Abstract
Cancer is a global public health issue that becomes the second primary cause of death globally. Considering the side effects of radio- or chemo-therapy, natural phytochemicals are promising alternatives for therapeutic interventions to alleviate the side effects and complications. Ginsenoside Rh2 (GRh2) is the main phytochemical extracted from Panax ginseng C.A. Meyer with anticancer activity. GRh2 could induce apoptosis and autophagy of cancer cells and inhibit proliferation, metastasis, invasion, and angiogenesis in vitro and in vivo. In addition, GRh2 could be used as an adjuvant to chemotherapeutics to enhance the anticancer effect and reverse the adverse effects. Here we summarized the understanding of the molecular mechanisms underlying the anticancer effects of GRh2 and proposed future directions to promote the development and application of GRh2.
Keywords: ginsenoside Rh2, anticancer, apoptosis, anti-proliferation
Graphical abstract
1. Introduction
Cancer has become a global public health issue with its increasing morbidity and mortality [1]. The operation, radio- and chemo-therapy are major current cancer therapeutic strategies. However, the operation is only therapeutic in early-stage cancer, and radio- or chemo-therapy still exists short-term toxicity and long-term consequences, such as alopecia [2], cognitive decline [3], skin erythema, mucositis, nausea, and diarrhea [4].
It has been generally recognized in recent years that natural phytochemicals are promising alternatives for therapeutic interventions intended to alleviate the side effects and complications in conventional cancer therapy. Ginseng, the root of Panax ginseng C.A. Meyer, is widely used as a natural health supplement in Asian countries. Ginsenosides are the main bioactive compounds extracted from ginseng. To date, there are more than one hundred naturally ginsenosides identified in ginseng [5]. And these ginsenosides have been proved to have multiple pharmacological activities (eg. anti-diabetes [6], reversing myocardial Ischemia-reperfusion injury [7], promoting cerebral angiogenesis [8], and improving chronic inflammatory arthritis [9]).
In preclinical studies, several types of ginsenosides have also been found to exert anticancer function in vivo and in vitro [10], and some have demonstrated the potential clinical application on therapy for non-small cell lung cancer [11] and rectal cancer [12]. Ginsenosides exert the anticancer effects mainly via inducing apoptosis, autophagy, and inhibiting cell proliferation, metastasis, and angiogenesis [13]. A large number of studies have revealed that the typical signaling pathways or molecules (eg. PI3K/Akt/mTOR, ERK/MAPK, Wnt/β-Catenin, and STAT3) have participated in the anticancer effects induced by ginsenosides (eg. Rg3, 20(S)-25-OCH3-PPD and CK) [[13], [14], [15]]. However, the mechanisms for triggering these pathways are still unclear, and the relationships between each independent molecules have not been well elucidated, which becomes an obstacle for further clinic trials.
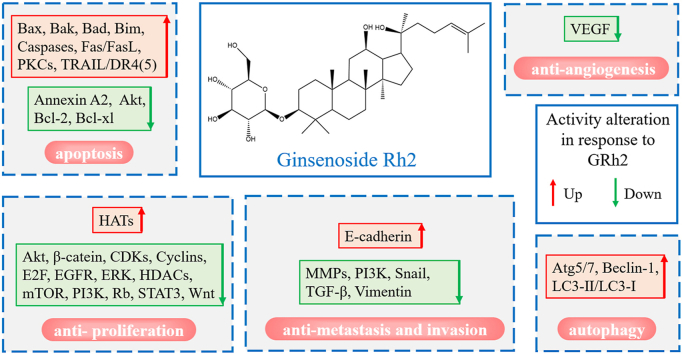
The same dilemma occurred on studies on ginsenoside Rh2, a protopanaxadiol-type ginsenoside. Ginsenoside Rh2 has been shown to have superior cytotoxic potency to cancer cells among various types of ginsenosides [16]. Certainly, ginsenoside Rh2 can regulate various signaling pathways associated with anticancer [17]. However, the clinical applications of ginsenoside Rh2 are still under restrictions due to the inexplicit mechanisms underlying its anticancer effects. Therefore, the regulation patterns of ginsenoside Rh2 on anticancer-associated molecules should be summarized systematically and logically. This review (1) focuses on recent advances in the understanding of the molecular mechanisms of Rh2-induced apoptosis, anti-proliferation, autophagy, anti-angiogenesis, and anti-metastasis effects on cancer cells, and summarizes associated key mediators into cascades, (2) introduces the combinations of ginsenoside Rh2 and other anticancer agents, (3) discusses the potential modification of ginsenoside Rh2 to improve its performance on anticancer, (4) proposes the potential applications of ginsenoside Rh2.
2. The stereoisomerism of ginsenoside Rh2
Ginsenoside Rh2 is one of the triterpene saponins and exists as two stereoisomers: 20(S)-ginsenoside Rh2 and 20(R)-ginsenoside Rh2. The two stereoisomeric pairs were differentiated by R- or S- configuration at carbon-20 (Table 1). This isomerism seems to have no effects on the physicochemical properties of ginsenoside Rh2 (Table 1). However, the stereoisomerism might determine the disparity in pharmacological effects between these two stereoisomers. The 20(S)-ginsenoside Rh2 showed stronger anticancer effects than its 20(R)-stereoisomer in various cancer cell lines [[18], [19], [20]]. The differences on pharmacokinetics might contribute to the differences on the pharmacology. It was reported that the uptake rate of 20(S)-ginsenoside Rh2 was 3-fold higher than 20(R)-ginsenoside Rh2 in Caco2 cell model, and the efflux ratio of 20(S)-ginsenoside Rh2 was significantly lower than the 20(R)-isomer [21]. And in rat model, the plasma concentration of 20(S)-ginsenoside Rh2 was 10-fold higher after intragastric administration with the same dose as the 20(R)-isomer [22]. The protopanoxadiol (PPD), as the important metabolite of Rh2 in vivo, has been proved to possess better anticancer activity than Rh2 [20]. And literature have demonstrated that 20(S)-PPD, but not 20(R)-PPD, was observed in plasma of rats which were administered with 20(S)-ginsenoside Rh2, and the plasma concentration ratio of 20(S)-PPD: 20(S)-ginsenoside Rh2 was about 1:4 [22]. On the contrary, PPD could be hardly detected in the rat plasma after administration with the 20(R)-ginsenoside Rh2. Taken together, higher absoption rates and higher concentration of metabolite PPD might contribute to the better anticancer activity of 20(S)-ginsenoside Rh2 (hereinafter referred to as GRh2). However, more evidences should be provided by further in vivo studies to certify this inference.
Table 1.
The Structure and Physicochemical Properties of Ginsenoside Rh2
| 20(S)-ginsenoside Rh2 | 20(R)-ginsenoside Rh2 | |
|---|---|---|
| Structure | 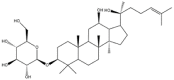 |
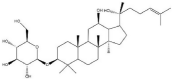 |
| CAS Registry Number | 67400-17-3 | 112246-15-8 |
| Molecular Formula | C36H62O8 | C36H62O8 |
| Molecular Weight | 622.873 | 622.873 |
| Density | 1.21 g/cm3 | 1.20 g/cm3 |
| Melting point | 225 °C | 228 °C |
| Boiling Point | 726.4 ± 60.0 °C at 760 mmHg | 726.4 ± 60.0 °C at 760 mmHg |
| Flashing Point | 365 ± 28 °C | 365 ± 28 °C |
| Water solubility | 65.5 μmol/L | 65.3 μmol/L |
| LogP | 5.62 | 5.62 |
| Surface Tension | 54.5 dyn/cm | 54.5 dyn/cm |
| Polarizability | 67.7 Å3 | 67.7 Å3 |
| Index of Refraction | 1.572 | 1.572 |
The data is from PubChem and United States Environmental Protection Agency.
3. Pharmacology of GRh2 in cancer model
3.1. GRh2-induced apoptosis
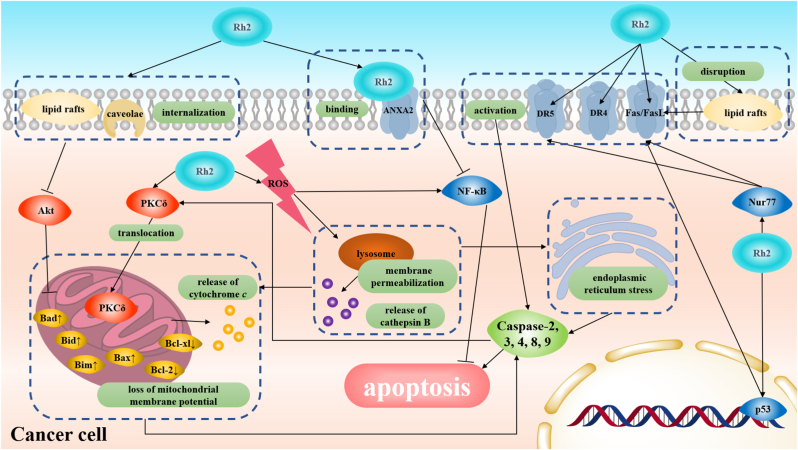
The apoptosis induced by GRh2 could be observed in a majority of common cancer models, and like other conventional anticancer drugs, caspases regulation plays a critical role in this apoptotic process (Fig. 1) [[23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33]]. Caspases are a conserved family of enzymes that can cleave an aspartate residue in their substrates [34]. Once effector caspases (which include caspase-2, -4, -8, -9) are activated [25,27,35], the caspase cascade will be initiated and effector caspases like caspase-3 will cleave various cellular targets like poly ADP-ribose polymerase (PARP) [30], which results in cell death eventually. However, the activation of the caspase-dependent pathway seems not the only way for GRh2 to trigger apoptosis, some caspase-independent pathways associated with NF-κB are also involved in GRh2-induced apoptosis [36,37].
Fig. 1.
Molecular mechanisms of GRh2-induced apoptosis.
3.1.1. Death-receptor (extrinsic) pathway
There have been a large number of studies reporting that GRh2-induced apoptosis was associated with activation of death receptors (and/or associated ligands) (eg. Fas/FasL, D4/TRAIL, and D5/TRAIL) and the following caspase cascades (Fig. 1 & Table 2). Recent evidences showed that GRh2 could activate death receptor-related proteins Fas/FasL and D5/TRAIL through up-regulating Nur77 expression in acute myeloid leukemia (AML) cell HL-60, which was followed by the activation of caspase-8 and caspase-3 (Fig. 1) [23]. Rather, it seems that the types of activated death receptors in GRh2-induced apoptosis can be cell-type-dependent. For example, GRh2 could only activate D4/TRAIL death receptor-related proteins and did not influence Fas/FasL pathway in lung adenocarcinoma A549 cells [27]. On the contrary, the GRh2 induced the overexpression of death receptor Fas not D4/D5 in HeLa cells but this up-regulating could be attenuated in p53-silence HeLa cells or p53-mutated SW480 cells (Fig. 1), which demonstrated GRh2-induced Fas overexpression was mediated by p53 [38]. However, the activation of p53 might not be solely responsible for Fas activation, as GRh2 can cause lipid rafts disruption which then initiates ligand-independent Fas activation [39]. The cell-type-dependent regulation of death receptors might be related to that the membrane protein composition differs from various cell lines, and death receptors are predominately located in the plasma membrane. Therefore, considering the different death receptors composition in the plasma membrane, different cell lines could show the distinguishing sensibility of death receptors in response to external stimulus.
Table 2.
Important Mediators of GRh2-Induced Anticancer Effects
| Key molecule(s) | Activity alteration in response to GRh2 | Pharmacological effects associated | References |
|---|---|---|---|
| Annexin A2 | DOWN | Apoptosis | [37] |
| Akt | DOWN | Apoptosis, Anti-proliferation | [31,45,49,59,72] |
| Atg5/7 | UP | Autophagy | [44,45,50,75] |
| Bax | UP | Apoptosis | [24,[31], [32], [33],35,36,45,46,52] |
| Bak | UP | Apoptosis | [32] |
| Bad | UP | Apoptosis | [31,36] |
| β-catenin | DOWN | Anti-proliferation | [53,73,75] |
| Bcl-2 | DOWN | Apoptosis | [24,30,32,33,35,36,45,46,52] |
| Bcl-xL | DOWN | Apoptosis | [32,36] |
| Beclin-1 | UP | Autophagy | [45,50] |
| Bim | UP | Apoptosis | [31,32] |
| Caspases | UP | Apoptosis | [[23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33],39] |
| CDKs | DOWN | Anti-proliferation | [26,27,43,52,54,56] |
| Cyclins | DOWN | Anti-proliferation | [26,27,45,[52], [53], [54],56] |
| E-cadherin | UP | Anti-metastasis and invasion | [50,73] |
| E2F | DOWN | Anti-proliferation | [26,54,61] |
| EGFR | DOWN | Anti-proliferation | [58,59,64] |
| ERK | DOWN | Anti-proliferation | [26,60] |
| Fas/FasL | UP | Apoptosis | [23,39] |
| HATs | UP | Anti-proliferation | [52] |
| HDACs | DOWN | Anti-proliferation | [52,70] |
| LC3-Ⅱ/LC3-Ⅰ | UP | Autophagy | [75] |
| mTOR | DOWN | Anti-proliferation | [45,49,59] |
| MMPs | DOWN | Anti-metastasis and invasion | [53,[70], [71], [72]] |
| PI3K | DOWN | Anti-proliferation, Anti-metastasis and invasion | [45,49,72] |
| PKCs | UP | Apoptosis | [29] |
| Rb | DOWN | Anti-proliferation | [27,54] |
| Snail | DOWN | Anti-metastasis and invasion | [50] |
| STAT3 | DOWN | Anti-proliferation | [71] |
| TGF-β | DOWN | Anti-metastasis and invasion | [50] |
| TRAIL/DR4(5) | UP | Apoptosis | [23,27] |
| VEGF | DOWN | Anti-angiogenesis | [[67], [68], [69]] |
| Vimentin | DOWN | Anti-metastasis and invasion | [50,73] |
| Wnt | DOWN | Anti-proliferation | [53,73] |
However, almost all GRh2 death-receptor pathway studies were only implemented as in vitro studies in human cancer cell lines, and a noteworthy issue that whether the death-receptor pathway is involved in GRh2-induced cancer cell death in mouse models still remains to be illuminated.
3.1.2. Mitochondrial (intrinsic) death pathway
A large number of studies have demonstrated that the mitochondrial death pathway plays a role in GRh2-induced apoptosis. When the mitochondrial death pathway is activated, cytochrome c can be released from mitochondrial intermembrane space, and then assemble with apoptotic protease-activating factor-1 (APAF-1) to form an apoptosome for the recruitment and autoactivation of caspase-9 [40]. Except for the proteins associated with the mitochondrial death pathway, the loss of mitochondrial membrane potential (a signal in the initial stage of the mitochondrial death pathway) has been also observed in GRh2-induced cancer cell death [24]. It is also worth mentioning that, not like the Fas/FasL pathway, the levels of mitochondrial-mediated caspase-9 expression and cytochrome c release are similar in both HeLa cells and p53-mutated SW480 cells (Fig. 1), indicating that the GRh2-induced mitochondrial death pathway is independent on p53 status in cervical cancer model [38]. Like a majority of anticancer drug, GRh2 triggers activation of the intrinsic apoptotic cascade in two possible ways (Table 2). One is that GRh2 regulates the expression of pro-apoptosis genes and pro-survival genes associated with mitochondria. The other one is that GRh2 regulates the up-steam signaling pathway of mitochondria. The possible mechanisms in the following three sections have been confirmed in the GRh2-induced mitochondrial death pathway.
3.1.2.1. Activation of caspase-3-dependent protein kinase C δ
It has been confirmed that activation of protein kinase C δ (PKCδ) could lead to translocation of PKCδ into the mitochondria followed by mitochondrial dysfunction and cytochrome c release [41]. Pre-treatment with the specific PKCδ inhibitor rottlerin could suppress the activation of caspase-9 and caspase-3, resulting in inhibiting GRh2-induced apoptosis (Fig. 1). And it has been also proved that caspase-3 could activate PKCδ selectively in GRh2-induced apoptosis [29]. These discoveries indicate that there exists a positive feedback loop mechanism of caspase-3 and PKCδ activation in GRh2-induced apoptosis.
3.1.2.2. Selective activation or induction of BH3-only/Bcl-2 family proteins
BH3-only proteins (Bcl-2 homology domain 3 only proteins), as pro-apoptotic proteins, are sensors to apoptotic signals derived from the various extracellular stimulus and intracellular processes. When BH3-only proteins are activated, they will interact with anti- and pro-apoptotic B cell lymphoma 2 (Bcl-2) family proteins to facilitate the apoptosis process [42]. The proapoptotic Bcl-2 family proteins like Bax and Bak cause mitochondrial outer membrane permeabilization and activate the mitochondrial death pathway. Conversely, anti-apoptotic Bcl-2 family proteins including Bcl-2, Bcl-xl, and Mcl-1 can inhibit the activation of pro-apoptotic Bcl-2 family proteins [43]. Accordingly, the activation of BH3-only proteins or Bcl-2 family proteins in response to GRh2-induced initiation of the mitochondrial death pathway has been investigated. Bax, Bcl-xl, and Bcl-2 have been irrefutably implicated in the GRh2-induced mitochondrial death pathway, the activation of Bax, and the inhibition of Bcl-xl and Bcl-2 have been observed in various cancer cell lines with GRh2-treatment (Fig. 1) [32,35,36]. Concerning the BH3-only proteins, Bim, Bid and Bad have been proved to be activated during GRh2-induced apoptosis [29,32].
How GRh2 regulates BH3-only or Bcl-2 family proteins is a major confusion remaining to be specifically explained. There have been two possible models being proposed: regulation of ROS and Akt. Destabilized by ROS, the lysosomal membrane can become permeabilized, and cathepsin B can be released in hepatoma HepG2 cells treated with GRh2, which contributes to the cleavage and activation of Bid (Fig. 1) [44]. In human epidermoid carcinoma A431 cells, GRh2 could down-regulate Akt activation by inducing the internalization of lipids rafts and caveolae [31]. And the Akt activation is responsible for the inhibition of the interaction between Bad and Bcl-2 family proteins (Fig. 1) [31,45]. Additionally, overexpression of voltage-dependent anion channel 1 also made contributions to the translocation of Bcl-2 and Bax into mitochondria [46].
Taken together, these studies illustrated the mechanisms of BH3-only and Bcl-2 family proteins in activating the mitochondrial death pathway in cancer cell lines with GRh2 treatment.
3.1.2.3. Regulation of ROS activity
Reactive oxygen species (ROS) can be detected after treatment with GRh2, and treatment with antioxidant N-acetyl-L-cysteine can alleviate GRh2-induced apoptosis. Moreover, the increase in ROS level contributes to the depletion of mitochondrial membrane potential and lysosomal membrane permeabilization (Fig. 1) [24,47]. Additionally, previous studies have shown that GRh2-induced ROS could initiate endoplasmic reticulum stress, leading to the activation of the caspase cascade (Fig. 1) [25]. However, the issue of how ROS level was elevated in GRh2-induced apoptosis currently remains unresolved. Interestingly, ROS functions as double-edged swords in GRh2-induced cancer cell death. GRh2-induced ROS can activate the NF-κB pathway, and treatment with NF-κB pathway inhibitor PS-1145 promote the GRh2-induced cell death, which indicates that the ROS-induced NF-κB pathway suppresses the GRh2-induced apoptosis (Fig. 1) [36]. Even though a large number of studies have demonstrated that cellular ROS was associated with the activation of various cytoplasmic signaling pathways which have been proved to be involved in GRh2-induced apoptosis such as protein kinase C (PKC), c-Jun N-terminal kinase (JNK), p38 kinase (p38MAPK) and PI3K/Akt pathways [48]. There is no sufficient evidence proving that it is ROS that directly mediates these pathways in the GRh2-induced apoptosis model.
3.1.3. Other pathways associated
As mentioned above, GRh2-induced ROS can activate NF-κB to suppress apoptosis. Fortunately, GRh2 has found another way to suppress the activation of the NF-κB pathway. Once activated by Annexin A2, NF-κB could promote the expression of anti-apoptosis genes like c-IAP1, c-IPA2, X-IAP, and Survivin. GRh2 is found to bind with Annexin A2, and then inhibit the interaction between Annexin A2 and NF-κB, consequently, down-regulates NF-кB activity (Fig. 1) [37]. In human lung adenocarcinoma A549 cells, GRh2 can induce JNK activation which contributes to the increases in expression and activity of downstream targeted genes like AP-1 and promotes cell apoptosis [26]. However, how GRh2 activated the JNK pathway remains to be explored. In addition, it is worth noticing that the regulation of gut microbiota and the immune system by GRh2 might be also associated with GRh2-induced apoptosis of cancer cells [49].
3.2. Anti-proliferation
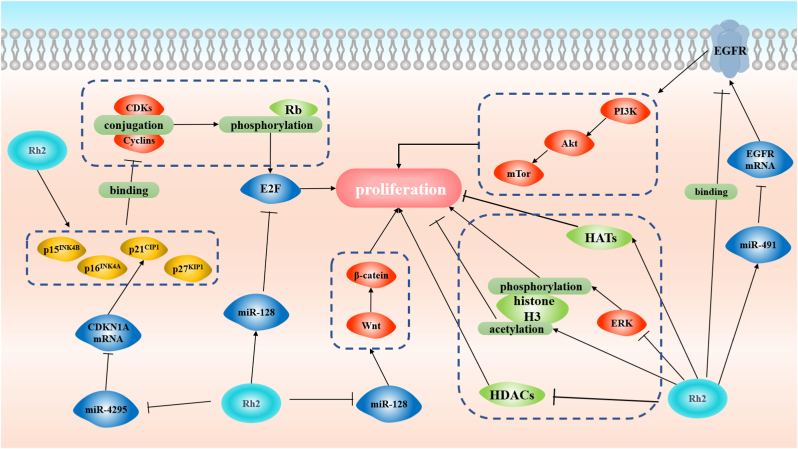
The growth of the tumor can also be suppressed by GRh2 (Fig. 2). In uterine leiomyoma cells, GRh2 can induce the activation of p38 MAPK and inhibition of c-Src, which both suppress the phosphorylation of estrogen receptor alpha (a proliferative effects initiator) [50]. Moreover, GRh2-induced anti-proliferation of cancer cells was found mainly associated with the cell-cycle arrest at the G0/G1 and G1/S boundary. A large number of studies demonstrate that the retinoblastoma tumor suppressor protein (Rb), epidermal growth factor (EGF), microRNA (miR), and histone are all involved in GRh2-inhibited proliferation (Table 2).
Fig. 2.
Molecular mechanisms of GRh2-induced anti-proliferation.
3.2.1. Regulation of Rb-related pathway
Cyclin-dependent kinases (CDKs) and their cyclin partners (Cyclins) are critical regulatory factors in cell proliferation. Cyclin D–CDK4/6 and Cyclin E–CDK2 complexes can activate the Rb phosphorylation which enables the E2F transcription factors to initiate transcription of genes promoting the cell cycle progression [51]. A large number of studies have proved that GRh2 could suppress the phosphorylation of Rb and the levels of CDKs, Cyclins, and E2F transcription factors in cancer cells [26,27,[52], [53], [54], [55], [56]]. GRh2 can up-regulate the levels of cyclin-dependent kinase inhibitors (including p15INK4B, p16INK4A, p21CIP1, and p27KIP1) which bind to CDKs or CDK-cyclin complexes and inhibit the kinase activity of CDKs [27,[52], [53], [54], [55], [56]]. Consequently, the phosphorylation of Rb and activation of E2F transcription factors are inhibited.
3.2.2. Inhibition of EGF-induced proliferation
EGF receptors (EGFR) are overexpressed in human carcinomas, which results in an ungovernable clinical behavior [57]. As a result, it becomes a rational way for anticancer treatment to block or abolish the functions of EGFR. The activation of EGFR is inhibited in response to GRh2-treatment in various cell lines [[58], [59], [60]]. GRh2 can bind to EGFR, thereby, depleting the sensitivity of EGFR to EGF, and inhibiting following PI3k/Akt/mTor signaling cascades (Fig. 2) [59]. However, GRh2 is unlikely to restrain the activity of EGF directly.
3.2.3. Regulation of miRs
According to all the studies to date, GRh2 can regulate the expression of more than 30 miRs [61], and some of them have been proved to be involved in the regulation of Rb-related pathways and suppression of EGFR. Concerning E2F transcription factors, GRh2 can suppress E2F3a via up-regulating the expression of miR-128 (Fig. 2) [62]. MiR-4295 can bind to 3′-UTR of CDKN1A mRNA (encoding p21CIP1), then promote phosphorylation of Rb, which has been found to be inhibited by GRh2 treatment (Fig. 2) [63]. Up-regulation of miR-491 is another way for GRh2 to suppress EGFR. The miR-491 can suppress EGFR protein translation through binding to 3′-UTR of EGFR mRNA (Fig. 2) [64,65]. Other pathways that have been directly implicated in GRh2-regulated miR expression include the Wnt/β-catenin signaling pathway which promotes cell proliferation. The Wnt/β-catenin signaling pathway is inhibited in response to GRh2 treatment: GRh2 can suppress the miR-31 expression, whereas miR-31 overexpression makes contributions to the activation of Wnt/β-catein signaling pathway (Fig. 2) [53]. Moreover, a recent research revealed that long non-coding RNA (like LNRNA C3orf67-AS1) also participated in GRh2-exerted anti-proliferation [39].
3.2.4. Modification of histone
Histone deacetylases (HDACs) and histone acetyltransferases (HATs) act as regulators of gene expression during cell proliferation by modifying chromatin. Through regulating HDACs and HATs, GRh2 can break the balance between acetylation and deacetylation of histone, leading to cell-cycle arrest in the cancer model (Fig. 2) [52]. A study by Liu et al showed that HDAC activity was decreased while HAT activity was increased in human leukemia K562 and KG1-a cells treated with GRh2 [28]. To be specific, GRh2 decreased the expression of HDAC1, 2 and 6, and promoted acetylation of histone H3 both in vivo and in vitro [28]. On the contrary, the phosphorylation of H3 was inhibited by GRh2 in HCT116 colon cancer cells due to the inhibition of ERK2 phosphorylation and PDZ-binding kinase/T-LAK cell-originated protein kinase pathway (Fig. 2) [36]. However, how GRh2-induced modifications of histone regulated the cell cycle remained to be unclear until Li et al discovered the relationship between H3K27me3 modification and cell proliferation [55]. GRh2 could decrease expression of H3k27me3 and EZH2 (a histone methyltransferase of H3K27me3), and inhibit their recruitment in p14, p15, and p16 genes promoter regions, and consequently, promote mRNA expression of the CDKN2A-2B gene cluster (encoding CDKs inhibitors) [55]. These findings revealed that GRh2 might function as a histone deacetylases inhibitor. In theory, histone deacetylases inhibitor could also induce apoptosis [66], but there is no sufficient evidence to support that the GRh2-induced apoptosis is associated with HDACs (Fig. 1).
3.3. Tumor angiogenesis, metastasis, and invasion
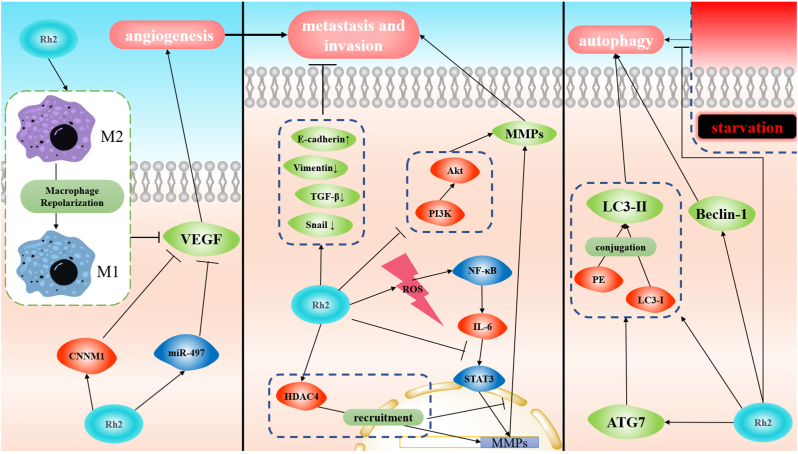
GRh2 has anti-angiogenic, anti-metastatic, and anti-invasive activities that play critical roles in the inhibition of tumor development and progression (Fig. 3 & Table 2). Anti-angiogenesis blocks the ways for the tumor to obtain sufficient nutrient supply, which inhibits tumor metastasis. The anti-angiogenic effect of GRh2 is achieved by inhibiting the expression of vascular endothelial growth factor (VEGF) which can promote tumor outgrowth and invasion. To be specific, GRh2-induced up-regulation of cyclin and CBS domain divalent metal cation transport mediator 1, the increase in miR-497 level and the reversion from the phenotype of M2 macrophages to M1 subtype all contribute to inhibition of VEGF (Fig. 3) [[67], [68], [69]].
Fig. 3.
Molecular mechanisms of GRh2-induced angiogenesis, autophagy, anti-metastasis, and anti-invasion.
The suppression of matrix metalloproteinases (MMPs) is the key to the GRh2-induced anti-metastatic effect. Four different ways have been reported to inhibit the expression of MMPs with GRh2 treatment. GRh2 can increase HDAC4 level and lead to the recruitment of HDAC4 to the MMP-3 promoter site and the binding site of proximal analyze activator protein 1, which inhibits the transcription of MMP-3 (Fig. 3) [70]. MicroRNAs are also involved in GRh2-induced inhibition of MMPs. The overexpression of miR-31 increased MMP-2 and MMP-9 levels, consequently reversed the anti-metastatic effect of GRh2 [53]. As mentioned above, GRh2-induced ROS can activate the NF-кB pathway, and then the IL-6 level was up-regulated. IL-6 can promote the expression of MMPs via activating the STAT3 signaling pathway, and GRh2 can reverse the overexpression of MMPs by inhibiting the interaction between IL-6 and its receptor (Fig. 3) [71]. Moreover, the MMP-13 was found inhibited owing to the suppression of the PI3k/Akt pathway induced by GRh2 in glioblastoma multiforme [72]. The increase in E-cadherin level and the decrease in epithelial-mesenchymal transition (EMT)-related proteins like vimentin, TGF-β, and Snail were observed under GRh2 treatment (Fig. 3), which indicated that EMT-mediated tumor metastasis was inhibited by GRh2 [73]. However, the mechanism underlying GRh2-inhibited EMT remains to be elusive.
3.4. GRh2-induced autophagy
The GRh2-induced autophagy has been associated with an increased expression level of autophagy-related protein (Table 2). During the vesicle nucleation stage of autophagy, Beclin-1 can scaffold class III PI3K complex [74], and GRh2 treatment can increase the Beclin-1 level in various cell lines [75,76]. LC3-II, the conjugation of LC3-I and PE, is an autophagosome marker that is mediated by ATG7 [77]. The higher ATG7 level and LC3-Ⅱ/LC3-Ⅰ ratio can be observed in cells with GRh2 treatment than in control groups (Fig. 3) [75,76]. Taken together, these studies provide pieces of evidences supporting the role of GRh2 in inducing autophagy through regulating of the expression of autophagy-related proteins. Interestingly, GRh2 was found to inhibit autophagy caused by starvation (free-serum cultivation), and starvation-induced autophagy could suppress the apoptosis of cancer cells (Fig. 3) [78].
4. Combining GRh2 with other drugs
GRh2 has shown its potential to be a single-anticancer agent. However, accumulated empirical experiments revealed that chemotherapeutic combination could achieve synergistic effects or attenuate drug toxicity.
4.1. Synergistic effects
4.1.1. With chemotherapeutic drugs
Synergistic death of various cancer cells could be observed following treatment with GRh2 in a combination of chemotherapeutics. Initially, GRh2 was found to yield synergistic activity in lowing cell viability when combined with paclitaxel, mitoxantrone, or docetaxel [79,80]. Then studies on the molecular level revealed that BH3-only/Bcl-2 family proteins and caspase family proteins played important roles in the synergistic effect.
In the human colorectal carcinoma cells model, GRh2 and sodium selenite could increase the Bax/Bcl2 ratio and the caspase-3 expression synergistically [81]. Given that both regorafenib and GRh2 can inhibit VEGF or VEGF receptors, they could function synergistically when downregulating survivin and overexpressing caspase-3, thereby, exhibited a synergistic inhibitory effect on HepG2 cells [82]. GRh2 could synergize with SMI-4a in increasing the LC3-Ⅱ expression and caspase 3/caspase 7 activity in melanoma cells [83].
4.1.2. With phytochemicals
The treatment with a combination of GRh2 and other phytochemicals (which have not been utilized in clinic) could also cause the synergistic death of cancer cells. Li et al extracted and purified corilagin from longan, and found that the combination of GRh2 and corilagin exerted synergistic cytotoxicity on SKOv3ip and Hey cells [84]. Li et al found that the combination treatment with GRh2 and betulinic acid could increase the expression of cleaved caspase-8, cleaved caspase-9, tBid, and the release of cytochrome c in Hela cells [85]. GRh2 could also synergize with its derivative – protopanaxadiol on antiproliferative activity in human breast cancer cells, and the phosphorylation of BAD, p53 and p38 protein has been proved to contribute to this synergistic effect [86].
GRh2 has already been proved to function synergistically with chemotherapeutic drugs or other phytochemicals. However, in many instances, the mechanisms of drug combinations involving GRh2 were still hypotheses, and the related experiments still could not elucidate why the combination could function synergistically.
4.2. Reversal effect on drug resistance
During chemotherapy, the acquisition of drug resistance is the major reason for the decline in therapeutic efficacy. Fortunately, previous reports proved that GRh2 could downregulate the expression of drug-resistance genes and proteins (eg. MRP1, MDR1, LRP, and GST) in 5-FU-resistant colorectal carcinoma cells, consequently, enhance the cytotoxicity of 5-FU to 5-FU-resistant colorectal carcinoma cells [87]. Drug efflux is the major mechanism for the acquisition of drug resistance. The over-expression of P-gp accounts for the efflux, and the GRh2 could inhibit P-gp via regulating the pentose phosphate pathway and redox balance [88]. Consequently, GRh2 could enhance the accumulation of adriamycin in nuclei, mitochondria, and cytosol in the adriamycin-resistant MCF-7 cells [89].
4.3. Decreasing side effects of chemotherapy
Highly proliferated cells would be inhibited or eliminated during chemotherapy, however, the benign cells, tissue, and organ could be impaired at the same time. Normal cellular senescence is the main side effect of chemotherapy due to DNA impairment. Previous reports indicated that GRh2 could inhibit the DNA damage induced by cyclophosphamide [90]. Subsequently, GRh2 was proved to inhibit cellular senescence-associated migration and invasion of human breast cells [91]. These effects could be explained by Hou et al who found GRh2 could suppress the phosphorylation of MEK1, MAPK p38, STAT3, and NF-κB p65 in senescent breast cells induced by doxorubicin [92]. Hou et al also found that GRh2 could regulate the mitochondrial dynamic, eliminate ROS level, promote mitophagy, and subsequently inhibit senescence-associated secretory phenotype in senescent breast cells [91].
5. Discussion and future perspectives
GRh2 is a traditional anticancer phytochemical that induce tumor cell death, differentiation, and cell-cycle arrest. These effects attribute to a large number of molecular events (eg. the activation of death-receptor, mitophagy, oxidative stress, and the secretion of cytokine) and participation of various molecules (including proteins and RNAs). Moreover, GRh2 could synergize with other anticancer agents, decrease the side effects of chemotherapy, and reverse the drug resistance.
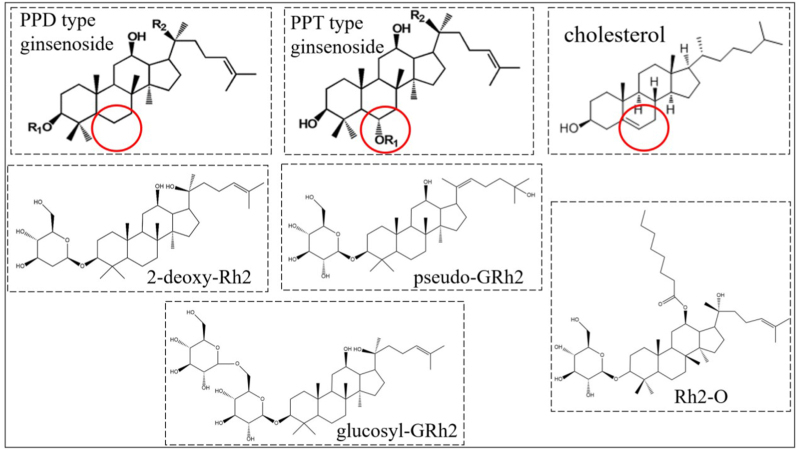
When GRh2 exerts its anticancer effects, lipid rafts disruption and ROS generation seem to play a significant role. This could be its unique mechanism when compared with other phytochemicals like polyphenols. The structure of GRh2 might be the reason. It is acknowledged that cholesterol could induce apoptosis and autophagy through generating ROS [93]. And GRh2 (a protopanaxadiols type ginsenoside a protopanaxadiols type ginsenoside which has no C-6 substituents) possesses a similar structure with cholesterol (Fig. 4), thereby, might exert similar biological activity (eg. generating ROS). Meanwhile, with a similar structure, GRh2 shows a good affinity to cholesterol which is an important substance to constitute lipid rafts in the plasma membrane. Therefore, GRh2 could insert into lipid rafts and interact with cholesterol, consequently, disrupt the internalization of lipid rafts [94]. This reminds us that GRh2 might exert better functions if the structure of GRh2 could be modified to possess better compatibility with the plasma membrane. And the function of GRh2 might be extended based on the biological effects of cholesterol, for example, GRh2 might regulate the lipid metabolism in the cancer cell.
Fig. 4.
The structures of cholesterol, ginsenoside and their derivative.
Synergistic effects between drugs are usually attributed to that 1) they can interact with the same target and the interaction of one drug with the target can promote the interaction of other drugs with the target, or 2) they are regulated in different pathways, but these pathways can interact with each other. The current studies mainly focus on the typical pathways GRh2 and other drugs both regulate. However, considering the chemotherapeutic agents are well-acknowledged to regulate the typical anticancer pathways, it is more worthwhile to study other associated pathways (eg. metabolism-associated pathways) GRh2 might regulate during exerting synergistic effects.
The side effects of drugs were mainly due to their non-organ-specific or non-tissue-specific. The biological processes in benign cells would be disrupted. GRh2 and its derivative have been proved that they could modulate abnormal biological processes into normal levels (eg. reversing the immunosuppression) [95]. Therefore, the use of GRh2 in adjuvant treatment or polypharmacy would be gradually accepted.
Obviously, there are still several aspects limiting the use of GRh2:
-
1)
There are few clinical trial data that could prove the anti-cancer effects of GRh2. The studies on GRh2 were still in the pre-clinical stage (cell model and mouse model). Considering GRh2 could synergize with many anticancer agents, it would be promising to use GRh2 as an adjuvant agent during cancer therapy if the clinical trial could provide enough evidence for the effectiveness and the safety of GRh2.
-
2)
The aqueous solubility and the bioavailability of GRh2 are relatively low, which limits its function in the human body. According to Lipinski's rule of five, the logP of the compounds should be lower than 5 (from 0-3, to be specific) to obtain high oral bioavailability. However, the logP of GRh2 is 5.62, so the structure of GRh2 needs to be modified. Esterification might be an effective way to increase the lipophilic solubility of GRh2 by inserting a carbon chain [96], thereby increasing the oral bioavailability of GRh2. Zhang et al [97] synthesized octyl ester derivative of ginsenoside Rh2 (Rh2–O) with ethyl acetate and GRh2 (Fig. 4). And the Rh2–O was found to possess higher absorption rate than GRh2 in both Caco2 and HepG2 cell models [97,98]. And then the Rh2–O has been proved to exert better anti-hepatoma activity than GRh2 in vitro and in vivo [98]. And other derivatives of GRh2 have also been proved to improve the anticancer activity of GRh2. Qian et al synthesized b-D-Glucopyranoside,(3b,12b,20E)-12,25-dihydroxydammar-20(22)-en-3-yl (pseudo-GRh2) (Fig. 4) and the pseudo-GRh2 exerted better anticancer activity than GRh2 in various cell lines [99]. The modification of the sugar moieties (eg. decreasing the number of hydroxyls) is another way to increase the lipophicity. Gao et al [93] replaced the glucose moiety of GRh2 by 2-deoxy-glucose (Fig. 4), and found this modification increased the toxicities of GRh2 to various cancer cell lines. However, considering that the glycosyl addition decrease the lipophicity, the glucosyl-GRh2 exerted poorer anticancer activity than GRh2 [100].
-
3)
the mechanisms of GRh2 exerting the anti-cancer effect have not been completely elucidated yet. There were amounts of typical cell signaling pathways (eg. PI3K/Akt/p-mTOR pathway and ERK/JNK pathway) that participated in GRh2-induced anticancer progress. However, how GRh2 triggered these pathways remain unclear. In general, drugs can bind to target proteins in the cytomembrane or cytosol to activate the signaling cascade. Nevertheless, the target of GRh2 for anticancer has not been discovered adequately, only a few proteins (eg. Annexin A2 and EGFR) have been proved to be the binding target of GRh2. The transcriptomics and proteomics which the current studies on GRh2 lacked might help to discover some novel targets. With these problems solved, the use of GRh2 for adjuvant therapy in cancer might have a bright future. For example, GRh2 could be used as an anti-angiogenesis agent to combine with immune checkpoint blockade for cancer treatment. And as a P-gp inhibitor, GRh2 could be used to improve the absorption of other oral drugs (not only for chemotherapeutics). Moreover, even if GRh2 could not be applicated in clinic at present, it could still be added as a functional phytochemical into the dietary intervention for cancer patients.
Declaration of competing interest
The author declare no conflict of interest.
Acknowledgment
We would like to thank the following funding sources: The National Natural Science Foundation of China (NO. 81860578), and the Academic and Technical Leaders Training Program of Major Disciplines in Jiangxi Province—Young Talents Programme (NO. 20204BCJ23025).
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Munzone E., Bagnardi V., Campenni G., Mazzocco K., Pagan E., Tramacere A., Masiero M., Iorfida M., Mazza M., Montagna E., et al. Preventing chemotherapy-induced alopecia: a prospective clinical trial on the efficacy and safety of a scalp-cooling system in early breast cancer patients treated with anthracyclines. Br J Cancer. 2019;121(4):325–331. doi: 10.1038/s41416-019-0520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Razzak M., Marshall L. Radiotherapy toxicity. Nat Rev Dis Primers. 2019;5(1):14. doi: 10.1038/s41572-019-0068-1. [DOI] [PubMed] [Google Scholar]
- 4.Bentzen S.M. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. 2006;6(9):702–713. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- 5.Kim Y.J., Zhang D., Yang D.C. Biosynthesis and biotechnological production of ginsenosides. Biotechnol Adv. 2015;33(6 Pt 1):717–735. doi: 10.1016/j.biotechadv.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Zhou P., Xie W., He S., Sun Y., Meng X., Sun G., Sun X. Ginsenoside Rb1 as an anti-diabetic agent and its underlying mechanism analysis. Cells. 2019;8(3) doi: 10.3390/cells8030204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng Q., Bao X.Y., Zhu P.C., Tong Q., Zheng G.Q., Wang Y. Ginsenoside Rb1 for myocardial ischemia/reperfusion injury: preclinical evidence and possible mechanisms. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/6313625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J., Zhang X., Liu X., Zhang C., Shang W., Xue J., Chen R., Xing Y., Song D., Xu R. Ginsenoside Rg1 promotes cerebral angiogenesis via the PI3K/Akt/mTOR signaling pathway in ischemic mice. Eur J Pharmacol. 2019;856 doi: 10.1016/j.ejphar.2019.172418. [DOI] [PubMed] [Google Scholar]
- 9.Hou T., Liu Y., Wang X., Jiao D., Xu H., Shi Q., Wang Y., Li W., Wu T., Liang Q. Ginsenoside Rg1 promotes lymphatic drainage and improves chronic inflammatory arthritis. J Musculoskelet Neuronal Interact. 2020;20(4):526–534. [PMC free article] [PubMed] [Google Scholar]
- 10.Ratan Z.A., Haidere M.F., Hong Y.H., Park S.H., Lee J.-O., Lee J., Cho J.Y. Pharmacological potential of ginseng and its major component ginsenosides. J of Ginseng Res. 2020;45(2):199–210. doi: 10.1016/j.jgr.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y., Wang Y., Niu K., Chen X., Xia L., Lu D., Kong R., Chen Z., Duan Y., Sun J. Clinical benefit from EGFR-TKI plus ginsenoside Rg3 in patients with advanced non-small cell lung cancer harboring EGFR active mutation. Oncotarget. 2016;7(43):70535–70545. doi: 10.18632/oncotarget.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiing J.H., Chen Y.Q., Ji M.X., Zhu S.G., Gong X.Q. Clinical study on effect of ginsenoside in inducing rectal cancer cell apoptosis. Chinese Journal of Integrated Traditional and Western Medicine. 2001;21(4):260–261. [in Chinese] [PubMed] [Google Scholar]
- 13.Chen X.J., Zhang X.J., Shui Y.M., Wan J.B., Gao J.L. Anticancer activities of protopanaxadiol- and protopanaxatriol-type ginsenosides and their metabolites. Evid Based Complement Alternat Med. 2016;2016 doi: 10.1155/2016/5738694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun M., Ye Y., Xiao L., Duan X., Zhang Y., Zhang H. Anticancer effects of ginsenoside Rg3 (review) Int J Mol Med. 2017;39(3):507–518. doi: 10.3892/ijmm.2017.2857. [DOI] [PubMed] [Google Scholar]
- 15.Kim D., Park M., Haleem I., Lee Y., Koo J., Na Y.C., Song G., Lee J. Natural product ginsenoside 20(S)-25-Methoxyl-Dammarane-3beta, 12beta, 20-triol in cancer treatment: a review of the pharmacological mechanisms and pharmacokinetics. Front Pharmacol. 2020;11:521. doi: 10.3389/fphar.2020.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong H., Bai L.P., Wong V.K., Zhou H., Wang J.R., Liu Y., Jiang Z.H., Liu L. The in vitro structure-related anti-cancer activity of ginsenosides and their derivatives. Molecules. 2011;16(12):10619–10630. doi: 10.3390/molecules161210619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X., Chu S., Lin M., Gao Y., Liu Y., Yang S., Zhou X., Zhang Y., Hu Y., Wang H., et al. Anticancer property of ginsenoside Rh2 from ginseng. Eur J Med Chem. 2020;203 doi: 10.1016/j.ejmech.2020.112627. [DOI] [PubMed] [Google Scholar]
- 18.Cheong J.H., Kim H., Hong M.J., Hong M.J., Yang M.H., Yang M.H., Kim J.W., Kim J.W., Yoo H., Yoo H., et al. Stereoisomer-specific anticancer activities of ginsenoside Rg3 and Rh2 in HepG2 cells: disparity in cytotoxicity and autophagy-inducing effects due to 20(S)-epimers. Biol Pharm Bull. 2015;38(1):102–108. doi: 10.1248/bpb.b14-00603. [DOI] [PubMed] [Google Scholar]
- 19.Liu J., Shimizu K., Yu H., Yu H., Zhang C., Zhang C., Jin F., Jin F., Kondo R., Kondo R. Stereospecificity of hydroxyl group at C-20 in antiproliferative action of ginsenoside Rh2 on prostate cancer cells. Fitoterapia. 2010;81(7):902–905. doi: 10.1016/j.fitote.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 20.Dong H., Bai L.P., Wong V.K.W., Wong V.K., Zhou H., Zhou H., Wang J.R., Wang J.R., Liu Y., Liu Y., et al. The in vitro structure-related anti-cancer activity of ginsenosides and their derivatives. Molecules. 2011;16(12):10619–10630. doi: 10.3390/molecules161210619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu Y., Wang G.J., Wu X.L., Wu X.L., Zheng Y.T., Zheng Y.T., Zhang J.W., Zhang J.W., Ai H., Ai H., et al. Intestinal absorption mechanisms of ginsenoside Rh2: stereoselectivity and involvement of ABC transporters. Xenobiotica. 2010;40(9):602–612. doi: 10.3109/00498254.2010.500744. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J., Zhou F., Niu F., Lu M., Wu X., Sun J., Wang G. Stereoselective regulations of P-glycoprotein by ginsenoside Rh2 epimers and the potential mechanisms from the view of pharmacokinetics. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0035768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang C., He H., Dou G., Li J., Zhang X., Jiang M., Li P., Huang X., Chen H., Li L., et al. Ginsenoside 20(S)-Rh2 induces apoptosis and differentiation of acute myeloid leukemia cells: role of orphan nuclear receptor Nur77. J Agric Food Chem. 2017;65(35):7687–7697. doi: 10.1021/acs.jafc.7b02299. [DOI] [PubMed] [Google Scholar]
- 24.Xia T., Wang Y.N., Zhou C.X., Wu L.M., Liu Y., Zeng Q.H., Zhang X.L., Yao J.H., Wang M., Fang J.P. Ginsenoside Rh2 and Rg3 inhibit cell proliferation and induce apoptosis by increasing mitochondrial reactive oxygen species in human leukemia Jurkat cells. Mol Med Rep. 2017;15(6):3591–3598. doi: 10.3892/mmr.2017.6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ge G., Yan Y., Cai H. Ginsenoside Rh2 inhibited proliferation by inducing ROS mediated ER stress dependent apoptosis in lung cancer cells. Biological & Pharmaceutical Bulletin. 2017;40(12):2117–2124. doi: 10.1248/bpb.b17-00463. [DOI] [PubMed] [Google Scholar]
- 26.Liu X., Sun Y., Yue L., Li S., Qi X., Zhao H., Yang Y., Zhang C., Yu H. JNK pathway and relative transcriptional factor were involved in ginsenoside Rh2-mediated G1 growth arrest and apoptosis in human lung adenocarcinoma A549 cells. Genet Mol Res. 2016;15(3) doi: 10.4238/gmr.15039003. [DOI] [PubMed] [Google Scholar]
- 27.Cheng C.C., Yang S.M., Huang C.Y., Chen J.C., Chang W.M., Hsu S.L. Molecular mechanisms of ginsenoside Rh2-mediated G1 growth arrest and apoptosis in human lung adenocarcinoma A549 cells. Cancer Chemother Pharmacol. 2005;55(6):531–540. doi: 10.1007/s00280-004-0919-6. [DOI] [PubMed] [Google Scholar]
- 28.Park J.A., Lee K.Y., Oh Y.J., Kim K.W., Lee S.K. Activation of caspase-3 protease via a Bcl-2-insensitive pathway during the process of ginsenoside Rh2-induced apoptosis. Cancer Letters. 1998;121(1):73–81. doi: 10.1016/s0304-3835(97)00333-9. [DOI] [PubMed] [Google Scholar]
- 29.Oh J.I., Chun K.H., Joo S.H., Oh Y.T., Lee S.K. Caspase-3-dependent protein kinase C delta activity is required for the progression of Ginsenoside-Rh2-induced apoptosis in SK-HEP-1 cells. Cancer Lett. 2005;230(2):228–238. doi: 10.1016/j.canlet.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 30.Kim J.H., Choi J.S. Effect of ginsenoside Rh-2 via activation of caspase-3 and Bcl-2-insensitive pathway in ovarian cancer cells. Physiol Res. 2016;65(6):1031–1037. doi: 10.33549/physiolres.933367. [DOI] [PubMed] [Google Scholar]
- 31.Park E.K., Lee E.J., Lee S.H., Koo K.H., Sung J.Y., Hwang E.H., Park J.H., Kim C.W., Jeong K.C., Park B.K., et al. Induction of apoptosis by the ginsenoside Rh2 by internalization of lipid rafts and caveolae and inactivation of Akt. Br J Pharmacol. 2010;160(5):1212–1223. doi: 10.1111/j.1476-5381.2010.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi S., Oh J.Y., Kim S.J. Ginsenoside Rh2 induces Bcl-2 family proteins-mediated apoptosis in vitro and in xenografts in vivo models. J Cell Biochem. 2011;112(1):330–340. doi: 10.1002/jcb.22932. [DOI] [PubMed] [Google Scholar]
- 33.Zhu S., Liu X., Xue M., Li Y., Cai D., Wang S., Zhang L. 20(S)-ginsenoside Rh2 induces caspase-dependent promyelocytic leukemia-retinoic acid receptor A degradation in NB4 cells via Akt/Bax/caspase9 and TNF-alpha/caspase8 signaling cascades. J Ginseng Res. 2021;45(2):295–304. doi: 10.1016/j.jgr.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riedl S.J., Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol. 2004;5(11):897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- 35.Chen F., Sun Y., Zheng S.L., Qin Y., Julian McClements D., Hu J.N., Deng Z.Y. Antitumor and immunomodulatory effects of ginsenoside Rh2 and its octyl ester derivative in H22 tumor-bearing mice. Journal of Functional Foods. 2017;32:382–390. [Google Scholar]
- 36.Li B., Zhao J., Wang C.Z., Searle J., He T.C., Yuan C.S., Du W. Ginsenoside Rh2 induces apoptosis and paraptosis-like cell death in colorectal cancer cells through activation of p53. Cancer Lett. 2011;301(2):185–192. doi: 10.1016/j.canlet.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y.S., Lin Y., Li H., Li Y., Song Z., Jin Y.H. The identification of molecular target of (20S) ginsenoside Rh2 for its anti-cancer activity. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-12572-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo X.X., Li Y., Sun C., Jiang D., Lin Y.J., Jin F.X., Lee S.-K., Jin Y.H. p53-dependent Fas expression is critical for Ginsenoside Rh2 triggered caspase-8 activation in HeLa cells. Protein & Cell. 2014;5(3):224–234. doi: 10.1007/s13238-014-0027-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yi J.S., Choo H.J., Cho B.R., Kim H.M., Kim Y.N., Ham Y.M., Ko Y.G. Ginsenoside Rh2 induces ligand-independent Fas activation via lipid raft disruption. Biochem Biophys Res Commun. 2009;385(2):154–159. doi: 10.1016/j.bbrc.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 40.Ow Y.P., Green D.R., Hao Z., Mak T.W. Cytochrome c: functions beyond respiration. Nat Rev Mol Cell Biol. 2008;9(7):532–542. doi: 10.1038/nrm2434. [DOI] [PubMed] [Google Scholar]
- 41.Mochly-Rosen D., Das K., Grimes K.V. Protein kinase C, an elusive therapeutic target? Nat Rev Drug Discov. 2012;11(12):937–957. doi: 10.1038/nrd3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Youle R.J., Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9(1):47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 43.Bock F.J., Tait S.W.G. Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol. 2020;21(2):85–100. doi: 10.1038/s41580-019-0173-8. [DOI] [PubMed] [Google Scholar]
- 44.Chen F., Zhang B., Sun Y., Xiong Z.X., Peng H., Deng Z.Y., Hu J.N. The octyl ester of ginsenoside Rh2 induces lysosomal membrane permeabilization via bax translocation. Nutrients. 2016;8(5):244. doi: 10.3390/nu8050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xia T., Zhang J., Zhou C., Li Y., Duan W., Zhang B., Wang M., Fang J. 20(S)-Ginsenoside Rh2 displays efficacy against T-cell acute lymphoblastic leukemia through the PI3K/Akt/mTOR signal pathway. J Ginseng Res. 2020;44(5):725–737. doi: 10.1016/j.jgr.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y., Wang J., Qiao J., Liu S., Wang S., Zhao D., Bai X., Liu M. Ginsenoside Rh2 inhibits HeLa cell energy metabolism and induces apoptosis by upregulating voltage-dependent anion channel 1. Int J Mol Med. 2020;46(5):1695–1706. doi: 10.3892/ijmm.2020.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen F., Deng Z., Xiong Z., Zhang B., Yang J., Hu J. A ROS-mediated lysosomal–mitochondrial pathway is induced by ginsenoside Rh2 in hepatoma HepG2 cells. Food Funct. 2015;6(12):3828–3837. doi: 10.1039/c5fo00518c. [DOI] [PubMed] [Google Scholar]
- 48.Perillo B., Di Donato M., Pezone A., Di Zazzo E., Giovannelli P., Galasso G., Castoria G., Migliaccio A. ROS in cancer therapy: the bright side of the moon. Exp Mol Med. 2020;52(2):192–203. doi: 10.1038/s12276-020-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xia T., Zhang B., Li Y., Fang B., Zhu X., Xu B., Zhang J., Wang M., Fang J. New insight into 20(S)-ginsenoside Rh2 against T-cell acute lymphoblastic leukemia associated with the gut microbiota and the immune system. Eur J Med Chem. 2020;203 doi: 10.1016/j.ejmech.2020.112582. [DOI] [PubMed] [Google Scholar]
- 50.Zhu Y., Xu J., Li Z., Xie S., Zhou J., Guo X., Zhou X., Li G., Zhong R., Ma A. Ginsenoside Rh2 suppresses growth of uterine leiomyoma in vitro and in vivo and may regulate ERα/c-Src/p38 MAPK activity. J Funct Foods. 2015;18:73–82. [Google Scholar]
- 51.Otto T., Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer. 2017;17(2):93–115. doi: 10.1038/nrc.2016.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Z.H., Li J., Xia J., Jiang R., Zuo G.W., Li X.P., Chen Y., Xiong W., Chen D.L. Ginsenoside 20(s)-Rh2 as potent natural histone deacetylase inhibitors suppressing the growth of human leukemia cells. Chem Biol Interact. 2015;242:227–234. doi: 10.1016/j.cbi.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 53.Chen Y., Shang H., Zhang S., Zhang X. Ginsenoside Rh2 inhibits proliferation and migration of medulloblastoma Daoy by down-regulation of microRNA-31. J Cell Biochem. 2018;119(8):6527–6534. doi: 10.1002/jcb.26716. [DOI] [PubMed] [Google Scholar]
- 54.Choi S., Kim T.W., Singh S.V. Ginsenoside Rh2-mediated G1 phase cell cycle arrest in human breast cancer cells is caused by p15 Ink4B and p27 Kip1-dependent inhibition of cyclin-dependent kinases. Pharm Res. 2009;26(10):2280–2288. doi: 10.1007/s11095-009-9944-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Q., Li B., Dong C., Wang Y., Li Q. 20(S)-Ginsenoside Rh2 suppresses proliferation and migration of hepatocellular carcinoma cells by targeting EZH2 to regulate CDKN2A-2B gene cluster transcription. Eur J Pharmacol. 2017;815:173–180. doi: 10.1016/j.ejphar.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 56.Lee K.Y., Park J.A., Chung E., You H.L., Lee S.K. Ginsenoside-Rh2 blocks the cell cycle of SK-HEP-1 cells at the G1/S boundary by selectively inducing the protein expression of p27kip1. Cancer Letters. 1996;110(1–2):193–200. doi: 10.1016/s0304-3835(96)04502-8. [DOI] [PubMed] [Google Scholar]
- 57.Mendelsohn J., Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2001;19(56):6550–6565. doi: 10.1038/sj.onc.1204082. [DOI] [PubMed] [Google Scholar]
- 58.Li S., Gao Y., Ma W., Guo W., Zhou G., Cheng T., Liu Y. EGFR signaling-dependent inhibition of glioblastoma growth by ginsenoside Rh2. Tumour Biol. 2014;35(6):5593–5598. doi: 10.1007/s13277-014-1739-x. [DOI] [PubMed] [Google Scholar]
- 59.Li S., Guo W., Gao Y., Liu Y. Ginsenoside Rh2 inhibits growth of glioblastoma multiforme through mTor. Tumour Biol. 2015;36(4):2607–2612. doi: 10.1007/s13277-014-2880-2. [DOI] [PubMed] [Google Scholar]
- 60.Yang J., Yuan D., Xing T., Su H., Zhang S., Wen J., Bai Q., Dang D. Ginsenoside Rh2 inhibiting HCT116 colon cancer cell proliferation through blocking PDZ-binding kinase/T-LAK cell-originated protein kinase. J Ginseng Res. 2016;40(4):400–408. doi: 10.1016/j.jgr.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu N., Wu G.C., Hu R., Li M., Feng H. Ginsenoside Rh2 inhibits glioma cell proliferation by targeting microRNA-128. Acta Pharmacol Sin. 2011;32(3):345–353. doi: 10.1038/aps.2010.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim Y.S., Jin S.H., Lee Y.H., Kim S., Park J.D. Ginsenoside Rh2 induces apoptosis independently of Bcl-2, Bcl-xL or Bax in C6Bu-1 cells. Archives of Pharmacal Research. 1999;22(5):448–453. doi: 10.1007/BF02979151. [DOI] [PubMed] [Google Scholar]
- 63.Gao Q., Zheng J. Ginsenoside Rh2 inhibits prostate cancer cell growth through suppression of microRNA-4295 that activates CDKN1A. Cell Prolif. 2018;51(3) doi: 10.1111/cpr.12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen W., Qiu Y. Ginsenoside Rh2 targets EGFR by up-regulation of miR-491 to enhance anti-tumor activity in hepatitis B virus-related hepatocellular carcinoma. Cell Biochem Biophys. 2015;72(2):325–331. doi: 10.1007/s12013-014-0456-9. [DOI] [PubMed] [Google Scholar]
- 65.Park J.A., Kim K.-W., Kim S.I., Lee S.K. Caspase 3 specifically cleaves p21WAF1/CIP1 in the earlier stage of apoptosis in SK-HEP-1 human hepatoma cells. European Journal of Biochemistry. 1998;257(1):242–248. doi: 10.1046/j.1432-1327.1998.2570242.x. [DOI] [PubMed] [Google Scholar]
- 66.Bolden J.E., Peart M.J., Johnstone R.W. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5(9):769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 67.Li S., Gao Y., Ma W., Cheng T., Liu Y. Ginsenoside Rh2 inhibits invasiveness of glioblastoma through modulation of VEGF-A. Tumour Biol. 2015;37:15477–15482. doi: 10.1007/s13277-015-3759-6. [DOI] [PubMed] [Google Scholar]
- 68.Li H., Huang N., Zhu W., Wu J., Yang X., Teng W., Tian J., Fang Z., Luo Y., Chen M., et al. Modulation the crosstalk between tumor-associated macrophages and non-small cell lung cancer to inhibit tumor migration and invasion by ginsenoside Rh2. BMC Cancer. 2018;18(1):579. doi: 10.1186/s12885-018-4299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang Y., Huang H., Han Z., Li W., Mai Z., Yuan R. Ginsenoside Rh2 inhibits angiogenesis in prostate cancer by targeting CNNM1. J Nanosci Nanotechnol. 2019;19(4):1942–1950. doi: 10.1166/jnn.2019.16404. [DOI] [PubMed] [Google Scholar]
- 70.Shi Q., Li J., Feng Z., Zhao L., Luo L., You Z., Li D., Xia J., Zuo G., Chen D. Effect of ginsenoside Rh2 on the migratory ability of HepG2 liver carcinoma cells: recruiting histone deacetylase and inhibiting activator protein 1 transcription factors. Mol Med Rep. 2014;10(4):1779–1785. doi: 10.3892/mmr.2014.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Han S., Jeong A.J., Yang H., Bin Kang K., Lee H., Yi E.H., Kim B.H., Cho C.H., Chung J.W., Sung S.H., et al. Ginsenoside 20(S)-Rh2 exerts anti-cancer activity through targeting IL-6-induced JAK2/STAT3 pathway in human colorectal cancer cells. J Ethnopharmacol. 2016;194:83–90. doi: 10.1016/j.jep.2016.08.039. [DOI] [PubMed] [Google Scholar]
- 72.Guan N., Huo X., Zhang Z., Zhang S., Luo J., Guo W. Ginsenoside Rh2 inhibits metastasis of glioblastoma multiforme through Akt-regulated MMP13. Tumour Biol. 2015;36(9):6789–6795. doi: 10.1007/s13277-015-3387-1. [DOI] [PubMed] [Google Scholar]
- 73.Zhang G., He L., Chen J., Xu B., Mao Z. Ginsenoside Rh2 activates alpha-catenin phosphorylation to inhibit lung cancer cell proliferation and invasion. Exp Ther Med. 2020;19(4):2913–2922. doi: 10.3892/etm.2020.8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Levy J.M.M., Towers C.G., Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17(9):528–542. doi: 10.1038/nrc.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang Z., Zhao T., Liu H., Zhang L. Ginsenoside Rh2 inhibits hepatocellular carcinoma through beta-catenin and autophagy. Sci Rep. 2016;6 doi: 10.1038/srep19383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li M., Zhang D., Cheng J., Liang J., Yu F. Ginsenoside Rh2 inhibits proliferation but promotes apoptosis and autophagy by down-regulating microRNA-638 in human retinoblastoma cells. Exp Mol Pathol. 2019;108:17–23. doi: 10.1016/j.yexmp.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 77.Mulcahy Levy J.M., Thorburn A. Autophagy in cancer: moving from understanding mechanism to improving therapy responses in patients. Cell Death Differ. 2020;27(3):843–857. doi: 10.1038/s41418-019-0474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang J., Bian S., Wang S., Yang S., Zhang W., Zhao D., Liu M., Bai X. Ginsenoside Rh2 represses autophagy to promote cervical cancer cell apoptosis during starvation. Chin Med. 2020;15(1):118. doi: 10.1186/s13020-020-00396-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Musende A.G., Eberding A., Jia W., Ramsay E., Bally M.B., Guns E.T. Rh2 or its aglycone aPPD in combination with docetaxel for treatment of prostate cancer. Prostate. 2010;70(13):1437–1447. doi: 10.1002/pros.21179. [DOI] [PubMed] [Google Scholar]
- 80.Xie X., Eberding A., Madera C., Fazli L., Jia W., Goldenberg L., Gleave M., Guns E.S. Rh2 synergistically enhances paclitaxel or mitoxantrone in prostate cancer models. J Urol. 2006;175(5):1926–1931. doi: 10.1016/S0022-5347(05)00891-8. [DOI] [PubMed] [Google Scholar]
- 81.Zhu C., Liu F., Qian W., Zhang T., Li F. Combined effect of sodium selenite and ginsenoside Rh2 on HCT116 human colorectal carcinoma cells. Arch Iran Med. 2016;19(1):23. [PubMed] [Google Scholar]
- 82.Wang B., Wang F., Ding A., Zhao H., Bu X. Regorafenib and ginsenoside combination therapy: inhibition of HepG2 cell growth through modulating survivin and caspase-3 gene expression. Clin Transl Oncol. 2020;22(9):1491–1498. doi: 10.1007/s12094-019-02283-9. [DOI] [PubMed] [Google Scholar]
- 83.Lv D.L., Chen L., Ding W., Zhang W., Wang H.L., Wang S., Liu W.B. Ginsenoside G-Rh2 synergizes with SMI-4a in anti-melanoma activity through autophagic cell death. Chin Med. 2018;13:11. doi: 10.1186/s13020-018-0168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li N., Lin Z., Chen W., Zheng Y., Ming Y., Zheng Z., Huang W., Chen L., Xiao J., Lin H. Corilagin from longan seed: identification, quantification, and synergistic cytotoxicity on SKOv3ip and hey cells with ginsenoside Rh2 and 5-fluorouracil. Food Chem Toxicol. 2018;119:133–140. doi: 10.1016/j.fct.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 85.Li Q., Li Y., Wang X., Fang X., He K., Guo X., Zhan Z., Sun C., Jin Y.H. Co-treatment with ginsenoside Rh2 and betulinic acid synergistically induces apoptosis in human cancer cells in association with enhanced capsase-8 activation, bax translocation, and cytochrome c release. Mol Carcinog. 2011;50(10):760–769. doi: 10.1002/mc.20673. [DOI] [PubMed] [Google Scholar]
- 86.Ren G., Wu C., Teng C., Yao Y. Synergistic effect of combined protopanaxatiol and ginsenoside Rh2 on antiproliferative activity in MDA-MB-231 human breast cancer cells in vitro. Food Agr Immunol. 2018;29(1):953–963. [Google Scholar]
- 87.Liu G.W., Liu Y.H., Jiang G.S., Ren W.D. The reversal effect of Ginsenoside Rh2 on drug resistance in human colorectal carcinoma cells and its mechanism. Hum Cell. 2018;31(3):189–198. doi: 10.1007/s13577-017-0189-3. [DOI] [PubMed] [Google Scholar]
- 88.Liu J., Cai Q., Wang W., Lu M., Liu J., Zhou F., Sun M., Wang G., Zhang J. Ginsenoside Rh2 pretreatment and withdrawal reactivated the pentose phosphate pathway to ameliorate intracellular redox disturbance and promoted intratumoral penetration of adriamycin. Redox Biol. 2020;32 doi: 10.1016/j.redox.2020.101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang J., Zhou F., Wu X., Zhang X., Chen Y., Zha B.S., Niu F., Lu M., Hao G., Sun Y., et al. Cellular pharmacokinetic mechanisms of adriamycin resistance and its modulation by 20(S)-ginsenoside Rh2 in MCF-7/Adr cells. Br J Pharmacol. 2012;165(1):120–134. doi: 10.1111/j.1476-5381.2011.01505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang Z., Zheng Q., Liu K., Li G., Zheng R. Ginsenoside Rh2 enhances antitumour activity and decreases genotoxic effect of cyclophosphamide. Basic Clin Pharmacol Toxicol. 2006;98(4):411–415. doi: 10.1111/j.1742-7843.2006.pto_348.x. [DOI] [PubMed] [Google Scholar]
- 91.Hou J., Yun Y., Xue J., Jeon B., Kim S. Doxorubicin-induced normal breast epithelial cellular aging and its related breast cancer growth through mitochondrial autophagy and oxidative stress mitigated by ginsenoside Rh2. Phytother Res. 2020;34(7):1659–1669. doi: 10.1002/ptr.6636. [DOI] [PubMed] [Google Scholar]
- 92.Hou J.G., Jeon B.M., Yun Y.J., Cui C.H., Kim S.C. Ginsenoside Rh2 ameliorates doxorubicin-induced senescence bystander effect in breast carcinoma cell MDA-MB-231 and normal epithelial cell MCF-10a. Int J Mol Sci. 2019;20(5):1244. doi: 10.3390/ijms20051244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gao H., Liang D., Li C., Xu G., Jiang M., Li H., Yin J., Song Y. 2-Deoxy-Rh2: a novel ginsenoside derivative, as dual-targeting anti-cancer agent via regulating apoptosis and glycolysis. Biomed Pharmacother. 2020;124 doi: 10.1016/j.biopha.2020.109891. [DOI] [PubMed] [Google Scholar]
- 94.Verstraeten S.L., Lorent J.H., Mingeot-Leclercq M.P. Lipid membranes as key targets for the pharmacological actions of ginsenosides. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.576887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu H.C., Hu Q.R., Luo T., Wei W.C., Wu H.J., Li J., Zheng L.F., Xu Q.Y., Deng Z.Y., Chen F. The immunomodulatory effects of ginsenoside derivative Rh2-O on splenic lymphocytes in H22 tumor-bearing mice is partially mediated by TLR4. Int Immunopharmacol. 2021;101 doi: 10.1016/j.intimp.2021.108316. [DOI] [PubMed] [Google Scholar]
- 96.Wang Z., Ding M., Lin Z., He C., Zhao Y. Esterified derivatives of panaxadiol and their inhibitory effect on HL-60, THP-1, and PC-3 cell lines. Chem Biodivers. 2019;16(8) doi: 10.1002/cbdv.201900188. [DOI] [PubMed] [Google Scholar]
- 97.Zhang B., Ye H., Zhu X.M., Hu J.N., Li H.Y., Tsao R., Deng Z.Y., Zheng Y.N., Li W. Esterification enhanced intestinal absorption of ginsenoside Rh2 in Caco-2 cells without impacts on its protective effects against H₂O₂-induced cell injury in human umbilical vein endothelial cells (HUVECs) J Agric Food Chem. 2014;62(9):2096–2103. doi: 10.1021/jf404738s. [DOI] [PubMed] [Google Scholar]
- 98.Chen F., Deng Z.Y., Zhang B., Xiong Z.X., Zheng S.L., Tan C.L., Hu J.N. Esterification of ginsenoside Rh2 enhanced its cellular uptake and antitumor activity in human HepG2 cells. J Agric Food Chem. 2016;64(1):253–261. doi: 10.1021/acs.jafc.5b05450. [DOI] [PubMed] [Google Scholar]
- 99.Qian G., Wang Z., Zhao J., Li D., Gao W., Wang B., Sui D., Qu X., Chen Y. Synthesis and anti-cancer cell activity of pseudo-ginsenoside Rh2. Steroids. 2014;92:1–6. doi: 10.1016/j.steroids.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 100.Wang D.D., Kim Y.J., Baek N.I., Mathiyalagan R., Wang C., Jin Y., Xu X.Y., Yang D.C. Glycosyltransformation of ginsenoside Rh2 into two novel ginsenosides using recombinant glycosyltransferase from Lactobacillus rhamnosus and its in vitro applications. J Ginseng Res. 2021;45(1):48–57. doi: 10.1016/j.jgr.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]