Abstract
Background
Ginsenoside Rg2 (Rg2) has a variety of pharmacological activities and provides benefits during inflammation, cancer, and other diseases. However, there are no reports about the relationship between Rg2 and atherosclerosis.
Methods
We used 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to detect the cell viability of Rg2 in vascular smooth muscle cells (VSMCs) and human umbilical vein endothelial cells (HUVECs). The expression of inflammatory factors in HUVECs and the expression of phenotypic transformation-related marker in VSMCs were detected at mRNA levels. Western blot method was used to detect the expression of inflammation pathways and the expression of phenotypic transformation at the protein levels. The rat carotid balloon injury model was performed to explore the effect of Rg2 on inflammation and phenotypic transformation in vivo.
Results
Rg2 decreased the expression of inflammatory factors induced by lipopolysaccharide in HUVECs–without affecting cell viability. These events depend on the blocking regulation of NF-κB and p-ERK signaling pathway. In VSMCs, Rg2 can inhibit the proliferation, migration, and phenotypic transformation of VSMCs induced by platelet derived growth factor-BB (PDGF-BB)–which may contribute to its anti-atherosclerotic role. In rats with carotid balloon injury, Rg2 can reduce intimal proliferation after injury, regulate the inflammatory pathway to reduce inflammatory response, and also suppress the phenotypic transformation of VSMCs.
Conclusion
These results suggest that Rg2 can exert its anti-atherosclerotic effect at the cellular level and animal level, which provides a more sufficient basis for ginseng as a functional dietary regulator.
Keywords: ginsenoside Rg2, atherosclerosis, endothelial cell, inflammation, signaling pathway
Graphical abstract
1. Introduction
Cardiovascular diseases (CVDs) have a high morbidity and fatality rate worldwide. Atherosclerosis (AS) is a chronic, progressive disease that underlies a variety of cardiovascular diseases, including sudden death, stroke, and myocardial infarction. Improvement in the living standard of people, change in their living habits, aging of the population are gradually increasing the incidence of CVDs based on atherosclerosis, and gradually showing a younger trend, thus burdening the societal and economic development [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11]].
Endothelial cells (ECs) and vascular smooth muscle cells (VSMCs) are essential for the occurrence of atherosclerosis. The inner surface of all types of blood vessels in the body are covered by ECs–which maintain the oxygen supply and nutritional demand of the tissue–thus protecting the vascular wall and the underlying tissue from damage [[7], [8], [12], [13]]. Hypertension, diabetes, and smoking can activate ECs leading to their increased permeability–which leads to the production of inflammatory cytokines, culminating in to a series of inflammatory cascading amplifications. The role of VSMCs in atherosclerosis cannot be ignored. In normal physiological regulation, VSMCs exist in the media of blood vessels while maintaining vascular homeostasis and normal cardiovascular functions–including regulating vasoconstriction, blood pressure and blood flow [[14], [15]]. The lesion of VSMCs in atherosclerosis is mainly a phenotypic transformation–that further leads to cell migration and gradual accumulation in the intima to form lesions. Platelet-derived growth factor (PDGF) is reported to promote phenotypic transformation of VSMCs. The main contractile markers related to phenotypic transformation of VSMCs are α-smooth muscle actin (α-SMA), smooth muscle myosin heavy chain (SM-MHC) and calponin.
Ginseng–a traditional medicine used since ancient times–is regarded as a universal medicine [[16], [17], [18], [19]]. Ginsenoside Rg2 (Rg2)–one of the main active ingredients of ginseng [20] regulates the immune function of the body [[21], [22]], and plays a role in neuroprotection and cardiovascular protection [[23], [24], [25]]. Furthermore, Rg2 attenuated the inflammatory response to LPS-induced macrophages-induced acute liver and kidney injury. However, there are no reports on the relationship between Rg2 and underlying atherosclerosis in CVDs.
The present study investigated the protective effect of Rg2 in alleviating the occurrence and progression of atherosclerosis via regulating the biological function of ECs and VSMCs, and explored the underlying mechanisms.
2. Materials and methods
2.1. Preparation of ginsenoside Rg2 and atorvastatin calcium
Rg2 was purchased from Chengdu Ruifensi Biotechnology Co., Ltd., Sichuan Province, China. Its chemical structure is C42H72O13, molecular weight is 785.01, and purity is more than 98%. Atorvastatin calcium (Ator) was purchased from Omer Biotechnology Co., Ltd (Nanjing, China).
2.2. Cell culture
The vascular smooth muscle cells (VSMCs) were cultured in Dulbecco's modified Eagle's medium (Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS) (ExCell Bio, Shanghai, China) in a moist atmosphere at 37 °C and 5% CO2. VSMCs is a human aortic smooth muscle cell line (ATCC, Manassas, VA, USA). The human umbilical vein endothelial cells (HUVECs) (Cell Bank, Shanghai, China) were cultured in DMEM/F-12 medium (MeilunBio, Dalian, China) containing 10% FBS.
2.3. Cell viability analysis
To evaluate the cytotoxic effect of Rg2, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed. The HUVECs and VSMCs were seeded in 96-well plates (5 × 103 cells/well), cultured to 80%-90%, and then treated with Rg2 (10-40 μM) for another 24 h, respectively. The original culture medium was replaced with fresh culture medium without FBS and incubated for 4 hours after adding 10 μL MTT. Thereafter, the culture medium was discarded, dimethyl sulfoxide (DMSO) was added to each well, vibrated on the oscillator for 15 mins, and then detected under the microplate reader.
2.4. Cell proliferation analysis
The HUVECs and VSMCs were inoculated in a 96-well plate overnight and cultured with serum-free medium containing Rg2 (10 μM and 20 μM) for 12 h, followed by a stimulation for 12 h with 20 ng/mL PDGF-BB (ab9706, Abcam), respectively. Then the original culture medium was replaced with 100 μL fresh culture medium supplemented with Cell Counting Kit-8 solution (CCK8) (Yeasen, Shanghai, China), followed by incubation of 1 h at 37°C. The Optical density (OD) at 450 nm was measured using a microplate reader, which detected the effect of Rg2 on the proliferation of PDGF-BB-induced HUVECs and VSMCs.
2.5. Wound healing assay
The VSMCs were planted into a 6-well plate. On reaching 70% confluence, the cells were pretreated with Rg2 (10 μM and 20 μM) for 12 h followed by an addition of 20 ng/mL PDGF-BB. After the 12 h, a scratch was made using a 1000 μL pipette tip and the incubation was continued at 37 °C and 5% CO2. Images for migration evaluation were captured using microscopy (Nikon, Tokyo, Japan) at 0, 12 and 24 h. The total migration ratio was analyzed with the Image J software.
2.6. Transwell migration assay
The HUVECs and VSMCs were first treated with or without Rg2 in a 12-well plate, respectively, followed by PDGF-BB stimulation for 12 h. The cells underwent pancreatic enzyme digestion with culture medium containing FBS and were centrifuged in a 1.5 ml centrifuge tube at 300 g for 5 min, and then to resuscitate the cells. Using the cell counting board, 3 × 104 cells were planted in the upper chamber of 24-well transwells, and the lower chamber contained media with 10% FBS. Continue to incubate the chamber at in a moist atmosphere at 37 °C and 5 % CO2. After 12 hours, the cells were stained using crystal violet for 30 minutes. The cells were pictured and counted under microscope.
2.7. Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from the cultured cells and tissue using TRIzol (Invitrogen, Carlsbad, CA, USA). The PrimeScript™RT Master Mix (Takara, Kyoto, Japan)–which reversed 1 μg total RNA extracted to cDNA–was used for mRNA detection and SYBR Premix Ex TaqTM (Yeasen, Shanghai, China) was used for analysis. The expression of relative mRNA levels by GAPDH was determined using the standard curve method (2−ΔΔCT) (Table 1).
Table 1.
Primer Sequences Used in This Study
| Gene | Primer | Synthesized sequences (5' -> 3′) |
|---|---|---|
| Human genes | ||
| GAPDH | Forward | 5′-AAGAAGGTGGTGAAGCAGGC-3′ |
| Reverse | 5′-TCCACCACCCAGTTGCTGTA-3′ | |
| Calponin | Forward | 5′-CCAACGACCTGTTTGAGAACACC-3′ |
| Reverse | 5′-ATTTCCGCTCCTGCTTCTCTGC-3′ | |
| SM-MHC | Forward | 5′-CGCCAAGAGACTCGTCTGG-3′ |
| Reverse | 5′-TCTTTCCCAACCGTGACCTTC-3′ | |
| α-SMA | Forward | 5′-GTGTTGCCCCTGAAGAGCAT-3′ |
| Reverse | 5′-GCTGGGACATTGAAAGTCTCA-3 | |
| TNFα | Forward | 5′-TGACAAGCCTGTAGCCCATGTT-3′ |
| Reverse | 5′-AGGGCAATGATCCCAAAGTAGA-3′ | |
| IL-6 | Forward | 5′-AGGGCTCTTCGGCAAATG-3′ |
| Reverse | 5′-GAAGGAATGCCCATTAACAACAA-3′ | |
| IL-8 | Forward | 5′-CACCGGAAGGAACCATCT-3′ |
| Reverse | 5′-AGAGCCACGGCCAGCTT-3′ | |
| IL-1β | Forward Reverse |
5′-GCCAGTGAAATGATGGCTTATT-3′ 5′-AGGAGCACTTCATCTGTTTAGG-3′ |
| Rat genes | ||
| GAPDH | Forward | 5′-CAAGGCTGTGGGCAAGGTCATC-3′ |
| Reverse | 5′-GTGTCGCTGTTGAAGTCAGAGGAG-3′ | |
| Calponin | Forward | 5′-AAGAGAAGGGCGGAACATCATTGG-3′ |
| Reverse | 5′-ACTCGGGCGTCAGGCAGTAC-3′ | |
| SM-MHC | Forward | 5′-GCAGCCAGCATTAAGGAGGAGAAG-3′ |
| Reverse | 5′-GTTGACCACCACGCAGAAGAGG-3′ | |
| α-SMA | Forward | 5′-AGAACACGGCATCATCACCAACTG-3′ |
| Reverse | 5′-AGTCACACCATCTCCAGAGTCCAG-3′ | |
2.8. Western blot
Cell samples and tissue were acquired from rat treated with RIPA buffer lysis (Solarbio, Beijing, China). The proteins were then heated in SDS-PAGE loading buffer (Solarbio, Beijing, China) at 98 °C for 10 min, where their equal quantities were isolated using 10% SDS-PAGE by imprinting onto a 0.45-μm polyvinylidene fluoride (PVDF) membrane. The membrane was then sealed with 5% milk in Tris buffer saline containing 0.1% Tween-20 for 1 h. It was incubated with the first antibody overnight at 4 °C. Antibodies included Calponin (24855-1-AP, 1:3000, Proteintech), SM-MHC (ab53219, 1:2000, Abcam, Cambridge, MA, USA), α-SMA (ab5694, 1:2000, Abcam), p-IκBα (abs130622, 1:1000, Absin), p-ERK1/2 (abs 130614, 1:1000, Absin), and NF-κB-p-p65 (#8242, 1:1000, cell signaling), β-actin antibody (1:1000, #4967, Cell Signaling Technology, USA). The secondary antibodies were purchased from Abcam. Enhanced chemiluminescence reagents were used to visualize the immune response band that were imaged by a luminescent image analyzer. The samples were normalized to β-actin using Image J software.
2.9. Experiment protocol and rat carotid artery balloon injury model
Male Sprague-Dawley rats (280 g) were randomly divided into four groups (n = 5 per group): control group, injury group, and two Rg2 treatment groups (low and high dose). The two Rg2 groups were treated with 8 mg/kg/d and 40 mg/kg/d Rg2 for 14 d. First, a carotid balloon injury model in rats was constructed using a balloon catheter (Mini TREK, Abbott Laboratories, Chicago, IL, USA) inserted into the common carotid artery through the left external carotid artery, and was swollen five times with 0.9% saline. The common carotid artery was ligated after pulling out the catheter. Post-surgery, rats in all the groups were administered carboxyl methyl cellulose sodium (CMC-Na) and Rg2 by gavage for two weeks. The day after the last administration, an intraperitoneal injection of 10% chloral hydrate was administered and the blood vessels of the left common artery were extracted for morphological examination and subsequent experiments–frozen section staining, Western blot analysis, and total RNA.
2.10. Sectioning and hematoxylin and eosin (H&E) staining
A continuous cross section of the carotid artery was obtained from the injured area, and sections were collected from each vessel for morphological analysis. The H&E staining was carried out according to the instructions of H&E staining kit (Meilunbio, Dalian, China), and the images were taken by Nikon Ti–S inverted phase contrast microscope (Olympus,Tokyo,Japan), followed by ruler addition by Image J software.
2.11. Statistical analysis
The data are presented as mean and standard deviation. The GraphPad Prism 8 software was used for statistical analysis of t-test and one-way ANOVA test (for comparison between three or more groups) or analysis according to the specific situation. The value of P < 0.05 was statistically significant. Each experiment was repeated at least three times.
3. Results
3.1. Effects of Rg2 on the viability, anti-proliferation and anti-migration of HUVECs and VSMCs
To determine the Rg2 toxicity toward HUVECs and VSMCs, and the optimal concentration needed in the follow-up experiments, the MTT detection was used to set Rg2 concentrations at 0, 10, 20, 30 and 40 μM for 24 h with HUVECs and VSMCs. After treating the cells with Rg2, at 10 μM concentration the cell activity did not decrease, but at 20 μM, 30 μM, and 40 μM, it decreased by 8%, 14%, and 30%, respectively. Therefore, the concentration of Rg2 was determined at 10 and 20 μM (Fig. 1A, left panel). In addition, the effect of Rg2 on the viability of VSMCs was tested using MTT method as well. The 10 μM and 20 μM concentrations of Rg2 were used for subsequent experiments during to their low cytotoxicity (Fig. 1A, right panel). Excessive proliferation and migration of ECs and VSMCs can lead to subsequent vascular remodeling, which leads to various pathological conditions, including endothelial-mesenchymal transition (EndMT) [[26], [27]]. Atherosclerosis development is promoted by EndMT–including intimal hyperplasia, plaque formation and vascular stenosis. Here PDGF-BB was used to treat HUVECs and VSMCs as it was reported to induce cell proliferation and migration, respectively [28]. The CCK8 method was used to detect Rg2 after 12 h of pre-processing with 10 and 20 μM concentrations, followed by 12 h exposure to PDGF-BB and testing by enzyme labeling. As the proliferation ability of Rg2 treated vessel cells decreased significantly, this inferred that Rg2 also inhibited cell proliferation in a concentration-dependent manner (Fig. 1B). To further prove the effect of Rg2 on the migration ability of VSMCs, scratch wound healing experiments was performed. The wound healing increased in cells after their first exposure to Rg2 (10 μM and 20 μM) for 12 h and PDGF-BB after 12 h of re-stimulation, while Rg2 reduced PDGF-BB induced migration (Fig. 1C and D). Moreover, the inhibitory effect of Rg2 on HUVECs and VSMCs migration were tested by transwell chamber method. Under the induction of PDGF-BB pretreated with 10 μM and 20 μM Rg2 for 12 h, the migration of HUVECs was observed to be less than that of the group without Rg2 pretreatment, which again revealed the inhibitory effect of Rg2 on endothelial cells migration and its potential inhibitory effect on atherosclerotic plaque formation (Fig. 1E and F). Also, the transwell assay was performed to confirm suppression of VSMCs migration which showed consistent results (Fig. 1G and H).
Fig. 1.
Effects of Rg2 on the viability, anti-proliferation and anti-migration of HUVECs and VSMCs. (A) The effect of Rg2 on the viability of HUVECs (left panel) and VSMCs (right panel) by MTT. (B) The effect of Rg2 on the proliferation of HUVECs (left panel) and VSMCs (right panel) induced by PDGF-BB was studied by CCK8. (C) The migration of the wound scratch. (D) The quantification of the migration of the wound scratch. (E) The result of transwell assay in HUVECs. (F) The quantification of transwell assay in HUVECs. (G) The result of transwell assay in VSMCs. (H) The quantification of transwell assay in VSMCs. The value of P < 0.05 between different groups in each experiment indicated that there was a significant difference. #, is compared with the control group, #P < 0.05 and ##P < 0.01; ∗, is compared with the PDGF-BB group, ∗P < 0.05, ∗∗∗P < 0.001 and ∗∗∗∗P < 0.0001.
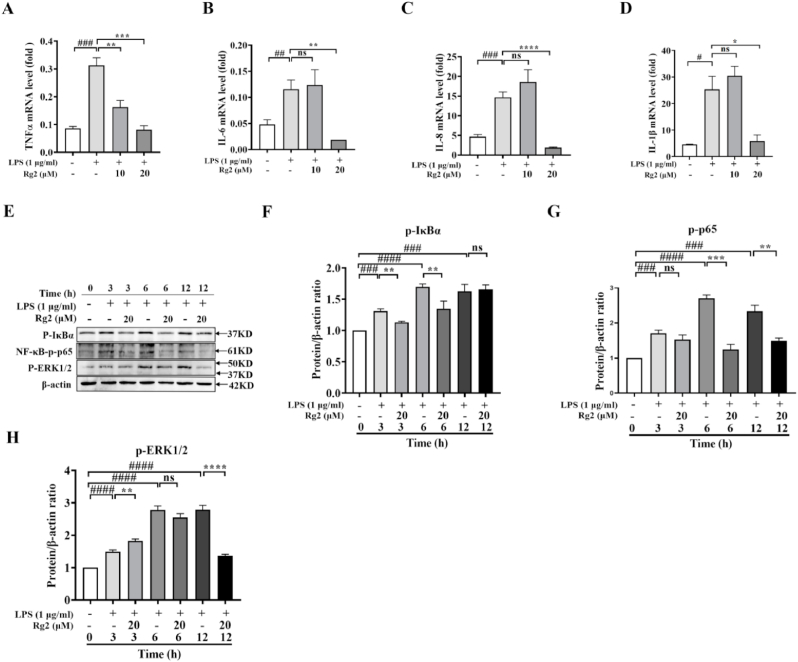
3.2. Rg2 attenuated LPS-induced HUVECs inflammation via NF-κB pathway and ERK1/2 phosphorylation
Inflammation is important in promoting the development of atherosclerosis, and ECs are also affected by inflammatory factors. This study found that TNFα, IL-6, IL-8 and IL-1β can be regulated by Rg2. To detect the expression of these key inflammatory factors at mRNA level at 10 μM and 20 μM Rg2 concentrations, LPS (1 μg/mL) was used to stimulate the HUVECs. The results showed that Rg2 decreased the expression of TNFα, IL-6 and IL-8 at mRNA level. Inhibition of TNFα was done by pretreatment under Rg2 concentrations both at 10 μM and 20 μM (Fig. 2A), while IL-6 (Fig. 2B), IL-8 (Fig. 2C) and IL-1β (Fig. 2D) were significantly inhibited at 20 μM concentration.
Fig. 2.
Rg2 attenuated LPS-induced HUVECs inflammation via NF-κB pathway and ERK1/2 phosphorylation. (A) The relative mRNA expression of TNFα. (B) The relative mRNA expression of IL-6. (C) The relative mRNA expression of IL-8. (D) The relative mRNA expression of IL-1β. (E) The protein expression of NF-κB p-p65, p-IκBα and p-ERK1/2. (F)The standard quantitative data of p-IκBα relative to β-actin. (G) The standard quantitative data of NF-κB p-p65 relative to β-actin. (H) The standard quantitative data of p-ERK1/2 relative to β-actin. #, is compared with the control group, ##P < 0.01, ###P < 0.001, and ####P < 0.0001; ∗, is compared with the LPS group, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001.
The phosphorylation of nuclear factor-κB-p65 (NF-κB-p65) subunit is essential for its transcriptional activity, so the protein expression levels of p-p65 and the protein expression level of p-IκBα were detected. After pretreating with 20 μM Rg2 for 1 h followed by induction with 1 μg/mL LPS, the results showed that the expression level of p-p65 treated with LPS alone was significantly higher than that of the control group at 3 h, 6 h, and 12 h after treatment, while the expression level of p-p65 was significantly decreased after LPS pretreatment by Rg2 at 6 h and 12 h (Fig. 2E and F). Moreover, the expression level of phosphorylated-IkBα increased remarkably after advance stimulation with LPS for 1 h. The expression level of p-IKBα at 3 h and 6 h after Rg2 treatment was decreased significantly (Fig. 2E and G), thus reversing the effect of phosphorylation.
Moreover, as the MAPK signaling pathway is important in inflammatory response, the expression levels of p38, c-Jun N-terminal kinase (JNK), and extracellular regulated protein kinases1/2 (ERK1/2) were detected. Interestingly, Rg2 had regulatory effect on ERK1/2, but no significant effect on p38 and JNK, thus the data did not show the expression of p38 and JNK. In addition, Rg2 could reduce the protein expression of p-ERK1/2 induced by LPS at 12 h (Fig. 2E and H) after treatment. Above all, the data showed that Rg2 can inhibit the occurrence of inflammatory events in HUVECs.
3.3. Rg2 reversed the phenotypic transformation in VSMC
Phenotypic transformation of VSMCs plays an important role in the pathogenesis of AS. The phenotypic transformation of VSMCs–from contraction to synthesis–can be induced by PDGF-BB–that induces differentiation to dedifferentiation. The VSMCs were first pretreated with various concentrations of Rg2, followed by an addition of 20 ng/mL PDGF-BB. The data showed that Rg2 can reverse the expression of markers related to phenotypic transformation of VSMCs induced by PDGF-BB. The expression of SM-MHC at mRNA level was reversed by Rg2 (Fig. 3A). Surprisingly, ɑ-SMA can significantly improve the levels of mRNA (Fig. 3B). The expression of calponin was also improved at transcription level (Fig. 3C). Regretly, the expression of these contractile markers was examined at protein level, whereas there was no significant recovery of SM-MHC observed after Rg2 treatment (Fig. 3D and E). Interestingly, ɑ-SMA was expressed at protein level in the convalescent stage after Rg2 treatment (Fig. 3D and F). Calponin also recovered to a certain extent at protein level (Fig. 3D and G), but the effect was not as strong as that of Rg2 on the regulation of protein ɑ-SMA.
Fig. 3.
Rg2 reversed the phenotypic transformation in VSMCs. (A) The relative mRNA expression of SM-MHC. (B) The relative mRNA expression of α-SMA. (C) The relative mRNA expression of calponin. (D)The protein expression of SM-MHC, α-SMA and calponin. (E)The standard quantitative data of SM-MHC relative to β-actin. (F) The standard quantitative data of α-SMA relative to β-actin. (G) The standard quantitative data of calponin relative to β-actin. #, is compared with the control group, #P < 0.05, ##P < 0.01, ###P < 0.001, and ####P < 0.0001; ∗, is compared with the PDGF-BB group, ∗∗P < 0.01 and ∗∗∗P < 0.001.
3.4. Rg2 repressed intimal hyperplasia and regulated inflammation via NF-κB and p-ERK signaling, and Rg2 suppressed VSMC phenotype transformation in vivo
In order to observe the effect of Rg2 on intimal formation and phenotypic transformation of VSMCs in vivo, a carotid balloon injury model was established in rats. Twenty-five male SD rats were divided into five groups: control group, injury group, low-concentration treatment group after injury (8 mg/kg/d), high-concentration treatment group after injury (40 mg/kg/d) and the Ator treatment group (4 mg/kg/d) (the positive drug). Thereafter, the control group and injury group were fed normally, while for the three treatment groups, Rg2 and Ator were dissolved in CMC-Na for 14 d, and the rats were anesthetized with chloral hydrate on the 14th d. The blood vessels of the left carotid artery of rats were removed and preserved in O·C.T. Compound for frozen section to observe the injury and treatment morphology of rats by HE staining. The remaining tissue was stored under −80 °C for extracting tissue RNA and protein for detecting the intervention effect of Rg2 on inflammatory events, and its ability to regulate phenotypic transformation. As shown in HE staining, it was observed that the proliferation, migration and intimal hyperplasia were strongly reversed after Rg2 treatment compared with injury group (Fig. 4A and Fig. 4B). In addition, the NF-κB-IκBɑ-p65 signal pathway (Fig. 4C–E) was detected which can reduce intimal inflammation in rats treated with Rg2. The MAPK signal pathway was also detected, however, at present, it was only found that Rg2 can inhibit the phosphorylation of ERK1/2 in vivo (Fig. 4C and F). Furthermore, ability of Rg2 in regulating the phenotypic transformation in rats was examined, and Ator was used as a positive control drug. After treatment with Rg2 and Ator, the expression of α-SMA (Fig. 4G), calponin (Fig. 4H) and SM-MHC (Fig. 4I) were all clearly reversed at mRNA level, especially at the high concentration treatment group. In addition, the tissue protein was extracted and its contractile protein was expressed. The protein expression of SM-MHC decreased significantly after injury, and increased after treatment with different concentrations of Rg2 (Fig. 4J and K). The protein expression of α-SMA in vivo greatly increased to the normal level after treatment (Fig. 4J and L). Moreover, the tissue protein level of calponin also decreased after injury, and its expression increased after exposure to Rg2 at 40 mg/kg/d (Fig. 4J and M).
Fig. 4.
Rg2 repressed intimal hyperplasia and regulated inflammation via NF-κB and p-ERK signaling, and Rg2 suppressed VSMC phenotype transformation in vivo.
4. Discussion
Unhealthy living habits are one of the leading causes of cardiovascular diseases [[29], [30], [31], [32], [33], [34], [35], [36]], while physical activity and a balanced diet can reduce their risk [[37], [38]]. Dietary fiber as supplements can be used to increase statins and lipid-lowering drugs for reducing lipoprotein and improving the side effects of statins [39]. Ginseng is now more widely used in dietary supplements, including ginseng candy containing vitamin C and ginseng white chocolate. The extract of Panax quinquefolium has a certain effect on glucose-induced oxidative stress and endothelial cell damage [40]. The detection of the compound or the certain element conferring the protection in this treatment is still lacking. Thus, in order to show that a substance has a functional therapeutic effect on atherosclerosis, its effect on the occurrence and development of the disease should be studied [3].
Normally, ECs are located in the innermost layer of the blood vessel wall, which is in a static state separates blood from tissue. Normal vascular endothelial cells can sense hemodynamic changes, and vascular endothelium-derived relaxing and contractile factors are beneficial to maintain the vasodilation function. After vascular endothelial dysfunction, such as hypoxia or injury, ECs get activated and cause angiogenesis–they further over-proliferate, migrate, and their permeability gets enhanced. Many growth factors, chemokines, and others also promote endothelial inflammation, and eventually lead to arterial stenosis [[41], [42], [43]]. As previously reported, Rg2 is shown to reduce the expression of adhesion molecules, including ICAM-1 and VCAM-1, to reduce vascular inflammation [44]. In addition, the latest research shows that the combination of Rh1 and Rg2 can protect liver function by inhibiting TAK1 and STAT3-mediated inflammatory activity and Nrf2/ARE-mediated antioxidant signaling pathway [45]. Therefore, this study is interested in detecting the expression of inflammatory factors in LPS-induced ECs. Surprisingly, Rg2 can significantly inhibit the expression of TNFα, IL-6, IL-8 and IL-1β through NF-κB and MAPK signaling pathways induced by LPS. Moreover, among the MAPK signaling pathways it was detected that Rg2 can only inhibit the phosphorylation of ERK1/2 partially. Therefore, the subsequent studies can focus on target inhibition by Rg2 in the MAPK signal pathway to reduce inflammation and more strongly support the results of this study.
The VSMCs located in media are important in the occurrence and development of AS [[46], [47]] During tissue injury and endothelial dysfunction, reactive oxygen species, inflammatory factors and growth factors are released, which lead to the proliferation, migration and phenotypic transformation of VSMCs–thus aggravating plaque formation [48]. Located in media of blood vessels and surrounded by basement membrane, the VSMCs on stimulation undergo extracellular proteolysis, that aids in their proliferation and migration–thus changing their phenotype from contractile to synthetic type. The expression of VSMCs-related contractile markers–such as α-SMA, SM-MHC–decreased, and the synthesis of extracellular matrix increased. These VSMCs accumulate and migrate to intimal lesions induced by PDGF, basic fibroblast growth factor (bFGF)and transforming growth factor-β (TGF-β) [46]. Ginsenoside Rg1 and ginsenoside Re could suppress the proliferation of VSMCs induced by PDGF-BB [[49], [50]]. Total ginsenosides may reduce neointimal hyperplasia of carotid artery after balloon injury through antioxidation and inhibition of VSMCs proliferation by NO/cGMP [51]. Moreover, compound K, a metabolite of ginsenoside, can not only inhibit the proliferation and migration of VSMCs induced by PDGF-BB, but also reduces the intimal hyperplasia after balloon injury in rats. These reports suggest the potential therapeutic effect of ginseng soap on atherosclerosis and also its development as a clinical drug for the treatment of restenosis. The data from this study showed that Rg2 inhibits PDGF-BB-induced proliferation and migration, and it increases the expression of contractile markers–especially α-SMA–induced by PDGF-BB at mRNA and protein levels. These results showed that Rg2 can alleviate the progress of atherosclerosis by regulating the proliferation, migration and phenotypic transformation of VSMCs. This suggests that Rg2 can also have potential benefits for atherosclerotic coronary heart disease, stroke, and myocardial infarction.
In addition, Ator is a commonly used clinical drug for the treatment of chronic inflammatory diseases such as atherosclerosis, and several studies have shown that Ator can reduce intimal hyperplasia and phenotypic transformation after carotid balloon injury in animal [[52], [53], [54], [55]].This study used balloons to injure the carotid arteries of rats. Therefore, we used Ator as the positive control drug in animal experiment. On treating rats with different concentrations of Rg2 and Ator, neointimal hyperplasia was reduced–as observed by HE staining. NF-κB signal pathway and part of MAPK signal pathway were inhibited–which regulate the inflammatory response after carotid artery injury. At the same time, the effect of Rg2 on the phenotypic transformation of VSMCs in vivo was examined, and it was observed that Rg2 can also regulate the expression of contractile markers at mRNA and protein levels. The in vivo therapeutic effect of α-SMA is of significance, as it suggests that the VSMCs-related diseases caused by abnormal α-SMA can be improved by Rg2 treatment. Ginsenoside Rg3 can reduce endothelial dysfunction in ApoE−/− mice and prevent atherosclerosis [56]. Compared with Rg3, Rg2 has better anti-inflammatory effect after the ECs are treated with a lower concentration. Ginsenoside F1 can also reduce the inflammatory response–such as adhesion between endothelial cells and monocytes induced by ox-LDL–through NF-κB signal pathway [57], and it can also inhibit the apoptosis of ECs, which suggests the possible detection of apoptosis inhibition of ECs or VSMCs by Rg2 and further regulation of AS by oxidative stress.
5. Conclusions
In summary, these results strongly support that Rg2 can inhibit the excessive proliferation and migration of ECs and reduce the inflammatory response induced by LPS through NF-κB pathway and the phosphorylation of ERK1/2 (Fig. 5). Meanwhile, Rg2 can also suppress the proliferation, migration and phenotypic transformation of VSMCs (Fig. 5). More importantly, Rg2 can reduce intimal proliferation and inhibit the phenotypic transition of VSMCs in rats with carotid balloon injury. As Rg2 has low toxicity and can inhibit vascular inflammation in the process of AS, it is suggested that the development of natural products as functional health food is a good strategy for the treatment of chronic diseases and provides a new way for the treatment of chronic inflammatory diseases.
Fig. 5.
Summary of anti-atherosclerotic function by Rg2. Rg2 exerts its anti-atherosclerotic roles via regulating biological functions of endothelial cells and vascular smooth muscle cells, which provides a more sufficient basis for ginseng as a functional dietary regulator.
Author Contributions
T.Y. and Y. Y. designed the framework for this study; Q.X., and L.Z. carried out the cell and protein experiment, X.L., Y.L. and M.L. carried out animal experiments, Q.X., Z.W., and X.F. participated in the data analysis and drafted the manuscript, T.Y., Y.Y. and J.Y.C. conceived of and designed the study, and participated in the data analysis and coordination, and helped to revise the manuscript. All authors read and approved the final manuscript.
Declaration of competing interest
The authors have declared that no competing interest exists.
The carotid artery of rats was injured by balloon, and the results were as follow. (A) The H&E staining of carotid artery in rat, which grouped as control, injury, injury + Rg2 (8 mg/kg/d), injury + Rg2 (40 mg/kg/d) and injury + Ator (4 mg/kg/d), black arrow represents proliferation, green arrow represents migration and the blue box shows the intimal hyperplasia. (B) The quantification of intimal/media. (C) The protein expression of NF-κB p-p65, p-IκBα and p-ERK1/2 in vivo. (D) The standard quantitative data of p-IκBα relative to β-actin. (E) The standard quantitative data of NF-κB p-p65 relative to β-actin. (F) The standard quantitative data of p-ERK1/2 relative to β-actin. (G) The relative mRNA expression of α-SMA calponin. (H) The relative mRNA expression of calponin. (I) The relative mRNA expression of SM-MHC. (J) The protein expression of SM-MHC, α-SMA and calponin induced by balloon injury in rat. (K) The standard quantitative data of SM-MHC relative to β-actin. (L) The standard quantitative data of α-SMA relative to β-actin. (M) The standard quantitative data of calponin relative to β-actin. Note: the value is expressed as the mean + standard deviation obtained from 5 rats. # is compared with the control group, #P < 0.05, ##P < 0.01, ###P < 0.001, and ####P < 0.0001; ∗, is compared with the treatment group, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001.
Acknowledgements
This work was supported by The National Natural Science Foundation of China (grant no. 81870331), China, and The Qingdao municipal science and technology bureau project (grant no. 21-1-4-rkjk-12-nsh), China.
Contributor Information
Qianqian Xue, Email: 1406007461@qq.com.
Tao Yu, Email: yutao0112@qdu.edu.cn.
Zhibin Wang, Email: m17853291291@163.com.
Xiuxiu Fu, Email: fuxiuxiu999@163.com.
Xiaoxin Li, Email: xiaoxindoc@163.com.
Lu Zou, Email: fennellime@qq.com.
Min Li, Email: mlee0317@163.com.
Jae Youl Cho, Email: jaecho@skku.edu.
Yanyan Yang, Email: yangyy1201@qdu.edu.cn.
References
- 1.Song P., Fang Z., Wang H., Cai Y., Rahimi K., Zhu Y., et al. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: a systematic review, meta-analysis, and modelling study. Lancet Glob Health. 2020;8:e721–e729. doi: 10.1016/S2214-109X(20)30117-0. [DOI] [PubMed] [Google Scholar]
- 2.Orrapin S., Rerkasem K. Carotid endarterectomy for symptomatic carotid stenosis. Cochrane Database Syst Rev. 2017;6:CD001081. doi: 10.1002/14651858.CD001081.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moss J.W., Ramji D.P. Nutraceutical therapies for atherosclerosis. Nat Rev Cardiol. 2016;13:513–532. doi: 10.1038/nrcardio.2016.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li D., Yang Y., Wang S., He X., Liu M., Bai B., et al. Role of acetylation in doxorubicin-induced cardiotoxicity. Redox Biol. 2021;46 doi: 10.1016/j.redox.2021.102089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu X., Zong T., Yang P., Li L., Wang S., Wang Z., et al. Nicotine: Regulatory roles and mechanisms in atherosclerosis progression. Food Chem Toxicol. 2021;151 doi: 10.1016/j.fct.2021.112154. [DOI] [PubMed] [Google Scholar]
- 6.Yang X., Yang Y., Guo J., Meng Y., Li M., Yang P., et al. Targeting the epigenome in in-stent restenosis: from mechanisms to therapy. Mol Ther Nucleic Acids. 2021;23:1136–1160. doi: 10.1016/j.omtn.2021.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang N., Jiang S., Yang Y., Liu S., Ponnusamy M., Xin H., et al. Noncoding RNAs as therapeutic targets in atherosclerosis with diabetes mellitus. Cardiovasc Ther. 2018;36 doi: 10.1111/1755-5922.12436. [DOI] [PubMed] [Google Scholar]
- 8.Zou Y., Yang Y., Fu X., He X., Liu M., Zong T., et al. The regulatory roles of aminoacyl-tRNA synthetase in cardiovascular disease. Mol Ther Nucleic Acids. 2021;25:372–387. doi: 10.1016/j.omtn.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y., Yang Y., Ju H., He X., Sun P., Tian Y., et al. Comprehensive profile of circRNAs in formaldehyde induced heart development. Food Chem Toxicol. 2022;162 doi: 10.1016/j.fct.2022.112899. [DOI] [PubMed] [Google Scholar]
- 10.Yang P., Yang Y., He X., Sun P., Zhang Y., Song X., et al. miR-153-3p Targets betaII Spectrin to Regulate Formaldehyde-Induced Cardiomyocyte Apoptosis. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.764831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X., Qi H., Cui W., Wang Z., Fu X., Li T., et al. Recent advances in targeted delivery of non-coding RNA-based therapeutics for atherosclerosis. Mol Ther. 2022 doi: 10.1016/j.ymthe.2022.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu X., Cui Y., Li C., Wang Y., Cheng J., Chen S., et al. SETD3 Downregulation Mediates PTEN Upregulation-Induced Ischemic Neuronal Death Through Suppression of Actin Polymerization and Mitochondrial Function. Mol Neurobiol. 2021;58:4906–4920. doi: 10.1007/s12035-021-02459-x. [DOI] [PubMed] [Google Scholar]
- 13.Cui Y., Zhang Z., Zhou X., Zhao Z., Zhao R., Xu X., et al. Microglia and macrophage exhibit attenuated inflammatory response and ferroptosis resistance after RSL3 stimulation via increasing Nrf2 expression. J Neuroinflammation. 2021;18:249. doi: 10.1186/s12974-021-02231-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashraf J.V., Al Haj Zen A. Role of Vascular Smooth Muscle Cell Phenotype Switching in Arteriogenesis. Int J Mol Sci. 2021;22 doi: 10.3390/ijms221910585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yap C., Mieremet A., de Vries C.J.M., Micha D., de Waard V. Six Shades of Vascular Smooth Muscle Cells Illuminated by KLF4 (Kruppel-Like Factor 4) Arterioscler Thromb Vasc Biol. 2021;41:2693–2707. doi: 10.1161/ATVBAHA.121.316600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baeg I.H., So S.H. The world ginseng market and the ginseng (Korea) J Ginseng Res. 2013;37:1–7. doi: 10.5142/jgr.2013.37.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J., Wang H., Mou X., Luan M., Zhang X., He X., et al. The Advances on the Protective Effects of Ginsenosides on Myocardial Ischemia and Ischemia-Reperfusion Injury. Mini Rev Med Chem. 2020;20:1610–1618. doi: 10.2174/1389557520666200619115444. [DOI] [PubMed] [Google Scholar]
- 18.Li M., Yang Y., Zong J., Wang Z., Jiang S., Fu X., et al. miR-564: A potential regulator of vascular smooth muscle cells and therapeutic target for aortic dissection. J Mol Cell Cardiol. 2022;170:100–114. doi: 10.1016/j.yjmcc.2022.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Zhan Q., Yi K., Qi H., Li S., Li X., Wang Q., et al. Engineering blood exosomes for tumor-targeting efficient gene/chemo combination therapy. Theranostics. 2020;10:7889–7905. doi: 10.7150/thno.45028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biswas T., Mathur A.K., Mathur A. A literature update elucidating production of Panax ginsenosides with a special focus on strategies enriching the anti-neoplastic minor ginsenosides in ginseng preparations. Appl Microbiol Biotechnol. 2017;101:4009–4032. doi: 10.1007/s00253-017-8279-4. [DOI] [PubMed] [Google Scholar]
- 21.Ru W., Wang D., Xu Y., He X., Sun Y.E., Qian L., et al. Chemical constituents and bioactivities of Panax ginseng (C. A. Mey.) Drug Discov Ther. 2015;9:23–32. doi: 10.5582/ddt.2015.01004. [DOI] [PubMed] [Google Scholar]
- 22.Irfan M., Kim M., Rhee M.H. Anti-platelet role of Korean ginseng and ginsenosides in cardiovascular diseases. J Ginseng Res. 2020;44:24–32. doi: 10.1016/j.jgr.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou L., Wang F., Sun R., Chen X., Zhang M., Xu Q., et al. SIRT5 promotes IDH2 desuccinylation and G6PD deglutarylation to enhance cellular antioxidant defense. EMBO Rep. 2016;17:811–822. doi: 10.15252/embr.201541643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao F., Xue Q., Li K., Cao X., Sun L., Liu Y. Phenolic Compounds and Ginsenosides in Ginseng Shoots and Their Antioxidant and Anti-Inflammatory Capacities in LPS-Induced RAW264.7 Mouse Macrophages. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20122951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X., Yang Y., Wang Z., Jiang S., Meng Y., Song X., et al. Targeting non-coding RNAs in unstable atherosclerotic plaques: Mechanism, regulation, possibilities, and limitations. International Journal of Biological Sciences. 2021;17:3413–3427. doi: 10.7150/ijbs.62506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohr T., Haudek-Prinz V., Slany A., Grillari J., Micksche M., Gerner C. Proteome profiling in IL-1beta and VEGF-activated human umbilical vein endothelial cells delineates the interlink between inflammation and angiogenesis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0179065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Souilhol C., Harmsen M.C., Evans P.C., Krenning G. Endothelial-mesenchymal transition in atherosclerosis. Cardiovasc Res. 2018;114:565–577. doi: 10.1093/cvr/cvx253. [DOI] [PubMed] [Google Scholar]
- 28.Son J.E., Jeong H., Kim H., Kim Y.A., Lee E., Lee H.J., et al. Pelargonidin attenuates PDGF-BB-induced aortic smooth muscle cell proliferation and migration by direct inhibition of focal adhesion kinase. Biochem Pharmacol. 2014;89:236–245. doi: 10.1016/j.bcp.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 29.Kohler J., Teupser D., Elsasser A., Weingartner O. Plant sterol enriched functional food and atherosclerosis. Br J Pharmacol. 2017;174:1281–1289. doi: 10.1111/bph.13764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santos H.O., Earnest C.P., Tinsley G.M., Izidoro L.F.M., Macedo R.C.O. Small dense low-density lipoprotein-cholesterol (sdLDL-C): Analysis, effects on cardiovascular endpoints and dietary strategies. Prog Cardiovasc Dis. 2020;63:503–509. doi: 10.1016/j.pcad.2020.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Li M., Yang Y., Wang Z., Zong T., Fu X., Aung L.H.H., et al. Piwi-interacting RNAs (piRNAs) as potential biomarkers and therapeutic targets for cardiovascular diseases. Angiogenesis. 2021;24:19–34. doi: 10.1007/s10456-020-09750-w. [DOI] [PubMed] [Google Scholar]
- 32.Yu T., Wang Z., Jie W., Fu X., Li B., Xu H., et al. The kinase inhibitor BX795 suppresses the inflammatory response via multiple kinases. Biochem Pharmacol. 2020;174 doi: 10.1016/j.bcp.2020.113797. [DOI] [PubMed] [Google Scholar]
- 33.Zong T., Yang Y., Lin X., Jiang S., Zhao H., Liu M., et al. 5′-tiRNA-Cys-GCA regulates VSMC proliferation and phenotypic transition by targeting STAT4 in aortic dissection. Mol Ther Nucleic Acids. 2021 doi: 10.1016/j.omtn.2021.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qi H., Shan P., Wang Y., Li P., Wang K., Yang L. Nanomedicines for the Efficient Treatment of Intracellular Bacteria: The "ART" Principle. Front Chem. 2021;9 doi: 10.3389/fchem.2021.775682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qi H., Wang Y., Fa S., Yuan C., Yang L. Extracellular Vesicles as Natural Delivery Carriers Regulate Oxidative Stress Under Pathological Conditions. Front Bioeng Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.752019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qi H., Yang J., Yu J., Yang L., Shan P., Zhu S., et al. Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes. 2022;11:1511–1524. [Google Scholar]
- 37.Fernstrom M., Fernberg U., Eliason G., Hurtig-Wennlof A. Aerobic fitness is associated with low cardiovascular disease risk: the impact of lifestyle on early risk factors for atherosclerosis in young healthy Swedish individuals - the Lifestyle, Biomarker, and Atherosclerosis study. Vasc Health Risk Manag. 2017;13:91–99. doi: 10.2147/VHRM.S125966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malhotra A., Redberg R.F., Meier P. Saturated fat does not clog the arteries: coronary heart disease is a chronic inflammatory condition, the risk of which can be effectively reduced from healthy lifestyle interventions. Br J Sports Med. 2017;51:1111–1112. doi: 10.1136/bjsports-2016-097285. [DOI] [PubMed] [Google Scholar]
- 39.Soliman G.A. Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients. 2019;11 doi: 10.3390/nu11051155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel S., Rauf A. Adaptogenic herb ginseng (Panax) as medical food: Status quo and future prospects. Biomed Pharmacother. 2017;85:120–127. doi: 10.1016/j.biopha.2016.11.112. [DOI] [PubMed] [Google Scholar]
- 41.Michiels C. Endothelial cell functions. J Cell Physiol. 2003;196:430–443. doi: 10.1002/jcp.10333. [DOI] [PubMed] [Google Scholar]
- 42.He X., Lian Z., Yang Y., Wang Z., Fu X., Liu Y., et al. Long Non-coding RNA PEBP1P2 Suppresses Proliferative VSMCs Phenotypic Switching and Proliferation in Atherosclerosis. Mol Ther Nucleic Acids. 2020;22:84–98. doi: 10.1016/j.omtn.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zong T., Yang Y., Zhao H., Li L., Liu M., Fu X., et al. tsRNAs: Novel small molecules from cell function and regulatory mechanism to therapeutic targets. Cell Prolif. 2021;54 doi: 10.1111/cpr.12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cho Y.S., Kim C.H., Ha T.S., Lee S.J., Ahn H.Y. Ginsenoside rg2 inhibits lipopolysaccharide-induced adhesion molecule expression in human umbilical vein endothelial cell. Korean J Physiol Pharmacol. 2013;17:133–137. doi: 10.4196/kjpp.2013.17.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen T.L.L., Huynh D.T.N., Jin Y., Jeon H., Heo K.S. Protective effects of ginsenoside-Rg2 and -Rh1 on liver function through inhibiting TAK1 and STAT3-mediated inflammatory activity and Nrf2/ARE-mediated antioxidant signaling pathway. Arch Pharm Res. 2021;44:241–252. doi: 10.1007/s12272-020-01304-4. [DOI] [PubMed] [Google Scholar]
- 46.Grootaert M.O.J., Moulis M., Roth L., Martinet W., Vindis C., Bennett M.R., et al. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc Res. 2018;114:622–634. doi: 10.1093/cvr/cvy007. [DOI] [PubMed] [Google Scholar]
- 47.Yang Y., Li M., Liu Y., Wang Z., Fu X., He X., et al. The lncRNA Punisher Regulates Apoptosis and Mitochondrial Homeostasis of Vascular Smooth Muscle Cells via Targeting miR-664a-5p and OPA1. Oxid Med Cell Longev. 2022;2022 doi: 10.1155/2022/5477024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bennett M.R., Sinha S., Owens G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ Res. 2016;118:692–702. doi: 10.1161/CIRCRESAHA.115.306361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao Y., Deng J., Yu X.F., Yang D.L., Gong Q.H., Huang X.N. Ginsenoside Rg1 inhibits vascular intimal hyperplasia in balloon-injured rat carotid artery by down-regulation of extracellular signal-regulated kinase 2. J Ethnopharmacol. 2011;138:472–478. doi: 10.1016/j.jep.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 50.Watson J., Aarden L.A., Lefkovits I. The purification and quantitation of helper T cell-replacing factors secreted by murine spleen cells activated by concanavalin A. J Immunol. 1979;122:209–215. [PubMed] [Google Scholar]
- 51.Yu X.F., Deng J., Yang D.L., Gao Y., Gong Q.H., Huang X.N. Total Ginsenosides suppress the neointimal hyperplasia of rat carotid artery induced by balloon injury. Vascul Pharmacol. 2011;54:52–57. doi: 10.1016/j.vph.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 52.Colak G., Pougovkina O., Dai L., Tan M., Te Brinke H., Huang H., et al. Proteomic and Biochemical Studies of Lysine Malonylation Suggest Its Malonic Aciduria-associated Regulatory Role in Mitochondrial Function and Fatty Acid Oxidation. Mol Cell Proteomics. 2015;14:3056–3071. doi: 10.1074/mcp.M115.048850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hua J., Xu Y., He Y., Jiang X., Ye W., Pan Z. Wnt4/beta-catenin signaling pathway modulates balloon-injured carotid artery restenosis via disheveled-1. Int J Clin Exp Pathol. 2014;7:8421–8431. [PMC free article] [PubMed] [Google Scholar]
- 54.Tian T., Yu K., Zhang M., Shao X., Chang L., Shi R., et al. Huotan Jiedu Tongluo Decoction Inhibits Balloon-Injury-Induced Carotid Artery Intimal Hyperplasia in the Rat through the PERK-eIF2alpha-ATF4 Pathway and Autophagy Mediation. Evid Based Complement Alternat Med. 2021;2021 doi: 10.1155/2021/5536237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu L., Zhang W., Li H., Chen B.Y., Zhang G.M., Tang Y.H., et al. Inhibition of aortic intimal hyperplasia and cell cycle protein and extracellular matrix protein expressions by BuYang HuanWu Decoction. J Ethnopharmacol. 2009;125:423–435. doi: 10.1016/j.jep.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 56.Geng J., Fu W., Yu X., Lu Z., Liu Y., Sun M., et al. Ginsenoside Rg3 Alleviates ox-LDL Induced Endothelial Dysfunction and Prevents Atherosclerosis in ApoE(-/-) Mice by Regulating PPARgamma/FAK Signaling Pathway. Front Pharmacol. 2020;11:500. doi: 10.3389/fphar.2020.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qin M., Luo Y., Lu S., Sun J., Yang K., Sun G., et al. Ginsenoside F1 Ameliorates Endothelial Cell Inflammatory Injury and Prevents Atherosclerosis in Mice through A20-Mediated Suppression of NF-kB Signaling. Front Pharmacol. 2017;8:953. doi: 10.3389/fphar.2017.00953. [DOI] [PMC free article] [PubMed] [Google Scholar]