Abstract
Background
The intestinal microbiota is an important regulator of bone health. In previous studies we have shown that intestinal microbiota dysbiosis, induced by treatment with broad spectrum antibiotics (ABX) followed by natural repopulation, results in gut barrier dysfunction and bone loss. We have also shown that treatment with probiotics or a gut barrier enhancer can inhibit dysbiosis-induced bone loss. The overall goal of this project was to test the effect of Korean Red Ginseng (KRG) extract on bone and gut health using antibiotics (ABX) dysbiosis-induced bone loss model in mice.
Methods
Adult male mice (Balb/C, 12-week old) were administered broad spectrum antibiotics (ampicillin and neomycin) for 2 weeks followed by 4 weeks of natural repopulation. During this 4-week period, mice were treated with vehicle (water) or KRG extract. Other controls included mice that did not receive either antibiotics or KRG extract and mice that received only KRG extract. At the end of the experiments, we assessed various parameters to assess bone, microbiota and in vivo intestinal permeability.
Results
Consistent with our previous results, post-ABX- dysbiosis led to significant bone loss. Importantly, this was associated with a decrease in gut microbiota alpha diversity and an increase in intestinal permeability. All these effects including bone loss were prevented by KRG extract treatment. Furthermore, our studies identified multiple genera including Lactobacillus and rc4-4 as well as Alistipes finegoldii to be potentially linked to the effect of KRG extract on gut-bone axis.
Conclusion
Together, our results demonstrate that KRG extract regulates the gut-bone axis and is effective at preventing dysbiosis-induced bone loss in mice.
Keywords: Korean Red Ginseng, bone loss, osteoporosis, microbiota, gut-bone axis
Graphical abstract
1. Introduction
Osteoporosis is a pathological condition characterized by decreased bone mass and/or altered bone quality/structure. The detrimental consequence of osteoporosis is an increased risk for bone fracture which can lead to increase in morbidity and mortality and decrease in independence and quality of life. The economic burden of fractures related to osteoporosis accounts for ∼$17 billion in the US [1]. Current medications to treat osteoporosis have limitations such as off-target effects and unwillingness of patients to take medications out of fear of these off-target effects. Thus, a critical unmet medical need is the identification of novel strategies to prevent or treat osteoporosis without significant side effects (reviewed in [2,3]).
Microbiota refers to collective consortium of microorganisms (including bacteria, viruses, and fungi) found in a particular niche. Based on studies over the last decade, we now know that intestinal microbiota and its metabolites are important in regulating diverse physiological processes [4]. Importantly, altered composition of microbiota or a decrease in bacterial diversity has now been shown to be closely linked with the pathogenesis of several disease processes including IBD, obesity and diabetes [5]. We have shown that pathogenic bacteria such as H. hepaticus causes bone loss [6]. Conversely, we and others have shown that beneficial bacteria (eg. probiotics) can enhance bone health and prevent bone loss in various mouse models of osteoporosis (reviewed in [2]). In recent studies we showed that when mice are administered oral broad-spectrum antibiotics for 2 weeks followed by 4 weeks of natural repopulation, microbiota composition is significantly altered and this is associated with gut barrier dysfunction and significant bone loss [7,8]. This suggests that directly perturbing a healthy microbiota with antibiotics can lead to bone loss in mice. We further demonstrated that probiotic bacteria L. reuteri can prevent bone loss induced by post-antibiotic dysbiosis. In this present study we tested the effect of Korean Red Ginseng (KRG) on bone loss induced by post-antibiotic dysbiosis. Although KRG has been shown to be beneficial for preventing or attenuating a number of diseases including bone loss [9], the effect of KRG on gut-bone axis has not been investigated.
Ginseng, known as the “king of herbs” is an herbal root. Korean Red Ginseng (KRG) belongs to the family of Araliaceae and is officially called the Panax ginseng Meyer. Its cultivation in Korea started in ∼11 B.C. The ginseng plant contains many active ingredients including saponins, and ginsenosides. Recent studies have identified ∼128 ginsenosides in Panax ginseng. Ginsenosides have been reported to have multiple activities including anti-diabetic, anti-cancer, anti-oxidant and anti-adipocyte properties (reviewed in [10]). In addition to these properties some previous studies have shown anti-osteoporotic activities. Previous studies have shown that ginseng and its ingredients are beneficial to bone health [[11], [12], [13], [14], [15]]. However, none of these studies have examined the effect of KRG on gut-bone axis using the antibiotic-dysbiosis model in mice. In this study we tested the hypothesis that microbiota dysbiosis-induced barrier dysfunction and bone loss will be prevented in KRG treated mice.
2. Materials and methods
2.1. Materials
Korean Red Ginseng (KRG) extract was obtained from Korea Ginseng Corp. (Daejeon, Korea), and the major components of the KRG extract are shown below as reported previously [16].
| Ingredient | Amount/g |
|---|---|
| Ginsenoside-Rg1 | 5.5 mg |
| Ginsenoside-Rg2 | 5.5 mg |
| Ginsenoside-Rg3 | 5.5 mg |
| Carbohydrates | 0.33 g |
2.2. Animals and experimental design
All animal procedures were approved by Michigan State University Institutional Animal Care and Use Committee and conformed to NIH guidelines. Eleven-week-old male Balb/C mice (#C0009615) were obtained from Charles River Laboratories (Wilmington, MA, USA) and were allowed to acclimate to the facility for one week prior to beginning experiments. Animals were housed at 4 mice per cage, on a 12:12 hour light-dark cycle, and had ad libitum access to sterilized standard chow (Teklad 2019, Teklad, Madison, WI, USA) and water. Upon reaching 12 weeks of age, mice were treated with oral broad-spectrum antibiotics (2-wks of ampicillin/neomycin, 160 and 80 mg/kg/day in sterilized drinking water), to deplete gram (+) and (−) bacteria [7]. Following ABX treatment, mice were given 4 weeks to naturally repopulate their intestinal microbiome. Korean Red Ginseng extract (KRG extract) @ 500 mg/kg/d was orally (by gavage) administered once daily during the duration of the 4-week treatment as follows: 1. Vehicle (H2O); 2. KRG extract 500 mg/kg/d (4 wk); 3. ABX (2 wk)+ vehicle(4 wk); 3. ABX (2 wk)+KRG extract 500 mg/kg/d (4 wk).
2.3. Microcomputed tomography (μCT) bone analysis
2.3.1. Femurs and vertebrae collected during harvest were scanned in a GE Explore
Locus μCT (GE Healthcare, Piscataway, NJ, USA) at a resolution of 20 μm obtained from 720 views and were analyzed as described before [7,8,17]. The distal femur trabecular bone region was defined as 10% proximal to the distal growth plate based on total bone length and excluded cortical bone. Trabecular bone was also analyzed within the body of the L4 vertebrae. Trabecular bone parameter values including volume, thickness, spacing, and number were obtained using GE Healthcare MicroView software version 2.2.
2.4. Microbiota analysis
A Qiagen® PowerSoil® DNA extraction kit was used to extract DNA from the fecal pellets following standard protocol. The variable region 4 of the bacterial 16S rRNA gene was amplified with universal primers 515f/806r and sequenced on the Illumina MiSeq platform at the Michigan State University Sequencing Core. The raw sequences were processed using QIITA (qiita.ucsd.edu [18]), which is based on QIIME2 algorithms [19], and quality filtered to generate amplicon sequence variants (ASVs) through the Deblur method [20]. Alpha diversity was assessed by Shannon index using Qiita and the values were input into GraphPad Prism for statistical analysis. Beta diversity was assessed using Bray-Curtis dissimilarity metric using Qiita. For analysis of bacterial composition at various taxonomical ranks, the relative distribution at various taxonomical ranks calculated from ASVs were input into GraphPad prism to analyze the distribution of Phylum, Class, Order and Family in the various groups. To further understand the relationship of the abundance changes in the bacterial taxa to bone health, we performed correlation analysis of the various taxa to that of femur BV/TV. Based on these correlations, we then assessed if there are statistically significant differences in the relative abundance between the various treatment groups. For this we focused at the level of the genus and species.
2.5. In vivo intestinal permeability measure
For measuring whole intestinal permeability, mice were gavaged with 300 mg/kg of 4 kD fluorescein isothiocyanate dextran (FITC-dextran) in sterile PBS 4 hours prior to the time of death. Sterile blood was collected via cardiac puncture immediately after euthanasia. Serum fluorescence was analyzed using Tecan Infinite M 1000 fluorescent plate reader (Tecan, Mannedorf, Switzerland) at an excitation/emission wavelength of 485/530 nm. The rate of 4 kD FITC-dextran transfer into the serum was calculated as described before [7].
2.6. Statistical analyses
Data analysis was performed using GraphPad Prism software version 9 (GraphPad, San Diego, CA, USA). The statistical tests are indicated in the figure legends. Data are shown as violin plots with lines at the median and quartiles.
3. Results
3.1. Dysbiosis model and general body parameters
Adult male mice (12 week, Balb/c) received oral broad-spectrum antibiotics (2-wks of ampicillin/neomycin, 160 and 80 mg/kg/day in sterilized drinking water), to deplete gram (+) and (−) bacteria [7]. Following ABX treatment, mice were given 4 weeks to naturally repopulate their intestinal microbiome. Korean Red Ginseng extract (KRG extract) @ 500 mg/kg/d was orally administered during the duration of the 4-week treatment as follows: 1. Vehicle (H2O); 2. KRG extract 500 mg/kg/d (4 wk); 3. ABX (2 wk)+vehicle(4 wk); 3. ABX (2 wk)+KRG extract 500 mg/kg/d (4 wk). At the end of the experimental period, animals were euthanized, and various tissues collected. Analysis of bone, intestinal permeability and microbiota were performed as described in our previous studies and as described in the methods [7,17]. As shown in Supplementary Fig. 1, body weight was similar between the different groups at the end of the experiment. Similarly, spleen and kidney weights were not significantly different between the groups. Interestingly however, liver weight was significantly decreased in the ABX + Vehicle group (without KRG extract) compared to Vehicle. In addition, ABX + KRG extract group also showed decreased liver weight compared to Vehicle and ABX + Vehicle groups. The significance of this effect on liver is unclear.
3.2. Korean Red Ginseng (KRG) extract treatment prevents dysbiosis-induced bone loss
In previous studies we demonstrated that post-ABX dysbiosis causes bone loss in mice [7,8]. Consistent with that, we demonstrate here that natural repopulation following ABX treatment, i.e. post-ABX (ABX + Vehicle group) caused a significant bone loss as evident in femur BV/TV and vertebral BV/TV (Fig. 1 and Supplementary Fig. 2). Treatment with KRG extract during the repopulation period however, prevented this bone loss (both femur and vertebral BV/TV). Analysis of femoral bone microarchitecture revealed a decrease in trabecular thickness (Tb.Th.) in the ABX + Vehicle group that was prevented by KRG extract treatment. Trabecular number (Tb.N) and spacing (Tb.Sp) were not significantly altered in the femur of the ABX + Vehicle group. Analysis of the vertebral bone architecture revealed a significant increase in trabecular spacing (Tb.Sp) and a significant decrease in trabecular number (Tb.N) and thickness (Tb.Th) in the ABX + Vehicle group compared to the controls. KRG extract treatment significantly prevented the Tb.Sp. and Tb.Th. parameters in the vertebrae. Interestingly, treatment of control mice with KRG extract for 4 weeks significantly increased femur BV/TV but not vertebral BV/TV. Consistent with this, the femur bone architecture revealed an increase in trabecular number (Tb.N.) and a decrease in trabecular spacing (Tb.Sp.) but no significant change to trabecular thickness (Tb.Th.) in the KRG extract treated mice compared to control group. Together these results demonstrate that KRG extract treatment during the microbiota repopulation period following ABX treatment is beneficial to bone health. In addition, KRG extract treatment without any underlying disease conditions can increase the femoral bone volume in healthy mice.
Fig. 1.
Korean Red Ginseng (KRG) extract treatment prevents post-antibiotic dysbiosis-induced femoral trabecular bone loss in mice. Balb/c male mice (12 weeks old) were divided into four experimental groups: Sterile drinking water (Vehicle), sterile drinking water with Korean ginseng extract gavage for 4 weeks (KRG extract), Broad-spectrum antibiotics (ABX) for 2 weeks followed by natural repopulation for 4 weeks (ABX + Vehicle), or Broad-spectrum antibiotics (ABX) for 2 weeks followed by with Korean Red Ginseng extract gavage for 4 weeks (ABX + KRG extract). (A) Femoral trabecular bone volume fraction (BVF) and (B) BVF corrected for body weight. Representative μCT isosurface images from the different groups are shown above A and B. (C) Bone femur microarchitecture analyses. Violin plots show the distribution of the data and line at the median and quartiles. Statistical analyses were performed with one-way ANOVA with Tukey post-test. ∗∗∗∗p < 0.0001; ∗∗∗p < 0.001, ∗∗p < 0.01; ∗p < 0.05; ns = not significant.
3.3. Korean Red Ginseng (KRG) extract treatment modulates intestinal microbiota
To assess the effect of KRG extract on the intestinal microbiota, the relative abundance and composition of the fecal extract was determined prior to the start of the study (“pre” group) and at the end of the study (“post” group). Alpha diversity was assessed by calculating the Shannon index. As shown in Fig. 2, compared to the “pre” group, ABX treatment followed by natural repopulation caused a significant decrease in alpha diversity. KRG extract treatment during this natural repopulation period (ABX + KRG extract group) completely prevented this decrease in alpha diversity. None of the other treatment groups showed any significant changes when compared between the respective -pre and the -post groups. To understand the relationship of changes in alpha diversity to that of bone volume, we correlated Shannon index to that of femur and vertebral BV/TV. Shannon index from the post- microbiota samples showed a significant correlation to both femur and vertebral BV/TV (r = 0.3028, p = 0.0364 for femur and r = 0.2966, p = 0.0365 for vertebra) (Fig. 2). These results suggest that changes in alpha diversity following post-antibiotic dysbiosis likely predict femur and vertebral bone volumes.
Fig. 2.
Korean Red Ginseng (KRG) extract treatment prevents post-antibiotic decrease in alpha diversity (gut microbiota) in mice: Fecal microbiota from mice were collected before the start of the experiments in each group (“pre” in each group) or collected at euthanasia (“post” in each group). Alpha diversity was assessed by Shannon index using Qiita and the values were input into GraphPad Prism for graph and statistics (top graph). Violin plots show the distribution of the data and line at the median and quartiles. N = 14-16 for all groups except KRG extract group (n = 5). Statistical analyses were performed with Kruskal-Wallis test followed by Dunn's test for multiple comparison. ∗∗∗∗p < 0.0001; ns: not significant. Shannon index from post-microbiota samples and femur BV/TV or Vertebral BV/TV were analyzed for Pearson correlation (bottom graph).
Further analysis of beta diversity did not reveal any significant findings (not shown). We next analyzed bacterial composition at various taxonomical ranks. For this, the relative distribution at various taxonomical ranks calculated from ASVs were input into GraphPad prism to analyze the distribution of Phylum, Class, Order and Family in the various groups. For relative abundance graphs at each taxonomic ranks (Fig. 3 and Supplementary Fig. 3) the replicates from each treatment group were combined and the aggregate data is shown. At the Phylum level, there were some marked differences in their distribution between the groups. Bacteroidetes and Firmicutes were the predominant phyla in all groups (Fig. 3). KRG extract treatment reduced the abundance of Firmicutes in ABX treated (ABX + KRG extract-post) and untreated mice (KRG extract-post). Conversely, KRG extract treatment increased the abundance of Bacteroidetes in the KRG extract treated groups. Also, KRG extract treatment distinctly increased the abundance of Protobacteria only in the non-ABX treated mice (KRG extract-post). At the class level, Bacteroidia (of Phylum Bacteroidetes) and Clostridia (of Phylum Firmicutes) were predominant in all groups of mice, and these followed similar trends to that of the phyla when compared between the groups. Like the Class, at the level of the Order, Clostridiales (of Class Clostridia) and Bacteriodales (of Class Bacteroidia) were the predominant groups in all the mice, and these showed trends similar to Phyla and Class in terms of the effect of KRG extract treatment. Because Bacteroidetes and Firmicutes were the predominant groups, we focused on the relative abundance of these two Phyla at the Family level. As shown in Supplementary Fig. 3, the Bacteroidales group S24-7, Porphyomondaceae, Rikenellaceae and Bacteroidaceae were the predominant members among the different families in Bacteroidetes. While S24-7 increased in abundance in post-ABX mice (ABX + Vehicle-post) compared to control, KRG treatment did not affect the levels. Bacteroidaceae and Porphyromonadaceae were decreased in the post-ABX mice (ABX + Vehicle-post) compared to control and KRG extract treatment did not have any marked effect. KRG treatment markedly decreased the abundance of Rikenellaceae in post-ABX mice (ABX + KRG extract-post) compared to its respective controls (ABX + Vehicle-post or ABX + KRG extract-pre). In the Firmicutes, Ruminococcaceae, Lachnospiraceae, Paenibacillaceae and Lactobacillaceae were predominant. Interestingly, Peptostreptococcaceae was present only in the post-ABX group and was absent in all other groups. Also, KRG extract treatment appeared to increase the abundance of Lactobacillaceae in the KRG treated groups.
Fig. 3.
Effect of Korean Red Ginseng (KRG) extract treatment on relative abundance of microbiota at the Phylum, Class and Order levels: Fecal microbiota from mice were collected before the start of the experiments in each group (“pre” in each group) or collected at euthanasia (“post” in each group). Bar graphs showing relative abundance of bacteria at the level of Phyla (top), Class (middle) and Order (bottom). Data were analyzed using Qiita. Relative abundance values for each mouse group were averaged and graphed as shown. N = 14-16 for all groups except KRG extract alone group (n = 5).
To further understand the relationship of the abundance changes in the bacterial taxa to bone health, we performed correlation analysis of the various taxa to that of femur BV/TV. We found that the relative distribution of several bacterial taxa were either positively or negatively correlated with femur BV/TV (the ones that were correlated at various taxa up to the family level are shown in Supplementary Table 1. Data not shown for genus and species levels). Based on these correlations, we then assessed if there are statistically significant differences in the relative abundance between the various treatment groups. For this we focused at the level of the genus and species. At the genus level we found that Lactobacillus (family Lactobacillaceae), rc4-4 (family Peptococcaceae) and an unknown genus of the family S24-7 showed significant differences between the KRG extract treated and untreated in the post-ABX mice. Specifically, we found that Lactobacillus was suppressed in the post-ABX group (ABX + Vehicle-post) compared to its control (ABX + Vehicle-pre) (p = 0.07 based on ANOVA Holm-Sidak's multiple comparisons test; p = 0.0021 based on t-test) (Fig. 4). This decrease in Lactobacillus was markedly prevented in the KRG extract treated group (ABX + KRG extract-pre vs ABX + KRG extract-post); p = 0.9084. KRG extract did not have any effect on Lactobacillus in the non-ABX mouse groups. Abundance of genus rc4-4 was significantly suppressed by KRG extract treatment in the post-ABX mice when compared to post-ABX mice without KRG extract treatment (ABX + KRG extract-post vs ABX + Vehicle-post) (Fig. 4). Abundance of unknown genus in the family f_S24-7 was significantly increased by KRG extract treatment compared to its control (ABX + KRG extract-pre vs ABX + KRG extract-post). Overall, at the genus level, KRG extract treatment appears to regulate the abundance of these 3 genera. In addition, abundance of these 3 genera were significantly correlated with femur BV/TV (Lactobacillus and f_S24-7;g_ were positively correlated and rc4-4 was negatively correlated), suggesting a link between these bacteria and bone health.
Fig. 4.
Effect of Korean Red Ginseng (KRG) extract treatment on the relative abundance of bacteria shown at the genus level: Violin plots of genus Lactobacillus (top), genus Rc4-4 (middle) and f_s24-7;g_ (bottom) from the various mouse groups (as in Fig. 3) show the distribution of the data and line at the median and quartiles. N = 14-16 for all groups except KRG extract alone group (n = 5). Statistical analyses were performed using ANOVA with post Holm-Sidak's multiple comparisons test and P values from Holm-Sidak's are as shown. Based on t-test, #p = 0.0021, @p = 0.0309. Pearson correlation was performed between the respective genus vs femur BV/TV and shown on the side.
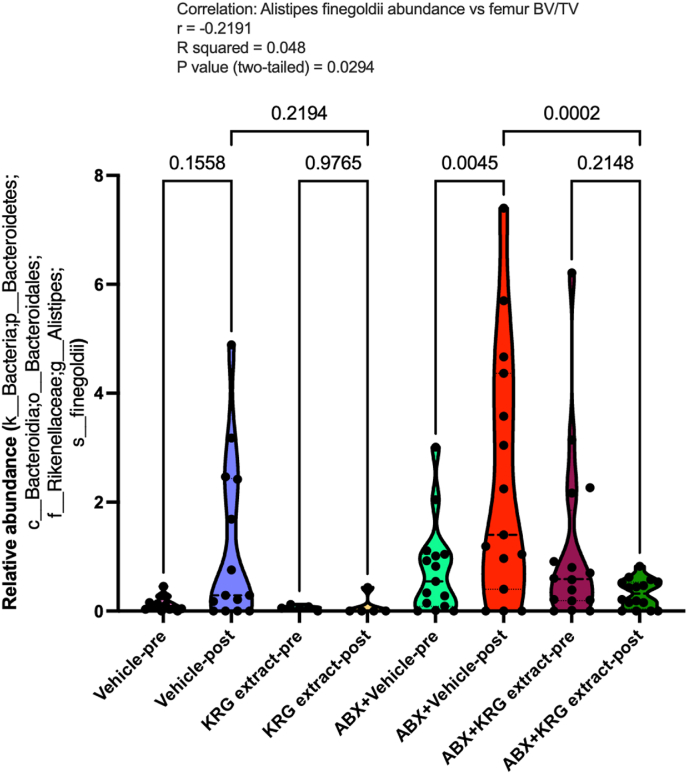
We further did a similar analysis at the species level taking into account all the identifiable species that showed significant correlation to femur BV/TV. We then analyzed to compare the abundance of each of those species in the different treatment groups. Interestingly, our results demonstrate that Alistipes finegoldii is significantly modulated by KRG extract treatment. As shown in Fig. 5, post-ABX dysbiosis increased the abundance of Alistipes finegoldii in the post-ABX mice (compared between ABX + Vehicle-pre vs ABX + Vehicle-post; p = 0.0045). KRG extract treatment significantly inhibited the abundance of this bacteria in the post-ABX mice (compared between ABX_Ginseng_Pre vs ABX_Ginseng_post; p = 0.2148). When compared between post-ABX mice with and without KRG treatment (ABX + Vehicle-post vs ABX + KRG extract-post), abundance of A. finegoldii was significantly lower in the KRG treated mice (p = 0.002). Importantly, abundance of A. finegoldii was significantly and negatively correlated to femur BV/TV (p = 0.0294). Together, microbiota analysis reveals important changes in bacterial composition in response to KRG extract treatment.
Fig. 5.
Korean Red Ginseng (KRG) extract treatment inhibits relative abundance of Alistipes finegoldii: Violin plots of relative abundance of Alistipes finegoldii from the various mouse groups (as shown in Fig. 3) show the distribution of the data and line at the median and quartiles. N = 14-16 for all groups except KRG extract alone group (n = 5). Statistical analyses were performed using ANOVA with post Holm-Sidak's multiple comparisons test. P values are as shown. Pearson correlation was performed between the respective species vs femur BV/TV and shown above the graph.
3.4. Korean Red Ginseng (KRG) extract treatment prevents dysbiosis-induced intestinal barrier leakage
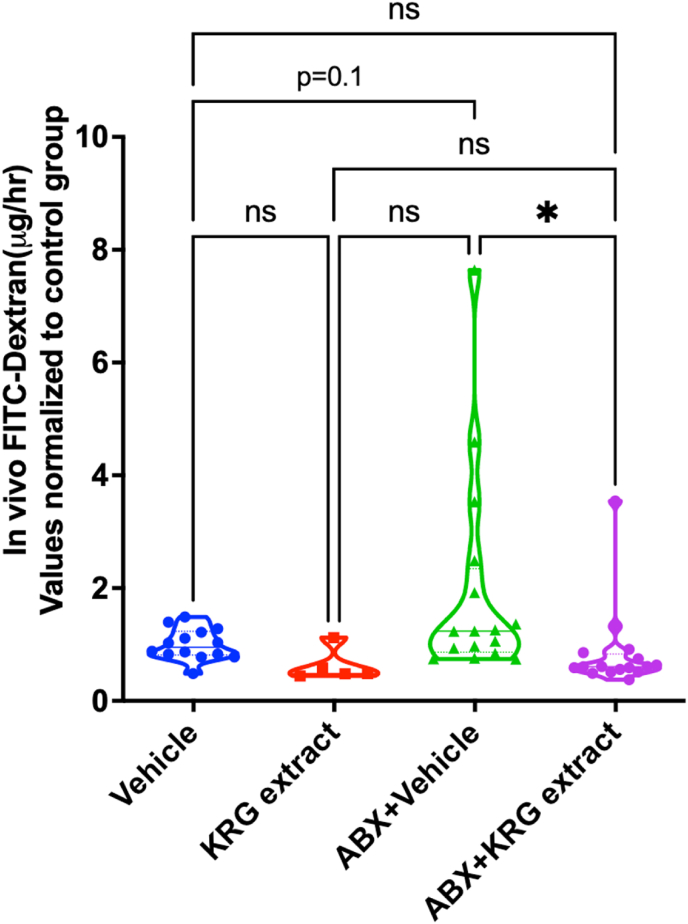
In previous studies we showed that intestinal barrier function is strongly correlated with bone health in the ABX-dysbiosis-induced bone loss model in mice [7]. To examine if KRG extract treatment prevents dysbiosis-induced barrier leakage, we assessed in vivo permeability using FITC-dextran (4 KDa). Although post-ABX dysbiosis group showed a modest increase in barrier leakage (as determined by serum FITC-dextran levels; p = 0.1), treatment with KRG extract significantly prevented the barrier leakage (Fig. 6). KRG extract treatment in control mice did not affect serum FITC-dextran levels. These results suggest that treatment with KRG extract prevents intestinal leakage induced by ABX-induced dysbiosis. To understand if these changes correlate with bone health, we analyzed correlation between serum FITC-dextran and femur and vertebral BV/TV. Our findings reveal a significant negative correlation between serum FITC-dextran and vertebral BV/TV (r = −0.3115; p = 0.0261). To further understand the mechanisms of changes in intestinal permeability, we assessed mRNA levels of various junction proteins in the distal colon and ileum. Except for Claudin-4 in the distal colon, none of the other genes were significantly altered in the ABX-treated group (without KRG extract treatment). KRG treatment of the post-ABX group did not alter any of these genes either in distal colon or ileum when compared to the post-ABX group (data not shown).
Fig. 6.
Korean Red Ginseng (KRG) extract treatment decreases intestinal permeability in post-antibiotic-dysbiosis in mice: (A) Intestinal in vivo flux (Serum FITC dextran) was measured by FITC dextran gavage. All groups and number of mice are as described in Fig. 1. Violin plots show the distribution of the data and line at the median and quartiles. Statistical analyses were performed with one-way ANOVA with Tukey post-test. ∗p < 0.05; ns = not significant.
4. Discussion
The focus of the current study is to understand whether Korean Red Ginseng (KRG) extract can prevent antibiotic dysbiosis-induced bone loss in mice as well as decipher the possible mechanisms of action on the gut-bone axis. As indicated earlier, previous studies have shown that ginseng prevents bone loss in other animal models. Kang et al [21]showed that co-administration of panax ginseng at 500 mg/kg/day along with Brassica oleracea (cabbage) for 10 weeks prevented ovariectomy-induced bone loss in mice. It is important to note that in this model, panax ginseng alone did not have any significant effect on either body weight or bone loss. Compared to these studies, Kim et al [22] showed that KRG could prevent glucocorticoid-induced osteoporosis (at 100 mg/Kg and 500 mg/kg). Similarly other studies have looked at the effect of ginseng in different models of bone loss and have found ginseng to be protective [23]. However, these studies did not look at the role of gut microbiota or barrier dysfunction in the context of bone loss. In our studies we used a dose of 500 mg/kg/day and observed significant effect on microbiota, barrier function and bone health (both femur and vertebrae) when mice were treated with KRG extract during post-ABX dysbiosis. Interestingly healthy control mice (without dysbiosis) treated with KRG extract for 4 weeks showed a significant increase in femur BV/TV but not vertebral BV/TV suggesting that different mechanisms could be at play in regulating femur and vertebral bone at least in control mice.
Even though previous studies have not examined the effect of ginseng on gut-bone axis, the role of ginseng on gut microbiota has been examined extensively (for review, see [24]). Han et al [25], showed that feeding white Korean ginseng to rats, increases Muc2 gene expression in the ileum and increases the number of Lactobacillus strains compared to control. Using human subjects, Song et al [26] have shown that treatment with panax ginseng (4 g twice a day for 8 weeks) was associated with some changes in gut microbiota. In another double-blind, placebo controlled human clinical trial [27], responses to KRG administration (at 6 g/day dose) were associated with changes in gut microbial composition. While there were some limitations to the study, the authors showed that KRG treatment decreased Firmicutes and Proteobacteria and increased Bacteroidetes and that patient responders that had higher abundance of Lachnospiraceae and Clostridiales showed decreases in serum total cholesterol and LDL. In a recent study, Ren et al [28] showed that the polysaccharide extract of the American ginseng can increase the relative richness of Lactobacillus and Bacteroides in an antibiotic-induced diarrhea model in rats. Ginsenoside Rk3, similarly improved antibiotic-induced diarrhea and enriched the beneficial microbiota in a mouse diarrhea model [29]. The antibiotic-induced dysbiosis model we have used in this study is not a diarrhea model but models a clinically relevant broad spectrum antibiotic treatment and subsequent dysbiosis. However, like the antibiotic-induced diarrhea model, KRG treatment in our studies also induced beneficial effects on the microbiota.
Our studies demonstrate a number of unique changes in microbiome with KRG extract treatment at various taxonomic levels. When examined at the genus level, our results reveal the importance of Lactobacillus, rc4-4 and an unknown genus under family S24-7 in the context of KRG extract treatment. In previous studies our lab and others have demonstrated the importance of Lactobacillus in different models of bone loss [7,8,17,[30], [31], [32]]. In particular, we have shown that Lactobacillus reuteri, a probiotic can beneficially influence the microbiota and bone health in multiple mouse models. Our results here show that the abundance of genus Lactobacillus is decreased with ABX-dysbiosis and that this decrease is markedly prevented by KRG treatment. In addition, abundance of Lactobacillus was positively correlated with femur BV/TV. Taken together based on our previous studies on Lactobacillus reuteri, our studies strongly suggest an important role for Lactobacillus in the beneficial effects of KRG extract treatment on bone health. The genus rc4-4 belongs to the Phylum Firmicutes, Class Clostridia and Family Peptococcaceae. Role of rc4-4 in modulating the effect of KRG extract is not well known and its effect on bone has not been studied. Our results reveal that the abundance of rc4-4 is negatively correlated with bone volume and that KRG extract treatment decreases its abundance in post-ABX mice. This suggests that presence of rc4-4 may not be beneficial to bone health. Further studies are needed to test this hypothesis. The abundance of an unknown genus in the family S24-7 was positively correlated with femur bone volume and KRG extract increased its abundance in post-ABX mice, suggesting that this bacteria may be beneficial to bone health. S24-7 belongs to the Phylum Bacteroidetes and Order Bacteroidales. Although the genus is unknown, the effect of S24-7 in bone health or ginseng treatments has not been well studied.
At the species level, our studies identify Alistipes finegoldii as a potentially important bacteria that is involved in the effects of KRG extract in the antibiotic-dysbiosis-induced bone loss model in mice. A. finegoldii negatively correlates with bone volume and its abundance increases with post-ABX dysbiosis. Importantly, A. finegoldii abundance is decreased with KRG treatment suggesting that the beneficial effect of KRG on bone in this model may be linked to a decrease in this bacterium. Role of bacteria of the genus Alistipes in terms of bone health is not well known, in part because it is a relatively recently described genus. There are 13 species in this genus including A. finegoldii [33]. Although the role of Alistipes in the pathogenesis of disease processes has been contrasting, A. finegoldii colonization has been shown to induce colitis-associated colon cancer via activation of IL-6/STAT3 pathway [34,35]. Thus, it is possible that A. finegoldii has potentially negative effects on bone health and will be the subject of future studies.
None of these studies however, examined the effect of gut microbiota changes in the context of gut permeability and bone health. In our study, we find that KRG prevents dysbiosis and gut permeability dysfunction, and importantly, these effects were associated with significant prevention of bone loss. We have previously shown that inhibiting an increase in intestinal permeability prevents post-ABX-dysbiosis induced bone loss in mice [7]. Thus, it is likely that KRG's effect on intestinal permeability in part explains the mechanism by which KRG extract prevents post-ABX dysbiosis-induced bone loss. Whether the changes induced by KRG on microbiota precedes gut permeability effects is not known and will be the subject of future studies.
Our studies for the first time demonstrate protective effect of KRG extract on post-ABX-dysbiosis-induced bone loss in mice. However, there are some limitations to our studies. We have used a single dose of KRG to test the effect on bone loss in mice. However, the dose we used in our mouse studies is similar to the human dose (3 g/day) used in a randomized, double-blind placebo-controlled trial that showed improvement in arthritis symptoms and serum osteocalcin concentrations over a 12-week period in osteopenic women [15]. Thus, we believe the dose we used in our study is clinically relevant. Our studies did not identify cellular and molecular mechanisms by which KRG affects gut-bone axis and this will be the focus of future studies.
Acknowledgement
The authors thank the staff of Campus Animal Resources for the excellent care of our animals. We also thank Dr. Sandra O'Reilly for helping with some mouse experiments. The work presented in this study was funded by the Korean Ginseng Society.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2022.08.006.
Contributor Information
Laura R. McCabe, Email: mccabel@msu.edu.
Narayanan Parameswaran, Email: narap@msu.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Clynes M.A., Harvey N.C., Curtis E.M., Fuggle N.R., Dennison E.M., Cooper C. The epidemiology of osteoporosis. Brit Med Bull. 2020;133:105–117. doi: 10.1093/bmb/ldaa005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCabe L.R., Parameswaran N. Advances in probiotic regulation of bone and mineral metabolism. Calcified Tissue International. 2018;1:1–9. doi: 10.1007/s00223-018-0403-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins F.L., Rios-Arce N.D., Schepper J.D., Parameswaran N., McCabe L.R. The potential of probiotics as a therapy for osteoporosis. Microbiol Spectr. 2017;5 doi: 10.1128/microbiolspec.bad-0015-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCabe L., Britton R.A., Parameswaran N. Prebiotic and probiotic regulation of bone health: role of the intestine and its microbiome. Current Osteoporosis Reports. 2015;13:363–371. doi: 10.1007/s11914-015-0292-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilbert J.A., Quinn R.A., Debelius J., Xu Z.Z., Morton J., Garg N., et al. Microbiome-wide association studies link dynamic microbial consortia to disease. Nature. 2016;535:94–103. doi: 10.1038/nature18850. [DOI] [PubMed] [Google Scholar]
- 6.Irwin R., Lee T., Young V.B., Parameswaran N., McCabe L.R. Colitis-induced bone loss is gender dependent and associated with increased inflammation. Inflammatory Bowel Diseases. 2013;19:1586–1597. doi: 10.1097/mib.0b013e318289e17b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schepper J.D., Collins F.L., Rios-Arce N.D., Raehtz S., Schaefer L., Gardinier J.D., et al. Probiotic Lactobacillus reuteri prevents postantibiotic bone loss by reducing intestinal dysbiosis and preventing barrier disruption. Journal of Bone and Mineral Research. 2019;34:681–698. doi: 10.1002/jbmr.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rios-Arce N.D., Schepper J.D., Dageanis A., Schaefer L., Daly-Seiler C.S., Gardinier J.D., et al. Post-antibiotic gut dysbiosis-induced trabecular bone loss is dependent on lymphocytes. Bone. 2020;134 doi: 10.1016/j.bone.2020.115269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang N., Liu D., Zhang X., Li J., Wang M., Xu T., et al. Effects of ginsenosides on bone remodelling for novel drug applications: a review. Chin Med-Uk. 2020;15:42. doi: 10.1186/s13020-020-00323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yun T.K. Brief introduction of Panax ginseng C. A. Meyer. J Korean Med Sci. 2001;16:S3–S5. doi: 10.3346/jkms.2001.16.s.s3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim H.-R., Cui Y., Hong S.-J., Shin S.-J., Kim D.-S., Kim N.-M., et al. Effect of ginseng mixture on osteoporosis in ovariectomized rats. Immunopharm Immunot. 2008;30:333–345. doi: 10.1080/08923970801949125. [DOI] [PubMed] [Google Scholar]
- 12.Lee J.-H., Lee H.-J., Yang M., Moon C., Kim J.-C., Bae C.-S., et al. Effect of Korean Red Ginseng on radiation-induced bone loss in C3H/HeN mice. Journal of Ginseng Research. 2013;37:435–441. doi: 10.5142/jgr.2013.37.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J., Lee H., Kang K.S., Chun K.-H., Hwang G.S. Protective effect of Korean Red Ginseng against glucocorticoid-induced osteoporosis in vitro and in vivo. J Ginseng Res. 2015;39:46–53. doi: 10.1016/j.jgr.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X., Huang F., Chen X., Wu X., Zhu J. Ginsenoside Rg3 attenuates ovariectomy-induced osteoporosis via AMPK/mTOR signaling pathway. Drug Develop Res. 2020;81:875–884. doi: 10.1002/ddr.21705. [DOI] [PubMed] [Google Scholar]
- 15.Jung S.-J., Oh M.-R., Lee D.Y., Lee Y.-S., Kim G.-S., Park S.-H., et al. Effect of ginseng extracts on the improvement of osteopathic and arthritis symptoms in women with osteopenia: a randomized, double-blind, placebo-controlled clinical trial. Nutrients. 2021;13:3352. doi: 10.3390/nu13103352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J.K., Shin K.K., Kim H., Hong Y.H., Choi W., Kwak Y.-S., et al. Korean Red Ginseng exerts anti-inflammatory and autophagy-promoting activities in aged mice. J Ginseng Res. 2021;45:717–725. doi: 10.1016/j.jgr.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schepper J.D., Collins F., Rios-Arce N.D., Kang H.J., Schaefer L., Gardinier J.D., et al. Involvement of the gut microbiota and barrier function in glucocorticoid-induced osteoporosis. J Bone Miner Res. 2020;35 doi: 10.1002/jbmr.3947. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez A., Navas-Molina J.A., Kosciolek T., McDonald D., Vázquez-Baeza Y., Ackermann G., et al. Qiita: rapid, web-enabled microbiome meta-analysis. Nat Methods. 2018;15:796–798. doi: 10.1038/s41592-018-0141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolyen E., Rideout J.R., Dillon M.R., Bokulich N.A., Abnet C.C., Al-Ghalith G.A., et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amir A., McDonald D., Navas-Molina J.A., Kopylova E., Morton J.T., Xu Z.Z., et al. Deblur rapidly resolves single-nucleotide community sequence patterns. Msystems. 2017;2 doi: 10.1128/msystems.00191-16. e00191-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang I.S., Agidigbi T.S., Kwon Y.M., Kim D.-G., Kim R.I., In G., et al. Effect of co-administration of Panax ginseng and Brassica oleracea on postmenopausal osteoporosis in ovariectomized mice. Nutrients. 2020;12:2415. doi: 10.3390/nu12082415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J., Lee H., Kang K.S., Chun K.-H., Hwang G.S. Protective effect of Korean Red Ginseng against glucocorticoid-induced osteoporosis in vitro and in vivo. Journal of Ginseng Research. 2015;39:46–53. doi: 10.1016/j.jgr.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siddiqi M.H., Siddiqi M.Z., Ahn S., Kang S., Kim Y.-J., Sathishkumar N., et al. Ginseng saponins and the treatment of osteoporosis: mini literature review. J Ginseng Res. 2013;37:261–268. doi: 10.5142/jgr.2013.37.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Z., Zhang Z., Liu J., Qi H., Li J., Chen J., et al. Gut microbiota: therapeutic targets of ginseng against multiple disorders and ginsenoside transformation. Front Cell Infect Mi. 2022;12 doi: 10.3389/fcimb.2022.853981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han K.-S., Balan P., Hong H.-D., Choi W.-I., Cho C.-W., Lee Y.-C., et al. Korean ginseng modulates the ileal microbiota and mucin gene expression in the growing rat. Food Funct. 2014;5:1506–1512. doi: 10.1039/c4fo00087k. [DOI] [PubMed] [Google Scholar]
- 26.Song M.-Y., Kim B.-S., Kim H. Influence of Panax ginseng on obesity and gut microbiota in obese middle-aged Korean women. Journal of Ginseng Research. 2014;38:106–115. doi: 10.1016/j.jgr.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seong E., Bose S., Han S.-Y., Song E.-J., Lee M., Nam Y.-D., et al. Positive influence of gut microbiota on the effects of Korean red ginseng in metabolic syndrome: a randomized, double-blind, placebo-controlled clinical trial. Epma J. 2021;12:177–197. doi: 10.1007/s13167-021-00243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren D., Li S., Lin H., Xia Y., Li Z., Bo P., et al. Panax quinquefolius polysaccharides ameliorate antibiotic-associated diarrhoea induced by lincomycin hydrochloride in rats via the MAPK signaling pathways. J Immunol Res. 2022;2022 doi: 10.1155/2022/4126273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bai X., Fu R., Duan Z., Wang P., Zhu C., Fan D. Ginsenoside Rk3 alleviates gut microbiota dysbiosis and colonic inflammation in antibiotic-treated mice. Food Res Int. 2021;146 doi: 10.1016/j.foodres.2021.110465. [DOI] [PubMed] [Google Scholar]
- 30.Quach D., Parameswaran N., McCabe L., Britton R.A. Characterizing how probiotic Lactobacillus reuteri 6475 and lactobacillic acid mediate suppression of osteoclast differentiation. Bone Reports. 2019 doi: 10.1016/j.bonr.2019.100227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collins F.L., Irwin R., Bierhalter H., Schepper J., Britton R.A., Parameswaran N., et al. Lactobacillus reuteri 6475 increases bone density in intact females only under an inflammatory setting. PloS One. 2016;11 doi: 10.1371/journal.pone.0153180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Britton R.A., Irwin R., Quach D., Schaefer L., Zhang J., Lee T., et al. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. Journal of Cellular Physiology. 2014;229:1822–1830. doi: 10.1002/jcp.24636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parker B.J., Wearsch P.A., Veloo A.C.M., Rodriguez-Palacios A. The genus Alistipes: gut bacteria with emerging implications to inflammation, cancer, and mental health. Front Immunol. 2020;11:906. doi: 10.3389/fimmu.2020.00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y., Jobin C. Novel insights into microbiome in colitis and colorectal cancer. Curr Opin Gastroen. 2017;33:422–427. doi: 10.1097/mog.0000000000000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moschen A.R., Gerner R.R., Wang J., Klepsch V., Adolph T.E., Reider S.J., et al. Lipocalin 2 protects from inflammation and tumorigenesis associated with gut microbiota alterations. Cell Host Microbe. 2016;19:455–469. doi: 10.1016/j.chom.2016.03.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.