Abstract
Mesenchymal stem cells (MSCs) are ubiquitous multipotent cells that exhibit significant therapeutic potentials in a variety of disorders. Nevertheless, their clinical efficacy is limited owing to poor survival, low rate of engraftment, and impaired potency upon transplantation. Spheroidal three-dimensional (3D) culture of MSCs (MSC3D) has been proven to better preserve their in vivo functional properties. However, the molecular mechanisms underlying the improvement in MSC function by spheroid formation are not clearly understood. NLRP3 inflammasomes, a key component of the innate immune system, have recently been shown to play a role in cell fate decision of MSCs. The present study examined the role of NLRP3 inflammasomes in the survival and potency of MSC spheroids. We found that MSC3D led to decreased activation of NLRP3 inflammasomes through alleviation of ER stress in an autophagy-dependent manner. Importantly, downregulation of NLRP3 inflammasomes signaling critically contributes to the enhanced survival rate in MSC3D through modulation of pyroptosis and apoptosis. The critical role of NLRP3 inflammasome suppression in the enhanced therapeutic efficacy of MSC spheroids was further confirmed in an in vivo mouse model of DSS-induced colitis. These findings suggest that 3D culture confers survival and functional advantages to MSCs by suppressing NLRP3 inflammasome activation.
Keywords: autophagy, ER tress, inflammasomes, MSC, spheroids
Graphical abstract

Spheroidal three-dimensional culture exhibits various advantages in the therapeutic application of MSCs. Downregulation of NLRP3 inflammasomes is essentially required for the enhanced therapeutic efficacy of MSC spheroids by modulating autophagy and ER stress. Accordingly, targeting NLRP3 inflammasomes might be a promising strategy for improving the effectiveness of MSC therapy.
Introduction
Mesenchymal stem cells (MSCs) are heterogeneous fibroblast-like cells possessing potent tissue-reparative and immunoregulatory characteristics along with multipotent differentiation capacity.1 A large body of evidence delineates that MSCs may serve as a promising cell-based therapy for treatment of various diseases, including cardiovascular diseases, inflammatory and autoimmune disorders, and malignancies.1,2 Although their therapeutic potential has been well established, the clinical efficacy of MSCs is limited by the poor survival, low engraftment rate, and impaired potency upon transplantation.3,4 For this reason, developing effective strategies to improve the therapeutic effectiveness of MSCs has received a great deal of attention in recent years. Common strategies aiming to enhance the lifespan in vivo, homing ability, and paracrine functions of MSCs are composed of preconditioning with various stimuli, such as hypoxia, serum deprivation, heat shock, genetic manipulation, and culture engineering.5
Spheroids, one of the most common three-dimensional (3D) culture methods, have been proven to better preserve the functional properties of MSCs. Indeed, the formation of MSC spheroidal aggregates may increase paracrine activities, enhance stemness, and confer a survival advantage to transplanted MSCs.6,7 Mechanistically, the enhanced cell survival and function of MSC spheroids were ascribed to the regulation of several key intracellular signaling pathways.8 The core of spheroids is hypoxic, and HIF-1α acts as a master transcription factor that regulates a wide range of genes related to cellular metabolic reprogramming, redox balance, and production of growth factors and cytokines in MSC spheroids.9 Recently, it has been documented that HIF-1α upregulation contributes to improved cell survival in MSC spheres through suppression of apoptosis in an autophagy-dependent manner.10 However, the molecular mechanisms underlying autophagy induction upon the 3D aggregation of MSCs and by which it contributes to the enhanced cell viability remain elusive. In addition to changes in the pattern of gene expression associated with hypoxia, interleukin (IL)-1 autocrine signaling and epigenetic modifications have been also shown to mediate upregulation of immunomodulatory factors and maintenance of stem cell multipotency, respectively, in MSC spheroids.11,12
Inflammasomes are multiprotein complexes that act as key players in the innate immune system.13 In response to various extracellular and intracellular pro-inflammatory signals, inflammasome components, including nucleotide-binding domain-like receptor protein (NLRP) and apoptotic speck protein (ASC), assemble and form a scaffold for the recruitment of pro-caspase-1. Caspase-1 is then activated by an autocleavage process and promotes the maturation and release of pro-inflammatory cytokines, such as IL-1β and IL-18.14 Initially discovered in myeloid cells, inflammasome components were further found in a variety of cell types including, but not limited to, hepatocytes, endothelial cells, adipocytes, and malignant cells where they participate in the pathogenesis of various diseases.15,16 Although the role of inflammasomes in MSCs has received little attention so far, there are some lines of evidence indicating that functional inflammasomes are expressed in MSCs. Chen et al. reported that activation of NLRP3 and non-canonical inflammasomes led to increased secretion of pro-inflammatory cytokines and induction of pyroptosis in bone marrow-derived MSCs.17 In addition, NLRP3 inflammasomes in human MSCs were activated upon LPS/palmitic acid stimulation, which results in decreased osteogenesis but increased adipogenesis.18 In contrast, a recent study has reported that human umbilical cord blood-derived MSCs preconditioned with LPS/ATP, which leads to activation of NLRP3 inflammasomes, show a better efficacy than naïve MSCs in alleviating dextran sulfate sodium (DSS)-induced colitis, presumably due to the enhanced immunosuppressive function of MSCs.19 These reports support a multifaceted role of NLRP3 inflammasomes in the regulation of MSC fate and function, which may depend on sources of MSC, stimulus types, and exposure duration.
MSCs can undergo various stressful conditions at transplanted sites, such as hypoxia, nutrient deprivation, and inflammation.20 Furthermore, oxidative stress, characterized by increased production of reactive oxygen species (ROS) in damaged tissues, compromises the function of MSCs through induction of anoikis and apoptosis.21,22 Given the role of ROS as upstream molecules in the inflammasome cascade,23 an unfavorable environment upon transplantation may trigger inflammasome activation, which in turn contributes to the modulation of transplanted MSC fate decision. Intriguingly, it has been previously shown that spheroid culture of MSCs downregulates the production of ROS but activates autophagy, a negative modulator of inflammasomes,10 raising the possibility that modulation of inflammasome activation critically contributes to the improved survival and therapeutic effectiveness of 3D-cultured MSCs. In the current study, we observed that NLRP3 inflammasome activation occurred in 2D-cultured MSCs under serum deprivation but was significantly suppressed by spheroid aggregation. We also found that the modulation of inflammasome activation by 3D culture was mediated by induction of autophagy and downregulation of the ER stress-thioredoxin interacting protein (TXNIP) axis. Importantly, blockage of NLRP3 inflammasome signaling in MSCs by RNA interference enhanced the viability and therapeutic efficacy of MSCs in a DSS-induced colitis mouse model, highlighting the key role of NLRP3 inflammasome signaling in determining the survival and therapeutic functions of MSCs.
Results
The NLRP3 inflammasome activation is downregulated in 3D spheroid cultured MSCs
To examine whether NLRP3 inflammasomes play roles in the differential functions of MSCs in 2D and 3D cultures, we first compared the basal levels of NLRP3 inflammasome activation in human adipose tissue-derived MSCs under 2D and 3D culture and found that the levels of active caspase-1 and mature IL-1β were significantly decreased in 3D culture of MSCs (MSC3D) compared with those in MSC2D (Figures 1A and 1B). In addition, other components of inflammasomes, including NLRP3 and ASC, were markedly downregulated under 3D culture (Figures 1C and 1D). Likewise, decreased expression of NLRP3, ASC, and IL-1β were also observed at the transcriptional level (Figure 1E). Suppression of inflammasome activation and downregulation of NLRP3 and ASC were further confirmed in mouse adipose tissue-derived MSCs (Figures 1F–1I). To further confirm the modulation of NLRP3 inflammasomes under the 3D culture, we measured the formation of ASC specks, which are aggregates of NLRP3, ASC, and pro-caspase-1 formed during inflammasome activation and are regarded as a hallmark of inflammasome activation, by labeling ASC proteins with a fluorescent-conjugated antibody. As shown in Figure 1J, the proportion of ASC speck-positive cells was significantly higher in MSC2D than in MSC3D, as expected. Taken together, these findings clearly demonstrate that MSCs express a large number of functional NLRP3 inflammasomes in 2D culture, but 3D culture suppresses the inflammasome activity in MSCs.
Figure 1.
Three-dimensinal culture suppresses NLRP3 inflammasome activation in MSCs
(A–I) Human adipose-derived (A–E) or mouse adipose-derived MSCs (F–I) were cultured in monolayers (MSC2D) and spheroids (MSC3D) for 24 h. Expression levels of IL-1β, caspase-1, NLRP3, and ASC were examined by western blot analysis (A–D and F–I). The mRNA levels of NLRP3, ASC, and IL-1β in human MSCs were measured by RT-qPCR (E). For western blot analyses, representative images from three independent experiments were presented along with bar diagrams showing the quantification of band intensity by densitometric analysis. (J) MSC2D and sections prepared from MSC spheroids were incubated with anti-ASC antibody, followed by incubation with an Alexa 488-conjugated secondary antibody. Cells were counterstained with DAPI (blue). ASC specks presented as green dots are indicated by red arrows. Representative images from three independent experiments are shown along with quantification of the proportion of ASC speck-positive cells; scale bar, 20 μm. ∗Denotes p < 0.05 compared with the MSC2D (n = 3).
Downregulation of NLRP3 inflammasomes contributes to enhanced cell survival in 3D-cultured MSCs
We next asked if suppression of NLRP3 inflammasome activation was implicated in the enhanced viability of MSCs under 3D culture. We first confirmed that the viability of MSCs in spheroids was significantly higher than that in monolayers (Figure 2A). Consistently, cell death, as determined by lactate dehydrogenase (LDH) assay, was lower in MSC3D (Figure 2B). Notably, blockage of NLRP3 inflammasome signaling by MCC950, a selective inhibitor of NLRP3, or Ac-YVAD-cmk, a pharmacological inhibitor of caspase-1, enhanced cell viability in MSC2D (Figures 2C and 2D). Also, transient knockdown of NLRP3 led to a significant increase in the viability of 2D-cultured MSCs (Figure 2E). In contrast, ectopic expression of NLRP3 (R260W), a gain-of-function variant of NLRP3 that may trigger inflammasome activation in the absence of exogenous stimuli,24,25 caused decreased viability in MSC3D (Figure 2F). Likewise, activation of inflammasomes by treatment with LPS/Nigericin resulted in a prominent reduction in cell survival in MSC spheroids (Figure 2G). In line with these observations, cell death was significantly decreased in NLRP3-deficient MSC2D (Figure 2H) or MSC2D treated with inflammasome inhibitors (MCC905 and Ac-YVAD-cmk) (Figure 2I), whereas activation of NLRP3 inflammasomes by the overexpression of NLRP3 R260W variant or stimulation with LPS/Nigericin promoted cell death in MSC spheroids (Figures 2J and 2K). Since cell viability, as determined by MTS assay, is also determined by cell cycle, we further examined the role of NLRP3 inflammasomes in the modulation of cell proliferation. As shown in Figure S1A, overexpression of NLRP3 active variant did not affect cell cycle distribution in MSC3D. Likewise, there was no significant effect of NLRP3 small interfering RNA (siRNA) on cell cycle progression in MSC2D (Figure S1B). In addition, the immunocytochemistry analyses showed that gene silencing of NLRP3 did not lead to significant changes in nuclear Ki67 expression in MSC2D (Figure S1C), confirming that regulation of MSC viability by NLRP3 inflammasomes would not be mediated via modulation of cell cycle.
Figure 2.
Critical roles of NLRP3 inflammasome signaling in cell fate decision in MSCs
(A and B) MSC2D and MSC3D were cultured in serum-deprived condition for 48 h. (A) Cell viability was examined by MTS assay. (B) The amount of LDH in the media was measured to examine cell death (LDH release assay). (C and D) MSC2D was treated with MCC950, a pharmacological inhibitor of NLRP3 (C), or Ac-YVAD-cmk, a selective caspase-1 inhibitor (D). Cell viability was measured at indicated time points by MTS assay. (E) MSC2D was transfected with NLRP3 siRNA for 24 h, followed by incubation for an additional 48 h. Gene silencing efficiency was monitored by western blot analysis (upper panel). Cell viability was determined by MTS assay. (F) Cells were transfected with plasmid expressing R260W variant of NLRP3 (a gain-of-function) or empty pcDNA 3.1 vector, followed by spheroid generation. Activation of NLRP3 inflammasomes was analyzed after 24 h of transfection by measurement of NLRP3, IL-1β, and caspase-1 expression using western blot analysis (left panel). Cell viability was examined by MTS assay at 48 h upon transfection (right panel). (G) MSC3D was treated with LPS (0.5 μg/mL) for 4 h, followed by incubation with nigericin (5 μM) for the indicated time durations. MTS assay was used to check the cell viability. (H and I) MSC2D was transfected with NLRP3 siRNA or treated with MCC950 or Ac-YVAD-cmk as indicated. LDH release assay was performed at 48 h upon the treatments. (J and K) MSC3D was transfected with NLRP3 (R260W) plasmid (J) or treated with LPS/Nigericin for 48 h (K). Cell death was measured by LDH release assay. (L–O) Human MSCs were labeled with DiR (1 μM). MSC2D was transfected with 25 nM of NLRP3 siRNA (MSC2D-NLRP3−) or a scramble siRNA (MSC2D-scrb) for 24 h (L). Instead, MSC3D was transfected with NLRP3 (R260W) plasmid or a vector control, followed by spheroid aggregation (M). MSC2D (106 cells/mouse) and MSC3D (1,000 spheroids/mouse) were then subcutaneously transplanted in C57BL/6 mice. Cell engraftment was monitored by in vivo imaging right after transplantation, and 1 day and 3 days after transplantation. The representative images from one mouse per group are shown along with quantification of fluorescent intensity for all mice (5 mice/group). At day 3, tissues surrounding transplantation sites were excised and a proportion of human DNA was examined through measurement of human Alu expression using qPCR (N and O). ∗Denotes p < 0.05 compared with the control group in MSC2D or MSC3D (n = 3), except for in vivo experiments where five animals per group were used.
To validate these findings in an in vivo condition, we subcutaneously transplanted DiR-labeled MSC2D and MSC3D derived from human adipose tissue into C57BL/6 mice, and MSC engraftment at the transplantation site was monitored using in vivo imaging. While no significant effects were observed on day 1, gene silencing of NLRP3 prominently enhanced the number of engrafted MSCs 3 days after MSC2D transplantation (Figure 2L). On the contrary, in vivo MSC engraftment was drastically impaired following the transplantation of spheroids derived from MSCs overexpressing an active variant of NLRP3 (Figure 2M). To further confirm the negative role of NLRP3 inflammasomes in the viability of MSCs in 2D and 3D cultures, the amount of human DNA was measured in the transplanted tissues, which were collected at day 3 of transplantation. Consistent with the in vivo imaging data, the percentage of human DNA markedly increased in the group transplanted with NLRP3 knockdown MSC2D (Figure 2N). Likewise, transplantation of 3D-cultured MSCs overexpressing NLRP3 (R260W) caused decreased expression of human DNA compared with control spheroids (Figure 2O), indicating the lower engraftment efficiency of transplanted MSCs. In this study, the efficacies of gene silencing and overexpression of NLRP3 were confirmed in transplanted tissues using RT-qPCR (Figures S2A and S2B). Collectively, these observations demonstrate the critical role of NLRP3 inflammasomes in the modulation of MSC survival under in vitro and in vivo conditions.
Enhanced cell survival of MSC spheroids is mediated via suppression of pyroptosis and apoptosis
Inflammasome activation is associated with cell death in various types of cells. Compelling evidence delineates that inflammasome activation is typically involved in a form of pro-inflammatory cell death, called pyroptosis. We next characterized cell death in MSC2D and MSC3D and further investigated the involvement of inflammasomes in the modulation of cell death. Upon staining with annexin V and 7-AAD, we observed a considerable population of pyroptotic/late apoptotic cells in MSC2D that was drastically suppressed in 3D-cultured MSCs (Figure 3A). In addition, 3D culture decreased the cleavage of gasdermin D, considered a critical event in pyroptosis, suggesting that pyroptotic cell death is significantly suppressed in 3D-culture of MSCs. The direct involvement of NLRP3 inflammasomes in the modulation of pyroptosis in MSCs was confirmed by a series of experiments using genetic manipulation of NLRP3. As shown in Figure 3, inhibition of NLRP3 inflammasome activity by transfection with a specific siRNA targeting NLRP3 decreased the apoptotic/pyroptotic cell population in MSC2D (Figure 3C), accompanied by a reduction in gasdermin D cleavage (Figure 3D), whereas overexpression of NLRP3 led to an induction of cell apoptosis/pyroptosis in MSC3D (Figure 3E) along with the promotion of gasdermin D cleavage (Figure 3F). Given that 3D culture of MSCs also downregulated apoptosis, we examined the effects of manipulation of NLRP3 expression on the apoptosis markers. While knockdown of NLRP3 suppressed caspase-3 cleavage, decreased Bax expression, and increased Bcl2 levels in MSC2D (Figure 3G), NLRP3 overexpression substantially enhanced the levels of caspase-3 and Bax, but reduced Bcl2 expression in MSC3D (Figure 3H). Essentially similar results were observed in mouse tissues transplanted with MSC2D and MSC3D (Figures 3I and 3J, respectively), further validating the modulatory role of NLRP3 inflammasomes in apoptosis in vivo. In these experiments, NLRP3 knockdown or overexpression did not significantly affect the expression levels of cyclin D1, a typical marker of cell cycle progression, suggesting that NLRP3 inflammasomes would not affect the cell cycle in MSCs. Taken together, these results signify that NLRP3 inflammasome activation triggers both pyroptotic and apoptotic cell death in MSCs.
Figure 3.
Involvement of pyroptosis and apoptosis in NLRP3 inflammasome-mediated cell death in MSC2D and MSC3D
(A) 2D- and 3D-cultured MSCs were labeled with annexin V and 7-AAD. Early and late apoptotic cells were detected by flow cytometry analysis. (B) The expression levels of total and cleaved gasdermin D in MSCs were measured by western blot analysis. (C and D) MSC2D was transfected with an siRNA targeting NLRP3 or a scrambled control. Apoptotic cell death was analyzed using annexin V staining assay (C) and gasdermin D cleavage was determined by western blot analysis (D). (E and F) MSCs were transfected with NLRP3 (R260W) plasmid followed by spheroid generation. Cell apoptosis and gasdermin D cleavage were assessed by annexin V staining assay (E) and western blot analysis (F). (G and H) MSC2D was transfected with NLRP3 siRNA (G) and MSC spheroids were generated from cells overexpressing NLRP3 (H). The protein expression levels of Bax and Bcl2, apoptotic regulators, were measured using western blot. For apoptotic and pyroptotic assays, MSC2D and MSC3D were cultured in serum-deprived media (containing 0.5% fetal bovine serum). (I and J) MSC2D (I) and MSC3D were subcutaneously transplanted in C57BL/6 mice. Tissues surrounding transplantation sites were excised and mRNA levels of Bcl2, Bax, and cyclin D1 were determined by RT-qPCR. ∗Denotes p < 0.05 compared with MSC2D or the control group (n = 3), except for in vivo experiments where five animals per group were used.
Suppression of inflammasome activation in MSC3D is mediated through modulation of ER stress and downregulation of TXNIP
ER stress has been reported to promote NLRP3 inflammasome activation under various conditions. To uncover the upstream regulatory mechanisms for inflammasome activation, we examined whether ER homeostasis is involved in the lower activity of NLRP3 inflammasomes in MSC spheroids. We found that markers of ER stress, including p-PERK, p-eIF2α, CHOP, GRP78/HSPA5, and XBP1s, were significantly downregulated in MSC3D compared with those in MSC2D (Figures 4A–4C). Notably, tunicamycin, an ER stress inducer, enhanced NLRP3 expression and the levels of active caspase-1 and IL-1β in MSC3D (Figure 4D), whereas inhibition of ER stress by treatment with tauroursodeoxycholic acid (TUDCA) prominently suppressed NLRP3 expression and the levels of active caspase-1 and IL-1β in MSC2D (Figure 4E). Likewise, gene silencing of PERK and CHOP resulted in robust decreases in NLRP3 expression, caspase-1 activation, and IL-1β maturation in MSC2D (Figures 4F and 4G), implying that the PERK arm in ER stress pathway is implicated in the modulation of inflammasome activation in MSCs. In ensuing experiments, we further observed that tunicamycin decreased the survival of MSC3D, whereas TUDCA enhanced cell viability in MSC2D, as determined by MTS assay (Figure S3), suggesting that ER stress is involved in the regulation of MSC viability by modulating NLRP3 inflammasome activation.
Figure 4.
Roles of ER stress-TXNIP axis in the modulation of NLRP3 inflammasome activation in MSC2D and MSC3D
(A and B) The expression levels of p-PERK, total PERK, p-eIF2α, total eIF2α (A), CHOP, and GRP78 (B) were measured in MSC2D and MSC3D by western blot analysis. (C) The mRNA levels of CHOP, XBP1s, and HSPA5 were measured in MSC2D and MSC3D using RT-qPCR. (D and E) MSC3D was treated with tunicamycin (1–5 μg/mL) for 8 h (D) and MSC2D was treated with TUDCA (100–500 nM) for 24 h (E). The expression levels of caspase-1 and IL-1β were measured by western blot analysis. (F and G) MSC2D was transfected with an siRNA targeting to PERK (F) or CHOP (G) for 48 h. Gene silencing efficiency was monitored by western bot analysis (upper panels of each blot). Protein levels of caspase-1 and IL-1β were determined by western blot analysis. (H and I) The messenger RNA (H) and protein levels (I) of TXNIP in MSC2D and MSC3D were determined using RT-qPCR and western blot analysis, respectively. (J) MSC3D was treated with tunicamycin for 8 h and TXNIP expression levels were measured. (K–M) MSC2D was treated with TUDCA for 24 h (K) or transfected with an siRNA targeting PERK (L) or CHOP (M) for 48 h. TXNIP expression levels were measured by western blot analysis. (N) MSC2D was transfected with TXNIP siRNA for 48 h. Gene silencing efficiency was monitored (upper panel) and the expression of caspase-1 and IL-1β was examined by western blot analysis. ∗Denotes p < 0.05 compared with MSC2D of control group (n = 3).
TXNIP has been reported to be a downstream target of ER stress that might directly trigger oligomerization and activation of NLRP3 inflammasomes.26,27 To further elucidate the mechanisms by which ER stress regulates NLRP3 inflammasome activation, we examined the mediating role of TXNIP in this process. As shown in Figures 4H and 4I, 3D culture of MSCs downregulated TXNIP at both mRNA and protein levels. Importantly, activation of ER stress with tunicamycin led to increased expression of TXNIP (Figure 4J), whereas TUDCA, an ER stress suppressor, reduced the TXNIP expression (Figure 4K). In addition, transient knockdown of PERK and CHOP caused downregulation of TXNIP, indicating that TXNIP expression in MSCs is regulated by ER stress (Figures 4L and 4M). Finally, we confirmed the functional role of TXNIP in the modulation of inflammasome activation by demonstrating that gene silencing of TXNIP prominently decreased NLRP3 inflammasome activation in MSC2D (Figure 4N). Taken together, these findings suggest that the reduced inflammasome activity in MSCs under 3D culture is mediated via downregulation of ER stress and TXNIP expression.
Autophagy induction contributes to relieved ER stress and downregulation of inflammasome activation in MSC spheroids
Autophagy is considered a critical modulator of inflammasomes. Since autophagy activation critically contributes to the enhanced cell survival in MSC spheroids,10 we examined the potential role of autophagy in the modulation of inflammasome activity in MSCs. For this, we first confirmed that 3D culture of MSCs led to induction of autophagy, as demonstrated by increased LC3-I/LC3-II conversion and upregulation of autophagy-related genes, such as Atg5 and Atg7, but reduced p62 level (Figure S4). Treatment with rapamycin, an autophagy inducer, suppressed the NLRP3 expression, caspase-1 activation, and IL-1β maturation in MSC2D (Figure 5A), as expected. On the contrary, blockage of autophagy using its pharmacological inhibitors 3-methyl adenine (3-MA) and bafilomycin A1, or gene silencing of LC3B prominently enhanced the levels of NLRP3, active caspase-1 and mature IL-1β (Figures 5B and 5C), indicating that autophagy induction mediates the suppression of inflammasome activation in MSC spheroids. Given the fact that inflammasome activity in MSCs is also modulated by ER stress, we further determined the regulatory role of autophagy in this process. To this end, we observed the effects of autophagy modulators on ER stress markers and found that autophagy activation by rapamycin suppressed phosphorylation of PERK and eIF2α, and decreased expression of GRP78 and CHOP in MSC2D (Figure 5D). In contrast, treatment with 3-MA and bafilomycin A1, or transfection with LC3B siRNA increased the expression of ER stress markers in MSC3D (Figures 5E and 5F), confirming a critical role of autophagy induction in the suppression of ER stress in MSCs under 3D culture. Furthermore, rapamycin downregulated TXNIP expression in MSC2D (Figure 5G), whereas 3-MA and bafilomycin A1 induced increase in expression of TXNIP (Figure 5H). Similarly, gene silencing of LC3B also caused robust induction of TXNIP (Figure 5I). Collectively, these data reveal that autophagy induction leads to suppression of ER stress and TXNIP expression in MSC3D, thereby preventing inflammasome activation.
Figure 5.
Roles of autophagy induction in the modulation of ER stress and inflammasome activation in MSC spheroids
(A and B) MSC2D was treated with rapamycin, a pharmacological autophagy inducer, for 24 h, while MSC3D was treated with 3-MA and Bafilomycin A1, inhibitors of autophagy, for 8 h. The expression levels of IL-1β and caspase-1 were determined using western blot analysis. (C) MSCs were transfected with LC3B siRNA, followed by generation of spheroids. Gene silencing efficiency (upper panel) and the levels of caspase-1 and IL-1β were measured by western blot analysis. (D–F) MSC2D was treated with rapamycin for 24 h (D). Instead, MSC3D was treated with 3-MA and Bafilomycin A1 for 3 h (E) or transfected with LC3B siRNA for 48 h (F). The phosphorylation of PERK, eIF2α, and the expression of CHOP and GRP78 was examined by western blot analysis. (G–I) MSC2D was treated with rapamycin for 24 h (G) and MSC3D was treated with 3-MA and Bafilomycin A1 for 8 h (H) or transfected with LC3B siRNA for 48 h (I). The expression of TXNIP was determined by western blot. ∗Denotes p < 0.05 compared with the control group (n = 3).
In vivo effects of MSC2D and MSC3D transplantation on the expression of the genes related to inflammasome and ER stress
To validate the effects of 3D culture on the modulation of inflammasomes and upstream signaling pathways under in vivo conditions, we checked the mRNA levels of the genes related to inflammasome and ER stress upon transplantation of human MSC2D and MSC3D. We first confirmed that the transplantation of MSC3D significantly enhanced the engraftment of MSCs after 3 and 7 days of transplantation, as determined by measurement of human DNA proportion (Figure 6A) and in vivo imaging (Figure 6B). In addition, consistent with in vitro observations, the expression of inflammasome-related genes, including NLRP3, ASC, and IL-1β, was suppressed in MSC3D-transplanted tissues (Figures 6C–6E). Likewise, ER stress markers, such as CHOP, XBP1s, and HSPA5, were significantly downregulated in the MSC3D-transplanted group (Figures 6F–6H). Moreover, TXNIP levels were also markedly lower upon transplantation of MSC3D compared with that in MSC2D (Figure 6I). Finally, expression of autophagy-related genes, including LC3B and Atg5, were found to be substantially higher in the MSC3D-transplanted tissues. Taken together, these results substantiate that transplantation of MSC3D suppresses NLRP3 inflammasome activation via modulation of ER stress and autophagy induction in vivo.
Figure 6.
Downregulation of the genes related to NLRP3 inflammasomes and ER stress in MSC3D upon transplantation in mice
(A) Human adipose-derived MSC2D and MSC3D were subcutaneously transplanted in C57BL/6 mice. Tissues surrounding the transplantation sites were collected at different timepoints, and human Alu DNA levels were determined by qPCR. The proportion of human DNA in the transplanted tissues was calculated based on a standard curve as described in the materials and methods section. (B) MSCs were labeled with DiR, followed by culturing in monolayers or spheroidal aggregations. One million MSC2D or 100 spheroids were then transplanted into subcutaneous layers of C57BL/6 mice. Cell engraftment was monitored by in vivo imaging right after transplantation and after 3 days of transplantation. The representative images of each group were presented along with quantification of fluorescent intensity from all animals (5 mice/group). (C–K) The tissues collected at day 1 and day 3 upon transplantation were used to examine the mRNA expression of the genes related with inflammasomes and ER stress, including NLRP3 (C), ASC (D), IL-1β (E), CHOP (F), XBP1s (G), HSPA5 (H), TXNIP (I), LC3B (J), and Atg 5 (K) by RT-qPCR. Human Alu gene was used as an internal control. ∗Denotes p < 0.05 compared with MSC2D (n = 5).
Downregulation of NLRP3 inflammasome activity is required for the improved therapeutic efficacy of MSC3D in a mouse model of DSS-induced colitis
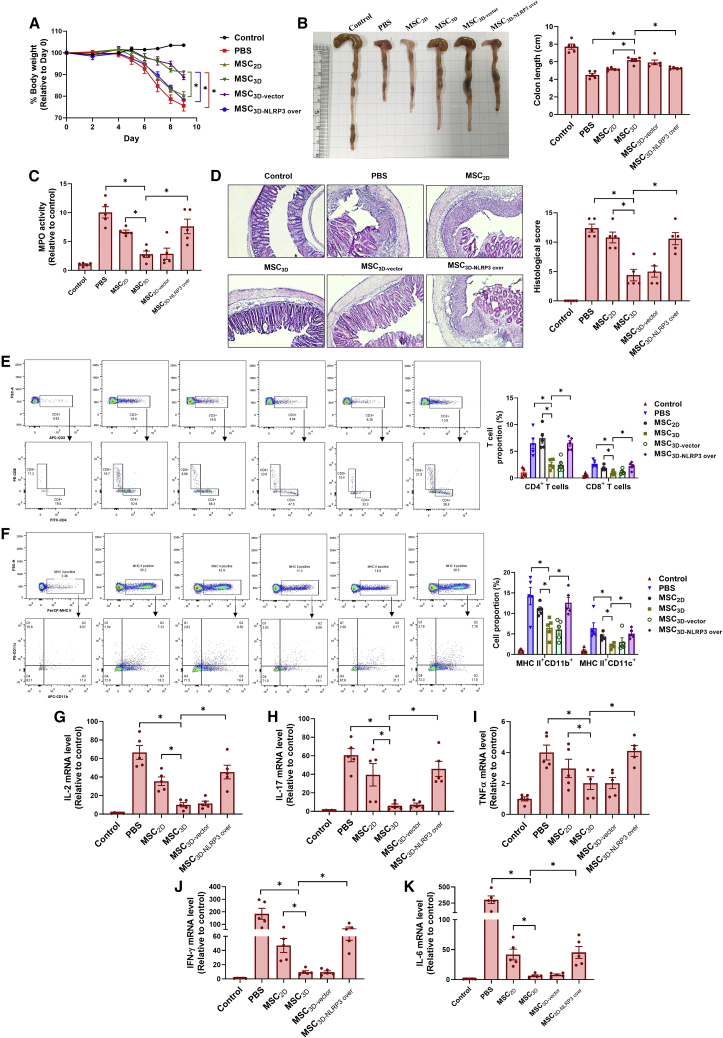
MSC spheroids have shown higher therapeutic effectiveness than MSC2D in various disease models, including DSS-induced colitis. Having demonstrated that 3D culture of MSCs enhanced cell survival through suppression of inflammasome activation, we further verify whether impaired NLRP3 inflammasome activity is involved in the enhanced therapeutic efficacy of MSC spheroids for the management of colitis. DSS-induced colitis mice were treated with MSC3D prepared from human MSCs with or without ectopic expression of an active NLRP3 variant and pathological severity was evaluated. As depicted in Figure 7A, the colitis-induced body weight loss and colon-length shortening were effectively prevented by MSC3D transplantation, which was higher than that of treatment with MSC2D. Interestingly, these effects were markedly reversed by NLRP3-overexpressing MSC3D (MSC3D-NLRP3 over) transplantation (Figures 7A and 7B). In addition, the severity of colonic inflammation, determined by myeloperoxidase (MPO) activity, was substantially reduced by MSC3D transplantation, and this effect of MSC3D was almost abrogated by overexpression of NLRP3 (Figure 7C). Likewise, histological analysis showed that treatment with MSC3D significantly suppressed the mucosal damage and infiltration of immune cells in colon tissues; however, overexpression of NLRP3 led to a robust reduction in the therapeutic effects of MSC3D (Figure 7D). The suppressive effects of MSC3D on colonic infiltration of immune cells were further confirmed by flow cytometry analyses, which showed that total number of T cells and immune cells positive to pan-macrophage markers (MHCII+CD11b+) and pan-dendritic markers (MHCII+CD11c+) were markedly reduced in colon tissues from the MSC3D-treated group (Figures 7E and 7F). Similarly, the immune modulatory effect by MSC3D was not observed upon overexpression of NLRP3. As a consequence of decreased immune cell recruitment, MSC3D transplantation prominently inhibited the production of pro-inflammatory cytokines, including IL-2 (Figure 7G), IL-17 (Figure 7H), tumor necrosis factor (TNF)α (Figure 7I), interferon (IFN)-γ (Figure 7J), and IL-6 (Figure 7K). Moreover, the suppressive effects of MSC3D on pro-inflammatory cytokine expression were not observed upon NLRP3 overexpression. Taken together, these data demonstrate that the modulation of NLRP3 inflammasome activity critically contributes to the improvements in the therapeutic effectiveness of MSC spheroids in DSS-induced colitis.
Figure 7.
Modulation of NLRP3 inflammasome activation critically contributes to enhanced therapeutic effects of MSC spheroids in DSS-induced colitis
A colitis model was established in C57BL/6 mice by administering 3% DSS in drinking water for 7 days. Mice were divided into six groups (five animals per group), including normal control and five DSS groups receiving one of the following treatments: PBS, MSC2D, MSC3D, MSC3D prepared from NLRP3 overexpressing MSCs, and MSC3D prepared from vector control transfected MSCs. After treatments, all the cells were administered via intraperitoneal injection at day 1 upon colitis induction. Two million single cells or 2,000 spheroids were used for each mouse. (A) Changes in body weight were monitored throughout the experiment. (B) Mice were killed at day 9 and colons were isolated. Representative images of colon were presented for each group (left panel) and the length of colon was recorded for all animals (right panel). (C) Colon tissues were homogenized and supernatants were used for the measurement MPO activity. (D) The tissue sections were prepared from colon samples, followed by H&E staining. Histological score was calculated based on epithelial cell destruction, loss of goblet cells, presence of hyperplasia, and immune cell infiltration. (E and F) Single cells were prepared from colon tissues as described in the materials and methods section and then used for analyzing surface markers using a flow cytometry. Representative cell populations for each group are shown in upper panels and the proportion of each cell population is given in the lower panel. CD3+CD4+ and CD3+CD8+ denote T CD4 and T CD8 populations, respectively (E). Macrophages and dendritic cells are characterized by MHCII+CD11b+ and MHCII+CD11c+ (F). (G–K) The mRNA levels of IL-2 (G), IL-17 (H), TNFα (I), IFNγ (J), and IL-6 (K) were measured using RT-qPCR. ∗Denotes p < 0.05 compared with the indicated group; n = 5.
Discussion
MSCs exhibit a high therapeutic potential for the treatment of various diseases. However, their therapeutic use has been limited owing to their low survival rates after transplantation.28,29 Although spheroid formation of MSCs in 3D culture has been demonstrated to increase the effectiveness of MSC therapy, the molecular mechanisms underlying these beneficial effects of 3D culture are far from elucidated. Compelling evidence delineates that inflammasomes critically contribute to cell fate decision in various cell types, other than participating in the regulation of inflammatory and immune responses.30,31 While a recent study reported that NLRP3 inflammasomes are present in MSCs and activated in response to inflammatory stimuli in vitro and in vivo,17 their roles in the modulation of cell death/survival and implication in the enhanced cell viability by 3D culture remains elusive. In the present study, we have elucidated the signaling mechanisms underlying modulation of NLRP3 inflammasomes in MSCs under 2D and 3D cultures. In particular, we found that NLRP3 inflammasomes activity was downregulated in MSC spheroids as a result of autophagy induction and ER stress amelioration. Downregulation of NLRP3 inflammasomes, in turn, conferred a survival advantage to MSC3D by attenuating cell apoptosis and pyroptosis. Notably, a lower level of inflammasome activation was required for the therapeutic benefits of MSC3D in a DSS-induced colitis mouse model.
Downstream effects of NLRP3 inflammasome activation are mainly mediated via caspase-1 and its cytokine substrates, such as IL-1β, IL-18, and IL-33.32 With respect to the role of inflammasomes in cell fate regulation, activation of NLRP3 inflammasomes is often considered an event leading to pyroptotic cell death, mostly orchestrated by caspase-1-dependent gasdermin D cleavage. In contrast, the release of IL-1β, a final product of inflammasome activation, differentially modulates cell survival, growth, and senescence in a complicated and context-dependent manner.33,34 Taking into consideration the multifaceted role of inflammasomes in cell fate decision, we sought to identify whether the modulation of NLRP3 inflammasomes contributes to the differential regulation of the survival rate of MSCs under 2D and 3D culture. Consistent with previous findings, we have found that NLRP3 inflammasome components are presented in mouse and human MSCs.17,19 Interestingly, activation of NLRP3 inflammasomes might occur in a higher proportion of 2D-cultured MSCs but was markedly suppressed upon spheroid formation. Moreover, downregulation of NLRP3 inflammasomes signaling was also observed in MSC spheroids after transplantation, convincingly demonstrating that 3D culture is an effective strategy to modulate NLRP3 inflammasome activation in MSCs.
Cell death and survival rate of MSCs is determined in a complex manner involving a variety of factors. While previous studies have suggested that enhanced viability of MSC3D is referred to the inhibition of apoptosis,10,35 given the various other factors affecting cell death and survival, we characterize the type of cell death mediated by inflammasome activation in MSCs under 2D and 3D cultures. Herein, we observed that pyroptosis, a pro-inflammatory type of cell death distinct from apoptosis and a direct consequence of inflammasome activation, was mediated by NLRP3 inflammasome activation (Figure 3). In addition, apoptotic cell death was also regulated by inflammasome activation (Figure 3), collectively indicating that both apoptotic and pyroptotic cell death are downregulated in MSC3D due to the suppressed inflammasome activity. To the best of our knowledge, this is the first report demonstrating that inhibition of pyroptosis, in addition to apoptosis, contributes to the improved survival of MSC spheroids. Herein, the co-occurrence of pyroptosis and apoptosis upon activation of NLRP3 inflammasomes possibly reflects the crosstalk between these programmed cell death pathways. In fact, previous studies have shown that caspase-1 may promote apoptosis in human keratinocytes and even gasdermin D-deficient macrophages.36,37,38 Moreover, caspase-8 is also recruited by NLRP3 inflammasomes to trigger apoptosis in parallel with caspase-1-dependent pyroptosis.39 Therefore, the balance between pyroptosis and apoptosis would depend on the cell type, specifically expression levels of pro-caspase-1 and gasdermin D, and the nature of the stimulators. Although inflammasome-mediated apoptosis of MSCs is also dependent on caspase-1, the implication of other pathways that trigger cell apoptosis upon NLRP3 inflammasome activation cannot be excluded.
Autophagy, an evolutionarily conserved self-digestive process, was supposed to protect MSCs from hypoxia/nutrition deprivation-induced oxidative stress.40 However, excessive activation of autophagy may also lead to cell death,41 indicating a multifaceted role of autophagy in the modulation of MSC survival. Notably, induction of autophagy was observed in MSCs after spheroid formation, which is required for their higher survival in response to oxidative stress and upon in vivo transplantation.10,42 In this study, we further clarify how autophagy induction contributes to the enhanced survival of MSC spheroids focusing on inflammasome modulation. It has been widely reported that autophagy plays a role as a negative modulator of inflammasomes through multiple mechanisms. For example, autophagy removes dysfunctional mitochondria that serve as scaffolds for inflammasomes.43 Autophagy also terminates inflammasome activation by directly degrading inflammasome components.44 Herein, our results also represent that autophagy induction suppresses NLRP3 inflammasome activation in MSCs, implying that autophagy acts as an important checkpoint of inflammasome activation in MSCs, particularly in 3D culture.
In an attempt to elucidate the mechanism by which autophagy induction results in the suppression of inflammasome activation in MSC spheroids, we found that 3D culture attenuated ER stress in an autophagy-dependent manner. ER stress, characterized by impaired capacity of the ER to protein folding, leads to accumulation of misfolded proteins. Protein unfolding/misfolding, in turn, initiates a signaling cascade termed the unfolded protein response (UPR) to restore the protein folding.45 Although ER stress and UPR act as adaptive processes in response to the stressful conditions, excessive or prolonged ER stress may promote apoptosis.46 By contrast, suppression of ER stress has been demonstrated to enhance cell survival under stressful conditions,47,48,49,50 suggesting that alleviation of ER stress is a potential strategy to improve the therapeutic potency of MSCs. UPR has been shown to trigger NLRP3 inflammasome activation through the PERK and/or IRE1 arm.51,52,53 Consistent with these reports, we observed a close correlation between downregulation of NLRP3 inflammasome activation and suppression of the PERK/eIF2/CHOP signaling pathway in MSC spheroids (Figure 4). Furthermore, we also demonstrated that TXNIP is a key mediator in the ER stress-inflammasome crosstalk. It has been generally accepted that TXNIP can directly interact with NLRP3 and trigger its oligomerization and activation.26 Moreover, TXNIP is a sensor of cellular ROS production that servers as a key signal in NLRP3 inflammasome activation process.54,55 Interestingly, since ER stress has been known to induce mitochondrial ROS (mtROS) generation,56 the production of mtROS may mediate the upregulation of TXNIP by ER stress. In fact, in this study, we observed that spheroid culture reduced mtROS levels (Figure S5A). Moreover, mitoQ, an inhibitor of mtROS, significantly decreased the expression of NLRP3, cleaved caspase-1, and mature IL-1β in MSC2D (Figure S5B), whereas treatment with antimycin, a potent inducer of mtROS production, resulted in NLRP3 inflammasome activation in MSC3D (Figure S5C), suggesting that downregulation of mtROS is critical for the suppression of NLRP3 inflammasome signaling by spheroid culture.
With potent anti-inflammatory and immunomodulatory properties, MSCs have been demonstrated to be useful in the treatment of inflammatory and autoimmune disorders, including graft versus host disease, inflammatory bowel disease (IBD), and others.57 While it is well established that an inflammatory environment activates anti-inflammatory and immunosuppressive properties of MSCs, the inflammatory response in MSCs may exert detrimental effects on their survival. Given the abundance of pathogen-associated molecular patterns (PAMP) and damage-associated molecular patterns (DAMP) in the inflammatory microenvironment, we assumed that protection of MSCs from inflammasome-mediated cell death may improve their therapeutic efficacy in inflammatory disorders, such as colitis. Furthermore, as activation of NLRP3 inflammasomes is thought to be a key player in the pathogenesis of IBD,58,59 genetic manipulation would allow selective silencing of NLRP3 inflammasome signaling in the transplanted MSCs without unexpected effects on inflammasome-mediated innate immune response. Here, we demonstrated that forced activation of NLRP3 inflammasomes in MSCs by ectopic expression of a gain-of-function NLRP3 variant completely abrogated the protective role of MSC spheroids in DSS-induced colitis, implying that downregulation of NLRP3 inflammasome activity is critically required for the enhanced therapeutic functions of MSC spheroids. The present findings provide novel insights into the molecular events underlying the improved therapeutic potency of MSC spheroids for the treatment of colitis and suggests that the control of NLRP3 inflammasome activation could be considered a new strategy to enhance the anti-inflammatory potency of MSCs.
In conclusion, we have elucidated the molecular mechanisms by which 3D culture enhances the survival, engraftment, and in vivo anti-inflammatory potency of MSCs. NLRP3 inflammasome activation contributes to induction of cell death after MSC transplantation. Spheroidal aggregation enhanced the viability of MSCs by downregulating inflammasome activation. Furthermore, impaired activation of NLRP3 inflammasomes in MSC spheroids was involved in the suppression of both apoptosis- and pyroptosis-mediated cell death. Mechanistically, 3D culture suppressed the PERK-eIF2α-CHOP arm of the UPR, which in turn decreased the expression of TXNIP and consequently inhibited the assembly of the NLRP3 inflammasome complex. In addition, autophagy induction was essential for the modulation of ER stress and inflammasome activation in MSC3D (Fig. 8). Given the critical role of NLRP3 inflammasomes in MSC fate decision, controlling inflammasome activation may be a promising strategy for improvement of MSC therapeutic efficacy.
Figure 8.
Proposed model for modulation of NLRP3 inflammasome activation in MSC spheroids
It has been postulated that the therapeutic efficacy of MSCs is enhanced by 3D spheroidal culture. The present study further elucidates the molecular mechanisms whereby spheroid formation leads to the improved viability and function of MSCs. 3D culture of MSCs using AgreeWell plates increased autophagy activation, which in turn alleviated ER stress and downregulated TXNIP expression. These molecular events resulted in downregulation of NLRP3 inflammasomes, a platform for caspase-1 activation, which played a critical role in cell fate decision in MSCs through the control of apoptotic and pyroptotic cell death. Using a DSS-induced colitis model, this study has demonstrated that downregulation of NLRP3 inflammasomes is essentially required for the enhanced therapeutic effectiveness of MSC spheroids.
Materials and methods
Materials
All reagents used for cell culture were acquired from HyClone Laboratories (South Logan, UT, USA). DSS (#216011080) was procured from MP Biomedical (Irvine, CA, USA). MCC950 (#s8930) was obtained from Selleck Chemicals LLC (Houston, TX, USA). Ac-YVAD-cmk (#SML0429), tunicamycin (#T7765), lipopolysaccharide (LPS; #L2630), and 3-methyl adenine (3-MA; #M9281) were purchased from Sigma-Aldrich (St. Louis, MO, USA). TUDCA (#HY-19696A) was provided by MedChemExpress LLC (Monmouth Junction, NJ, USA). Nigericin (#tlrl-nig) was acquired from InvivoGen (San Diego, CA, USA). Rapamycin (#9904) and bafilomycin A1 (#1829–50) were purchased from Cell Signaling Technology (Beverly, MA, USA) and Biovision (Milpitas, CA, USA), respectively. The following primary antibodies were procured from Cell Signaling Technology (Beverly, MA, USA): anti-IL-1β (#12242), anti-PERK (#3192), anti-phospho-eIF2α (9721S), anti-eIF2α (#9722S), anti-GRP78 (#3183), anti-LC3 (#2775), anti-Atg7 (26315), anti-p62 (#5114), anti-TXNIP (#14715), anti-Bax (#5023), anti-Bcl2 (#3498), anti-cleaved caspase-3 (#9664), and anti-gasdermin D (#39754). The primary antibodies against caspase-1 (#AG-20B-0048-C100) and ASC (#AG-25B-0006-C100) were provided by Adipogen Life Sciences (San Diego, CA, USA); antibodies against Atg5 (#PA1-46178) and β-actin (#MA5-15739) were procured from Thermo Scientific (Waltham, MA, USA); antibodies against NLRP3 (#MAB7578) and CHOP (#MA1-250) were purchased from R&D Systems (Minneapolis, MN, USA) and Santa Cruz Biotechnology (Dallas, TX, USA), respectively. The antibodies used for flow cytometry, including PerCP-conjugated anti-MHCII (#107623), PE-conjugated anti-CD11c (#117307), APC-conjugated anti-CD11b (#101212), FITC-conjugated anti-CD3 (#100204), FITC-conjugated anti-CD4 (#100510), and PE/Cy7-conjugated anti-CD8 (#100722) antibodies were procured from BioLegend (San Diego, CA, USA). The secondary antibodies against rabbit (#7074), mouse (#7076), and rat (#7077) immunoglobulin (Ig)G were obtained from Cell Signaling Technology (Beverly, MA, USA).
Animals
C57BL6 mice were obtained from Orient Ltd. (Osan, South Korea). The animals were maintained under pathogen-free conditions set up by the Institutional Animal Care and Use Committee (IACUC) of Yeungnam University. All animal experiments were carried out according to the protocols and experimental design approved by the IACUC (Protocol number: 2021–009).
Isolation and culture of MSCs
Human adipose tissue-derived MSCs (#STC002) were purchased from Stemore (Seoul, South Korea). MSCs were cultured in alpha-MEM media containing 10% fetal bovine serum and 1% penicillin/streptomycin. Cells at passage 4–7 were used for the experiments.
Mouse MSCs were isolated from the adipose tissues of 5- to 7-week-old C57BL6 mice. Briefly, mice were killed by CO2 asphyxiation and inguinal white adipose tissues were collected after removing the lymph nodes. The adipose tissues were digested with 0.1% collagenase P (#11213873001; Roche, Mannheim, Germany) for 1 h at 37°C. Cells were passed through a 40-μm cell strainer and then treated with RBC lysis buffer to obtain the stromal vascular fraction (SVF). Finally, single cells in SVF were plated in 100-mm dishes with complete alpha-MEM media and nonadherent cells were removed after 4 h. The phenotype of isolated MSCs was confirmed using surface markers, including CD29, CD90, CD105, CD44, and Sca-1 (positive), and CD11b, CD45, and CD34 (negative) (Figure S6). Mouse MSCs at passages between 3 and 5 were used for the experiments.
Preparation of MSC spheroids
MSC spheroids were prepared using AggreWell 400 plates (STEMCELL Technologies, Cambridge, MA, USA). Briefly, 1.2 × 106 cells in 2 mL of complete media were transferred into each well of the plate, followed by centrifugation and incubation for 6 h at 37°C in a humidified incubator. By this method, each spheroid contains 1,000 cells with the diameter ranging from 180–220 μm.
Cell viability assay
Cell viability was measured by MTS assay as described previously.60 In brief, 1 × 104 single cells or 10 spheroids were seeded into a 96-well plate. After overnight incubation, cells were treated as mentioned in the figure legends and then incubated with 20 μL of MTS reagent (#G3580; Promega Corporation, Madison, WI, USA) for 2 h at 37°C. Cell viability was monitored by measuring the absorbance at 490 nm using a Spark 10M multimode microplate reader (Tecan, Mannedorf, Switzerland).
Lactate dehydrogenase release assay
Cell death was evaluated through measurement of lactate dehydrogenase (LDH) release using the CytoTox 96 Non-Radioactive Cytotoxicity Assay Kit (#G1780; Promega, Madison, USA). MSCs (1×104) in monolayers or spheroids were seeded in a 96-well plate and incubated in serum-free media for 48 h. Culture media (50 μL) was then collected and transferred into the wells of a new 96-well plate. Subsequently, equal amounts of CytoTox 96 reagent were added into each well, and the plate was incubated for 30 min at 37°C. LDH activity was determined by monitoring the formation of the red formazan product through measurement of the absorbance at 490 nm.
Apoptosis assay
Apoptotic cells in spheroids and monolayers were detected using the Annexin V Apoptosis Detection Kit (#640922; BioLegend, San Diego, CA, USA). For single-cell preparation, spheroids were collected and broken up by trypsinization with pipetting for 5 min. Cells were then incubated with staining buffer containing FITC-annexin V and 7-AAD in annexin V binding buffer for 15 min at room temperature as per the manufacturer’s recommendations. Finally, the cells were subjected to flow cytometry analysis using a BD FACSCalibur (BD Biosciences, San Jose, CA, USA).
Cell cycle analysis
Single cells were collected from MSC monolayers and MSC spheroids by trypsinization. Cells were then fixed with 70% ice-cold ethanol for 2 h, followed by staining with propidium iodide and RNAse I inhibitor (#ab139418; Abcam, Cambridge, MA, USA) according to the manufacturer’s instructions. Finally, cells were analyzed using flow cytometry (BD FACSCalibur), and cell cycle distribution was calculated by FlowJo 7.6 software.
Measurement of mtROS
MSC2D- and MSC3D-derived single cells were prepared as described above. Subsequently, single cells were incubated with 5 μM of MitoSOX Red reagent (M36008; Thermo Scientific) for 30 min at 37°C. The oxidation of MitoSOX red was finally analyzed using flow cytometry.
Western blot analysis
Western blot analysis was carried out as described previously.61 Briefly, total cell lysates were obtained using radioimmunoprecipitation assay (RIPA) buffer containing protease and phosphatase inhibitors. Equal amounts of proteins were loaded onto a 7%–15% sodium dodecyl sulfate polyacrylamide gel, which was then subjected to electrophoresis. The separated proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane, followed by blocking with 5% skim milk and sequential incubation with a primary antibody against the protein of interest and a respective secondary antibody. Enhanced chemiluminescent method was used to detect the resultant immunocomplexes. The chemiluminescent images were acquired using a Fujifilm LAS-4000 mini system (Fujifilm, Tokyo, Japan).
RNA isolation, cDNA synthesis, and qPCR
The mRNA levels of the target genes were measured by RT-qPCR.62 Total RNA was extracted using Qiagen lysis reagent (#79306; Qiagen, Germantown, MD, USA). Complementary DNA (cDNA) was synthesized from 1 μg of total RNA using the GoScript Reverse Transcription Kit (#A5000; Promega Corporation). Finally, relative gene expression was determined by qPCR using the ABsolute qPCR SYBR Green Capillary Mix (Thermo Scientific) on a Roche LightCycler 2.0 (Mannheim, Germany). The sequences of all the primers used are summarized in Table S1.
siRNA and plasmid DNA transfection
For transient gene silencing, cells were grown to 50%–70% confluency. Cells were then transfected with an siRNA targeting the gene of interest or a scramble siRNA using Lipofectamine RNAiMAX Transfection Reagent (Thermo Scientific) according to the manufacturer’s instructions. Where necessary, the cells were subjected to spheroid generation after 6 h of transfection. Transfection efficiency was monitored by western blot analysis.
For overexpression of NLRP3, cells were transfected with pcDNA3-Myc-NLRP3 (R260W), which contains a gain-of-function variant of human NLRP3, using the FugeneHD transfection reagent (Promega). pcDNA3-Myc-NLRP3 (R260W) was a gift from Christian Stehlik (Addgene plasmid # 73956; Addgene, Watertown, MA, USA).
Immunocytochemistry
ASC speck formation and Ki67 expression were measured by immunocytochemistry according to a previously described protocol.63 MSCs in monolayers or spheroids were fixed with 4% paraformaldehyde. Frozen sections (10 μm) were prepared from the spheroids using a freezing sliding microtome (Microm HM 450, Thermo Scientific). Cells in monolayers or spheroid sections were permeabilized with 0.2% Triton X-100, followed by blocking with 2% normal goat serum. Then, cells were sequentially incubated with a primary antibody (4°C, overnight) and Alexa Fluor 488-conjugated anti-rabbit goat secondary antibody (room temperature, 90 min). The cells were then counterstained with DAPI, and the images were acquired using a confocal microscope (Confocal microscope A1; Nikon, Tokyo, Japan).
Measurement of cell engraftment capacity
Cells were first labeled with 1 μM of 1,1′- dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide (DiR) (#60017; Biotium, Fremont, CA, USA) for 15 min at 37°C, followed by washing with PBS three times. MSC2D and MSC3D were then prepared from the labeled cells, as described above. One million cells (or 1,000 spheroids) were subcutaneously transplanted into the thigh region of C57BL/6 mice. Cell engraftment was monitored through measurement of the fluorescent signal intensity at various timepoints upon transplantation using IVIS Lumina III In Vivo Imaging System (PerkinElmer Inc., Waltham, MA, USA).
Cell engraftment was examined by measuring the proportion of human genomic DNA at the transplantation site. In brief, subcutaneous tissues around the injection site were excised and chilled with dry ice, followed by homogenization using a mortar and pestle. DNA extracts were prepared using the AccuPREP Genomic DNA Extraction Kit (#K-3032; Bioneer, Daejeon, South Korea) according to the manufacturer’s instructions. Subsequently, qPCR was carried out to determine the level of human DNA using sample DNA (25 ng), SYBR green (5 μL), and ALU primer (37.5 nM). The percentage of human DNA was calculated from a calibration curve built on the basis of various concentrations of standard human DNA and mouse DNA.
Development of DSS-induced colitis model
The regulatory role of NLRP3 inflammasomes in the therapeutic potency of MSCs was validated in an in vivo DSS-induced colitis model developed in C57BL/6 mice. To induce colitis, 7-week-old mice were administered 3% DSS in the drinking water for 7 days. One day after DSS administration, the mice were treated with 2 × 106 MSC2D or 2,000 MSC spheroids via intraperitoneal injection. Changes in body weight and clinical symptoms were monitored daily throughout the study. Disease severity index was calculated based on stool consistency, body weight reduction, rectal bleeding, and the presence of hemoccult in the stool. At day 9, the mice were killed and colon samples were harvested for further analyses.
MPO activity measurement
For MPO activity assay to examine the extent of colonic neutrophil infiltration, colon tissues (10 mg) were homogenized in PBS (pH 6.0) containing 0.5% hexadecyltrimethylammonium bromide. After centrifugation, the supernatant was collected and used for MPO activity determination. The reaction mixture was composed of 20 μL of supernatant, 90 μL of 0.0001% v/v hydrogen peroxide, and 90 μL of 2 mg/mL O-dianisidine in a 96-well plate. The plate was incubated for 15 min at room temperature before absorbance was measured at 460 nm using a microplate reader.
Flow cytometry analysis for determination of colonic immune cell populations
The infiltration of immune cells, including T cells, macrophages, and dendritic cells, into colon tissues was examined by flow cytometry. Briefly, colon tissues were cut into small pieces using sterile scissors and digested in Hank’s balanced slat solution containing collagenase P (0.1 mg/mL) and DNase I (0.1 mg/mL) (#11284932001; Roche) for 1 h at 37°C with gentle shaking. After Fc receptor blocking, the cells were stained with antibody cocktails against different surface markers, including CD3, CD4, CD8, CD11b, CD11c, and MHCII for 20 min at 4°C. The cells were then subjected to flow cytometry analysis using a BD FACS Verse flow cytometer. Data were analyzed using FlowJo 7.6 software.
Hematoxylin and eosin staining
H&E staining was used for the histological study of the colon tissues. After collection, colon tissue was washed with ice-cold PBS and fixed with 4% paraformaldehyde for 24 h, followed by soaking in 30% sucrose solution for 48 h. A freezing sliding microtome (Microm HM 450; Thermo Scientific) was used to make 10-μm sections of the colon. The sections were mounted on the gelatin-coated glass slides and subjected to H&E staining using an H&E Staining Kit (#ab245880; Abcam, Cambridge, MA, USA). Images were acquired using an optical microscope (Nikon Eclipse Ti, Tokyo, Japan). The severity of the pathophysiology was determined based on epithelial cell destruction, loss of goblet cells, presence of hyperplasia, and immune cell infiltration.
Statistical analysis
All data are presented as the mean ± standard error from at least three experiments. Statistical analyses were performed using GraphPad Prism 8.02 (San Diego, CA, USA). Significant differences were examined using one-way ANOVA or t test, where p values below 0.05 were considered statistically significant.
Data availability statement
All data generated during the study are included in the article and supplemental information.
Acknowledgments
This work was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2021R1A2C1013132) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2020R1A6A1A03044512). The authors thank the Core Research Support Center for Natural Products and Medical Materials (CRCNM) for technical support regarding the confocal microscopic analysis
Author contributions
D.P. designed and conducted the experiments and wrote the manuscript; P.S. designed and conducted the experiments; T.N., J.P., and M.P. conducted the experiments; J.C., S.K., D.C., S.H., I.C., and G.P. conceived the idea and designed the experiments; and J.J. and P.P. supervised the project, designed the experiments, and wrote the manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2022.12.014.
Contributor Information
Jee-Heon Jeong, Email: jeeheon@skku.edu.
Pil-Hoon Park, Email: parkp@yu.ac.kr.
Supplemental information
References
- 1.Pittenger M.F., Discher D.E., Péault B.M., Phinney D.G., Hare J.M., Caplan A.I. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen. Med. 2019;4:22. doi: 10.1038/s41536-019-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heldring N., Mäger I., Wood M.J.A., Le Blanc K., Andaloussi S.E.L. Therapeutic potential of multipotent mesenchymal stromal cells and their extracellular vesicles. Hum. Gene Ther. 2015;26:506–517. doi: 10.1089/hum.2015.072. [DOI] [PubMed] [Google Scholar]
- 3.Moya A., Paquet J., Deschepper M., Larochette N., Oudina K., Denoeud C., Bensidhoum M., Logeart-Avramoglou D., Petite H. Human mesenchymal stem cell failure to adapt to glucose shortage and rapidly use intracellular energy reserves through glycolysis explains poor cell survival after implantation. Stem Cells. 2018;36:363–376. doi: 10.1002/stem.2763. [DOI] [PubMed] [Google Scholar]
- 4.Eggenhofer E., Luk F., Dahlke M.H., Hoogduijn M.J. The Life and fate of mesenchymal stem cells. Front. Immunol. 2014;5:148. doi: 10.3389/fimmu.2014.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silva L.H.A., Antunes M.A., Dos Santos C.C., Weiss D.J., Cruz F.F., Rocco P.R.M. Strategies to improve the therapeutic effects of mesenchymal stromal cells in respiratory diseases. Stem Cell Res. Ther. 2018;9:45. doi: 10.1186/s13287-018-0802-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cesarz Z., Tamama K. Spheroid culture of mesenchymal stem cells. Stem Cells Int. 2016;2016 doi: 10.1155/2016/9176357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kouroupis D., Willman M.A., Best T.M., Kaplan L.D., Correa D. Infrapatellar fat pad-derived mesenchymal stem cell-based spheroids enhance their therapeutic efficacy to reverse synovitis and fat pad fibrosis. Stem Cell Res. Ther. 2021;12:44. doi: 10.1186/s13287-020-02107-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeh H.-Y., Liu B.-H., Sieber M., Hsu S.-h. Substrate-dependent gene regulation of self-assembled human MSC spheroids on chitosan membranes. BMC Genomics. 2014;15:10. doi: 10.1186/1471-2164-15-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGettrick A.F., O'Neill L.A.J. The role of HIF in immunity and inflammation. Cell Metab. 2020;32:524–536. doi: 10.1016/j.cmet.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Regmi S., Raut P.K., Pathak S., Shrestha P., Park P.H., Jeong J.H. Enhanced viability and function of mesenchymal stromal cell spheroids is mediated via autophagy induction. Autophagy. 2021;17:2991–3010. doi: 10.1080/15548627.2020.1850608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartosh T.J., Ylöstalo J.H., Bazhanov N., Kuhlman J., Prockop D.J. Dynamic compaction of human mesenchymal stem/precursor cells into spheres self-activates caspase-dependent IL1 signaling to enhance secretion of modulators of inflammation and immunity (PGE2, TSG6, and STC1) Stem cells (Dayton, Ohio) 2013;31:2443–2456. doi: 10.1002/stem.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sui B.-D., Zheng C.-X., Li M., Jin Y., Hu C.-H. Epigenetic regulation of mesenchymal stem cell homeostasis. Trends Cell Biol. 2020;30:97–116. doi: 10.1016/j.tcb.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Guo H., Callaway J.B., Ting J.P.Y. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat. Med. 2015;21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broz P., Dixit V.M. Inflammasomes: mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016;16:407–420. doi: 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- 15.Pham D.-V., Park P.-H. Recent insights on modulation of inflammasomes by adipokines: a critical event for the pathogenesis of obesity and metabolism-associated diseases. Arch. Pharm. Res. 2020;43:997–1016. doi: 10.1007/s12272-020-01274-7. [DOI] [PubMed] [Google Scholar]
- 16.Paramel Varghese G., Folkersen L., Strawbridge R.J., Halvorsen B., Yndestad A., Ranheim T., Krohg-Sørensen K., Skjelland M., Espevik T., Aukrust P., et al. NLRP3 inflammasome expression and activation in human atherosclerosis. J. Am. Heart Assoc. 2016;5:e003031. doi: 10.1161/jaha.115.003031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y., Qin X., An Q., Yi J., Feng F., Yin D., An N., Liu Z., Weng L., Chen S., et al. Mesenchymal stromal cells directly promote inflammation by canonical NLRP3 and non-canonical caspase-11 inflammasomes. EBioMedicine. 2018;32:31–42. doi: 10.1016/j.ebiom.2018.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L., Chen K., Wan X., Wang F., Guo Z., Mo Z. NLRP3 inflammasome activation in mesenchymal stem cells inhibits osteogenic differentiation and enhances adipogenic differentiation. Biochem. Biophys. Res. Commun. 2017;484:871–877. doi: 10.1016/j.bbrc.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Ahn J.-S., Seo Y., Oh S.-J., Yang J.W., Shin Y.Y., Lee B.-C., Kang K.-S., Sung E.-S., Lee B.-J., Mohammadpour H., et al. The activation of NLRP3 inflammasome potentiates the immunomodulatory abilities of mesenchymal stem cells in a murine colitis model. BMB Rep. 2020;53:329–334. doi: 10.5483/BMBRep.2020.53.6.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee S., Choi E., Cha M.-J., Hwang K.-C. Cell adhesion and long-term survival of transplanted mesenchymal stem cells: a prerequisite for cell therapy. Oxid. Med. Cell. Longev. 2015;2015 doi: 10.1155/2015/632902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng W., Xiao J., Zheng G., Xing F., Tipoe G.L., Wang X., He C., Chen Z.-Y., Liu Y. Antioxidant treatment enhances human mesenchymal stem cell anti-stress ability and therapeutic efficacy in an acute liver failure model. Sci. Rep. 2015;5 doi: 10.1038/srep11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song H., Cha M.J., Song B.W., Kim I.K., Chang W., Lim S., Choi E.J., Ham O., Lee S.Y., Chung N., et al. Reactive oxygen species inhibit adhesion of mesenchymal stem cells implanted into ischemic myocardium via interference of focal adhesion complex. Stem Cells. 2010;28:555–563. doi: 10.1002/stem.302. [DOI] [PubMed] [Google Scholar]
- 23.Harijith A., Ebenezer D.L., Natarajan V. Reactive oxygen species at the crossroads of inflammasome and inflammation. Front. Physiol. 2014;5:352. doi: 10.3389/fphys.2014.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng G., Zhang F., Fuss I., Kitani A., Strober W. A mutation in the Nlrp3 gene causing inflammasome hyperactivation potentiates Th17 cell-dominant immune responses. Immunity. 2009;30:860–874. doi: 10.1016/j.immuni.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dorfleutner A., Bryan N.B., Talbott S.J., Funya K.N., Rellick S.L., Reed J.C., Shi X., Rojanasakul Y., Flynn D.C., Stehlik C. Cellular pyrin domain-only protein 2 is a candidate regulator of inflammasome activation. Infect. Immun. 2007;75:1484–1492. doi: 10.1128/iai.01315-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou R., Tardivel A., Thorens B., Choi I., Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 27.Oslowski C.M., Hara T., O'Sullivan-Murphy B., Kanekura K., Lu S., Hara M., Ishigaki S., Zhu L.J., Hayashi E., Hui S.T., et al. Thioredoxin-interacting protein mediates ER stress-induced β cell death through initiation of the inflammasome. Cell Metab. 2012;16:265–273. doi: 10.1016/j.cmet.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li L., Chen X., Wang W.E., Zeng C. How to improve the survival of transplanted mesenchymal stem cell in ischemic heart? Stem Cells Int. 2016;2016 doi: 10.1155/2016/9682757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou T., Yuan Z., Weng J., Pei D., Du X., He C., Lai P. Challenges and advances in clinical applications of mesenchymal stromal cells. J. Hematol. Oncol. 2021;14:24. doi: 10.1186/s13045-021-01037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strowig T., Henao-Mejia J., Elinav E., Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 31.Franz K.M., Kagan J.C. Innate immune receptors as competitive determinants of cell fate. Mol. Cell. 2017;66:750–760. doi: 10.1016/j.molcel.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma D., Kanneganti T.-D. The cell biology of inflammasomes: mechanisms of inflammasome activation and regulation. J. Cell Biol. 2016;213:617–629. doi: 10.1083/jcb.201602089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi L., Zhao Y., Fei C., Guo J., Jia Y., Wu D., Wu L., Chang C. Cellular senescence induced by S100A9 in mesenchymal stromal cells through NLRP3 inflammasome activation. Aging (Albany NY) 2019;11:9626–9642. doi: 10.18632/aging.102409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaneko N., Kurata M., Yamamoto T., Morikawa S., Masumoto J. The role of interleukin-1 in general pathology. Inflamm. Regen. 2019;39:12. doi: 10.1186/s41232-019-0101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee J.H., Han Y.-S., Lee S.H. Long-duration three-dimensional spheroid culture promotes angiogenic activities of adipose-derived mesenchymal stem cells. Biomol. Ther. 2016;24:260–267. doi: 10.4062/biomolther.2015.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sollberger G., Strittmatter G.E., Grossi S., Garstkiewicz M., Auf dem Keller U., French L.E., Beer H.D. auf dem Keller, U., French, L.E., and Beer, H.-D. (2015). Caspase-1 Activity Is Required for UVB-Induced Apoptosis of Human Keratinocytes. J. Invest. Dermatol. 2015;135:1395–1404. doi: 10.1038/jid.2014.551. [DOI] [PubMed] [Google Scholar]
- 37.Pierini R., Juruj C., Perret M., Jones C.L., Mangeot P., Weiss D.S., Henry T. AIM2/ASC triggers caspase-8-dependent apoptosis in Francisella-infected caspase-1-deficient macrophages. Cell Death Differ. 2012;19:1709–1721. doi: 10.1038/cdd.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsuchiya K., Nakajima S., Hosojima S., Thi Nguyen D., Hattori T., Manh Le T., Hori O., Mahib M.R., Yamaguchi Y., Miura M., et al. Caspase-1 initiates apoptosis in the absence of gasdermin D. Nat. Commun. 2019;10:2091. doi: 10.1038/s41467-019-09753-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sagulenko V., Thygesen S.J., Sester D.P., Idris A., Cridland J.A., Vajjhala P.R., Roberts T.L., Schroder K., Vince J.E., Hill J.M., et al. AIM2 and NLRP3 inflammasomes activate both apoptotic and pyroptotic death pathways via ASC. Cell Death Differ. 2013;20:1149–1160. doi: 10.1038/cdd.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu C., Zhao L., Wu D., Li L. Modulating autophagy in mesenchymal stem cells effectively protects against hypoxia- or ischemia-induced injury. Stem Cell Res. Ther. 2019;10:120. doi: 10.1186/s13287-019-1225-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee J.H., Yoon Y.M., Han Y.-S., Jung S.K., Lee S.H. Melatonin protects mesenchymal stem cells from autophagy-mediated death under ischaemic ER-stress conditions by increasing prion protein expression. Cell Prolif. 2019;52 doi: 10.1111/cpr.12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang C.-M., Huang Y.-J., Hsu S.-h. Enhanced autophagy of adipose-derived stem cells grown on chitosan substrates. Biores. Open Access. 2015;4:89–96. doi: 10.1089/biores.2014.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harris J., Lang T., Thomas J.P.W., Sukkar M.B., Nabar N.R., Kehrl J.H. Autophagy and inflammasomes. Mol. Immunol. 2017;86:10–15. doi: 10.1016/j.molimm.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 44.Shi C.-S., Shenderov K., Huang N.-N., Kabat J., Abu-Asab M., Fitzgerald K.A., Sher A., Kehrl J.H. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nat. Immunol. 2012;13:255–263. doi: 10.1038/ni.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin J.H., Walter P., Yen T.S.B. Endoplasmic reticulum stress in disease pathogenesis. Annu. Rev. Pathol. 2008;3:399–425. doi: 10.1146/annurev.pathmechdis.3.121806.151434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jing G., Wang J.J., Zhang S.X. ER stress and apoptosis: a new mechanism for retinal cell death. Exp. Diabetes Res. 2012;2012:589589. doi: 10.1155/2012/589589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei H., Li Z., Hu S., Chen X., Cong X. Apoptosis of mesenchymal stem cells induced by hydrogen peroxide concerns both endoplasmic reticulum stress and mitochondrial death pathway through regulation of caspases, p38 and JNK. J. Cell. Biochem. 2010;111:967–978. doi: 10.1002/jcb.22785. [DOI] [PubMed] [Google Scholar]
- 48.Yoon Y.M., Lee J.H., Yun S.P., Han Y.-S., Yun C.W., Lee H.J., Noh H., Lee S.-J., Han H.J., Lee S.H. Tauroursodeoxycholic acid reduces ER stress by regulating of Akt-dependent cellular prion protein. Sci. Rep. 2016;6 doi: 10.1038/srep39838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ulum B., Teker H.T., Sarikaya A., Balta G., Kuskonmaz B., Uckan-Cetinkaya D., Aerts-Kaya F. Bone marrow mesenchymal stem cell donors with a high body mass index display elevated endoplasmic reticulum stress and are functionally impaired. J. Cell. Physiol. 2018;233:8429–8436. doi: 10.1002/jcp.26804. [DOI] [PubMed] [Google Scholar]
- 50.Qiu T., He Y.-y., Zhang X., Ma X.-l. Novel role of ER stress and mitochondria stress in serum-deprivation induced apoptosis of rat mesenchymal stem cells. Curr. Med. Sci. 2018;38:229–235. doi: 10.1007/s11596-018-1870-9. [DOI] [PubMed] [Google Scholar]
- 51.Han C.Y., Rho H.S., Kim A., Kim T.H., Jang K., Jun D.W., Kim J.W., Kim B., Kim S.G. FXR inhibits endoplasmic reticulum stress-induced NLRP3 inflammasome in hepatocytes and ameliorates liver injury. Cell Rep. 2018;24:2985–2999. doi: 10.1016/j.celrep.2018.07.068. [DOI] [PubMed] [Google Scholar]
- 52.Lebeaupin C., Proics E., de Bieville C.H.D., Rousseau D., Bonnafous S., Patouraux S., Adam G., Lavallard V.J., Rovere C., Le Thuc O., et al. ER stress induces NLRP3 inflammasome activation and hepatocyte death. Cell Death Dis. 2015;6:e1879. doi: 10.1038/cddis.2015.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lv S., Li X., Wang H. The role of the effects of endoplasmic reticulum stress on NLRP3 inflammasome in diabetes. Front. Cell Dev. Biol. 2021;9:663528. doi: 10.3389/fcell.2021.663528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang X., Zhang J.H., Chen X.Y., Hu Q.H., Wang M.X., Jin R., Zhang Q.Y., Wang W., Wang R., Kang L.L., et al. Reactive oxygen species-induced TXNIP drives Fructose-mediated hepatic inflammation and lipid accumulation through NLRP3 inflammasome activation. Antioxid. Redox Signal. 2015;22:848–870. doi: 10.1089/ars.2014.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Han Y., Xu X., Tang C., Gao P., Chen X., Xiong X., Yang M., Yang S., Zhu X., Yuan S., et al. Reactive oxygen species promote tubular injury in diabetic nephropathy: the role of the mitochondrial ros-txnip-nlrp3 biological axis. Redox Biol. 2018;16:32–46. doi: 10.1016/j.redox.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bhattarai K.R., Riaz T.A., Kim H.-R., Chae H.-J. The aftermath of the interplay between the endoplasmic reticulum stress response and redox signaling. Exp. Mol. Med. 2021;53:151–167. doi: 10.1038/s12276-021-00560-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi Y., Wang Y., Li Q., Liu K., Hou J., Shao C., Wang Y. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat. Rev. Nephrol. 2018;14:493–507. doi: 10.1038/s41581-018-0023-5. [DOI] [PubMed] [Google Scholar]
- 58.Pandey A., Shen C., Man S.M. Inflammasomes in colitis and colorectal cancer: mechanism of action and therapies. Yale J. Biol. Med. 2019;92:481–498. [PMC free article] [PubMed] [Google Scholar]
- 59.Zhen Y., Zhang H. NLRP3 inflammasome and inflammatory bowel disease. Front. Immunol. 2019;10:276. doi: 10.3389/fimmu.2019.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raut P.K., Kim S.-H., Choi D.Y., Jeong G.-S., Park P.-H. Growth of breast cancer cells by leptin is mediated via activation of the inflammasome: critical roles of estrogen receptor signaling and reactive oxygen species production. Biochem. Pharmacol. 2019;161:73–88. doi: 10.1016/j.bcp.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 61.Pham D.-V., Raut P.K., Pandit M., Chang J.-H., Katila N., Choi D.-Y., Jeong J.-H., Park P.-H. Globular adiponectin inhibits breast cancer cell growth through modulation of inflammasome activation: critical role of Sestrin2 and AMPK signaling. Cancers. 2020;12:613. doi: 10.3390/cancers12030613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee S., Pham D.-V., Park P.-H. Sestrin2 induction contributes to anti-inflammatory responses and cell survival by globular adiponectin in macrophages. Arch. Pharm. Res. 2022;45:38–50. doi: 10.1007/s12272-021-01364-0. [DOI] [PubMed] [Google Scholar]
- 63.Tran V.T.-H., Pham D.-V., Choi D.-Y., Park P.-H. Mitophagy induction and aryl hydrocarbon receptor-mediated redox signaling contribute to the suppression of breast cancer cell growth by raloxifene via regulation of inflammasomes activation. Antioxid. Redox Signal. 2022;37:1030–1050. doi: 10.1089/ars.2021.0192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated during the study are included in the article and supplemental information.