Abstract
Vascular endothelial growth factor A (VEGF-A) has therapeutic cardiovascular effects, but delivery challenges have impeded clinical development. We report the first clinical study of naked mRNA encoding VEGF-A (AZD8601) injected into the human heart. EPICCURE (ClinicalTrials.gov: NCT03370887) was a randomized, double-blind study of AZD8601 in patients with left ventricular ejection fraction (LVEF) 30%–50% who were undergoing elective coronary artery bypass surgery. Thirty epicardial injections of AZD8601 (total 3 mg) or placebo in citrate-buffered saline were targeted to ischemic but viable myocardial regions mapped using quantitative [15O]-water positron emission tomography. Seven patients received AZD8601 and four received placebo and were followed for 6 months. There were no deaths or treatment-related serious adverse events and no AZD8601-associated infections, immune reactions, or arrhythmias. Exploratory outcomes indicated potential improvement in LVEF, Kansas City Cardiomyopathy Questionnaire scores, and N-terminal pro-B-type natriuretic peptide levels, but the study is limited in size, and significant efficacy conclusions are not possible from the dataset. Naked mRNA without lipid encapsulation may provide a safe delivery platform for introducing genetic material to cardiac muscle, but further studies are needed to confirm efficacy and safety in a larger patient pool.
Keywords: EPICCURE, VEGF-A, cardiac, mRNA, clinical trial, LVEF, regeneration, therapeutic mRNA, safety, tolerability, phase 2a, CABG
Graphical abstract
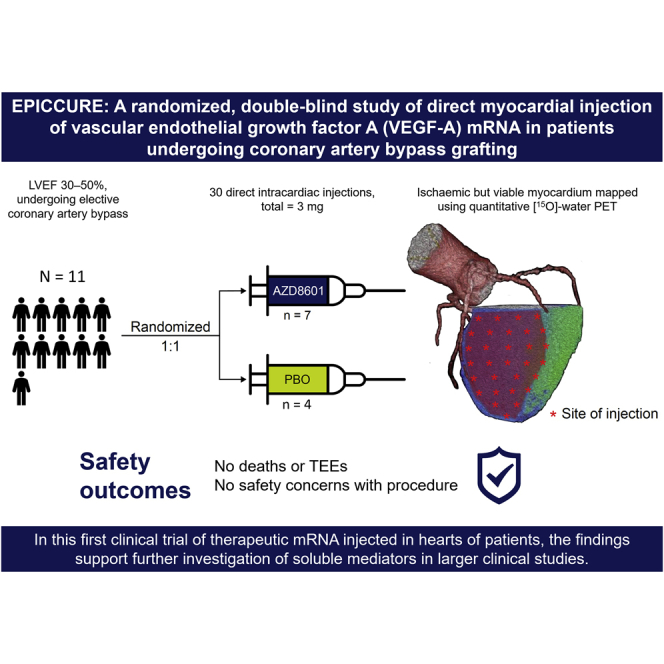
The manuscript describes the first clinical study of therapeutic mRNA encoding VEGF-A (AZD8601) injected into the heart. EPICCURE (ClinicalTrials.gov: NCT03370887) was a randomized, double-blind study of AZD8601 in patients undergoing elective coronary artery bypass surgery. The study is an important contribution to the mRNA research field demonstrating an encouraging safety profile.
Introduction
Ischemic heart disease remains a major threat to public health, with more than 9 million deaths and 197 million prevalent cases worldwide in 2019 and increasing mortality in many regions.1 The therapeutic potential of vascular endothelial growth factor A (VEGF-A) in patients with ischemic heart disease has been recognized since the 1990s.2,3 Efforts to translate this potential into clinically meaningful benefit have been hampered by the challenges of delivering VEGF-A to the correct regions of the heart in patients with ischemic heart disease.2,3
AZD8601 is an mRNA drug encoding VEGF-A and was the first mRNA-based drug encoding a secreted protein to enter clinical trials.4 AZD8601 uses the same modified mRNA technology as in the recently successful COVID-19 vaccines, with 1-methylpseudouridine as a substitution for uridine, but is not formulated as lipid nanoparticles. Instead, AZD8601 comprises VEGF-A mRNA formulated in a simple citrate-saline buffer. Porcine cardiomyocytes are transfected and transiently express VEGF-A protein following injection of naked VEGF-A mRNA into the myocardium, without activation of the innate immune system.5
The pulse-like kinetics of VEGF-A protein expression following intramyocardial injection of VEGF-A mRNA differ fundamentally from the kinetics of expression with previously unsuccessful gene therapy modalities.2,3,6 Evidence from animal models indicates that intramyocardial injection of VEGF-A mRNA increases capillary density and reduces fibrosis in the region surrounding the myocardial infarct and is associated with improvement in left ventricular ejection fraction and other cardiac functional parameters.5 VEGF-A delivered using mRNA has been shown to activate human endogenous epicardial progenitor cells in vitro and drive their proliferation and differentiation toward the endothelial lineage.7 Pleiotropic effects of VEGF-A mRNA have been observed in the mouse heart, with stimulation of the epicardial progenitor cell compartment and beneficial effects on left ventricular function after experimental myocardial infarction.6 In a phase 1 study in otherwise healthy volunteers with type 2 diabetes, intradermal injection of AZD8601 induced a rapid burst of local VEGF-A protein production and transiently enhanced basal skin blood flow.8 These findings in vitro and in vivo in animals and humans provided proof of the principle that AZD8601 may have potentially beneficial effects in patients with ischemic heart disease.9 The progression from preclinical studies to clinical trials illustrates the importance of building a comprehensive data package in a stepwise manner for a novel therapeutic modality such as mRNA.9
Here, we report the results of a phase 2a trial of AZD8601 in patients undergoing cardiac revascularization by coronary artery bypass grafting.10 This was the first study of therapeutic mRNA in cardiac patients to assess safety and tolerability.
Results
Patients
The study took place between February 2017 and June 2021. The study was terminated early because of slow enrollment before any patients were randomized to the high dose (30 mg) of AZD8601.
Of 15 patients enrolled, four failed screening, and 11 were randomized: seven to AZD8601 3 mg, and four to placebo. Eight patients were enrolled in Finland (AZD8601, n = 4; placebo, n = 4), and three were enrolled in Germany (AZD8601, n = 3; placebo, n = 0). All 11 randomized patients received study treatment, completed the study, and were included in the safety and exploratory efficacy analysis sets. The 11 patients had a median age of 69 years. Nine patients were male, all were White, and the majority were overweight or obese (Table 1).
Table 1.
Baseline characteristics
| AZD8601 3 mg (n = 7) | Placebo (n = 4) | Overall (N = 11) | |
|---|---|---|---|
| Age, years | |||
| Median (range) | 68 (61–77) | 69.5 (53–79) | 69 (53–79) |
| Sex | |||
| Male, n (%) | 7 (100) | 2 (50) | 9 (81.8) |
| Female, n (%) | 0 (0) | 2 (50) | 2 (18.2) |
| White, n (%) | 7 (100) | 4 (100) | 11 (100) |
| Body mass index category | |||
| Normal (<25 kg/m2), n (%) | 4 (57.1) | 1 (25) | 5 (45.5) |
| Overweight (25–30 kg/m2), n (%) | 2 (28.6) | 3 (75) | 5 (45.5) |
| Obese (>30 kg/m2), n (%) | 1 (14.3) | 0 (0) | 1 (9.1) |
| Medical history (at least 50% of patients) | |||
| Coronary artery disease, n (%) | 7 (100) | 4 (100) | 11 (100) |
| Hypertension, n (%) | 7 (100) | 3 (75) | 10 (90.9) |
| Dyslipidemia, n (%) | 4 (57.1) | 4 (100) | 8 (72.7) |
| Left ventricular ejection fraction (Simpson method), % | |||
| Median (range) | 50.24 (25.03, 63.30) | 50.72 (40.85, 55.40) | |
Safety and tolerability
There were no deaths or treatment-related serious adverse events during the study (Tables 2 and 3). During the treatment period, the only treatment-related adverse events were ventricular arrhythmia and ventricular extrasystoles in one patient in the AZD8601 3 mg group; these occurred before administration of the study drug and were judged by the investigator to be related to the surgery (Table 2). During the follow-up period, the only treatment-related adverse events were gynecomastia related to spironolactone use in one male patient in the AZD8601 3 mg group and an increased liver function test in one patient in the placebo group (Table 3).
Table 2.
Adverse events during the treatment period
| AZD8601 3 mg (n = 7) | Placebo (n = 4) | |
|---|---|---|
| Any adverse event, n (%) | 7 (100) | 4 (100) |
| Related to study druga | 1 (14.3) | 0 (0) |
| Fatal adverse event, n (%) | 0 (0) | 0 (0) |
| Serious adverse event, n (%)b | 2 (28.6) | 0 (0) |
| Related to study drug | 0 (0) | 0 (0) |
| Adverse event leading to discontinuation or withdrawal, n (%) | 0 (0) | 0 (0) |
| Anemia, n (%)c | 2 (28.6) | 1 (25) |
| Atrial fibrillation, n (%)d | 2 (28.6) | 2 (50) |
| Coagulopathy, n (%)c | 2 (28.6) | 0 (0) |
| Dizziness, n (%)c | 1 (14.3) | 0 (0) |
| Dyspepsia, n (%)c | 1 (14.3) | 0 (0) |
| Dyspnea, n (%)e | 1 (14.3) | 0 (0) |
| Hypotension, n (%)c | 1 (14.3) | 0 (0) |
| Nausea, n (%)e | 1 (14.3) | 0 (0) |
| Pleural effusion, n (%)c | 0 (0) | 2 (50) |
| Procedural pain, n (%)e | 0 (0) | 1 (25) |
| Pyrexia, n (%)e | 0 (0) | 1 (25) |
| Sinus bradycardia, n (%)e | 1 (14.3) | 0 (0) |
| Urinary tract infection, n (%)e | 0 (0) | 1 (25) |
| Ventricular arrhythmia, n (%)f | 1 (14.3) | 0 (0) |
| Ventricular extrasystoles, n (%)e | 1 (14.3) | 0 (0) |
Ventricular arrhythmia and ventricular extrasystoles before AZD8601 administration in one patient.
Anemia in one patient and coagulopathy in one patient.
Moderate maximum severity.
Moderate maximum intensity in three patients and mild in one (in the AZD8601 group).
Mild maximum intensity.
Severe maximum intensity.
Table 3.
Adverse events during the 6-month follow-up period
| AZD8601 3 mg (n = 7) | Placebo (n = 4) | |
|---|---|---|
| Any adverse event, n (%) | 5 (71.4) | 4 (100) |
| Related to study druga | 1 (14.3) | 1 (25) |
| Fatal adverse event, n (%) | 0 (0) | 0 (0) |
| Serious adverse event, n (%)b | 2 (28.6) | 1 (25) |
| Related to study drug | 0 (0) | 0 (0) |
| Anemiac | 0 (0) | 1 (25) |
| Cataractd | 1 (14.3) | 0 (0) |
| Chest paind | 1 (14.3) | 0 (0) |
| Dizzinessd | 0 (0) | 1 (25) |
| Dyslipidemiad | 0 (0) | 1 (25) |
| Dyspneac | 0 (0) | 1 (25) |
| Embolic cerebral infarctionc | 1 (14.3) | 0 (0) |
| Feeling coldd | 1 (14.3) | 0 (0) |
| Gynecomastiad | 1 (14.3) | 0 (0) |
| Incision site impaired healingc | 1 (14.3) | 0 (0) |
| Incision site inflammationc | 1 (14.3) | 0 (0) |
| Insomniad | 0 (0) | 1 (25) |
| Left ventricular dysfunctionc | 1 (14.3) | 0 (0) |
| Liver function test increasedd | 0 (0) | 1 (25) |
| Myalgiad | 0 (0) | 1 (25) |
| Non-cardiac chest painc | 0 (0) | 1 (25) |
| Pain in extremityc | 1 (14.3) | 0 (0) |
| Skin necrosise | 0 (0) | 1 (25) |
| Syncoped | 1 (14.3) | 0 (0) |
| Tachycardiac | 1 (14.3) | 0 (0) |
| Vascular graft occlusione | 1 (14.3) | 0 (0) |
| Vertigod | 1 (14.3) | 0 (0) |
| Wound infectionc | 0 (0) | 1 (25) |
Gynecomastia in one patient in the AZD8601 group and increased liver function test in one patient in the placebo group.
Embolic cerebral infarction and vascular graft occlusion in one patient in the AZD8601 group; incision site impaired healing and incision site inflammation in one patient in the AZD8601 group; and skin necrosis in one patient in the placebo group.
Moderate maximum intensity.
Mild maximum intensity.
Severe maximum intensity.
No adverse events of pyrexia or infection occurred among patients who received AZD8601 (Tables 2 and 3). There were no findings of concern in echocardiographic or electrocardiographic assessments, vital signs, clinical laboratory parameters, or physical examinations (Table S2; supplemental information). All leukocyte counts including monocyte counts returned to normal levels after surgery in both the AZD8601 and placebo groups.
Exploratory outcomes
Pharmacodynamics
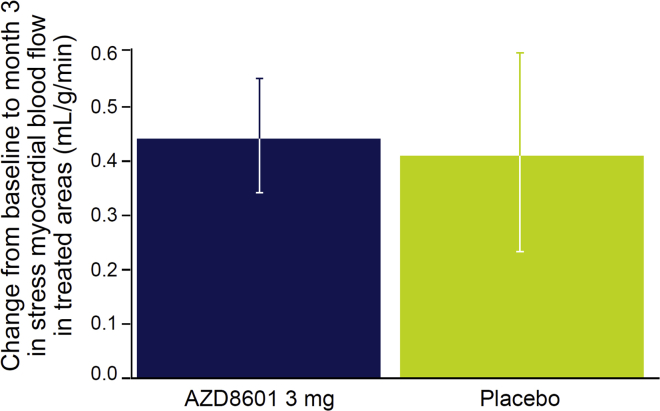
Patients in both groups had increases in stress myocardial blood flow in the treated area from similar mean baseline values (AZD8601 group, 1.28 mL/g/min; placebo group, 1.26 mL/g/min), generally peaking at 1 month after surgery, detected using 15O-water positron emission tomography (PET). Compared with the placebo group, there were no additional increases in stress myocardial blood flow in the treated area at 3 months after surgery in the AZD8601 group (Figure 1; Table 4). In statistical analyses, there were no significant differences between AZD8601 and placebo in myocardial blood flow or myocardial perfusion reserve in the treated area (Table 4). Systemic VEGF-A levels did not differ significantly between the AZD8601 and placebo groups, as expected after local intramyocardial injection and as previously observed with intradermal injection (Table S3; supplemental information),8 and is also in line with the data from the pig study with intracardiac injections.5
Figure 1.
Change in stress myocardial blood flow in the treated area from baseline to month 3 in each group
Data are mean and standard error of the mean.
Table 4.
Exploratory pharmacodynamic and efficacy outcome analysis results
| Change from baseline |
Difference, AZD8601 versus placebo (p) | ||
|---|---|---|---|
| AZD8601 3 mg (n = 7) | Placebo (n = 4) | ||
| Pharmacodynamics at 3 months | |||
| 15O-water PETa | |||
| Treated area stress myocardial blood flow, mL/g/min | 0.4342 | 0.4070 | 0.0272 (0.462) |
| Treated area myocardial flow reserve, ratio | 0.3729 | 0.3862 | −0.0133 (0.513) |
| Efficacy at 6 months | |||
| Echocardiographya | |||
| Left ventricular end-diastolic volume, mL | −12.329 | 0.853 | −13.182 (0.698) |
| Left ventricular end-systolic volume, mL | −11.919 | 9.099 | −21.018 (0.852) |
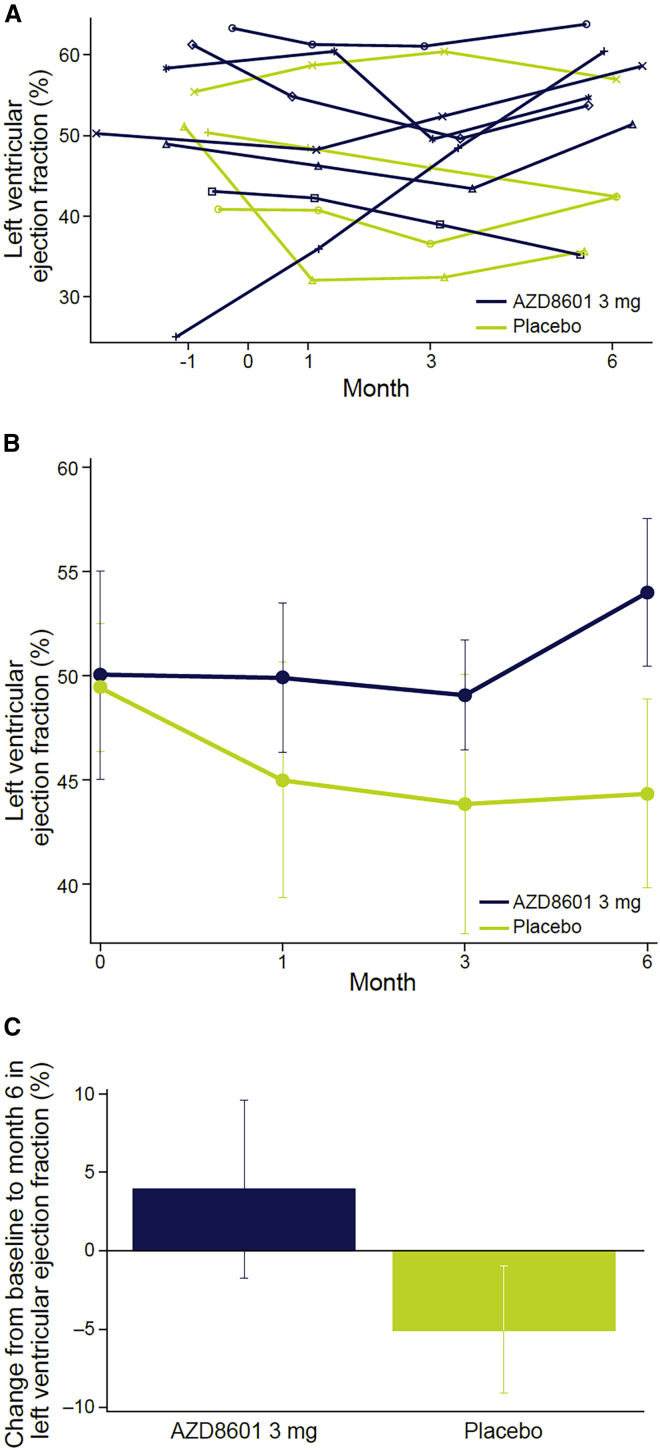
| Left ventricular ejection fraction, % | 4.072 | −5.296 | 9.367 (0.054) |
| Global longitudinal strain, % | 2.914 | 3.070 | −0.156 (0.539) |
| Patient-reported outcomesa | |||
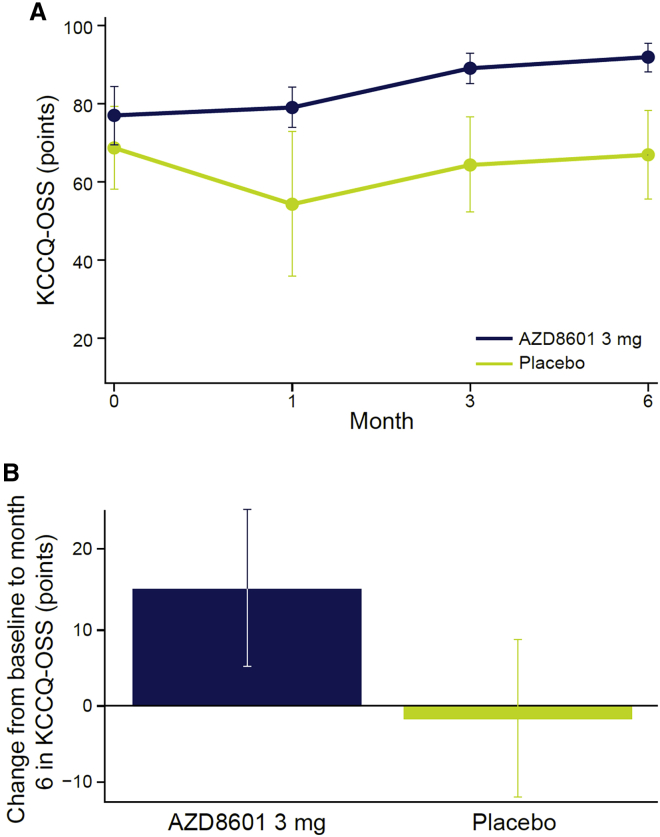
| KCCQ overall summary score | 17.04 | −5.55 | 22.59 (0.044) |
| SAQ physical limitation score | 18.39 | 10.18 | 8.21 (0.247) |
| SAQ angina stability score | 6.91 | 6.66 | 0.26 (0.492) |
| SAQ angina frequency score | 26.73 | 18.22 | 8.52 (0.225) |
| SAQ treatment satisfaction score | 7.47 | −4.21 | 11.68 (0.092) |
| SAQ quality of life score | 31.11 | 14.31 | 16.79 (0.145) |
| Cardiac biomarkerb | |||
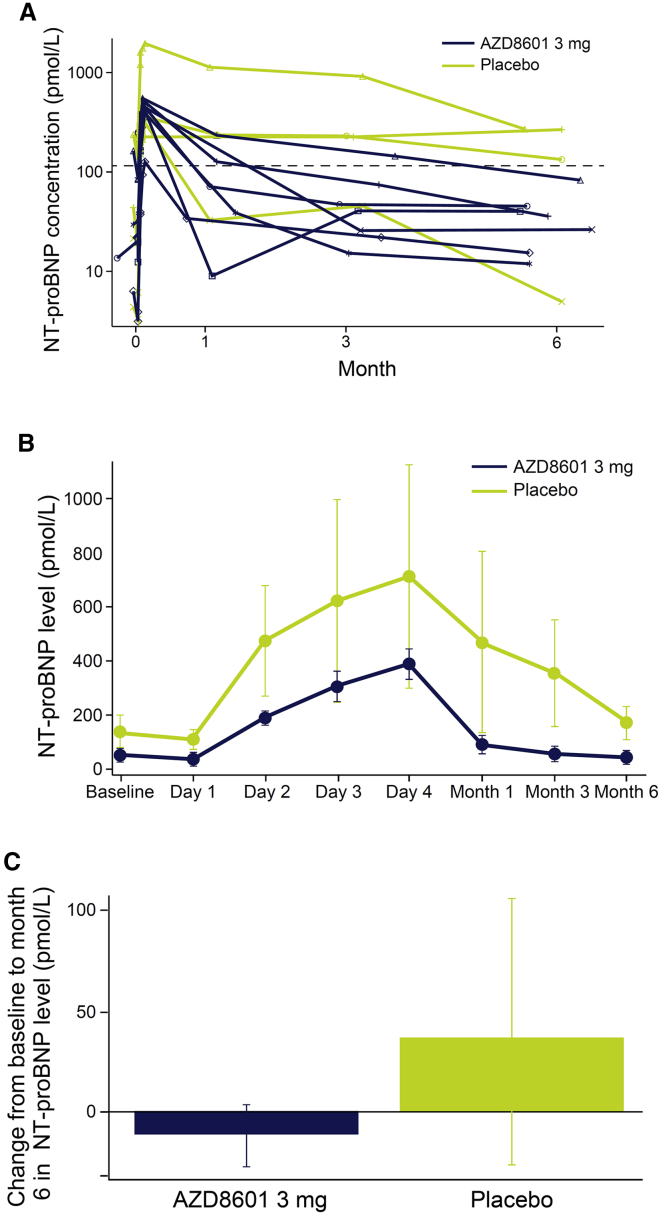
| N-terminal pro-B-type natriuretic peptide, pmol/L | 0.990 | 1.735 | 0.57 (0.108) |
Data are from mixed-model repeated measures with treatment, visit, and visit-by-treatment interaction as fixed factors, subject as random factor, and baseline value as covariate. One-sided nominal p values are presented. KCCQ, Kansas City Cardiomyopathy Questionnaire; PET, positron emission tomography; SAQ, Seattle Angina Questionnaire.
Least-squares mean change from baseline and least-squares mean difference versus placebo.
Ratio versus baseline and ratio versus placebo.
Echocardiography
A trend toward improvement in left ventricular ejection fraction in the AZD8601 group was noted (Figure 2), with a mean change at 6 months of +3.9% (standard error of the mean [SEM], 5.7) in the AZD8601 3 mg group versus a change of −5.1% (SEM, 4.1) from mean baseline values of approximately 50% in both groups (Figure 2). In statistical analyses, there were no significant differences between AZD8601 and placebo in any of the echocardiographic parameters assessed, including left ventricular ejection fraction (Table 4).
Figure 2.
Change in left ventricular ejection fraction
Left ventricular ejection fraction (A) over time in individual patients (B) over time in each group and (C) change from baseline to month 6. Data in (B) and (C) are mean and standard error of the mean.
Patient-reported outcomes
A trend toward improvement in Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary score was noted in the AZD8601 group but not in the placebo group, with a mean change from baseline at 6 months of +14.9 points (SEM, 9.9) in the AZD8601 3 mg group versus −1.8 points (SEM, 10.3) in the placebo group (Figure 3). In statistical analyses, there was a significant difference between AZD8601 and placebo in the change in KCCQ overall summary score from baseline to 6 months (p = 0.044; one-sided, nominal). There were no significant differences between AZD8601 and placebo in any of the five Seattle Angina Questionnaire (SAQ) domain scores (Table 4).
Figure 3.
Change in Kansas City Cardiomyopathy Questionnaire scores
Kansas City Cardiomyopathy Questionnaire overall summary score (A) over time in each group and (B) change from baseline to month 6. Data are mean and standard error of the mean. KCCQ-OSS, Kansas City Cardiomyopathy Questionnaire – overall summary score.
High-sensitivity troponin T
No persistent increases in high-sensitivity troponin T (hsTnT) levels were observed, and levels did not differ significantly between the AZD8601 and placebo groups in statistical analyses (Table S3; supplemental information).
N-terminal pro-B-type natriuretic peptide
N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels increased in the 4 days following surgery and then declined out to 6 months in both groups (Figure 4). There was a trend toward greater NT-proBNP elevations in the placebo group than in the AZD8601 group, but no significant difference was found in the statistical analysis (Table 4). NT-proBNP levels had fallen to below 125 pmol/L in the AZD8601 group, but not in the placebo group, at 6 months after surgery (Figure 4).
Figure 4.
Change in N-terminal pro-B-type natriuretic peptide levels
N-terminal pro-B-type natriuretic peptide levels (A) over time in individual patients (B) over time in each group and (C) change from baseline to month 6. Dashed line in (A) indicates 125 pmol/L. Data in (B) and (C) are mean and standard error of the mean. NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Discussion
AZD8601 VEGF-A mRNA (3 mg) administered as 30 separate intramyocardial injections during cardioplegia was safe and well tolerated in this phase 2a study (EPICCURE) in seven patients undergoing coronary artery bypass grafting for treatment of severe coronary artery disease with left ventricular dysfunction. There were no deaths, no treatment-related serious adverse events, no infections, no safety signals of concern, and no negative impact on cardiac function during 6 months of follow up among patients receiving AZD8601.
Recent studies have shown that mRNA-based prophylactic vaccines are effective and safe when administered intramuscularly.11 EPICCURE provides not only the first evidence on the safety of any therapeutic mRNA in patients but also the first evidence on the safety of mRNA injection into the human myocardium. The study was initiated in early 2017, before the development of mRNA-based COVID-19 vaccines. The findings expand the safety profile of this novel modality from the prophylactic setting using lipid nanoparticle encapsulation to the therapeutic setting using naked mRNA in a simple biocompatible buffer.
The smaller-than-planned sample size was a limitation of EPICCURE that resulted from slow enrollment and early termination of the study (partly due to COVID-19). The high dose of AZD8601 30 mg was therefore not tested as planned. The small sample size of 11 patients led to imbalance in baseline characteristics between the groups (e.g., no female patients in the AZD8601 group versus 50% in the placebo group). The small sample size also limited the interpretability of the efficacy findings.
VEGF-A has long been considered a potential therapeutic in patients with heart failure, but clinically tractable approaches for localized delivery to the human heart with appropriate kinetics have previously been lacking.2,3,6 Exploratory multidimensional assessment of efficacy was used in EPICCURE to capture potential therapeutic benefits in patients with heart failure, in accordance with the recommendations of the recent taskforce involving industry, academia, and the US Food and Drug Administration (FDA).12 The small sample size meant that the study was underpowered for efficacy. Marginal, non-significant trends for improvement in left ventricular ejection fraction, health-related quality of life, and biomarkers were noted at 6 months after treatment with AZD8601, and NT-proBNP biomarker levels reduced to below levels characteristic of heart failure. The improvement in left ventricular ejection fraction was small. Health-related quality of life was assessed using the KCCQ, in accordance with FDA guidance, but this patient-reported instrument may be susceptible to bias and placebo effects.
There was no appreciable additive effect of VEGF-A mRNA on blood flow in patients undergoing coronary artery bypass grafting. Although the study was underpowered because of the small sample size, the effect of surgical revascularization may also have masked potential effects of VEGF-A mRNA treatment. Myocardial blood flow was assessed at 1 and 3 months after surgery and treatment as an exploratory efficacy outcome that aimed to capture potential pharmacodynamic effects of VEGF-A mRNA injection. Moreover, the exploratory efficacy outcomes assessed at 6 months after treatment did not include myocardial blood flow, per the study protocol. It is possible that VEGF-A mediates transient enhancements in blood flow followed by long-term functional effects without long-term enhancement of blood flow.
VEGF-A is a potent soluble mediator that has previously been shown to stimulate proliferation and differentiation of epicardial progenitor cells toward an endothelial cell fate in addition to promoting angiogenesis.5,6 VEGF-A mRNA may therefore have potentially beneficial effects on cardiac function in the absence of detectable improvements in myocardial blood flow at late time points after treatment. Stress myocardial blood flow peaked at 1 month after surgery in EPICCURE among patients in both the AZD8601 group and the placebo group. The transient pharmacokinetic profile and rapid onset of VEGF-A protein expression may mean that potential alterations in myocardial blood flow occurred before the first assessment at 1 month after treatment in EPICCURE. The lack of any detectable additional effect of AZD8601 compared with placebo in patients undergoing surgical revascularization could be related to an incorrect time point of measurement, with the transient effect of VEGF-A expression having already passed, 15O-water PET discrepancy, and insufficient power of the study to detect differences in flow with the low patient numbers.
Unique to this study was the use of individualized 15O-water PET imaging to identify and localize target regions of ischemic but viable myocardium for injection with AZD8601. This came with the limitation that enrollment was slow because some of the patients who underwent elective coronary artery bypass grafting at study sites were unable to have required PET imaging performed. In addition, the frequency of elective surgery was substantially reduced after the COVID-19 pandemic started. As a result, no patients received the planned high dose of AZD8601 (30 mg) in this study because of early termination of the study.
The safety endpoints of EPICCURE were met for the 3 mg dose, supporting further clinical investigation of AZD8601 for treatment of ischemic heart disease. The safety findings also suggest that naked VEGF mRNA, without any lipid encapsulation, may represent a safe delivery system for genetically modifying the human heart with other mRNAs encoding new potential therapeutic proteins and platforms. The sample size was insufficient to allow any firm conclusions on the exploratory efficacy outcomes, which were not intended to be definitive. Improvements in delivery strategies and optimization of doses for administration of AZD8601 may be required to achieve clinically meaningful benefits of VEGF mRNA therapy in patients with ischemic heart disease. Having established initial safety data on AZD8601, we hypothesize that the use of VEGF mRNA could shift toward alternative ischemia-related diseases such as peripheral vascular disease.13 Furthermore, the vasculogenic and anti-fibrotic mechanisms of action of VEGF mRNA could enable expansion to indications such as wound healing, bone repair, and soft tissue augmentation.14,15,16 VEGF mRNA has been used to enhance engraftment and viability of human induced pluripotent stem-cell derived cardiomyocytes in an animal model of heart failure.17 We envision that the application of VEGF mRNA to these indications will continue to widen the use of synthetic mRNA as a novel drug for disabling injuries and disorders. Further development of alternative approaches to cardiac delivery of AZD8601 may allow administration via percutaneous intervention with cardiac catheters or via minimally invasive surgery (micro-thoracotomy).18 As the first clinical trial of therapeutic mRNA in human myocardium, EPICCURE paves the way for future larger clinical trials of soluble mediators delivered using this new modality in patients with ischemic heart disease.
Materials and methods
Overview and objectives
EPICCURE (ClinicalTrials.gov: NCT03370887) was a randomized, placebo-controlled, double-blind, phase 2a clinical trial of AZD8601 in patients with moderately reduced left ventricular ejection fraction (30%–50%) undergoing elective coronary artery bypass grafting surgery and has been performed with oversight by an institutional review board.10
The primary objective was to assess the safety and tolerability of AZD8601 administered via epicardial injections during surgery. Exploratory efficacy objectives including assessing the effect of AZD8601 on myocardial blood flow (using 15O-water PET), echocardiographic measures of cardiac function, clinical symptoms, biomarkers, and functional tests.
Patients
Eligible patients were men and women aged at least 18 years with left ventricular ejection fraction between 30% and 50% based on medical records at screening. Patients had to be enrolled 15–90 days before elective coronary artery bypass grafting surgery. Patients also had to be on stable doses of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, statins, or beta-blockers for at least 2 weeks before the screening visit if taking these medications. Men had to be surgically sterile or using barrier contraception, and women had to be surgically sterile or postmenopausal. Complete inclusion and exclusion criteria are provided in Table S1.
Randomization and blinding
Per study protocol, patients had to be enrolled sequentially into two ascending-dose cohorts (n = 12 per cohort) and randomized at least 14 days before coronary artery bypass grafting to receive AZD8601 (n = 8) or matching placebo (n = 4). The planned AZD8601 dose was 3 mg in the first cohort and 30 mg in the second cohort, resulting in a final planned randomization of 1:1:1 to AZD8601 3 mg, AZD8601 30 mg, or matching placebo in a total of 24 patients. Only the AZD8601 3 mg dose or matching placebo were tested in the study due to early termination.
Randomization codes were generated by Parexel using the AZRand solution and assigned by the AstraZeneca supply chain. Each cohort included two subgroups of two sentinel patients with safety review before progression to the next subgroup, as previously described.10 Patients and the study team were blinded to study treatment assignment throughout the study, investigators were blinded, and the safety review committee was unblinded.
Injection site mapping
Preoperative myocardial blood flow was quantified and mapped at baseline in enrolled patients by using 15O-water PET at rest and during adenosine stress. Ischemic regions were those with stress myocardial blood flow below 2.3 mL/g/min (±0.3 mL/g/min) or below 80% (±10%) of the segment with the highest stress myocardial blood flow.19,20 Regions targeted for injection were ischemic regions with resting myocardial blood flow above 0.6 mL/g/min, indicating potential viability. A perfusion map of myocardial blood flow and coronary anatomy was then generated by combining 15O-water PET scans with baseline contrast-enhanced coronary computed tomography angiograms. An individualized injection map was designed based on the perfusion map in a preoperative multidisciplinary conference. Each patient’s injection map identified 30 epicardial injection sites with approximately 1-cm spacing within the target regions of ischemic but viable myocardium.
Surgery and study drug administration
Patients received epicardial injections of randomized study treatment (either AZD8601 or placebo) under cardioplegia immediately after bypass grafting and before reperfusion. Thirty injections of 0.2-mL volume were given at the sites identified on the individualized injection map via separate 1-mL syringes with 30G × 13 mm hypodermic needles. Patients assigned to AZD8601 received 0.1 mg per injection site (0.2 mL of a 0.5 mg/mL solution), resulting in a total dose of 3 mg VEGF-A mRNA. Surgeons documented the injection procedure photographically, recorded actual injection sites on the individualized maps, and recorded comments on potential or suspected perforation, potential sustained bleeding at the injection site, and other adverse events.
Safety assessments
Patients stayed in the hospital for at least 4 days after surgery (treatment period) and then attended follow-up clinic visits at 1, 3, and 6 months after surgery (follow-up period). Echocardiography was performed 3–4 days after surgery to assess hemopericardium and tamponade. Adverse events were recorded from surgery until the end of follow up, and serious adverse events were recorded from informed consent until the end of follow up.
Exploratory assessments
At baseline and 1 and 3 months after surgery, 15O-water PET was used to assess regional and global myocardial blood flow during stress and myocardial flow reserve (the ratio of stress myocardial blood flow to resting myocardial blood flow). At baseline and 1, 3, and 6 months after surgery, echocardiography was used to assess left ventricular ejection fraction; patients completed the KCCQ21 and the SAQ22; and investigators classified patients according to the New York Heart Association criteria.23 Plasma samples were collected throughout the study for measurement of levels of the cardiac biomarkers hsTnT and NT-proBNP levels, as previously described.10 Plasma samples were also collected for measurement of VEGF-A protein levels.10
Statistical methods
The planned sample size of 24 patients (AZD8601 3 mg, n = 8; AZD8601 30 mg, n = 8; placebo, n = 8) was not based on a formal power calculation. Safety was assessed in all randomized patients who received at least one injection of AZD8601 or placebo. Exploratory efficacy was assessed in all randomized patients who received AZD8601 or placebo and for whom data were available at baseline and at least one follow-up visit.
Changes from baseline in exploratory outcome measures were assessed using mixed-model repeated measures (with treatment, visit, and interaction between treatment and visit as fixed effects and baseline as a covariate) and one-sided p values (two-sided p value for hsTNT). Changes from baseline in VEGF-A protein levels were assessed using analyses of covariance (with treatment as a fixed effect and baseline value as a covariate) and one-sided p values. Analyses were not adjusted for multiplicity, and all p values are nominal.
Ethics
The study was conducted in accordance with the principles of the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice, the AstraZeneca policy on Bioethics and Human Biological Samples, and all applicable regulatory requirements. Local ethics committees at all study sites reviewed and approved the study protocol, and all participants gave written informed consent before enrollment.
Data availability
Data underlying the findings described in this article may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Acknowledgments
We thank the patients and study site staff who took part in the study. This study was funded by AstraZeneca. We thank Meera Kodukulla, PhD, of AstraZeneca for providing critical input to the manuscript drafts. Under the direction of the authors, Matt Cottingham, PhD, of Oxford PharmaGenesis, Oxford, UK, provided medical writing support funded by AstraZeneca.
Declaration of interests
J.R., P.Z., A.R., P.G., C.W., M.N.P., R.F.-D., A.C., and L.-M.G. are employees and stockholders of AstraZeneca. V.A. has received a grant from AstraZeneca. A.S. has received consultancy or speaker fees from Abbott, Amgen, Astra Zeneca, Bayer, Boehringer Ingelheim, and Pfizer. J.K. has received speaker fees from GE Healthcare, Merck, Lundbeck, Pfizer, Boehringer Ingelheim, and Bayer and consultancy fees from AstraZeneca and GE Healthcare. M.H. has received speaker fees from Siemens Healthcare and GE. K.-L.L. has received advisory fees from AstraZeneca. M.K. is a physician proctor and a member of the medical advisory board for JOMDD, a physician proctor for Peter Duschek, and has received speaker fees from Medtronic and Terumo. A.J. has received consultancy or speaker fees from AstraZeneca, Werfen, Boehringer Ingelheim, Portola, Baxter, and LFB. K.R.C. is an advisor and chair of the External Science Panel for AstraZeneca and a member of the Karolinska Institutet/AstraZeneca Integrated Cardio Metabolic Center in Huddinge and receives support for these services, as well as research support through the Karolinska Institutet Center, and is a cofounder and equity holder of Moderna, Inc.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2022.11.017.
Supplemental information
References
- 1.Roth G.A., Mensah G.A., Johnson C.O., Addolorato G., Ammirati E., Baddour L.M., Barengo N.C., Beaton A.Z., Benjamin E.J., Benziger C.P., et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chien K.R., Zangi L., Lui K.O. Synthetic chemically modified mRNA (modRNA): toward a new technology platform for cardiovascular biology and medicine. Cold Spring Harb. Perspect. Med. 2014;5:a014035. doi: 10.1101/cshperspect.a014035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ylä-Herttuala S., Bridges C., Katz M.G., Korpisalo P. Angiogenic gene therapy in cardiovascular diseases: dream or vision? Eur. Heart J. 2017;38:1365–1371. doi: 10.1093/eurheartj/ehw547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mullard A. mRNA-based drug approaches Phase I milestone. Nat. Rev. Drug Discov. 2016;15:595. doi: 10.1038/nrd.2016.182. [DOI] [PubMed] [Google Scholar]
- 5.Carlsson L., Clarke J.C., Yen C., Gregoire F., Albery T., Billger M., Egnell A.C., Gan L.M., Jennbacken K., Johansson E., et al. Biocompatible, purified VEGF-A mRNA improves cardiac function after intracardiac injection 1 Week post-myocardial infarction in swine. Mol. Ther. Methods Clin. Dev. 2018;9:330–346. doi: 10.1016/j.omtm.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zangi L., Lui K.O., von Gise A., Ma Q., Ebina W., Ptaszek L.M., Später D., Xu H., Tabebordbar M., Gorbatov R., et al. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat. Biotechnol. 2013;31:898–907. doi: 10.1038/nbt.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lui K.O., Zangi L., Silva E.A., Bu L., Sahara M., Li R.A., Mooney D.J., Chien K.R. Driving vascular endothelial cell fate of human multipotent Isl1+ heart progenitors with VEGF modified mRNA. Cell Res. 2013;23:1172–1186. doi: 10.1038/cr.2013.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gan L.M., Lagerström-Fermér M., Carlsson L.G., Arfvidsson C., Egnell A.C., Rudvik A., Kjaer M., Collén A., Thompson J.D., Joyal J., et al. Intradermal delivery of modified mRNA encoding VEGF-A in patients with type 2 diabetes. Nat. Commun. 2019;10:871. doi: 10.1038/s41467-019-08852-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collén A., Bergenhem N., Carlsson L., Chien K.R., Hoge S., Gan L.M., Fritsche-Danielson R. VEGF-A mRNA for regenerative treatment of heart failure. Nat. Rev. Drug Discov. 2022;21:79–80. doi: 10.1038/s41573-021-00355-6. ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anttila V., Saraste A., Knuuti J., Jaakkola P., Hedman M., Svedlund S., Lagerström-Fermér M., Kjaer M., Jeppsson A., Gan L.M. Synthetic mRNA encoding VEGF-A in patients undergoing coronary artery bypass grafting: design of a phase 2a clinical trial. Mol. Ther. Methods Clin. Dev. 2020;18:464–472. doi: 10.1016/j.omtm.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butler J., Hamo C.E., Udelson J.E., Pitt B., Yancy C., Shah S.J., Desvigne-Nickens P., Bernstein H.S., Clark R.L., Depre C., et al. Exploring new endpoints for patients with heart failure with preserved ejection fraction. Circ. Heart Fail. 2016;9:e003358. doi: 10.1161/CIRCHEARTFAILURE.116.003358. [DOI] [PubMed] [Google Scholar]
- 13.Yu Z., Witman N., Wang W., Li D., Yan B., Deng M., Wang X., Wang H., Zhou G., Liu W., et al. Cell-mediated delivery of VEGF modified mRNA enhances blood vessel regeneration and ameliorates murine critical limb ischemia. J. Control Release. 2019;310:103–114. doi: 10.1016/j.jconrel.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Geng Y., Duan H., Xu L., Witman N., Yan B., Yu Z., Wang H., Tan Y., Lin L., Li D., et al. BMP-2 and VEGF-A modRNAs in collagen scaffold synergistically drive bone repair through osteogenic and angiogenic pathways. Commun. Biol. 2021;4:82. doi: 10.1038/s42003-020-01606-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu F., Witman N., Yan D., Zhang S., Zhou M., Yan Y., Yao Q., Ding F., Yan B., Wang H., et al. Human adipose-derived stem cells enriched with VEGF-modified mRNA promote angiogenesis and long-term graft survival in a fat graft transplantation model. Stem Cell Res. Ther. 2020;11:490. doi: 10.1186/s13287-020-02008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun N., Ning B., Hansson K.M., Bruce A.C., Seaman S.A., Zhang C., Rikard M., DeRosa C.A., Fraser C.L., Wågberg M., et al. Modified VEGF-A mRNA induces sustained multifaceted microvascular response and accelerates diabetic wound healing. Sci. Rep. 2018;8:17509. doi: 10.1038/s41598-018-35570-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ai X., Yan B., Witman N., Gong Y., Yang L., Tan Y., Chen Y., Liu M., Lu T., Luo R., et al. Transient secretion of VEGF protein from transplanted hiPSC-CMs enhances engraftment and improves rat heart function post MI. Mol. Ther. 2022 doi: 10.1016/j.ymthe.2022.08.012. S1525-0016(22)00500-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grankvist R., Jensen-Urstad M., Clarke J., Lehtinen M., Little P., Lundberg J., Arnberg F., Jonsson S., Chien K.R., Holmin S. Superselective endovascular tissue access using trans-vessel wall technique: feasibility study for treatment applications in heart, pancreas and kidney in swine. J. Intern. Med. 2019;285:398–406. doi: 10.1111/joim.12841. [DOI] [PubMed] [Google Scholar]
- 19.Kajander S.A., Joutsiniemi E., Saraste M., Pietilä M., Ukkonen H., Saraste A., Sipilä H.T., Teräs M., Mäki M., Airaksinen J., et al. Clinical value of absolute quantification of myocardial perfusion with (15)O-water in coronary artery disease. Circ. Cardiovasc. Imaging. 2011;4:678–684. doi: 10.1161/CIRCIMAGING.110.960732. [DOI] [PubMed] [Google Scholar]
- 20.Danad I., Uusitalo V., Kero T., Saraste A., Raijmakers P.G., Lammertsma A.A., Heymans M.W., Kajander S.A., Pietilä M., James S., et al. Quantitative assessment of myocardial perfusion in the detection of significant coronary artery disease: cutoff values and diagnostic accuracy of quantitative [(15)O]H2O PET Imaging. J. Am. Coll. Cardiol. 2014;64:1464–1475. doi: 10.1016/j.jacc.2014.05.069. [DOI] [PubMed] [Google Scholar]
- 21.Green C.P., Porter C.B., Bresnahan D.R., Spertus J.A. Development and evaluation of the Kansas city Cardiomyopathy Questionnaire: a new health status measure for heart failure. J. Am. Coll. Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 22.Spertus J.A., Winder J.A., Dewhurst T.A., Deyo R.A., Prodzinski J., McDonell M., Fihn S.D. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J. Am. Coll. Cardiol. 1995;25:333–341. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 23.The Criteria Committee of the New York Heart Association . 9th Ed. Little, Brown & Co; 1994. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data underlying the findings described in this article may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.