Abstract
Background
The beneficial effects of compound K (CK) on different chronic diseases have been shown to be at least related to antioxidant action. Nevertheless, since its antioxidant activity in human retinal pigment epithelial (RPE) cells is still unknown, here we investigated whether CK alleviates oxidative stress-stimulated damage in RPE ARPE-19 cells.
Methods
The cytoprotective consequence of CK in hydrogen peroxide (H2O2)-treated cells was evaluated by cell viability, DNA damage, and apoptosis assays. Fluorescence analysis and immunoblotting were performed to investigate the inhibitory action of CK on reactive oxygen species (ROS) production and mitochondrial dysfunction.
Results
H2O2-promoted cytotoxicity, oxidative stress, DNA damage, mitochondrial impairment, and apoptosis were significantly attenuated by CK in ARPE-19 cells. Furthermore, nuclear factor erythroid 2-related factor 2 (Nrf2) phosphorylation level and its shuttling to the nucleus were increased, which was correlated with upregulated activation of heme oxygenase-1 (HO-1). However, zinc protoporphyrin, a blocker of HO-1, significantly abrogated the preventive action of CK in H2O2-treated ARPE-19 cells.
Conclusion
This study indicates that activation of Nrf2/HO-1 signaling by CK plays an important role in rescuing ARPE-19 cells from oxidative cellular damage.
Keywords: Apoptosis, ARPE-19 cells, Compound K, Nrf2/HO-1, Oxidative stress
Graphical abstract
1. Introduction
Retinal pigment epithelial (RPE) cells consist of a monolayer between the photoreceptors and the choriocapillaris in the retina. They form an outer blood-retinal barrier to protect the retina and are involved in maintaining photoreceptor homeostasis [1]. Therefore, RPE cells function critically in the inhibition of retinal edema and neovascularization [2]. In particular, RPE cells are unprotected to an oxidative environment rich in reactive oxygen species (ROS) due to high oxygen consumption for vision formation [3]. Excessive oxidative stress can eventually induce irreversible degeneration and apoptosis of RPE cells, leading to onset and development of various eye disorders such as age-related macular degeneration (AMD) [2,3]. Meanwhile, various studies have indicated that the removal of ROS could effectively prevent RPE cells from oxidative damage [4]. In this context, it is necessary to prevent RPE cells from oxidative stress for the protection and treatment of ophthalmic diseases.
Ginsenosides, which are secondary metabolites of saponins in plant of the genus Panax (ginseng), are widely applied as therapeutic agents for various diseases [5]. Existing evidences have demonstrated that they have multiple beneficial effects on several chronic diseases [6]. Numerous previous research results have also shown that ginsenosides contains multiple physiological activities such as antioxidant and anti-cancer activities without showing any appreciable toxicity [7]. More importantly, although the accumulation of ROS can act as a main source for induction of cancer cell death by ginsenosides [7,8], their protective effects on cellular injury are associated with potent antioxidant activity [6,9]. Furthermore, it has been reported that ginsenosides can effectively block oxidative stress by acting as potent ROS scavengers through nuclear factor erythroid 2-related factor 2 (Nrf2) activation, a potent mediator of antioxidant signaling. For example, ginsenoside Rg1 antagonized particulate matter-stimulated oxidative condition in umbilical vein endothelial cells while suppressing ROS production, which was correlated the induction of heme oxygenase-1 (HO-1) expression, a representative downstream target of Nrf2 [10]. Their findings are well-agreed with the results of Li et al [11] that Nrf2/HO-1 axis activation was required for the inhibitory action of Rg1 on oxidative injury in cardiomyocytes. Activation of Nrf2 was also associated with the antioxidant potency of Rg1 shown in cisplatin-induced liver and ultraviolet (UV)-irradiated mouse skin injury models [12,13]. In addition, it has been recently suggested that Nrf2 activation by various ginsenosides was playing a direct role in the defense mechanism for oxidative stress-mediated neurodegeneration, hepatic inflammation, and ischemic stroke, and more other dis. [14,15]. Similar to previous results, Yang et al [16] addressed that the ginsenoside compound K (CK) could block neuronal cell death through activation of Nrf2 signaling. Recently, it was reported that topical administration of Rg1 protected the lens from cataracts caused by oxidative stress and improved hyperglycemia and hypoxia-induced RPE cell damage by eliminating ROS production [17,18]. Similarly, Rh3 blocked UV-induced RPE cell injury while inhibiting ROS generation through activation of Nrf2 signaling [19]. Nevertheless, studies on the antioxidant activity of CK in ocular cells such as RPE cells have not yet been reported. Thus, in our study, we investigated the preventive potential of CK for hydrogen peroxide (H2O2)-promoted oxidative damage in human RPE ARPE-19 cells and whether the Nrf2 signaling pathway activation regulates in this process.
2. Materials and methods
2.1. Materials, reagents and chemicals
Dulbecco's modified Eagle's medium/F-12 (DMEM/F12), fetal bovine serum (FBS) and antibiotics were purchased from WELGENE Inc. (Gyeongsan, Republic of Korea). CK, H2O2, zinc protoporphyrin (ZnPP), mitochondrial isolation kit and 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide (JC-1) were procured from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 2,7-dichlorofluorescin diacetate (DCF-DA) and NE-PER™ nuclear and cytoplasmic extraction reagents were obtained from Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Comet assay and annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis kits were procured from Trevigen, Inc. (Gaithersburg, MD, USA) and BD Biosciences (San Diego, CA, USA), respectively. Caspase-3 enzyme-linked immunosorbent assay (ELISA) kit and HO-1 ELISA kit were purchased from R&D Systems, Inc. (Minneapolis, MN, USA) and Abcam, Inc. (Cambridge, MA, USA), respectively. Antibodies were obtained from Thermo Fisher Scientific, Abcam, Inc., Santa Cruz Biotechnology (Santa Cruz, CA, USA) and Cell Signaling Technology (Beverly, MA, USA). All chemicals and reagents not mentioned were of analytical grade.
2.2. Cell culture and cell cytotoxicity assay
ARPE-19 cells (CRL-2302™, ATCC, Manassas, VA, USA) were cultured in DMEM/F12 medium (10% FBS and 100 U/mL penicillin/streptomycin) at 37°C and 5% CO2. To determine cytotoxicity, different concentrations of CK or H2O2 was treated for 24 h, or exposed to CK and/or ZnPP for 1 h prior to H2O2 treatment for 24 h. After the end of treatment, the MTT assay was adopted as described previously [20].
2.3. ROS generation measurement
To measure generated ROS, H2O2 was treated for 1 h or pretreated with CK and/or ZnPP for 1 h to cells before treatment with H2O2. The ROS levels were assessed using flow cytometry (BD Biosciences). In parallel, cells were counterstained with 4′,6-diamidino-2-phenylindol (DAPI), and then imaged under fluorescence microscopy according to the previous report [21].
2.4. Immunoblotting
Cells were stimulated with CK and/or ZnPP for 1 h before stimulation with H2O2 for 24 h. Isolation of total proteins was conducted using a RIPA buffer. Cytosol and mitochondrial portions were isolated by NE-PER™ extraction reagents. Subsequently, the obtained proteins were subject to immunoblotting as described previously [22].
2.5. Immunofluorescence staining for the detection of DNA damage
Expression of phosphorylated form of histone H2AX (p-γH2AX) in the nucleus was observed by immunofluorescence staining as described previously [23]. Finally, using fluorescence microscope, the images were acquired.
2.6. Comet assay
Using a comet assay kit, we determined whether CK could inhibit H2O2-promoted DNA damage. In brief, treated cells were coated with agarose and dispersed on comet slides following the product protocol guideline. After electrophoresis, EtBr-stained images of comet tail formation were taken using fluorescence microscopy [24].
2.7. Flow cytometry for apoptosis
After treatment, annexin V-FITC/PI solution was mixed with cells and then reacted following the product protocols. The proportions of apoptosis-occurred cells were evaluated using flow cytometry to express the proportion of the number of FITC-stained cells to the whole amounts of cells [25].
2.8. Caspase-3 activity assay
To evaluate activity of caspase-3, the amount of p-nitroanilide cleaved from the caspase-3 substrate by caspase-3 was analyzed by microplate reader at the absorbance value of 405 nm as described previously [26]. In detail, cells were lysed using lysis buffer and 200 μg of protein lysate was reacted with caspase-3 substrate. Total concentration of released p-nitroanilide was calculated based on the absorbance measure at 405 nm.
2.9. Mitochondrial membrane potential (MMP) assay
As described previously [23], cells pretreated with H2O2 for 24 h or with CK for 1 h and treated with H2O2 for 24 h were resuspended in JC-1 solution and incubated with periodic shaking. Changes in MMP were observed under a fluorescence microscope.
2.10. HO-1 activity assay
HO-1 activity was assessed following manufacturer's protocol of the activity assay kit. Briefly, the amount of bilirubin produced was calculated after the cell lysate was stirred with a buffer, and HO-1 activity was defined as picomoles bilirubin/mg protein.
2.11. Statistical analysis
The data from three or more independent assays are introduced as means ± standard deviation (SD). Comparative evaluation was conducted by Student's t-test found in GraphPad Prism (Ver 5.0) (GraphPad Software, Inc., San Diego, CA, USA) to determine the meaningful distinct among different experimental groups. A p value of <0.05 was cut-off to propose statistical distinction (∗p ˂ 0.05, ∗∗p ˂ 0.01 and ∗∗∗p ˂ 0.001 vs. control group; #p ˂ 0.05 and ###p ˂ 0.001 vs. H2O2-treated group; $p ˂ 0.05 and $$$p ˂ 0.001 vs. CK + H2O2 treatment group).
3. Results
3.1. CK alleviated H2O2-induced reduction of cell viability
The results of the MTT assay showed that no apparent inhibition of ARPE-19 cell growth was detected in cells stimulated below 10 μM CK, but markedly inhibited under stimulation above 20 μM CK (Fig. 1A). And H2O2 stimulation declined the cell viability with increasing concentrations, and cells stimulated with 500 μM H2O2 showed approximately 60% viability (Fig. 1B). Therefore, the pretreatment concentration of CK was selected to be 10 μM or less to avoid cytotoxicity and the treatment concentration of H2O2 was chosen to be 500 μM. To explore the protective action of CK on the inhibition of cell growth by H2O2, cells were pre-stimulated with CK for 1 h before exposure to H2O2 for 24 h. As demonstrated in Fig. 1C, the decline in cell viability due to H2O2 treatment was significantly inhibited in the presence of CK.
Fig. 1.
Effects of CK on the cell viability and ROS generation in H2O2-treated ARPE-19 cells. (A-C) Cell viability was investigated using the MTT assay. (D-F) ROS levels were detected using DCF-DA. (D) Representative results from flow cytometry analyses are demonstrated. (E) The values from flow cytometry are presented as averages. (F) The level of ROS (green) was detected using fluorescence microscope, and representative images are presented. Nuclei were co-stained with DAPI (blue). (A-C and E) Data are graphically presented as mean ± SD.
3.2. CK reduced H2O2-promoted ROS generation
As shown in Fig. 1D and E, results obtained from flow cytometry indicated that the ROS level was greatly elevated in H2O2-treated cells, and the ROS level in the cells stimulated with CK alone was not notably distinct from those in control cells. In contrast, pre-treating with CK gradually decreased H2O2-induced ROS generation with increasing concentration of CK. The protective activity of CK on ROS accumulation in H2O2-treated cells was also evident in DCF fluorescence images (Fig. 1F), indicating that CK-improved cell viability was associated with its antioxidant effects.
3.3. CK blocked H2O2-promoted DNA damage and apoptosis
To evaluate whether CK could protect DNA damage induced by H2O2, we investigated the level of p-γH2AX. As shown in Fig. 2A, the level of p-γH2AX was greatly escalated in H2O2-treated cells, whereas its level was downregulated to the control condition level in co-stimulated condition with CK. Immunofluorescence staining also revealed similar results that the level of p-γH2AX in the nucleus was markedly upregulated by H2O2 treatment, but was weakened by CK pretreatment (Fig. 2B). In addition, the results of comet assay demonstrated that H2O2 treatment greatly increased DNA tail length, but H2O2-induced comet tail formation in the presence of CK was clearly alleviated (Fig. 2C). To determine whether CK could protect cells from H2O2-induced apoptosis, we performed flow cytometry after staining with annexin V-FITC/PI and found that H2O2 treatment markedly increased the frequency of apoptosis induction, whereas it was clearly restored by CK pretreatment (Fig. 2D and E).
Fig. 2.
Protection of H2O2-induced DNA damage and apoptosis by CK in ARPE-19 cells. (A) The expression of p-γH2AX was assessed by immunoblotting. β-actin was for loading control. (B) Cells were double-stained with p-γH2AX (red) and DAPI (blue), and representative immunofluorescence images are shown. (C) Using comet assay, DNA damage was analyzed. (D and E) Flow cytometry with annexin V/PI staining was conducted. (D) Representative histograms and (E) quantitative analysis are shown.
3.4. CK restored H2O2-promoted mitochondrial dysfunction and caspase-3 activation
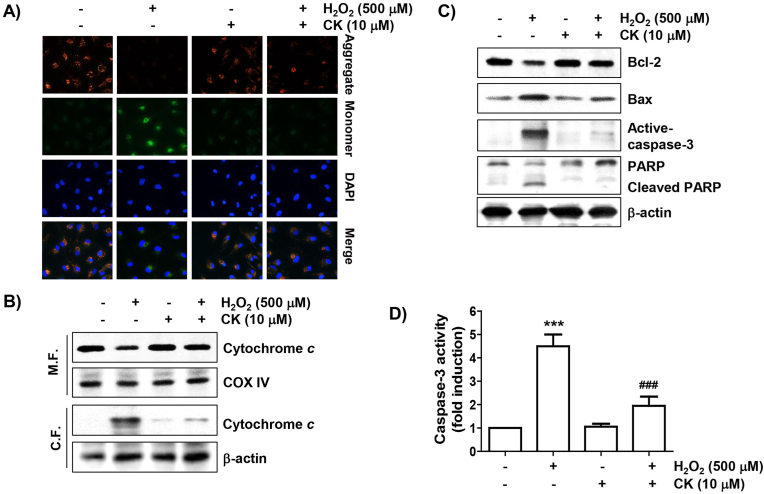
To investigate whether CK could ameliorate H2O2-induced mitochondrial dysfunction, we evaluated the MMP using JC-1. As indicated in Fig. 3A, red fluorescence, an aggregated form of the JC-1 complexes reflecting high levels of MMP in the mitochondrial matrix of healthy cells, was strongly expressed in control cells. In contrast, the intensity of green fluorescence, marking that JC-1 remained in the monomeric form, was increased in H2O2-treated cells. However, the JC-1 aggregate/monomer ratio was reversed with CK pretreatment, meaning that H2O2-induced mitochondrial dysfunction was blocked under co-treatment of CK. In addition, cytochrome c protein level was stronger in the cytosolic fraction of H2O2-stimulated cells than in the mitochondrial portion, suggesting that H2O2 treatment increased the release of cytochrome c into the cytoplasm (Fig. 3B). And, the expression of Bax was greatly increased by H2O2 treatment, while that of Bcl-2 was decreased, which was related to the caspase-3 activation and poly (ADP-ribose) polymerase (PARP) degradation (Fig. 3C and D). However, these alterations were clearly suppressed in the presence of CK (Fig. 4).
Fig. 3.
Attenuation of H2O2-stimulated mitochondrial dysfunction by CK in ARPE-19 cells. (A) MMPs were examined by changes in JC-1-derived fluorescence. The appearance of JC-1 aggregates (red) and monomers (green) in cells was detected using fluorescence microscopy. (B) To investigate the expression of cytochrome c, mitochondrial and cytosol portions were assessed. Cytochrome c oxidase (COX IV) and β-actin were used as control markers for the fractions, respectively. (C) Changes in expression of the indicated proteins were investigated and β-actin was presented as a loading control. (D) The caspase-3 activity was presented as a relative value by comparing the concentration of the released p-nitroanilide with the control.
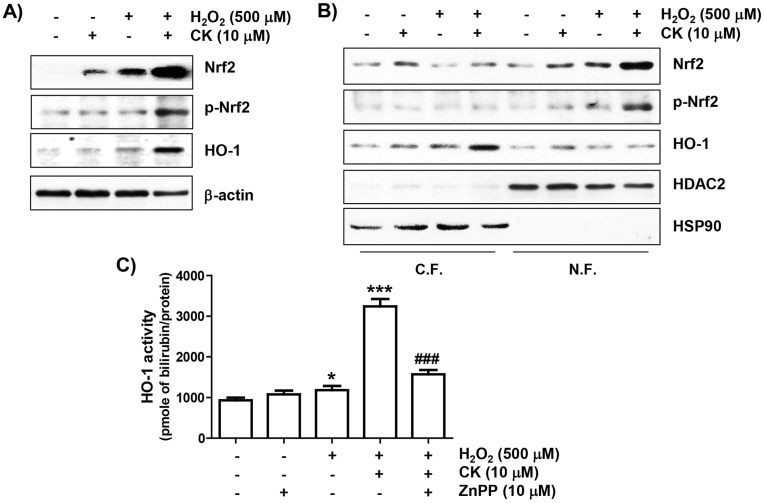
Fig. 4.
Activation of Nrf2/HO-1 signaling by CK in H2O2-stimulated ARPE-19 cells. (A) Total protein was extracted and analyzed using immunoblotting. (B) The cytoplasmic (C.F.) and nuclear (N.F.) fractions were used to evaluate the expression of the presented proteins. Histone deacetylase type 2 (HDAC2) and heat-shock protein 90 (HSP90) were used as markers, respectively. (C) The activity of HO-1 activity was measured by the HO-1 activity assay kit.
3.5. CK promoted the Nrf2/HO-1 signaling activation
We next investigated the relevance of Nrf2 activation in the antioxidant action of CK in H2O2-treated cells. As demonstrated in Fig. 4A, there was a modest upregulation in Nrf2 protein levels in cells with H2O2 treatment and CK alone compared to untreated cells. However, in the H2O2-treated cells with co-stimulation with CK, the expression of Nrf2 as well as its phosphorylated form (p-Nrf2) were greatly upregulated, and their expression was predominantly expressed in the nucleus (Fig. 4B), indicating that Nrf2 was activated and moved to the nucleus. Additionally, the HO-1 expression in the cytoplasm was further upregulated in cells co-stimulated with CK and H2O2. Furthermore, the enzymatic activity of HO-1 was slightly upregulated in cells stimulated with H2O2 alone, but was more activated when pretreated with CK (Fig. 4C). However, its activity was notably suppressed with HO-1 inhibitor ZnPP.
3.6. Suppression of HO-1 activity abrogated the cytoprotective action of CK against H2O2
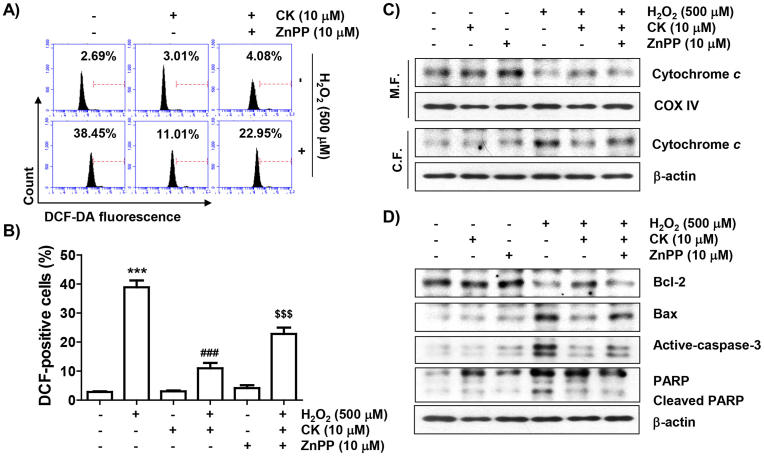
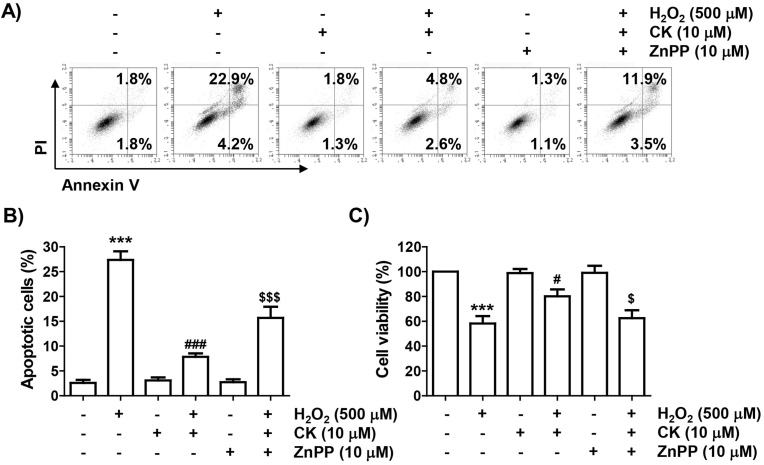
We further evaluated whether the inhibitory activity of CK against H2O2 in ARPE-19 cells was reliant on Nrf2-mediated HO-1 activation. As illustrated in Fig. 5, when given in combination with CK and ZnPP, the suppressive action of CK on H2O2-promoted ROS generation was evidently reversed. Additionally, the blocking effect of CK on the cytosolic release of cytochrome c by H2O2 was excluded by ZnPP, and the inhibitory effect on the change of Bax and Bcl-2, caspase-3 activation and PARP cleavage was also offset. Furthermore, the treatment of ZnPP mostly neutralized the apoptosis rescue effect of CK in H2O2-treated cells, and the inhibitory action of CK on the decrease in cell viability was evidently disappeared (Fig. 6).
Fig. 5.
Effect of ZnPP on the regulation of antioxidant activity and apoptosis factor expression of CK on H2O2 in ARPE-19 cells. (A and B) ROS levels were analyzed using DCF-DA staining. (A) Representative results of flow cytometry are demonstrated. (B) The graph represents the mean with SD. (C) Expression of cytochrome c and (D) indicated proteins was assessed by immunoblotting.
Fig. 6.
Abolition of cytoprotective action of CK against H2O2 by inhibition of HO-1 activity in ARPE-19 cells. Cells exposed to CK and/or ZnPP for 1 h were further stimulated with H2O2 for 24 h, and then flow cytometry with annexin V/PI dual staining was conducted. (A) Representative histograms and (B) quantitative analysis are shown. (C) Cell viability was examined for cells grown under the same conditions.
4. Discussion
Accumulated data through various experimental models demonstrated that CK, a metabolite of ginseng saponin, effectively protected cells from oxidative stress through ROS scavenging. For example, Kang et al [27] reported that CK could contribute to block articular cartilage degradation by blocking H2O2-induced ROS generation in osteoblasts. Li et al [28] also suggested that CK inhibited cardiac ischemia/reperfusion-induced cardiomyocyte apoptosis by blocking ROS accumulation due to mitochondrial damage. Additionally, numbers of previous researches have addressed that inhibition of ROS levels by CK critically function in ameliorating renal damage, ischemic stroke, and atherosclerosis, etc. [29,30]. Nevertheless, the efficacy of CK in protecting RPE cells from oxidative damage has not been studied. In current study, we analyzed the inhibitory action of CK on cellular damage based on antioxidant activity using the well-established H2O2-treated oxidative stress-mimicking ARPE-19 cell model.
Abnormal ROS overproduction in RPE cells due to reduced free radical scavenging ability and disruption of the redox balance could result in DNA disruption and apoptosis activation [4]. In accordance to the flow cytometry results and fluorescence image analysis by DCF-DA staining, CK significantly repressed ROS generation H2O2-treated ARPE-19 cells at concentrations in a non-cytotoxic range, which was associated with improved cell survival. These results were consistent with the findings of past studies using various cell types [[27], [28], [29], [30]], suggesting that CK may act as an oxidative stress inhibitor. Also, in agreement with a previous study [31] that CK was able to protect keratinocytes from apoptosis by reducing UV-specific DNA lesions, CK clearly attenuated H2O2-stimulated DNA damage and apoptosis. These data demonstrate that the blocking action of CK on H2O2-promoted DNA damage and apoptosis in ARPE-19 cells is directly related to blocking the production of ROS.
Oxidative stress-stimulated cell death in ARPE-19 cells is directly correlated with a deficit in mitochondrial function [32]. Of the two apoptosis pathways, the regulation of mitochondria-mediated-apoptosis, which is classified as an inherent apoptosis signaling pathway, is highly reliant on the activity of Bcl-2 family proteins [33]. Among them, when the expression of apoptosis-promoting protein such as Bax is increased, it is translocated to the mitochondria, unlike apoptosis-inhibiting protein including Bcl-2. In the outer mitochondrial membrane, Bax forms pores, which induces the breakdown of MMPs and promotes cytochrome c release into cytosol. Cytochrome c triggers the initiation of a caspase cascade in the cytoplasm, eventually terminating apoptosis [33]. In our study, the association between Bax/Bcl-2 ratio, expressed as increased Bax and decreased Bcl-2 levels, was reversed in H2O2-stimulated ARPE-19 cells compared to the results in untreated cells. Additionally, an increase in the cytochrome c expression in the cytoplasm of H2O2-stimulated cells and a concomitant loss of MMP were observed. However, all these changes were significantly offset by CK administration, indicating that CK prevented H2O2-induced mitochondrial damage through blockade of ROS production.
Recently, many previous studies have suggested that Nrf2 is an effective target for regulating oxidative stress-related retinal degeneration. For example, AMD-like retinal alterations have been shown to occur in Nrf2-deficient mice, indicating that negative modulation of Nrf2 is implied in the initiation and progression of maculopathy [34]. Furthermore, it was found that Nrf2-mediated activation of HO-1 critically functions in ameliorating maculopathy by preventing oxidative damage in RPE cells [32,34]. As is well known, Nrf2 is normally segregated by physically interacting to Kelch-like ECH-associated protein 1 (Keap1), a blocker of Nrf2, in the cytoplasm under no oxidative stress condition. However, upon oxidative stimulation or in the presence of Nrf2-stimulating factors, Nrf2 is dissociated from Keap1. Thereafter, Nrf2 must be phosphorylated in order to initiate the transcriptional activity of phase 2 detoxifying enzymes, including HO-1, in the nucleus [35]. In this regard, we investigated the function of Nrf2 in the antioxidant activity of CK and recognized that CK enhanced the expression and phosphorylation of Nrf2 in the nuclei of H2O2-stimulated ARPE-19 cells. Furthermore, CK upregulated HO-1 expression in the cytoplasm and also significantly increased its enzymatic activity, indicating that CK activated Nrf2-regulated HO-1 antioxidant signaling. However, when the activity of HO-1 was blocked with ZnPP, the ROS scavenging activity of CK was significantly canceled. Moreover, the blocking action of CK on the expression modification of the apoptosis regulatory proteins by H2O2 was abolished in the presence of ZnPP, and ultimately, the ability to improve viability along with the apoptosis inhibitory effect of CK was lost. These outcomes suggest that that the enhanced HO-1 activity contributed to the mitigating action of CK on oxidative stress-induced cellular disruption. In addition, our results support well with previous findings suggesting that the risk of ocular diseases associated with oxidative stress-induced retinal cell damage can be modulated by activating HO-1 [11,35]. Therefore, these findings indicate that CK can prevent ARPE-19 cells from H2O2-stimulated oxidative injury, at least by Nrf2-mediated HO-1 activation. However, further studies, including the role of intracellular signaling pathways involved in the phosphorylation of Nrf2 by CK, and its efficacy in in vivo animal models, need to be performed.
Collectively, in current study, we showed that CK attenuated DNA damage, mitochondrial impairment, and apoptosis while blocking ROS production in H2O2-stimulated RPE ARPE-19 cells. CK also markedly augmented the HO-1 expression and its enzymatic activity, along with phosphorylation and translocation of Nrf2 to the nucleus. However, these beneficial effects of CK were abolished when the activity of HO-1 was blocked. Taken together, our results provide evidence that CK as an ROS scavenger can rescue RPE cells from oxidative damage via Nrf2/HO-1 axis activation and potentially prevent retinal damage initiated by oxidative stress.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was funded by the National Research Foundation of Korea (NRF) grant funded by the Korea government (2021R1A2C200954911).
References
- 1.Ao J., Wood J.P., Chidlow G., Gillies M.C., Casson R.J. Retinal pigment epithelium in the pathogenesis of age-related macular degeneration and photobiomodulation as a potential therapy? Clin Exp Ophthalmol. 2018;46:670–686. doi: 10.1111/ceo.13121. [DOI] [PubMed] [Google Scholar]
- 2.Sadda S.R., Guymer R., Monés J.M., Tufail A., Jaffe G.J. Anti-vascular endothelial growth factor use and atrophy in neovascular age-related macular degeneration: systematic literature review and expert Opinion. Ophthalmology. 2020;127:648–659. doi: 10.1016/j.ophtha.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Eells J.T. Mitochondrial dysfunction in the aging retina. Biology (Basel) 2019;8:31. doi: 10.3390/biology8020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brook N., Brook E., Dharmarajan A., Chan A., Dass C.R. The role of pigment epithelium-derived factor in protecting against cellular stress. Free Radic Res. 2019;53:1166–1180. doi: 10.1080/10715762.2019.1697809. [DOI] [PubMed] [Google Scholar]
- 5.Chopra P., Chhillar H., Kim Y.J., Jo I.H., Kim S.T., Gupta R. Phytochemistry of ginsenosides: recent advancements and emerging roles. Crit Rev Food Sci Nutr. 2021;19:1–28. doi: 10.1080/10408398.2021.1952159. [DOI] [PubMed] [Google Scholar]
- 6.de Oliveira Zanuso B., de Oliveira Dos Santos A.R., Miola V.F.B., Guissoni Campos L.M., Spilla C.S.G., Barbalho S.M. Panax ginseng and aging related disorders: a systematic review. Exp Gerontol. 2022;161 doi: 10.1016/j.exger.2022.111731. [DOI] [PubMed] [Google Scholar]
- 7.Wan Y., Wang J., Xu J.F., Tang F., Chen L., Tan Y.Z., Rao C.L., Ao H., Peng C. Panax ginseng and its ginsenosides: potential candidates for the prevention and treatment of chemotherapy-induced side effects. J Ginseng Res. 2021;45:617–630. doi: 10.1016/j.jgr.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sodrul I.M.D., Wang C., Chen X., Du J., Sun H. Role of ginsenosides in reactive oxygen species-mediated anticancer therapy. Oncotarget. 2017;9:2931–2950. doi: 10.18632/oncotarget.23407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ratan Z.A., Haidere M.F., Hong Y.H., Park S.H., Lee J.O., Lee J., Cho J.Y. Pharmacological potential of ginseng and its major component ginsenosides. J Ginseng Res. 2021;45:199–210. doi: 10.1016/j.jgr.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li C.P., Qin G., Shi R.Z., Zhang M.S., Lv J.Y. Ginsenoside Rg1 reduces toxicity of PM2.5 on human umbilical vein endothelial cells by upregulating intracellular antioxidative state. Environ Toxicol Pharmacol. 2013;35:21–29. doi: 10.1016/j.etap.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Li L., Du G., Wang D., Zhou J., Jiang G., Jiang H. Overexpression of heme oxygenase-1 in mesenchymal stem cells augments their protection on retinal cells in vitro and attenuates retinal ischemia/reperfusion injury in vivo against oxidative stress. Stem Cells Int. 2017;2017 doi: 10.1155/2017/4985323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J., Liu D., Wu J., Zhang D., Cheng B., Zhang Y., Yin Z., Wang Y., Du J., Ling C. Ginsenoside Rg1 attenuates ultraviolet B-induced glucocortisides resistance in keratinocytes via Nrf2/HDAC2 signalling. Sci Rep. 2016;6 doi: 10.1038/srep39336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao Y., Chu S., Shao Q., Zhang M., Xia C., Wang Y., Li Y., Lou Y., Huang H., Chen N. Antioxidant activities of ginsenoside Rg1 against cisplatin-induced hepatic injury through Nrf2 signaling pathway in mice. Free Radic Res. 2017;51:1–13. doi: 10.1080/10715762.2016.1234710. [DOI] [PubMed] [Google Scholar]
- 14.Yao Y., Hu S., Zhang C., Zhou Q., Wang H., Yang Y., Liu C., Ding H. Ginsenoside Rd attenuates cerebral ischemia/reperfusion injury by exerting an anti-pyroptotic effect via the miR-139-5p/FoxO1/Keap1/Nrf2 axis. Int Immunopharmacol. 2022;105 doi: 10.1016/j.intimp.2022.108582. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen T.L.L., Huynh D.T.N., Jin Y., Jeon H., Heo K.S. Protective effects of ginsenoside-Rg2 and -Rh1 on liver function through inhibiting TAK1 and STAT3-mediated inflammatory activity and Nrf2/ARE-mediated antioxidant signaling pathway. Arch Pharm Res. 2021;44:241–252. doi: 10.1007/s12272-020-01304-4. [DOI] [PubMed] [Google Scholar]
- 16.Yang Q., Lin J., Zhang H., Liu Y., Kan M., Xiu Z., Chen X., Lan X., Li X., Shi X. Ginsenoside compound K regulates amyloid β via the Nrf2/Keap1 signaling pathway in mice with scopolamine hydrobromide-induced memory impairments. J Mol Neurosci. 2019;67:62–71. doi: 10.1007/s12031-018-1210-3. [DOI] [PubMed] [Google Scholar]
- 17.Zhang G., Zhang M., Yu J., Kang L., Guan H. Ginsenoside Rg1 prevents H2O2-induced lens opacity. Curr Eye Res. 2021;46:1159–1165. doi: 10.1080/02713683.2020.1869266. [DOI] [PubMed] [Google Scholar]
- 18.Shi Q., Chen X., Sun G., Wang L., Cui L. Ginsenoside Rg1 protects human retinal pigment epithelial ARPE-19 cells from toxicity of high glucose by up-regulation of miR-26a. Life Sci. 2019;221:152–158. doi: 10.1016/j.lfs.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Tang C.Z., Li K.R., Yu Q., Jiang Q., Yao J., Cao C. Activation of Nrf2 by Ginsenoside Rh3 protects retinal pigment epithelium cells and retinal ganglion cells from UV. Free Radic Biol Med. 2018;117:238–246. doi: 10.1016/j.freeradbiomed.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Kim M., Moon A. A curcumin analog CA-5f inhibits urokinase-type plasminogen activator and invasive phenotype of triple-negative breast cancer cells. Toxicol Res. 2022;38:19–26. doi: 10.1007/s43188-021-00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noh Y., Ahn J.H., Lee J.W., Hong J., Lee T.K., Kim B., Kim S.S., Won M.H. Brain factor-7 improves learning and memory deficits and attenuates ischemic brain damage by reduction of ROS generation in stroke in vivo and in vitro. Lab Anim Res. 2020;36:24. doi: 10.1186/s42826-020-00057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi M.J., Mukherjee S., Yun J.W. Loss of ADAMTS15 promotes browning in 3T3-L1 white adipocytes via activation of β3-adrenergic receptor. Biotechnol Bioprocess Eng. 2021;26:188–200. [Google Scholar]
- 23.Lee H., Kim D.H., Kim J.H., Park S.K., Jeong J.W., Kim M.Y., Hong S.H., Song K.S., Kim G.Y., Hyun J.W., et al. Urban aerosol particulate matter promotes necrosis and autophagy via reactive oxygen species-mediated cellular disorders that are accompanied by cell cycle arrest in retinal pigment epithelial cells. Antioxidants (Basel) 2021;10:149. doi: 10.3390/antiox10020149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volobaev V.P., Serdyukova E.S., Kalyuzhnaya E.E., Schetnikova E.A., Korotkova A.D., Naik A.A., Bach S.N., Prosekov A.Y., Larionov A.V. Investigation of the genotoxic effects of fluoride on a bone tissue model. Toxicol Res. 2020;36:337–342. doi: 10.1007/s43188-020-00039-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Z., Ouyang G., Lu W., Zhang H. Long non-coding RNA HOTAIR promotes hepatocellular carcinoma progression by regulating miR-526b-3p/DHX33 axis. Genes Genomics. 2021;43:857–868. doi: 10.1007/s13258-021-01098-9. [DOI] [PubMed] [Google Scholar]
- 26.Choi Y.H. Activation of the Nrf2/HO-1 signaling pathway contributes to the protective effects of platycodin D against oxidative stress-induced DNA damage and apoptosis in C2C12 myoblasts. Gen Physiol Biophys. 2020;39:519–530. doi: 10.4149/gpb_2020030. [DOI] [PubMed] [Google Scholar]
- 27.Kang S., Siddiqi M.H., Yoon S.J., Ahn S., Noh H.Y., Kumar N.S., Kim Y.J., Yang D.C. Therapeutic potential of compound K as an IKK inhibitor with implications for osteoarthritis prevention: an in silico and in vitro study. Vitro Cell Dev Biol Anim. 2016;52:895–905. doi: 10.1007/s11626-016-0062-9. [DOI] [PubMed] [Google Scholar]
- 28.Li X., Huang Q., Wang M., Yan X., Song X., Ma R., Jiang R., Zhao D., Sun L. Compound K inhibits autophagy-mediated apoptosis through activation of the PI3K-Akt signaling pathway thus protecting against ischemia/reperfusion injury. Cell Physiol Biochem. 2018;47:2589–2601. doi: 10.1159/000491655. [DOI] [PubMed] [Google Scholar]
- 29.Duan H., Zhang Q., Liu J., Li R., Wang D., Peng W., Wu C. Suppression of apoptosis in vascular endothelial cell, the promising way for natural medicines to treat atherosclerosis. Pharmacol Res. 2021;168 doi: 10.1016/j.phrs.2021.105599. [DOI] [PubMed] [Google Scholar]
- 30.Huang Q., Lou T., Wang M., Xue L., Lu J., Zhang H., Zhang Z., Wang H., Jing C., Zhao D., et al. Compound K inhibits autophagy-mediated apoptosis induced by oxygen and glucose deprivation/reperfusion via regulating AMPK-mTOR pathway in neurons. Life Sci. 2020;254 doi: 10.1016/j.lfs.2020.117793. [DOI] [PubMed] [Google Scholar]
- 31.Cai B.X., Luo D., Lin X.F., Gao J. Compound K suppresses ultraviolet radiation-induced apoptosis by inducing DNA repair in human keratinocytes. Arch Pharm Res. 2008;31:1483–1488. doi: 10.1007/s12272-001-2134-x. [DOI] [PubMed] [Google Scholar]
- 32.Hong Y., Liang Y.P., Chen W.Q., You L.X., Ni Q.F., Gao X.Y., Lin X.R. Protective effects of upregulated HO-1 gene against the apoptosis of human retinal pigment epithelial cells in vitro. Int J Ophthalmol. 2021;14:649–655. doi: 10.18240/ijo.2021.05.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Senichkin V.V., Pervushin N.V., Zuev A.P., Zhivotovsky B., Kopeina G.S. Targeting Bcl-2 family proteins: what, where, when? Biochemistry (Mosc) 2020;85:1210–1226. doi: 10.1134/S0006297920100090. [DOI] [PubMed] [Google Scholar]
- 34.Ulasov A.V., Rosenkranz A.A., Georgiev G.P., Sobolev A.S. Nrf2/Keap1/ARE signaling: towards specific regulation. Life Sci. 2022;291 doi: 10.1016/j.lfs.2021.120111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tavakkoli A., Iranshahi M., Hasheminezhad S.H., Hayes A.W., Karimi G. The neuroprotective activities of natural products through the Nrf2 upregulation. Phytother Res. 2019;33:2256–2273. doi: 10.1002/ptr.6427. [DOI] [PubMed] [Google Scholar]