testing for SARS-CoV-2 was positive, and later confirmed by our laboratory on January 21, 2022. Patient death was recorded 17 days after admission (January 31, 2022).
Sequencing was performed on the nasopharyngeal swab in the context of routine surveillance and monitoring for SARS-CoV-2 variants. Sequence analysis showed an unusually low number of mutations (N = 17) compared with the circulating lineages on our territory at the time, Delta (N ≈ 45) and Omicron (N > 60). In addition, several mutations were detected in a lower fraction of the viral population (variant fraction, 70-90%). All the analysis softwares used for lineage characterization yielded inconclusive results.
Because this mutational pattern was highly suggestive of a double viral population, the primary sample was re-tested to exclude sequencing errors or contaminations. At the same time, viral culture on the specimen was paired with plaque assay to attempt to isolate and characterize the two populations.
Methods
Sequencing
Whole genome sequencing was performed on the original sample using an amplicon-based approach. We implemented the CleanPlex SARS-CoV-2 Panel (Paragon Genomics, Inc., Hayward, CA, USA) for target enrichment and library preparation, which involves multiplex polymerase chain reaction (PCR) reactions. The sequencing step was conducted on a MiSeq platform (Illumina, Inc., San Diego, CA, USA).
Bioinformatic tools
The data analysis for the consensus sequence generation and mutation calling was performed according to the supplier's recommendations using SOPHiA-DDM-v4 (SOPHiA Genetics, Lausanne, Switzerland). The software operates a cut-off, which excludes from reporting all the mutations detected below 70%. The consensus sequences were submitted to Pangolin and NextClade for lineage assignment. In addition, the raw data from the primary sample was aligned and analyzed using Lasergene SeqMan Ultra software (DNASTAR Inc, Madison, WI, USA) to detect mutations below 70%. Each mutation identified was analyzed compared with the database of all samples sequenced in our laboratory to date, comprising 2668 Delta sequences, 1043 Omicron, and 1500 Alpha at the time of the analysis. The mutations were considered markers of a specific lineage if they were significantly present within it (>90% samples) and absent in all others (<10%).
Plaque assay
Viral isolates were propagated from the residual specimen on Vero E6 cell cultures (American Type Culture Collection CRL-1586), as recommended [17]. A total of 500 µl of viral transport media were used to infect a cell monolayer at confluency, allowing a 1-hour adsorption and a 72-hour incubation. Viral replication was then assessed by reverse transcription-PCR. Serial dilutions of viral isolate were cultured using 0.5% agarose added to the medium to obtain visible, immobilized focuses of infection (plaque assay). Each focus was then separately eluted, cultured, and sequenced, performing data analysis as previously described.
Characterization of coinfection and recombinant strain identification
The double analysis on the primary sample, collected on January 21, 2022, highlighted the presence of respectively 17 and 21 mutations compared with the reference. Most mutations are traceable either to Delta (italics) or Omicron (bold) lineages (Table 1 ), and several were detected in an unusually low fraction of the viral population (70-90%). The low number of mutations cannot be attributed to data loss as the genome coverage was 99.8% and 99.9%, respectively. The most likely explanation is a higher number of mutations with a variant fraction below 70%, which would be hidden by the software cut-off. Marker mutations of multiple lineages, low variant fractions, and fluctuating mutational patterns are all hallmarks of coinfections [18].
Table 1.
Sequence profiles from the analysis of separate aliquots of the same primary sample and of the viral isolates. Only mutations above 70% are reported. Most mutations are referable either to Delta (italics) or Omicron (bold). The mutational pattern in the primary sample is consistent with the presence of two separate viral lineages within the specimen. Conversely, in the viral isolates the ORF1ab portion is Delta-like (italics), the rest of the genome is Omicron-like (bold). This mutational pattern is consistent with a single viral population, deriving from the recombination of two separate lineages.
| Sequence 1 |
Sequence 2 |
Plaque assay |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| gene | protein | depth | var fraction % | protein | depth | var fraction (%) | gene | protein | depth | var fraction (%) | gene | protein | depth | var fraction (%) | gene | protein | depth | var fraction (%) |
| ORF1ab | Ile695Val | 1702 | 73.6 | ORF1ab | Ile695Val | 1515 | 99.7 | Spike | Ala67Val | 2995 | 99.8 | ORF3a | Thr64= | 2786 | 99.8 | |||
| Phe924= | 2101 | 99.7 | Phe924= | 1777 | 99.3 | Phe924= | 1871 | 99.5 | His69_Val70del | 3001 | 99.9 | E gene | Thr9Ile | 1556 | 100 | |||
| Pro2046Leu | 1017 | 71.5 | Gly934Val | 1856 | 99.5 | Thr95Ile | 3931 | 99.9 | M gene | Asp3Gly | 2015 | 99.9 | ||||||
| Pro2287Ser | 2874 | 74.1 | Asn1076= | 1771 | 99.9 | Gly142_Tyr145delinsAsp | 4445 | 99.9 | Gln19Glu | 2013 | 99.9 | |||||||
| Ala2529Val | 1633 | 74.3 | Ala1306Ser | 3610 | 99.9 | Asn211_Leu212delinsIle | 1308 | 100 | Ala63Thr | 2158 | 99.9 | |||||||
| Thr3255Ile | 1690 | 100 | Thr3255Ile | 1616 | 99.8 | Tyr1873= | 1737 | 99.8 | Arg214_Asp215insGluProGlu | 1305 | 97.5 | ORF6 | Arg20= | 459 | 99.1 | |||
| Ala3645= | 3997 | 72.2 | Pro2046Leu | 1217 | 99.4 | Gly339Asp | 2524 | 99.8 | ORF7b | Leu18= | 3204 | 99.8 | ||||||
| Thr3646Ala | 3997 | 72.2 | Pro2287Ser | 4221 | 99.6 | Ser371Pro | 134 | 100 | N gene | Pro13Leu | 5706 | 99.9 | ||||||
| Leu3674_Gly3676del | 744 | 100 | Leu3674_Gly3676del | 525 | 100 | Ala2529Val | 2973 | 99.7 | Ser371Phe | 134 | 100 | Glu31_Ser33del | 2189 | 99.7 | ||||
| Val3689= | 746 | 100 | Val3689= | 1199 | 99.7 | Asp2907= | 2428 | 99.6 | Ser373Pro | 134 | 100 | Arg203Lys | 1771 | 99.8 | ||||
| Pro4715Leu | 2651 | 99.7 | Pro4715Leu | 2368 | 99.9 | Val2930Leu | 522 | 99.2 | Ser375Phe | 134 | 100 | Arg203= | 1771 | 99.9 | ||||
| Gly5063Ser | 6005 | 73.2 | Gly5063Ser | 5473 | 75.3 | Thr3255Ile | 2100 | 99.9 | Lys417Asn | 584 | 100 | Gly204Arg | 1771 | 99.9 | ||||
| Pro5401Leu | 5085 | 74.3 | Pro5401Leu | 6446 | 76.2 | Ala3645= | 8379 | 99.8 | Asn440Lys | 4132 | 99.9 | |||||||
| Ala6319Val | 471 | 71.5 | Thr3646Ala | 8379 | 99.8 | Gly446Ser | 4131 | 99.9 | ||||||||||
| Spike | Ala67Val | 1323 | 73.5 | Val3689= | 2176 | 99.9 | Thr547Lys | 2721 | 99.7 | |||||||||
| His69_Val70del | 1323 | 73.3 | Pro4715Leu | 2930 | 99.8 | Asp614Gly | 5187 | 99.9 | ||||||||||
| Thr95Ile | 2156 | 99.9 | Thr95Ile | 2304 | 99.9 | Gly5063Ser | 5078 | 99.9 | His655Tyr | 4796 | 99.8 | |||||||
| Gly142_Tyr145delinsAsp | 1211 | 100 | Gly142_Tyr145delinsAsp | 1181 | 100 | Pro5401Leu | 9979 | 99.9 | Asn679Lys | 3369 | 100 | |||||||
| Glu156_Arg158delinsGly | 334 | 98.2 | Glu156_Arg158delinsGly | 252 | 100 | Ala6319Val | 2302 | 100 | Pro681His | 3368 | 99.8 | |||||||
| Leu452Arg | 159 | 100 | Leu452Arg | 106 | 100 | Ala701Val | 231 | 99.6 | ||||||||||
| Thr478Lys | 314 | 100 | Thr478Lys | 210 | 100 | Asn764Lys | 1232 | 99.5 | ||||||||||
| Asp614Gly | 2780 | 99.9 | Asp614Gly | 3559 | 100 | Asp796Tyr | 408 | 100 | ||||||||||
| Asp950Asn | 651 | 79.7 | Asp950Asn | 1003 | 90.1 | Asn856Lys | 3903 | 99.2 | ||||||||||
| Gln954His | 1544 | 99.8 | ||||||||||||||||
| Asn969Lys | 872 | 100 | ||||||||||||||||
| Leu981Phe | 884 | 99.5 | ||||||||||||||||
| Asp1146= | 1428 | 99.7 | ||||||||||||||||
ORF, open reading frame.
This hypothesis was confirmed through analysis with a second alignment software to categorize all the mutations below the initial cut-off. As expected, we found, across the whole viral genome, a very high number of mutations previously undetected and well below 70%, pertaining to both lineages. In two instances, we were able to identify the simultaneous presence of two marker mutations, respectively for Delta and Omicron, at the same genomic position (Tables 2 and 3 ). Once analyzed below 70%, the two runs yielded very similar results. In no case, however, we found patient-specific mutations.
Table 2.
Sequence profiles from the analysis of aliquot 1 from primary sample. Mutations below 70% are reported. Most mutations are referable either to Delta (italics) or Omicron (bold). The mutational pattern in the primary sample is consistent with the presence of two separate viral lineages within the specimen.
|
Sequence 1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| gene | protein | depth | var fraction % | gene | protein | depth | var fraction % | gene | protein | depth | var fraction % |
| Spike | Thr19Arg | 26 | 800 | ||||||||
| ORF1ab | Ile695Val | 69 | 2025 | Ala67Val | 73 | 1323 | ORF3a | Ser26Leu | 34 | 815 | |
| Lys856Arg | 35 | 1214 | His69_Val70del | 73 | 1323 | Thr64= | 66 | 1102 | |||
| Phe924= | 100 | 2096 | Thr95Ile | 100 | 2156 | Asp155Tyr | 41 | 2114 | |||
| Gly934Val | 58 | 2094 | Gly142_Tyr145delinsAsp | 1211 | 100 | ||||||
| Asn1076= | 36 | 598 | Glu156_Arg158delinsGly | 334 | 98 | E gene | Thr9Ile | 60 | 602 | ||
| Ala1306Ser | 40 | 2672 | Gly339Asp | 64 | 4471 | ||||||
| Ala1707= | 66 | 739 | Ser371Pro | 67 | 109 | M gene | Asp3Gly | 62 | 2196 | ||
| Pro2046Leu | 66 | 1148 | Ser371Phe | 67 | 109 | Gln19Glu | 63 | 1996 | |||
| Pro2287Ser | 69 | 2533 | Ser373Pro | 67 | 109 | Ala63Thr | 56 | 1982 | |||
| Ala2529Val | 68 | 1501 | Ser375Phe | 65 | 109 | Ile82Thr | 45 | 1983 | |||
| Ala2710Thr | 37 | 696 | Lys417Asn | 58 | 952 | ||||||
| Asp2907= | 64 | 2293 | Leu452Arg | 100 | 159 | ORF6 | Arg20= | 48 | 591 | ||
| Thr3255Ile | 100 | 1690 | Thr478Lys | 100 | 314 | ||||||
| Pro3395His | 34 | 2457 | Thr547Lys | 50 | 3938 | ORF7a | Thr120Ile | 50 | 2243 | ||
| Ala3645= | 68 | 3282 | Asp614Gly | 100 | 2780 | ||||||
| Thr3646Ala | 68 | 3282 | His655Tyr | 49 | 4559 | ORF7b | Leu18= | 57 | 3203 | ||
| Leu3674_Gly3676del | 744 | 100 | Asn679Lys | 53 | 2032 | Thr120Ile | 27 | 961 | |||
| Val3689= | 100 | 746 | Pro681Arga | 47 | 949 | ||||||
| Ile3758Val | 43 | 1940 | Pro681Hisa | 53 | 1079 | N gene | Pro13Leu | 45 | 7798 | ||
| Val4310= | 36 | 3859 | Asn764Lys | 57 | 1805 | Asp63Gly | 53 | 1143 | |||
| Pro4715Leu | 100 | 2651 | Asn856Lys | 62 | 3616 | Arg203Meta | 58 | 1949 | |||
| Asn4992= | 29 | 2756 | Asp950Asn | 80 | 649 | Arg203Lysa | 42 | 1389 | |||
| Gly5063Ser | 73 | 6005 | Gln954His | 20 | 650 | Gly204Arg | 42 | 3339 | |||
| Pro5401Leu | 74 | 5086 | Asn969Lys | 58 | 1121 | Gly215Cys | 58 | 8679 | |||
| Ile5967Val | 32 | 1998 | Leu981Phe | 57 | 1124 | Asp377Tyr | 51 | 4223 | |||
| Asp1146= | 66 | 1679 | |||||||||
In two instances, we were able to identify the simultaneous presence of two marker mutations, respectively for Delta and Omicron, at the same genomic position.
Table 3.
Sequence profiles from the analysis of aliquot 2 from primary sample. Mutations below 70% are reported. Most mutations are referable either to Delta (italics) or Omicron (bold). The mutational pattern in the primary sample is consistent with the presence of two separate viral lineages within the specimen.
|
Sequence 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| gene | protein | depth | var fraction % | gene | protein | depth | var fraction % | gene | protein | depth | var fraction % |
| ORF1ab | Ile695Val | 74 | 1702 | Spike | Thr19Arg | 1024 | 30 | ORF3a | Ser26Leu | 974 | 39 |
| Lys856Arg | 33 | 1561 | Ala67Val | 504 | 55 | Thr64= | 1294 | 62 | |||
| Phe924= | 100 | 1765 | His69_Val70del | 504 | 55 | Asp155Tyr | 1839 | 44 | |||
| Gly934Val | 61 | 1773 | Thr95Ile | 2304 | 100 | ||||||
| Asn1076= | 30 | 725 | Gly142_Tyr145delinsAsp | 1181 | 100 | E gene | Thr9Ile | 788 | 53 | ||
| Ala1306Ser | 34 | 2851 | Glu156_Arg158delinsGly | 252 | 100 | ||||||
| Ala1707= | 72 | 922 | Arg214_Asp215insGluProGlu | 540 | 67 | M gene | Asp3Gly | 1619 | 62 | ||
| Pro2046Leu | 72 | 1017 | Gly339Asp | 4855 | 64 | Gln19Glu | 1415 | 60 | |||
| Pro2287Ser | 74 | 2874 | Ser371Pro | 170 | 65 | Ala63Thr | 1681 | 53 | |||
| Ala2529Val | 74 | 1633 | Ser371Phe | 170 | 65 | Ile82Thr | 1681 | 47 | |||
| Ala2710Thr | 27 | 897 | Ser373Pro | 170 | 65 | ||||||
| Asp2907= | 69 | 2072 | Ser375Phe | 170 | 65 | ORF6 | Arg20= | 334 | 51 | ||
| Thr3255Ile | 100 | 1616 | Lys417Asn | 508 | 50 | ||||||
| Pro3395His | 27 | 2849 | Leu452Arg | 106 | 100 | ORF7a | Thr120Ile | 2315 | 54 | ||
| Ala3645= | 72 | 3997 | Thr478Lys | 210 | 100 | ||||||
| Thr3646Ala | 72 | 3997 | Thr547Lys | 3698 | 41 | ORF7b | Leu18= | 3123 | 52 | ||
| Leu3674_Gly3676del | 100 | 525 | Asp614Gly | 3559 | 100 | Thr120Ile | 811 | 30 | |||
| Val3689= | 100 | 1199 | His655Tyr | 5317 | 45 | ||||||
| Ile3758Val | 39 | 2381 | Asn679Lys | 2604 | 45 | N gene | Pro13Leu | 8973 | 46 | ||
| Val4310= | 32 | 4634 | Pro681Arga | 1438 | 55 | Asp63Gly | 1574 | 50 | |||
| Pro4715Leu | 100 | 2368 | Pro681Hisa | 1161 | 45 | Arg203Meta | 1711 | 55 | |||
| Asn4992= | 29 | 2079 | Asn764Lys | 1804 | 52 | Arg203Lysa | 1378 | 45 | |||
| Gly5063Ser | 75 | 5473 | Asn856Lys | 4261 | 65 | Gly204Arg | 3096 | 45 | |||
| Pro5401Leu | 76 | 6446 | Asp950Asn | 1003 | 90 | Gly215Cys | 10837 | 57 | |||
| Ile5967Val | 28 | 2662 | Asn969Lys | 933 | 53 | Asp377Tyr | 5012 | 48 | |||
| Ala6319Val | 72 | 470 | Leu981Phe | 938 | 53 | ||||||
| Asp1146= | 1276 | 65 | |||||||||
In two instances, we were able to identify the simultaneous presence of two marker mutations, respectively for Delta and Omicron, at the same genomic position.
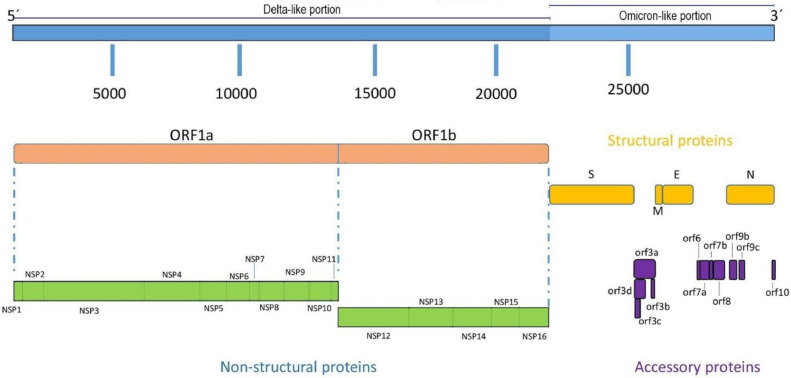
Conversely, the sequences of the initial viral propagation and of eight separate plaques of infection all yielded next-to-identical results, summarized with a single sequence illustrated in Table 1 (EPI_ISL_12870564) and Figure 1 . Variant fractions for all detected mutations are nearing 100%, a strong indicator of a single viral population. Furthermore, the open reading frame (ORF1ab) portion is generally consistent with a Delta lineage and specifically bears the marker mutation for AY.4 (Ala2529Val) [19]; the rest of the genome is comparable to BA.1.
Figure 1.
Schematic figure representing the recombinant structure with respect to the different lineages. NSP, nonstructural protein; ORF, open reading frame.
These results are compatible with a recombinant strain deriving from Delta and Omicron lineages. The sequence analysis with NextClade offered further confirmation, illustrating a clear breakpoint between ORF1ab and spike (approximate breakpoint site: 20418-21618).
The sequence was initially classified as XF, which caused a small cluster in the United Kingdom in February 2022 [20,21], but it has now been regrouped as XS by the lineage assignment softwares. Both XF and XS are recombinant strains deriving from AY.4 and BA.1, differing in the position of the breakpoint site. The first XS sequence has been deposited on the Global Initiative on Sharing Avian Influenza Data (GISAID) on February 02, 2022, coming from North America, as all sequences currently considered XS on GISAID (n = 61). This number may be underestimated, as sequences coming from recombinant strains are often difficult to assign and require much longer investigation.
Discussion
The ability of SARS-CoV-2 to generate new variants is an intricate process determined by the interplay among different mechanisms on the molecular, organism, and population scale. Although the development of point mutations has played a big role, recombination is a huge driving force in determining the virus genetic variability. To allow for homologous recombination, coinfection of genetically different viruses must occur in the same host cell [22].
Here, we describe the characterization of a peculiar SARS-CoV-2 sequence found in an immunocompromised patient, suggestive of coinfection. A more accurate bioinformatic analysis on the sequences deriving from the primary sample supports the hypothesis of a double viral population within the host. On the other hand, the sequencing of separate focuses of infection in vitro highlighted identical mosaic structures. The result is a recombinant SARS-CoV-2 strain derived from the combination of AY.4 (Delta) and BA.1 (Omicron), currently categorized as XS, derived from the coexistence of the two lineages.
It would be very interesting to establish whether the recombination event happened within the host or in vitro. This could be done in two ways: first, through the identification of sequencing reads containing markers for both lineages and second, through the generation of PCR products overlapping the putative recombination site. Neither of these methods are feasible in our context because the last Delta marker was identified at position 20418 and the first Omicron marker at 21618; there are no reads long enough to contain both. As for the detection of recombinant PCR products, it is obvious from Tables 2 and 3 that there is a very high presence of parent lineages in the primary sample, as indicated from the balanced percentage of markers of both lineages at the same genomic position; in this context, a negative result would be no indication of a later recombination event because it could very well stem from a low percentage of recombinant virus in an interfering environment.
Both Delta-Omicron coinfections and recombinants have now been reported and/or isolated multiple times [8], [9], [10], [11], [12], [13],23]. Recombinant strains are examined accurately for their epidemic potential and ability to escape neutralization as they have shown resistance to monoclonal antibodies, such as Sotrovimab [12], whereas the parent lineages are not. However, it is very difficult to monitor the exact moment of the strain generation. At present, and to the best of our knowledge, only Burel and colleagues were able to monitor a coinfection until its evolution in a recombinant strain over the course of 14 months [14] and culture it.
Our report aims to expand the body of work on the subject. Given the very short time span between first sequencing and patient death, there is a lack of sequential sampling providing more detailed information on viral evolution, which is the main weakness of the study. On the other hand, this also raises the question of a potential rapid development of recombinants under the right environmental conditions.
The generation of mutated strains in hosts who are immunocompromised is very well characterized as linked to their higher likelihood to be long carriers, which in turn increases the chance of subsequent coinfection and mutation events [16,[24], [25], [26]. This is especially related to the variants created through the accumulation of point mutations, while it only takes one mutational step to generate a single breakpoint recombinant. It is worth mentioning that contexts of partial immune control favor evolutionary jumps not only through very long infections that cannot be overcome, but also acting as selective pressure [16,24,25].
Furthermore, the region between ORF1ab and the Spike gene is a very frequent breakpoint site not only in Delta-Omicron recombinants, (usually with ORF1ab Delta region and an Omicron region encompassing Spike's receptor binding domain and C-terminal regions, [10], [11], [12], [13],23]) but dating as far back as Alpha recombinants [5]. This has been linked to the phenomenon of template switching by viral polymerase during normal transcription, where the polymerase pauses at a transcription-regulatory sequence after transcribing the last open reading frame of one subgenomic RNA and switches to a similar regulatory sequence, omitting a looped-out region of the template RNA, which contains at least ORF1ab in the case of SARS-CoV-2 [5]. In the context of coinfections, the availability of alternative template RNA molecules provides an environment that is highly conducive to homologous recombination.
This study expands on SARS-CoV-2 recombinants and especially on the advantages of pairing sequencing and bioinformatic analysis with culture to monitor and characterize coinfections and any newly generated strain. Despite our impossibility to pinpoint the time of recombination, it is worth noting the speed with which XS emerged and substituted its parent lineages in vitro. Considering the combination of favorable conditions for a recombinant strain to be generated in relatively short times, this study further stresses the necessity of monitoring patients who are immunocompromised carefully, especially in contexts of co-circulation between the different lineages.
Declaration of competing interest
The authors have no competing interests to declare.
Acknowledgments
Funding
This research was supported by European funding within the NextGenerationEU-MUR PNRR Extended Partnership initiative on Emerging Infectious Diseases (Project n. PE00000007, INF-ACT).
Ethical approval
Written informed consent was obtained from the participant to have the results of this work published. The information on clinical history, treatment, and SARS-CoV-2 quantitative PCR test results were obtained from medical records.
Author contributions
Study design and conceptualization: S.Z., M.B., M.M.M., G.D., V.S. Data collection: A.D., A.M., F.T., V.A., M.M., A.B., L.G., A.S., G.D. Data analysis: S.Z., M.B., M.M.M., G.G. Writing: S.Z.
References
- 1.Gribble J, Stevens LJ, Agostini ML, Anderson-Daniels J, Chappell JD, Lu X, et al. The coronavirus proofreading exoribonuclease mediates extensive viral recombination. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstein SA, Brown J, Pedersen BS, Quinlan AR, Elde NC. Extensive recombination-driven coronavirus diversification expands the pool of potential pandemic pathogens. Genome Biol. Evol. 2022;14:evac161. doi: 10.1093/gbe/evac161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makino S, Keck JG, Stohlman SA, Lai MM. High-frequency RNA recombination of murine coronaviruses. J Virol. 1986;57:729–737. doi: 10.1128/JVI.57.3.729-737.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banner LR, Lai MM. Random nature of coronavirus RNA recombination in the absence of selection pressure. Virology. 1991;185:441–445. doi: 10.1016/0042-6822(91)90795-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson B, Boni MF, Bull MJ, Colleran A, Colquhoun RM, Darby AC, et al. Generation and transmission of interlineage recombinants in the SARS-CoV-2 pandemic. Cell. 2021;184:5179–5188. doi: 10.1016/j.cell.2021.08.014. .e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francisco RDS, Jr, Benites LF, Lamarca AP, de Almeida LGP, Hansen AW, Gularte JS, et al. Pervasive transmission of E484K and emergence of VUI-NP13L with evidence of SARS-CoV-2 co-infection events by two different lineages in Rio Grande do Sul, Brazil. Virus Res. 2021;296 doi: 10.1016/j.virusres.2021.198345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taghizadeh P, Salehi S, Heshmati A, Houshmand SM, InanlooRahatloo K, Mahjoubi F, et al. Study on SARS-CoV-2 strains in Iran reveals potential contribution of co-infection with and recombination between different strains to the emergence of new strains. Virology. 2021;562:63–73. doi: 10.1016/j.virol.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rockett RJ, Draper J, Gall M, Sim EM, Arnott A, Agius JE, et al. Co-infection with SARS-CoV-2 Omicron and Delta variants revealed by genomic surveillance. Nat Commun. 2022;13:2745. doi: 10.1038/s41467-022-30518-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zayet S, Vuillemenot JB, Josset L, Gendrin V, Klopfenstein T. Simultaneous co-infection with Omicron (B.1.1.529) and Delta (21A/478K.V1) SARS-CoV-2 variants confirmed by whole genome sequencing. Int J Infect Dis. 2022;124:104–106. doi: 10.1016/j.ijid.2022.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colson P, Fournier PE, Delerce J, Million M, Bedotto M, Houhamdi L, et al. Culture and identification of a "Deltamicron" SARS-CoV-2 in a three cases cluster in southern France. J Med Virol. 2022;94:3739–3749. doi: 10.1002/jmv.27789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lacek KA, Rambo-Martin BL, Batra D, Zheng XY, Hassell N, Sakaguchi H, et al. SARS-CoV-2 Delta-omicron recombinant viruses, United States. Emerg Infect Dis. 2022;28:1442–1445. doi: 10.3201/eid2807.220526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duerr R, Zhou H, Tada T, Dimartino D, Marier C, Zappile P, et al. Delta-Omicron recombinant escapes therapeutic antibody neutralization. iScience. 2023;26 doi: 10.1016/j.isci.2023.106075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arora P, Zhang L, Rocha C, Graichen L, Nehlmeier I, Kempf A, et al. The SARS-CoV-2 Delta-Omicron recombinant lineage (XD) exhibits immune-escape properties similar to the Omicron variant. Int J Mol Sci. 2022;23:14057. doi: 10.3390/ijms232214057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burel E, Colson P, Lagier JC, Levasseur A, Bedotto M, Lavrard-Meyer P, et al. Sequential appearance and isolation of a SARS-CoV-2 recombinant between two major SARS-CoV-2 variants in a chronically infected immunocompromised patient. Viruses. 2022;14:1266. doi: 10.3390/v14061266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sonnleitner ST, Prelog M, Sonnleitner S, Hinterbichler E, Halbfurter H, Kopecky DBC, et al. Cumulative SARS-CoV-2 mutations and corresponding changes in immunity in an immunocompromised patient indicate viral evolution within the host. Nat Commun. 2022;13:2560. doi: 10.1038/s41467-022-30163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi B, Choudhary MC, Regan J, Sparks JA, Padera RF, Qiu X, et al. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med. 2020;383:2291–2293. doi: 10.1056/NEJMc2031364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ammerman NC, Beier-Sexton M, Azad AF. Growth and maintenance of Vero cell lines. Curr Protoc Microbiol. 2008 doi: 10.1002/9780471729259.mca04es11. Appendix:Appendix 4E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pedro N, Silva CN, Magalhães AC, Cavadas B, Rocha AM, Moreira AC, et al. Dynamics of a dual SARS-CoV-2 lineage co-infection on a prolonged viral shedding COVID-19 case: insights into clinical severity and disease duration. Microorganisms. 2021;9:300. doi: 10.3390/microorganisms9020300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pango Network. New AY lineages and an update to AY.4-AY.12, 2021. https://www.pango.network/new-ay-lineages-and-an-update-to-ay-4-ay-12/; [accessed 29 October 2022].
- 20.UK Health security Agency . 2022. SARS-CoV-2 variants of concern and variants under investigation in England - Technical briefing 39.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1063424/Tech-Briefing-39-25March2022_FINAL.pdf [accessed 28 October 2022] [Google Scholar]
- 21.European Centre for Disease Prevention and Control. SARS-CoV-2 variants of concern as of 24 March 2022, https://www.ecdc.europa.eu/en/covid-19/variants-concern-; [accessed 25 October 2022].
- 22.Kemp SA, Collier DA, Datir RP, Ferreira IATM, Gayed S, Jahun A, et al. SARS-CoV-2 evolution during treatment of chronic infection. Nature. 2021;592:277–282. doi: 10.1038/s41586-021-03291-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolze A, Basler T, White S, Dei Rossi A, Wyman D, Dai H, et al. Evidence for SARS-CoV-2 Delta and Omicron co-infections and recombination. Med (N Y) 2022;3:848–859. doi: 10.1016/j.medj.2022.10.002. .e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corey L, Beyrer C, Cohen MS, Michael NL, Bedford T, Rolland M. SARS-CoV-2 variants in patients with immunosuppression. N Engl J Med. 2021;385:562–566. doi: 10.1056/NEJMsb2104756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weigang S, Fuchs J, Zimmer G, Schnepf D, Kern L, Beer J, et al. Within-host evolution of SARS-CoV-2 in an immunosuppressed COVID-19 patient as a source of immune escape variants. Nat Commun. 2021;12:6405. doi: 10.1038/s41467-021-26602-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brandolini M, Zannoli S, Gatti G, Arfilli V, Cricca M, Dirani G, et al. Viral population heterogeneity and fluctuating mutational pattern during a persistent SARS-CoV-2 infection in an immunocompromised patient. Viruses. 2023;15:291. doi: 10.3390/v15020291. [DOI] [PMC free article] [PubMed] [Google Scholar]