Abstract
Lysosomal storage diseases (LSDs) are multisystem inherited metabolic disorders caused by dysfunctional lysosomal activity, resulting in the accumulation of undegraded macromolecules in a variety of organs/tissues, including the central nervous system (CNS). Treatments include enzyme replacement therapy, stem/progenitor cell transplantation, and in vivo gene therapy. However, these treatments are not fully effective in treating the CNS as neither enzymes, stem cells, nor viral vectors efficiently cross the blood-brain barrier. Here, we review the latest advancements in improving delivery of different therapeutic agents to the CNS and comment upon outstanding questions in the field of neurological LSDs.
Keywords: lysosomal storage diseases, LSDs, central nervous system, CNS, blood-brain barrier, BBB, enzyme replacement therapy, ERT, hematopoietic stem/progenitor cell transplantation, HSC, gene therapy
Graphical abstract

Benedetti and colleagues provide a comprehensive overview of therapeutic strategies targeting the CNS for the treatment of neurological lysosomal storage disorders, a group of severe metabolic diseases. The review focuses on the latest advancements in improving delivery of therapeutics across the blood-brain barrier and comment upon outstanding questions in the field.
Introduction
Lysosomal storage diseases (LSDs) are a group of more than 70 inherited metabolic disorders characterized by deficient function of lysosomes, organelles whose function is to catabolize macromolecules. The lysosome contains an array of hydrolytic enzymes that, together with transporters, lysosomal membrane proteins, and targeting motifs are accountable for the proper functioning of the cell-recycling apparatus. Defects in any of these components result in the aberrant accumulation of undegraded macromolecules, or “storage products,” disruption of cell homeostasis, cell dysfunction, and, in some cases, cell death.1 Prevalence of each LSD is very low; however, when considered as a group they affect a significant minority of live births (12.1–25 per 100,0002). LSDs are genetically heterogeneous, and can be classified into subcategories depending upon the type of macromolecule involved (reviewed by Platt et al.1). These are multisystem diseases that affect different tissues and organs to a variable degree depending on lysosome/substrate distribution, and expression profile of the causative gene(s). Clinical symptoms range in severity depending upon the extent to which a specific LSD affects each cell type, tissue, or organ; however, 50%–70%3,4 significantly affect the central nervous system (CNS), resulting in severe and progressive neurodegeneration. Brain damage commonly begins in early infancy but can also occur during adulthood in late onset forms. Neurological LSDs (summarized in Table 1) are often fast-progressing fatal diseases, therefore substantial effort has been made to develop effective treatments.
Table 1.
Neurological LSDs
Summary of neurological LSDs, including details of defective gene, primary protein involved, and associated lysosomal storage product (adapted from Platt et al.1).
| Neurological LSD (gene) | Primary defective protein (substrate/storage product) |
|---|---|
| Mucopolysaccharidoses (MPS) | |
| MPS I, also known as Hurler syndrome (IDUA) | α-L-iduronidase (dermatan sulfate, heparan sulfate) |
| MPS II (IDS) | iduronate 2-sulfatase (dermatan sulfate, heparan sulfate) |
| MPS III | |
| Type A (SGSH) | N-sulfoglucosamine sulfohydrolase (heparan sulfate) |
| Type B (NAGLU) | N-acetyl-α-glucosaminidase (heparan sulfate) |
| Type C (HGSNAT) | heparan-α-glucosaminide-N-acetyltransferase (heparan sulfate) |
| Type D (GNS) | N-acetylglucosamine-6-sulfatase (heparan sulfate) |
| MPS VII (GUSB) | β-glucuronidase (dermatan sulfate, heparan sulfate, chondroitin 6-sulfate) |
| Sphingolipidoses | |
| Fabry disease (GLA) | α-galactosidase A (globotriaosylceramide) |
| Gaucher disease type II, III, and perinatal lethal form (GBA) | β-glucocerebrosidase (glucocerebroside and glucosylsphingosine) |
| GM1 gangliosidosis types I-III (GLB1) | β-galactosidase (GM1 ganglioside, keratan sulfate, and oligosaccharides) |
| GM2 gangliosidosis | |
| Tay-Sachs (HEXA) | β-hexosaminidase (GM2 ganglioside, glycosphingolipids, and oligosaccharides) |
| Sandhoff (HEXB) | β-hexosaminidase (GM2 ganglioside, GA2 glycolipid, and oligosaccharides) |
| GM2 activator deficiency (GM2A) | GM2 ganglioside activator (GM2 ganglioside and glycosphingolipids) |
| Krabbe disease, also known as globoid cell leukodystrophy (GALC) | galactosylceramidase (galactocerebroside and psychosine) |
| Metachromatic leukodystrophy (ARSA and PSAP) | arylsulfatase A and prosaposin (sulfatides) |
| Niemann-Pick disease type A (SMPD1) | sphingomyelin phosphodiesterase (sphingomyelin) |
| Glycoproteinoses | |
| α-Mannosidosis types I, II, and III (MAN2B1) | lysosomal α-mannosidase (mannose-rich oligosaccharides) |
| β-Mannosidosis (MANBA) | β-mannosidase (Man(β1>4) N-acetylglucosamine) |
| Fucosidosis (FUCA1) | α-L-fucosidase (fucose-rich oligosaccharides, glycoproteins, and glycolipids) |
| Aspartylglucosaminuria (AGA) | aspartoglucosaminidase (aspartylglucosamine) |
| Schindler disease: types I–III (NAGA) | α-N-acetylgalactosaminidase (sialylated or asialo glycopeptides and glycosphingolipids) |
| Sialidosis type II (NEU1) | neuraminidase-1 (sialylated oligosaccharides and glycopeptides, LAMP1, and amyloid precursor protein) |
| Glycogen storage diseases (GSD) | |
| GSD II, also known as Pompe disease (GAA) | lysosomal α-glucosidase, also known as acid maltase (glycogen) |
| Lipid storage diseases | |
| Acid lipase deficiency, also known as Wolman disease (LIPA) | lysosomal acid lipase/cholesteryl ester hydrolase (cholesteryl esters, triglycerides, and other lipids) |
| Post-translational modification defects | |
| Mucolipidosis type II, also known as Inclusion-cell disease (GNPTAB) | N-acetylglucosamine-1-phosphotransferase subunits α/β (oligosaccharides, glycosaminoglycans, and glycosphingolipids) |
| Integral membrane protein disorders | |
| Danon disease (LAMP2) | lysosomal associated membrane protein 2 (cytoplasmic debris and glycogen) |
| Action myoclonus-renal failure syndrome (SCARB2) | lysosomal integral membrane protein 2 (unknown) |
| Sialic acid storage disease (SLC17A5) | sialin (sialic acids) |
| Niemann-Pick disease type C (NPC1 and NPC2) | NPC intracellular cholesterol transporter 1 and 2 (cholesterol and sphingolipids) |
| Mucolipidosis type IV (MCOLN1) | mucolipin 1 (lipids and mucopolysaccharides) |
| Neuronal ceroid lipofuscinoses (largely unknown heterogeneous mix of substrates) | |
| CLN1 (PPT1) | palmitoyl-protein thioesterase 1 (lipidated thioesters and saposins A and D) |
| CLN2 (TPP1) | tripeptidyl peptidase 1 (subunit c of mitochondrial ATP synthase) |
| CLN3 (CLN3) | battenin (subunit c of mitochondrial ATP synthase) |
| CLN4 (DNAJC5) | cysteine string protein (subunit c of mitochondrial ATP synthase) |
| CLN5 (CLN5) | ceroid-lipofuscinosis neuronal protein 5 (subunit c of mitochondrial ATP synthase) |
| CLN6 (CLN6) | transmembrane ER protein (subunit c of mitochondrial ATP synthase) |
| CLN7 (MFSD8) | major facilitator superfamily domain containing 8 (subunit c of mitochondrial ATP synthase) |
| CLN8 (CLN8) | ceroid-lipofuscinosis neuronal protein 8 (subunit c of mitochondrial ATP synthase) |
| CLN9 (gene unknown) | protein unknown (substrate unknown) |
| CLN10 (CTSD) | cathepsin D (saposins A and D) |
| CLN11 (GRN) | granulin (unknown) |
| CLN12 (ATP13A2) | cation-transporting ATPase 13A2 (inorganic cations) |
| CLN13 (CTSF) | cathepsin F (unknown) |
| CLN14 (KCTD7) | potassium channel tetramerization domain containing 7 (unknown) |
| Lysosome-related organelle disorders | |
| Chédiak-Higashi disease (LYST) | lysosomal trafficking regulator (size and movement of lysosomes) |
Currently, there are several experimental and clinical treatments available for specific LSDs with the collective aim of restoring enzyme function. Standards of care include (1) enzyme replacement therapy (ERT) to deliver exogenous enzyme directly to the patient5; (2) hematopoietic stem/progenitor cell (HSC) transplantation, in which patients receive either allogeneic or autologous HSCs, which are genetically modified ex vivo (HSC gene therapy) and are able to engraft the CNS, providing a source of functional enzyme6,7; and (3) substrate reduction therapy that utilizes small molecules to attenuate accumulation of specific macromolecules.8 Ongoing clinical trials are evaluating improved standard of care approaches, especially for HSC gene therapy, while also testing alternative approaches. These include in vivo gene therapy, which delivers a healthy copy of the defective gene directly to patients’ cells9; and chaperone therapy to guide correct protein folding of patients’ aberrant enzymes to improve their catalytic function.10
Despite some of these treatment strategies being successful for specific forms of LSDs,11 there are still a number of drawbacks. Each treatment has different limitations: ERT is immunogenic, must be administered regularly, and has limited efficacy in some organs5; HSC transplantation (HSCT) necessitates chemotherapeutic pre-conditioning and has a risk of transplant-associated morbidity and mortality6,7; substrate reduction therapy, like ERT, does not correct the primary defect and some molecules are associated with undesirable secondary side effects8; and a range of gene therapy vectors can be immunogenic.12
One limitation that is common to all these strategies is the inability, or limited ability, of therapeutic agents to cross the blood-brain barrier (BBB) and reach the CNS or, in the specific case of HSCT therapy, to engraft rapidly enough and in optimal numbers to prevent the rapid neurological deterioration that occurs in some LSDs. Consequently, in recent years there has been a strong focus on increasing delivery of therapeutic agents to the CNS. Innovations in CNS delivery have been recently discussed from the perspective of nanoparticles13 and small molecules.14 However, methods to improve delivery of enzymes, stem cells, and viral vectors to the CNS have not been reviewed in recent years. This review focuses upon methods to increase delivery across the BBB, with emphasis on the latest advancements in targeting HSCT, ERT, and viral vectors to the CNS, and discusses the future of CNS-directed LSD therapy.
The blood-brain barrier (BBB)
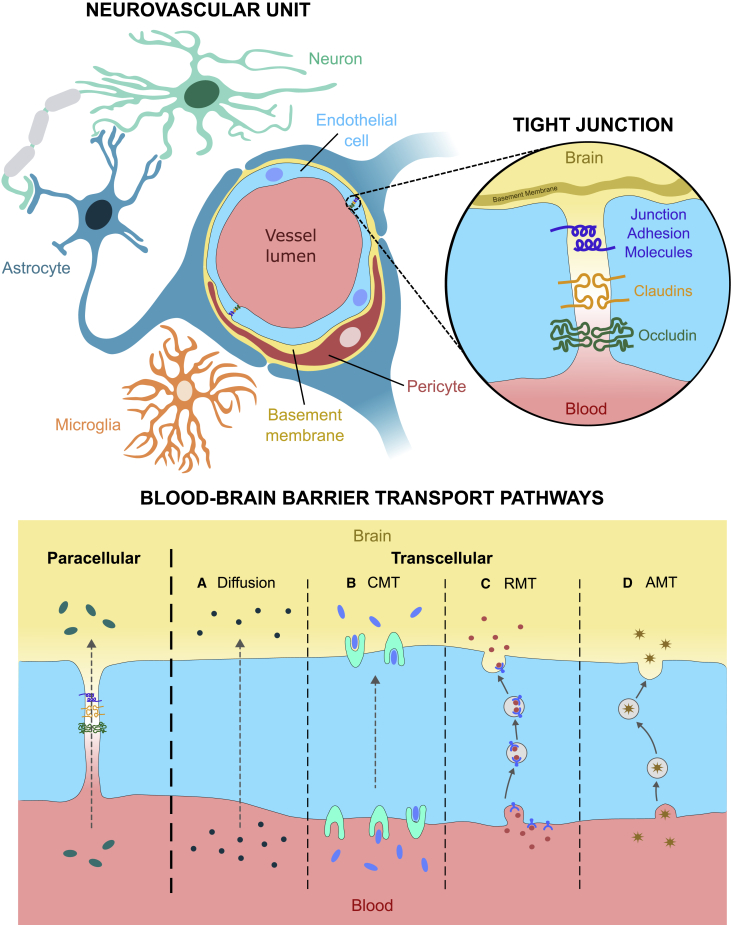
The BBB is a selectively permeable barrier between the CNS and the systemic circulation that controls exchange of solutes and protects the brain from toxins and potential pathogens circulating in the bloodstream.15 It is comprised of neurovascular units, in which brain cells closely interact with the vasculature. The neurovascular unit involves multiple cell types: endothelial cells, pericytes, astrocytes, neurons, and microglia. Endothelial cells are the primary component and are supported by pericytes, perivascular cells that embrace the vessels and provide them with stability. Astrocytic endfeet ensheath almost the entire abluminal surface of microvessels,16 and neurons and perivascular microglia interact with these cells to establish the neurovascular unit (Figure 1). Brain endothelial cells are especially vital for restricting BBB permeability, and have particular properties that enable them to perform this function, including (1) reduced transcellular flux, (2) lack of fenestrations, (3) greater mitochondrial density to assist rapid metabolism, (4) specialized transport systems, and (5) high electrical resistance as a result of an increased number of tight junctions between endothelial cells compared with other tissues and organs17 (Figure 1). Multiple proteins are involved in tight junctions, namely junctional adhesion molecules, claudins, zonular occludens, and occludin.15 Under normal conditions, they prevent molecules from leaking across the BBB through the paracellular transport pathway, which represents one of the two main transport routes across the BBB (Figure 1). Alternatively, molecules can move transcellularly with some crossing the BBB by passive diffusion, while most require assistance from carrier proteins (carrier-mediated transcytosis), receptors (receptor-mediated transcytosis), or vesicles (adsorptive-mediated transcytosis).17
Figure 1.
The neurovascular unit and blood-brain barrier transport pathways
Graphical depiction of the neurovascular unit (NVU), the fundamental anatomical and functional unit of the blood-brain barrier (BBB), including the key protein components of tight junctions (TJs) between brain endothelial cells which control the paracellular transport pathway. Alternatively, molecules may be transported transcellularly via passive diffusion (A), carrier-mediated transcytosis (CMT) (B), receptor-mediated transcytosis (RMT) (C), or adsorptive-mediated transcytosis (AMT) (D).
However, in pathological conditions BBB integrity can be disrupted, allowing passage of substances that would normally not be able to cross. In the case of some CNS diseases (including Alzheimer’s and Parkinson’s), systemic diseases (e.g., diabetes mellitus and chronic cerebrovascular disease), and viral infections (e.g., viral encephalitis), disruptive remodeling of tight junctions results in reduced BBB integrity, leading to neuroinflammation, which further contributes to increased BBB permeability.18,19,20,21,22,23 A greater understanding of the role of neurovascular units and tight junctions in the transport of therapeutic agents across the BBB, and being able to manipulate transport to increase delivery, may be vital for the effective delivery of therapeutic agents to treat the neurological component of LSDs. In the following sections, we explore how current treatments have been modified to improve stem cell, enzyme, and viral vector delivery across the BBB, including methods that exploit aspects of BBB transport pathways or bypass the barrier altogether.
Blood-brain barrier (BBB) manipulation
Several methods have been employed to disrupt the BBB with the aim of temporarily increasing permeability for LSD therapeutic agents (reviewed by Hersh et al.24).
Here, we briefly explore both non-selective and selective methods that assist delivery of specific cells and/or enzymes.
Focused ultrasound
The use of magnetic resonance thermometry to guide focused ultrasound pulses in the presence of microbubbles is able to briefly compromise BBB permeability. Ultrasound pulses cause the microbubbles to expand and contract, temporarily separating endothelial tight junctions, which facilitates passage of therapeutics without allowing pathological events to occur.25 In relation to neurological LSDs, the method has been used to deliver GFP-labeled neural stem cells to wild-type rat brains,26 and to transport enzyme across the BBB in an MPS I murine model, restoring 75% of normal enzyme activity in the treated brain hemisphere.27 Investigation of this method’s safety and feasibility is underway in a number of neurological diseases and is employed in a phase I trial delivering Cerezyme (an analog of the β-glucocerebrosidase enzyme, which is also defective in the LSD Gaucher disease) across the BBB in Parkinson’s disease patients (ClinicalTrials.gov NCT04370665).
Hyperosmotic agents
Intravenously delivered hyperosmotic agents increase BBB permeability by shrinkage of brain endothelial cells and consequent tight junction widening.28 This temporarily augmented permeability allows a generalized increase in migration of substances from the bloodstream. The hyperosmotic agent mannitol has been used in murine models to deliver adeno-associated viral (AAV) vectors to the CNS in Sandhoff disease,29 MPS IIIB,30,31 and CLN2 deficiency,32 showing enhanced delivery and greater therapeutic effect. However, the potential for toxic substances to cross the BBB during the period of non-selectively enhanced permeability, or for cerebral edema to occur if mannitol enters the brain, has limited its use in patients despite its clinical safety profile.33
Receptor stimulation
Receptor stimulation can increase delivery of enzymes across the BBB by relocalizing receptors to the luminal surface of brain endothelial cells. Studies in the LSD field have predominantly focused on the mannose 6-phosphate (M6P) receptor, a transport mechanism in the brain present during early post-natal development but lost during maturation.34 Murine studies have shown that administration of epinephrine35 or retinoic acid34 stimulates M6P receptors and significantly elevates M6P-mediated transport of the lysosomal enzyme β-glucuronidase (P-GUS, defective in MPS VII) across the BBB. Further work involving direct stimulation of specific adrenoreceptors with α1/2 agonists suggested that increased enzyme uptake was likely due to redistribution of M6P receptors from an intracellular pool to the intra-luminal surface of brain microvascular endothelial cells.34,36 These studies suggest that manipulation of receptor-mediated transport is a viable method for increasing selective delivery of enzymes across the BBB.
Enzyme replacement therapy (ERT)
The concept of ERT as a potential treatment for LSDs (reviewed in Solomon and Muro5) first arose in the mid-1960s; however, a further three decades of development were required to generate the first effective, clinically approved ERT. ERT entails administration of fully functional exogenous enzyme to the patient, mainly via intravenous (i.v.) injection. The enzyme is taken up by patients’ cells via endocytosis and trafficked to the lysosomes, where it compensates for endogenous enzyme dysfunction. ERT’s limitations have been extensively reviewed elsewhere5; the major one of relevance to neurological LSDs is the inability to treat organs that are difficult to access—particularly the musculoskeletal, cardiovascular, ocular, and central nervous systems.1 In the following sections, we review strategies employed to circumvent this limitation.
Enzyme modification
Fusion proteins
Modification of the therapeutic enzyme with an exogenous protein subunit might enable interaction with a specific receptor to increase CNS uptake. Multiple fusion proteins have been tested for efficacy in augmenting CNS delivery in LSD murine and/or primate models, including an acidic amino acid tag,37 the fat-binding apolipoprotein E,38,39,40 and importantly antibody conjugates targeting endogenous BBB transport receptors, including the insulin receptor41,42,43 and the transferrin receptor.44 Antibody-conjugated enzymes harness the receptor-mediated transport pathway to cross the BBB into the CNS. Results from in vivo studies demonstrated reduction of substrates and neuroinflammation in MPS II murine and primate models,43,44 and highlighted a pharmacological safety profile.41,42 A number of clinical trials (NCT03128593, NCT03568175, NCT04573023) have pursued this further; following a successful phase I/II trial of iduronate-2-sulfatase fused with an anti-human transferrin receptor antibody in MPS II patients,45 results of a phase II/III study showed significantly reduced substrate accumulation in both the CNS and peripheral tissue, in addition to positive neurocognitive changes, while demonstrating a clinical safety profile consistent with current standards of care.46 This strategy has now been approved for clinical use in Japan.47
Chemical modification
An alternative to fusion proteins is the chemical modification of lysosomal enzymes to alter receptors’ affinity, allowing an elevated blood concentration of the therapeutic enzyme to maintain a high concentration at the BBB for prolonged periods. This approach has been tested in MPS VII48,49 and MPS IIIA50,51,52 murine models, showing significant reduction in CNS lysosomal storage biomarkers.48,49,50,51,52
Delivery
Injection routes
A range of different injection routes have been tested for ERT to improve enzyme delivery (Figure 2). Beyond traditional i.v. injection, intracerebroventricular (i.c.v.) injection53,54 has been reported most extensively in recent literature in comparison with intrathecal (i.t., lumbar and cisternal),53 or i.v. injection,55 or to control conditions.54,56,57,58,59,60,61,62,63 A number of studies have reported that i.c.v. is effective for ERT in multiple neurological LSD animal models.53,54,55,57,58,59,60,61,62 However, these studies raise an important issue as sufficient enzyme delivery for therapeutic effect59 remains a challenge. Treleaven et al. observed that less than 1% of the total ERT dose reached the CNS of a Niemann-Pick type A (NPA) murine model. Higher doses did not increase this percentage, suggesting that the enzyme uptake mechanism is saturated. However, while this is an extremely small proportion, it was distributed widely throughout the CNS, and previous work in the same murine model61 demonstrated significant reduction of storage product levels and partial alleviation of motor abnormalities. The study proposed ERT scaling by CNS weight to maintain this therapeutic level in larger rodents.59 Work in the NPA model raised a second potential limitation with i.c.v. delivery; despite therapeutic effect on the CNS as a whole, they observed a steep gradient in therapeutic enzyme from outer to inner brain regions, raising the possibility that therapeutic correction may be less successful in deeper tissue.60 However, elevating the concentration of therapeutic enzyme may trigger an immune response against the exogenous enzyme, as was observed in a few MPS I mice given high-dose i.v. ERT.64 Thorough investigation of toxic effects of high-dose ERT, and the impact upon CNS therapeutic correction, are required.
Figure 2.
Clinically relevant delivery routes for LSD therapeutic agents
Graphical summary of the injection routes used to deliver enzyme replacement therapy (ERT), stem cells, and viral vectors in neurological LSDs.
Other CNS-targeted ERT injection routes, including i.t., intranasal (i.n.), and intracisternal (i.c.), have been tested to varying degrees. i.t. and i.c. methods have been trialled in a similar range of animal models to i.c.v.,56,65,66,67,68,69,70,71,72,73,74,75,76 with i.t. being shown to have greater benefit over i.v. in a single MPS IA patient.65 i.n. delivery has only been tested in a murine model of MPS I,77,78 and, similar to i.c.v. injection, only a very minimal percentage of the total dose of therapeutic enzyme (estimated 0.001%) reached the brain.78 Despite studies reporting a predominantly positive effect on the neurological pathology, these injection routes entail reduced quantity of administered enzyme and consequently a reduced effect in deep brain tissues.56,66,68,70,73,77,78
Few studies have directly compared the effect of different ERT injection routes on LSD CNS pathology. i.c.v. proved more therapeutically effective than i.c. injection in two studies53,55 despite being the most invasive.53 Comparison between i.c.v. and i.t. has shown mixed results; in a canine model of MPS II, i.c.v. injection of ERT was superior, with correction of deep brain tissues55; however, in wild-type non-human primates and dogs i.t. delivery gave better results,67 which was supported by a small-scale trial of i.t. injection in MPS II mice by the same group; however, no mice were injected using the i.c.v. route, limiting direct comparison in the disease model setting.67 Altogether, these studies suggest that i.c.v. injection is the most effective for delivering ERT to the CNS in LSD models; however, the concerns regarding non-homogenous delivery throughout the brain and the limited percentage of treatment delivered to the CNS (which, albeit small, is sufficient to exert a therapeutic effect) suggest that other strategies may need to be employed.
Delivery vehicles
Delivery vehicles, such as nanoparticles,13 extracellular vesicles,79 polymersomes,80,81 and quantum dots,82 have been explored to improve enzyme delivery to the CNS in LSDs. While quantum dots have only been investigated in an in vitro setting,82 successful in vivo studies have been conducted with polymersomes,81 extracellular vesicles,79 and nanoparticles; of these, nanoparticles have been researched most extensively. Multiple studies have employed nanoparticles to successfully deliver therapeutic enzymes to the CNS of Gaucher disease,83 Krabbe disease,84 and MPS II murine models, reporting reduction of storage products to non-pathological levels85 (for a thorough review of the role of nanoparticles in LSD treatment up to 2016, please refer to Martín-Banderas et al.13). However, it is important to note that not all CNS LSDs are amenable to treatment using nanoparticles; three different nanoparticle formulations tested in an MLD murine model showed no increase in CNS enzyme levels, perhaps due to the therapeutic enzyme itself interfering with the targeting of the nanoparticles to the CNS.86 The authors speculate that this could be due to interference of the enzyme’s charge or side-chain oligosaccharides with surfactant coating or apolipoprotein recruitment, which are reported to be key mechanisms in BBB transport of nanoparticles. Therefore, it is possible that other delivery vehicles may also be limited by this issue.
Delivery devices
Initially, subcutaneous delivery devices were designed to enable continuous delivery of therapeutic enzymes to the CNS.87 Devices that deliver therapeutic enzymes via the i.c.v.88,89,90,91 or i.t. route92,93 were tested in the past decade and proved effective in MPS,88,89,93 MLD,90 and NCL91 murine models. In 2017, an infusion pump that delivers into the cerebrospinal fluid was tested in a canine model,87 but the study concluded that repeated i.c. or intra-spinal delivery was more effective. Furthermore, continuous delivery of therapeutics via these devices necessitates storage of the enzyme at body temperature for prolonged periods, which is likely to compromise enzyme stability and therefore limit utility. Consequently, research focus has now shifted toward devices with no indwelling enzyme reservoir. An i.t. drug delivery device utilized for monthly dosing was tested in a clinical trial for MPS II patients92 (ClinicalTrials.gov Identifier NCT02055118); early results indicated a promising 80% reduction in storage substrate; however, over 50% of the trial participants had their device removed because of significant adverse events,92 either due to the device breaking or the infusion cannula migrating away from the delivery site. Recent trials of a new i.c.v. device (Ommaya reservoir) in MPS IIIB patients have proven more effective (EudraCT 2017-003083-13; ClinicalTrials.gov NCT02754076 and NCT03784287), and the device has been applied to delivery of an ERT approved for i.c.v. dosing in MPS II patients.94
Convection enhanced delivery
One alternative strategy that has predominantly been applied to augment i.c.v. delivery for brain tumor treatment is convection-enhanced delivery,95 where catheters are stereotactically inserted and, using image guidance, directed into the interstitial spaces before an infusion pump is used to drive delivery, therefore not requiring a high concentration of the therapeutic agent.95 The only in vivo application of this strategy for Gaucher disease96 ERT showed progressive and complete filling of the CNS target regions with therapeutic enzyme, while a trial in a single patient with type 2 Gaucher disease demonstrated safety.96 Another clinical study evaluated safety of convection-enhanced delivery for gene therapy agents in late infantile NCL patients, reporting no adverse effects of the procedure and enzyme infusion rates between 50% and 90%.97 However, there has been very limited further testing of this method in neurological LSDs, perhaps due to the range of risks associated with this procedure, primarily backflow, air bubbles, and flow within brain tissue.95
Stem and progenitor cell transplantation
The requirement for a permanent, long-term fix that delivers lysosomal enzyme to all affected tissues in LSD patients has pushed scientists to look at other treatments beyond ERT. One promising alternative is stem and progenitor cell transplantation, which can generate lifelong tissue-resident sources of functional lysosomal enzyme that can relieve both somatic and neurological pathology.6 Stem and progenitor cells are injected into the patient, where they engraft in affected tissues, contribute to the patients’ resident cell populations, and secrete functional enzyme. The ability of stem and progenitor cells to potentially cross the BBB and provide cross-correction in the CNS has led to this strategy being trialled for a range of neurological LSDs. To date, hematopoietic stem and progenitor cells (HSCs) have been most commonly trialed in LSD animal models, and also human patients.6,7 Other stem cells have been used for transplantation specifically targeting the CNS in LSDs, including neural stem cells and, to a lesser extent, mesenchymal stem cells.
Analysis of neural and mesenchymal stem cell transplants for CNS LSDs can be found in a number of recent reviews98,99,100; herein, we focus on HSC transplantation (HSCT) as the most promising strategy. HSCs can either be isolated from a healthy donor (allogeneic transplantation) or in an autologous manner using the patients’ own genetically modified cells to provide a healthy copy of the mutated/non-functional gene.6
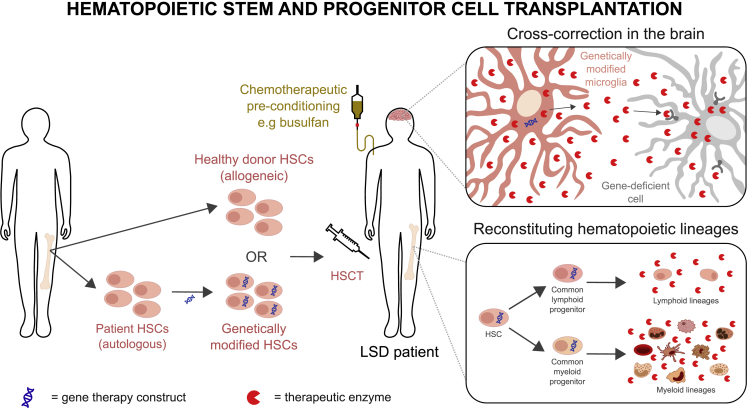
A yet-to-be-identified subpopulation of transplanted HSCs is able to cross the BBB following the use of specific chemotherapy or irradiation-based conditioning regimes and replace tissue resident microglia in the CNS.101,102 The newly generated microglia secrete functional lysosomal enzyme, which can be taken up by neighboring enzyme-deficient brain cells in a process called cross-correction (Figure 3, “cross-correction in the brain” panel).6,7,98,99,103 At the same time, differentiation of HSCs (which do not engraft the CNS) reconstitutes the entire hematopoietic system, thereby providing a peripheral source of therapeutic enzyme (Figure 3, “reconstituting hematopoietic lineages” panel). However, treatment of the CNS remains a challenge. In HSCT, cellular engraftment is not instantaneous, and gradual expansion of the transplanted cell population is required before lysosomal enzyme activity can be restored.6,99 During this period, neurological symptoms often progress, which significantly reduces the impact of HSCT.6 Furthermore, efficacy of HSCT in the CNS can be limited by (1) insufficient quantity of transplanted cells being trafficked to the CNS or (2) not enough functional lysosomal enzyme from engrafted cells being expressed in the CNS.99 At present, most studies in this field are designed to improve the ability of stem cells to secrete functional enzyme once they have engrafted the CNS, rather than increasing the absolute number which cross the BBB, because this aim is more achievable with current knowledge and technologies. In the coming sections, we explore innovative strategies targeted to the CNS pathology of LSDs.
Figure 3.
Overview of HSCT approaches
Graphical summary of HSCT using either allogeneic donor cells, or genetically modified patient cells to secrete a supraphysiological level of the defective enzyme. HSCT mediates therapeutic effect in the central nervous system by an HSC subpopulation crossing the BBB, engrafting the CNS, and generating genetically modified microglia, which provides a source of therapeutic enzyme to cross-correct neighboring enzyme-deficient brain cells.
Pre-conditioning agent
In bone marrow transplants, a pre-transplantation chemotherapeutic conditioning regime is essential to deplete patients’ resident HSCs and, possibly, resident microglia,102,104 which facilitates engraftment of an HSC subpopulation in the CNS following transplantation102 (Figure 3). The most widely used pre-conditioning agent, busulfan, has been demonstrated to deplete resident microglia more effectively than alternative conditioning regimes (irradiation or treosulfan) in mice,102 specifically by causing microglial senescence and exhaustion of their regenerative ability.105 Some studies suggest that busulfan could also be responsible for vascular injury and BBB disruption,106,107 hypothesizing that a perturbed BBB could be accountable for the increased HSC engraftment. However, recent work by Cartier and colleagues suggests a non-inflammation- or non-BBB-disruption-induced permissive engraftment following busulfan conditioning.105 Busulfan is associated with significant systemic toxicity in patients108; in addition, in mice it has been shown to cause a permanent inhibition of adult neurogenesis, suggesting a potential cognitive deficit for patients undergoing this regime105 and emphasizing need for the future development of alternative pre-conditioning strategies with lower toxicity.
In this direction, antibody-based pre-conditioning regimes with reduced toxicity have been tested in mice,109,110,111 and regimes that specifically target the hematopoietic lineages have successfully been used in severe combined immunodeficiency patients,112 or immunocompetent mice and dogs.113,114,115 However, the ability of antibody-based regimes to deplete resident microglia in the CNS and allow neurological engraftment of transplanted HSCs has not been determined.
Another option might be brain-targeted conditioning; this could potentially improve treatment efficacy in the CNS of neurological LSD patients. A newly synthetized and highly selective brain penetrant CSF1R inhibitor, PLX5622, has been used for extensive and specific microglial elimination in a murine model of Alzheimer’s disease.116 Moreover, in a recent study wild-type mice were pre-treated with PLX5622, lethally irradiated, and then received a bone marrow transplant. Mice receiving the CSF1R inhibitor showed a depletion of microglia and subsequent microglia replacement at the CNS-wide scale (around 90%) compared with non-treated mice, which show a minimal engraftment only in specific regions.117
Ex vivo stem cell gene therapy enhancement
When considering the two sources of therapeutic enzyme generated by HSCT, namely the peripheral cells of the reconstituted hematopoietic system and the tissue-resident macrophages, ex vivo gene therapy of autologous HSCs can be utilized in two ways to deliver a greater level of therapeutic benefit to the CNS. Firstly, by engineering vectors so that each genetically corrected cell secretes a supraphysiological level of enzyme, therapeutic benefit might be achieved in the CNS even with a limited number of engrafted cells. Moreover, studies have demonstrated that even a modest increase in enzyme activity in the CNS can provide therapeutic benefit; for example, restoring enzyme expression to 3.7% of wild-type levels in MPS II mice following HSCT was sufficient to correct CNS disease phenotype.118 Secondly, modifying the enzyme sequence in the gene therapy construct so that therapeutic enzyme produced by peripheral hematopoietic cells can cross the BBB more easily also potentially enhances therapeutic effect in the CNS. Many of the methods of HSCT gene construct modification overlap with previously discussed enzyme modifications. In addition, HSC gene therapy for LSDs has been reviewed in depth by Biffi,6 therefore we only briefly discuss it here.
Enzyme modification has been achieved in the HSC gene therapy setting by improving, before HSC transduction, the characteristics of the viral vectors used, or the therapeutic genes they contain. Similar to in ERT, fusion proteins have been included in the gene therapy constructs to increase uptake by the CNS.38,118 Other modifications of the therapeutic construct have focused on careful choice of promoters of gene expression. Appropriate choice and manipulation of the promoter could achieve production and secretion of supraphysiological levels of functional enzyme, and potentially increase its uptake by enzyme-deficient brain cells.119,120 For example, utilization of the myeloid promoter CD11b to promote expression of the codon-optimized therapeutic enzyme specifically in myeloid cells (including microglia) and not in progenitors or other hematopoietic cells (to avoid potential toxicity) has proven beneficial in the CNS of MPS IIIA121 and MPS IIIB122 murine models, and has been taken further for MPS IIIA treatment with completed pre-clinical safety studies.123
An alternative approach to construct modification, which has been applied to a gene therapy construct but not yet combined with HSC gene therapy, focuses on promoting enzyme secretion and increasing post-translational activation speed in MPS IIIA124,125 and MPS VII126 mice. Further investigation is required to ascertain whether these concepts could perhaps be applied to HSC gene therapy for neurological LSDs. Both modification of the enzyme, or promoting its expression via editing of the gene therapy construct, have resulted in improved pathology correction in neurological murine LSD models, and represent a valid approach to targeting the CNS in LSDs. However, neither of these methods assist infiltration of the CNS by stem and progenitor cells.
Overall HSC gene therapy has shown to be effective in targeting the neurological pathology in LSDs127,128,129,130,131,132 (and reviewed in Biffi6). HSC gene therapy clinical trials in MLD127,128,129 and adrenoleukodystrophy (ALD)130,131,132 patients showed high levels of therapeutic enzyme expression, reduction of storage products, and improvement of the clinical phenotype. Based on the efficacy and safety profile, the European Commission granted approval for marketing of HSC gene therapies for MLD and ALD at the end of 2020 and 2021, respectively.133,134
Administration routes
There has been less extensive investigation of transplantation administration routes in HSCT than in ERT for neurological LSDs; however, similar injection sites have been tested for stem and progenitor cell delivery to the CNS (Figure 2). Studies in MPS VII135 and MPS I136 murine models support the use of i.c.v. delivery to increase therapeutic effect in the CNS. Work by Capotondo et al. provided fundamental insight into the success of engraftment and fate of transplanted cells, demonstrating that HSCs do engraft the CNS, and give rise to microglia-like cells with biochemical characteristics matching bona fide microglia.101 Comparison with conventional i.v. delivery provided evidence for i.c.v. injection leading to more rapid engraftment of the CNS and a greater abundance of therapeutic enzyme in a murine model of MLD.102 Combined, these studies support i.c.v. delivery to improve therapeutic benefit in the CNS of LSD patients.
In vivo gene therapy
Whilst we have already discussed using viral vectors for ex vivo gene therapy, we have not yet considered them as an independent treatment option. In vivo gene therapy involves delivering the therapeutic gene directly to patients’ cells using a viral vector. In LSDs, gene therapy facilitates expression of therapeutic concentrations of functional lysosomal enzyme by directly modifying a subset of patients’ own cells.9 A large range of viral vectors have been trialed for this purpose. In the last decade or so, AAVs emerged as the most useful vectors for CNS-directed gene therapy due to their transduction efficiency, wide tropism, and relative safety profile. In particular, direct administration of small, non-enveloped, and non-integrating AAVs, named recombinant adeno-associated viral vectors (rAAVs), has been trialed both systemically and locally. A comprehensive overview of retroviral, lentiviral, and adenoviral-based vectors together with a discussion of their pros and cons for in vivo gene therapy and CNS targeting has been provided in recent reviews.9,137,138 Here we focus on the most relevant pre-clinical and clinical data, specifically discussing how to increase AAV-mediated CNS-targeted expression.
Use of different AAV serotypes and capsids
One of the greatest advantages of rAAVs over other viral vectors is the possibility to choose different serotypes—for example, those with CNS-tropism can be utilized with the aim of improving in vivo gene therapy outcome for neurological LSD patients. Several in vivo studies showed that serotypes 5, 8, and 9, and the recombinant human (rh)10, can cross the BBB, each to a different extent, allowing transduction of the CNS following systemic administration.139,140,141,142 For example, AAV9 was shown to be able to cross the BBB and improve neurological symptoms after systemic administration in LSD animal models.143,144 Two open-label, dose-escalation, phase 1/2 global clinical trials assessing AAV9 technology via a single-dose i.v. infusion are currently underway for young (2 years old or less) and asymptomatic (development quotient >60) MPS IIIA (NCT02716246) and MPS IIIB (NCT03315182) patients, called ABO-102 and ABO-101, respectively. For the MPS IIIA trial, data collected at different time points (6, 12, and 24 months post-treatment) from the three dose-escalating groups highlighted a provisional safety profile in all patients, with time- and dose-dependent statistically significant reductions in cerebrospinal fluid and plasma heparan sulfate levels, and stabilization or improvement of adaptive behavior and/or cognitive function.145,146 Another trial on MPS IIIA patients in middle and advanced phases of the disease receiving the highest dose of ABO-102 (3 × 10e13 vg/kg) has recently terminated due to lack of efficacy (NCT04088734).147 Preliminary results from the MPS IIIB trial were promising, with multiple disease biomarkers providing clear evidence of a biological effect in patients.148
Indeed, use of serotypes able to naturally target the CNS, such as AAV9, has been pivotal in providing access to the CNS. However, the low efficiency and lack of target specificity mean that high vector load needs to be used, potentially leading to toxicity. Generation of novel capsids would be important in increasing AAVs’ specificity and efficiency. Years of capsid engineering efforts using different platforms have now yielded a number of improved CNS capsids for rodents that are undergoing pre-clinical testing.149,150,151 In one recently published study, Chen et al. evolved a family of AAV capsid variants that can efficiently transduce both the central and peripheral nervous system in rodents. Both vectors also enable efficient targeting in non-human primates.152
Increased AAV dosing
Historically, serotypes AAV8 and AAV9 have preferential tropism for liver and muscle,153 but, when used at higher doses, these serotypes might achieve more widespread tissue expression, including in the CNS. However, dose-related neurotoxicity has been reported in large animal models treated with high doses of AAV9.154 Severe adverse events have been described in at least three clinical trials for other genetic disorders where high doses of the vector were administered, including increased serum transaminase (NCT03306277), complement activation and acute kidney injury (NCT03362502), and sepsis-induced deaths (NCT03199469).155 These observations highlight the need to gather further safety data and, even when this has been obtained, these findings must be considered carefully because the severe immune response observed in these patients was not seen previously in animal models, making the outcome of this strategy to increase widespread tissue targeting unpredictable.156
Local delivery of AAVs
Local AAV delivery may allow BBB circumvention and enhanced delivery of therapeutics to the CNS. As described before for ERT and HSCT, there are several routes of administration to exploit (Figure 2) and the choice of one over another takes into account several factors, such as injection route difficulty and its prime therapeutic sites, the type of enzyme to express, cell type(s) to target, and their localization and distribution within the CNS. As direct CNS administration routes were discussed previously (sections “injection routes” and “administration routes”) and have been extensively reviewed elsewhere,157 only a few relevant examples are discussed here.
Preliminary results of clinical trials for CLN2 deficiency (Batten disease) pediatric patients based on intracerebral injection of AAV2 (NCT00151216)158 or AAV2/rh10 (NCT01161576, NCT01035424, and NCT01414985)159 have demonstrated a slower rate of gray matter loss and a significantly reduced rate of neurological decline, including motor and language function. Intracerebral administration of AAV2/rh10 and AAV2/5 has also been trialed for MPS IIIA (NCT01474343, NCT03612869)160 and MPS IIIB (EudraCT 2012–000856-33),161 respectively, showing moderate improvement in neuropsychological evaluations of behavior, attention, and sleep. Furthermore, a phase I/II clinical trial for intracerebral delivery of AAV2/rh10 for early onset MLD has reached completion and results should be available soon (NCT01801709). In several of the children treated in these clinical trials, a mild systemic immune response was observed,159 while others presented with abnormal MRI results and experienced seizures,159 or AAV vector was present in urine.160 These observations perhaps suggest leakage from the CNS injection site into the periphery, triggering the immune response and hampering overall in vivo gene therapy efficacy. Transient immunosuppression by neonatal AAV-mediated systemic expression of a therapeutic gene prior to CNS-targeted in vivo gene therapy, and induction of liver-mediated tolerance,142,156,162 have been trialed in MPS IIIA patients to address these concerns, with promising results.160,163
In terms of other injection routes, a small number of clinical trials based on i.t./i.c. administration of AAV9 serotype for MPS IIIA (EudraCT 2015–000359-26), MPS I (NCT03580083), and MPS II (NCT03566043) are currently underway. Only a small number of pre-clinical studies of i.c.v. injection have been conducted to date; pre-clinical studies in CLN2-deficient dogs with AAV2164,165 showed delay of neurological progression and prolonged lifespan;165 however, one animal experienced impaired cardiac function, likely due to augmented storage deposition in the heart.164 In other pre-clinical studies performed in Niemann-Pick C,166 MPS IIIA,167 and MPS I mice,166 animals treated i.c.v. with AAV2/9 showed reduced neurodegeneration, increased motor function, and extended lifespan.
Optimization of gene therapy cassette and AAV engineering
An indirect method to target the CNS is to engineer systemically delivered AAVs to produce enzymes that have an enhanced ability to cross the BBB. This can be achieved by including fusion proteins in the therapeutic construct (as discussed extensively in “enzyme modification”). Alternatively, the use of tissue-specific promoters, secreting peptides and optimized gene sequences can increase expression, secretion, and uptake of the therapeutic enzyme respectively.168 This strategy might also overcome the limitation of using serotypes that have restricted CNS tropism. In addition, bioinformatics-guided design of lysosomal enzymes may not only improve enzyme production/secretion/uptake, but also reduce immunogenicity.169
Conclusions and future perspectives
Here, we have explored strategies to increase the ability of enzymes, stem cells, or viral particles to engraft the damaged CNS of neurological LSD patients (Figure 4).
Figure 4.
Summary of the different strategies used to improve delivery of therapeutic agents to the CNS in the treatment of LSDs
(A) Blood-brain barrier (BBB) disruption strategies: (I) ultrasound or (II) hyperosmotic agents can be used to disrupt the integrity of the BBB; (III) stimulation of receptors can increase passage of enzymes and/or stem cells across the BBB. (B) Enzyme replacement therapy can be targeted to the CNS by modifying enzymes directly (I) or using delivery vehicles to facilitate easier passage across the BBB (II). A range of delivery methods (III) including convection-enhanced delivery, direct injection routes, and delivery vehicles can be used to target the CNS. (C) Ex vivo genetic modification of stem cells using gene therapy (I), different pre-conditioning regimes/agents (II), and different injection routes (III) have been trialled to improve CNS targeting in stem and progenitor cell transplantation. (D) Modifications of gene therapy constructs, including optimization of the gene cassette (I) and selection of viral serotype with CNS tropism (II), and specific injection routes (III) can be utilized to target the CNS with in vivo gene therapy approaches
While choosing the appropriate therapy for each LSD is of vital importance, timing of the intervention is almost as critical. Treatments administered when patients are still asymptomatic have proven to be more effective in both animal models170,171,172 and patients,173,174,175,176,177 highlighting the need for early intervention and implementation of newborn screening for more LSDs. In this direction, in utero intervention may circumvent the BBB selectivity issue, as at this developmental stage the BBB is not yet functional; moreover, transplanted cells can engraft and occupy the microglial niche during the same developmental time frame as resident cells,178 removing the need for pre-conditioning. To this end, a pre-clinical study in MPS VII mice showed that in utero delivery of ERT or HSCT improved neurological symptoms.178 In utero HSCT has been successfully applied in small-scale clinical trials for severe combined immunodeficiency patients, and less successfully for thalassemia patients.179 However, a careful benefit/risk assessment of in utero procedures must be performed and further pre-clinical and clinical studies would be required to support routine application.
Among all the strategies described here, BBB manipulation techniques are relatively easy and cheap compared with others; however, they provide non-selective permeability, posing the risk of toxicity.24 For clinical application to be a realistic prospect, toxicity must be limited, and patients would require strict monitoring for adverse events.
Immunogenicity of therapeutics needs to be considered carefully too, as this can trigger the immune system and subsequently reduce treatment efficacy. In the case of ERT, the repeated infusion of enzyme often results in immune reaction against the enzyme itself64 and furthermore negatively affects therapeutic impact in the CNS; following ERT in an MPS I canine model, animals with a high titer of antibody against the therapeutic enzyme showed less-significant reduction of storage product accumulation in the brain than those with lower antibody titers.180 Similarly, despite rAAVs for in vivo gene therapy having several advantages over other viral vectors, including relatively low immunogenicity,181 long-term gene expression,182,183 and wider tissue tropism, they still trigger the immune system; T cell responses to the transgene might appear after AAV-based vector administration.184,185 Moreover, as the majority of humans have already been exposed to several wild-type AAV serotypes, neutralizing anti-capsid antibodies might be either present in patients prior to treatment186,187,188 or arise quickly following the first administration, rendering vector re-administration not a viable option.189 Continued efforts to minimize the immunogenicity of all therapeutics is vital to the success of ERT and gene therapy, especially in the CNS where prolonged inflammation can have particularly severe negative consequences, as shown, for example, in the case of viral encephalitis, in Alzheimer’s patients, and in association with diabetes.18,19,21,22,23
Another important point is the need for efficient targeting. A notable disadvantage of rAAVs for CNS-targeted gene therapy is that they can only efficiently transduce neurons, and no other disease-relevant brain cells, such as microglia, oligodendrocytes, or astrocytes.140,190 However, not all cells must be corrected for treatment to be able to exert therapeutic effect due to so called “cross-correction,” especially if therapeutics have been modified to deliver supraphysiological levels of enzyme.
Among the most successful and safe strategies for neurological LSDs is HSCT gene therapy. In the last 2 years, two medicinal products based on HSC gene therapy strategies have been approved in Europe; Libmeldy129 for MLD and Skysona191 for ALD (NCT01896102, NCT03852498, NCT02698579). Skysona also received accelerated FDA approval in September 2022.192 This has brought great enthusiasm and renewed hope to neurological LSD patients.
A further consideration for wide adoption of these single administration gene therapies in healthcare systems is pricing and reimbursement. Current models of payment for chronic therapies, such as ERT, accept regular costs year on year for the lifetime of an individual; the cumulative costs of which can be considerable with a recent cost-analysis estimate between €9.3 and €9.7 million (£8.1 and £8.5 million) for LSD treatment.193 This needs to be balanced against a once off payment for single-administration cell and gene therapies, where, although the initial price may be considerable, this is deemed appropriate given the long-term overall clinical benefit.194,195
Even though HSC gene therapy holds great potential, one main issue remains for its clinical suitability, namely the suboptimal, and in some case minimal, engraftment of HSCs in the CNS. The goal is to engraft a sufficient number of HSC-derived cells able to differentiate into microglia and act as a constant and never-ending source of enzyme secretion. A crucial role for a successful CNS engraftment is played by the conditioning regime chosen to clear the niche (by depletion of the native microglia) for donor HSCs. Engraftment in the CNS is significantly improved by busulfan conditioning compared with irradiation,102 with busulfan being the regime of choice for neurological LSD patients196 before transplantation. However, busulfan is associated with a substantial systemic toxicity,108 and alternative strategies based on CNS-targeted microglial depletion may represent less toxic and safer pre-conditioning alternatives for neurological LSD patients in the longer term.
Another way to increase CNS engraftment would be to focus on improving stem cells’ crossing of the BBB; studies aiming to understand what HSC subpopulation engraft the CNS and the mechanisms they use to cross the BBB would be helpful in devising new strategies to increase BBB cell permeability.
At the moment, no single therapeutic approach discussed here provides the perfect solution for every neurological LSD,197 supporting the idea that, for these neurometabolic disorders, the CNS component remains a significant challenge. However, in these monogenic severe disorders, where there is a clear genetic component and pathway to be addressed, there is a unique opportunity to develop therapeutics that can have significant impact and which, if successful, may have wider application to more common forms of neurodegeneration.
Acknowledgments
This work was funded by the NIHR Biomedical Research Centre at Great Ormond Street Hospital for Children NHS Foundation Trust, Child Health Research Charitable Incorporated Organisation, Great Ormond Street Hospital Children's Charity, Krabbe Disease UK, and Sparks. The views expressed are those of the authors and not necessarily those of the NIHR.
Author contributions
B.J.C. wrote the manuscript’s draft under the supervision of H.B.G. and S.B. S.B. coordinated the work, contributed to the draft, finalized the manuscript, and acquired funding.
Declaration of interests
H.B.G. is the CEO of Orchard Therapeutics. The other authors declare no competing interests.
References
- 1.Platt F.M., d'Azzo A., Davidson B.L., Neufeld E.F., Tifft C.J. Lysosomal storage diseases. Nat. Rev. Dis. Primers. 2018;4:27. doi: 10.1038/s41572-018-0025-4. [DOI] [PubMed] [Google Scholar]
- 2.Poupetová H., Ledvinová J., Berná L., Dvoráková L., Kozich V., Elleder M. The birth prevalence of lysosomal storage disorders in the Czech Republic: comparison with data in different populations. J. Inherit. Metab. Dis. 2010;33:387–396. doi: 10.1007/s10545-010-9093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muro S. New biotechnological and nanomedicine strategies for treatment of lysosomal storage disorders. Wiley Interdiscip. Rev. Nanomed Nanobiotechnol. 2010;2:189–204. doi: 10.1002/wnan.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gritti A. Gene therapy for lysosomal storage disorders. Expert Opin. Biol. Ther. 2011;11:1153–1167. doi: 10.1517/14712598.2011.582036. [DOI] [PubMed] [Google Scholar]
- 5.Solomon M., Muro S. Lysosomal enzyme replacement therapies: historical development, clinical outcomes, and future perspectives. Adv. Drug Deliv. Rev. 2017;118:109–134. doi: 10.1016/j.addr.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biffi A. Hematopoietic stem cell gene therapy for storage disease: current and new indications. Mol. Ther. 2017;25:1155–1162. doi: 10.1016/j.ymthe.2017.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan E.Y., Boelens J.J., Jones S.A., Wynn R.F. Hematopoietic stem cell transplantation in inborn errors of metabolism. Front. Pediatr. 2019;7:433. doi: 10.3389/fped.2019.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coutinho M.F., Santos J.I., Alves S. Less is more: substrate reduction therapy for lysosomal storage disorders. Int. J. Mol. Sci. 2016;17:1065. doi: 10.3390/ijms17071065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagree M.S., Scalia S., McKillop W.M., Medin J.A. An update on gene therapy for lysosomal storage disorders. Expert Opin. Biol. Ther. 2019;19:655–670. doi: 10.1080/14712598.2019.1607837. [DOI] [PubMed] [Google Scholar]
- 10.Parenti G., Andria G., Valenzano K.J. Pharmacological chaperone therapy: preclinical development, clinical translation, and prospects for the treatment of lysosomal storage disorders. Mol. Ther. 2015;23:1138–1148. doi: 10.1038/mt.2015.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desnick R.J., Astrin K.H., Schuchman E.H. In: Emery and Rimoin's Principles and Practice of Medical Genetics and Genomics. Seventh Edition. Pyeritz R.E., Korf B.R., Grody W.W., editors. Academic Press; 2019. 7 - Therapies for Lysosomal Storage Diseases; pp. 205–227. [DOI] [Google Scholar]
- 12.Shirley J.L., de Jong Y.P., Terhorst C., Herzog R.W. Immune responses to viral gene therapy vectors. Mol. Ther. 2020;28:709–722. doi: 10.1016/j.ymthe.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martín-Banderas L., Holgado M.A., Durán-Lobato M., Infante J.J., Álvarez-Fuentes J., Fernández-Arévalo M. Role of nanotechnology for enzyme replacement therapy in lysosomal diseases. A focus on gaucher's disease. Curr. Med. Chem. 2016;23:929–952. doi: 10.2174/0929867323666160210130608. [DOI] [PubMed] [Google Scholar]
- 14.Thomas R., Kermode A.R. Enzyme enhancement therapeutics for lysosomal storage diseases: current status and perspective. Mol. Genet. Metab. 2019;126:83–97. doi: 10.1016/j.ymgme.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Villabona-Rueda A., Erice C., Pardo C.A., Stins M.F. The evolving concept of the blood brain barrier (BBB): from a single static barrier to a heterogeneous and dynamic relay center. Front. Cell. Neurosci. 2019;13:405. doi: 10.3389/fncel.2019.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathiisen T.M., Lehre K.P., Danbolt N.C., Ottersen O.P. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58:1094–1103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- 17.Tajes M., Ramos-Fernández E., Weng-Jiang X., Bosch-Morató M., Guivernau B., Eraso-Pichot A., Salvador B., Fernàndez-Busquets X., Roquer J., Muñoz F.J. The blood-brain barrier: structure, function and therapeutic approaches to cross it. Mol. Membr. Biol. 2014;31:152–167. doi: 10.3109/09687688.2014.937468. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y., Rosenberg G.A. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011;42:3323–3328. doi: 10.1161/STROKEAHA.110.608257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kook S.Y., Seok Hong H., Moon M., Mook-Jung I. Disruption of blood-brain barrier in Alzheimer disease pathogenesis. Tissue Barriers. 2013;1:e23993. doi: 10.4161/tisb.23993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee H., Pienaar I.S. Disruption of the blood-brain barrier in Parkinson’s disease: curse or route to a cure? Front. Biosci. 2014;19:272–280. doi: 10.2741/4206. [DOI] [PubMed] [Google Scholar]
- 21.Prasad S., Sajja R.K., Naik P., Cucullo L. Diabetes mellitus and blood-brain barrier dysfunction: an overview. J. Pharmacovigil. 2014;2:125. doi: 10.4172/2329-6887.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonney S., Seitz S., Ryan C.A., Jones K.L., Clarke P., Tyler K.L., Siegenthaler J.A. Gamma interferon alters junctional integrity via rho kinase, resulting in blood-brain barrier leakage in experimental viral encephalitis. mBio. 2019;10 doi: 10.1128/mBio. e01675-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsieh J.T., Rathore A.P.S., Soundarajan G., St John A.L. Japanese encephalitis virus neuropenetrance is driven by mast cell chymase. Nat. Commun. 2019;10:706. doi: 10.1038/s41467-019-08641-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hersh D.S., Wadajkar A.S., Roberts N., Perez J.G., Connolly N.P., Frenkel V., Winkles J.A., Woodworth G.F., Kim A.J. Evolving drug delivery strategies to overcome the blood brain barrier. Curr. Pharm. Des. 2016;22:1177–1193. doi: 10.2174/1381612822666151221150733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fishman P.S., Fischell J.M. Focused ultrasound mediated opening of the blood-brain barrier for neurodegenerative diseases. Front. Neurol. 2021;12:749047. doi: 10.3389/fneur.2021.749047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgess A., Ayala-Grosso C.A., Ganguly M., Jordão J.F., Aubert I., Hynynen K. Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood-brain barrier. PLoS One. 2011;6:e27877. doi: 10.1371/journal.pone.0027877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu Y.H., Liu R.S., Lin W.L., Yuh Y.S., Lin S.P., Wong T.T. Transcranial pulsed ultrasound facilitates brain uptake of laronidase in enzyme replacement therapy for Mucopolysaccharidosis type I disease. Orphanet J. Rare Dis. 2017;12:109. doi: 10.1186/s13023-017-0649-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rapoport S.I. Osmotic opening of the blood–brain barrier: principles, mechanism, and therapeutic applications. Cell. Mol. Neurobiol. 2000;20:217–230. doi: 10.1023/a:1007049806660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bourgoin C., Emiliani C., Kremer E.J., Gelot A., Tancini B., Gravel R.A., Drugan C., Orlacchio A., Poenaru L., Caillaud C. Widespread distribution of beta-hexosaminidase activity in the brain of a Sandhoff mouse model after coinjection of adenoviral vector and mannitol. Gene Ther. 2003;10:1841–1849. doi: 10.1038/sj.gt.3302081. [DOI] [PubMed] [Google Scholar]
- 30.Fu H., Kang L., Jennings J.S., Moy S.S., Perez A., Dirosario J., McCarty D.M., Muenzer J. Significantly increased lifespan and improved behavioral performances by rAAV gene delivery in adult mucopolysaccharidosis IIIB mice. Gene Ther. 2007;14:1065–1077. doi: 10.1038/sj.gt.3302961. [DOI] [PubMed] [Google Scholar]
- 31.McCarty D.M., DiRosario J., Gulaid K., Muenzer J., Fu H. Mannitol-facilitated CNS entry of rAAV2 vector significantly delayed the neurological disease progression in MPS IIIB mice. Gene Ther. 2009;16:1340–1352. doi: 10.1038/gt.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foley C.P., Rubin D.G., Santillan A., Sondhi D., Dyke J.P., Crystal R.G., Gobin Y.P., Ballon D.J. Intra-arterial delivery of AAV vectors to the mouse brain after mannitol mediated blood brain barrier disruption. J. Control Release. 2014;196:71–78. doi: 10.1016/j.jconrel.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shawkat H., Westwood M.M., Mortimer A. Mannitol: a review of its clinical uses. Continuing Education Anaesth. Crit. Care Pain. 2012;12:82–85. doi: 10.1093/bjaceaccp/mkr063. [DOI] [Google Scholar]
- 34.Urayama A., Grubb J.H., Sly W.S., Banks W.A. Pharmacologic manipulation of lysosomal enzyme transport across the blood-brain barrier. J. Cereb. Blood Flow Metab. 2016;36:476–486. doi: 10.1177/0271678X15614589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Urayama A., Grubb J.H., Banks W.A., Sly W.S. Epinephrine enhances lysosomal enzyme delivery across the blood–brain barrier by up-regulation of the mannose 6-phosphate receptor. PNAS. 2007;104:12873–12878. doi: 10.1073/pnas.0705611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urayama A., Dohgu S., Robinson S.M., Sly W.S., Grubb J.H., Banks W.A. Alpha adrenergic induction of transport of lysosomal enzyme across the blood-brain barrier. PLoS One. 2015;10:e0142347. doi: 10.1371/journal.pone.0142347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montaño A.M., Oikawa H., Tomatsu S., Nishioka T., Vogler C., Gutierrez M.A., Oguma T., Tan Y., Grubb J.H., Dung V.C., et al. Acidic amino acid tag enhances response to enzyme replacement in mucopolysaccharidosis type VII mice. Mol. Genet. Metab. 2008;94:178–189. doi: 10.1016/j.ymgme.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Wang D., El-Amouri S.S., Dai M., Kuan C.Y., Hui D.Y., Brady R.O., Pan D. Engineering a lysosomal enzyme with a derivative of receptor-binding domain of apoE enables delivery across the blood-brain barrier. Proc. Natl. Acad. Sci. USA. 2013;110:2999–3004. doi: 10.1073/pnas.1222742110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meng Y., Sohar I., Sleat D.E., Richardson J.R., Reuhl K.R., Jenkins R.B., Sarkar G., Lobel P. Effective intravenous therapy for neurodegenerative disease with a therapeutic enzyme and a peptide that mediates delivery to the brain. Mol. Ther. 2014;22:547–553. doi: 10.1038/mt.2013.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Böckenhoff A., Cramer S., Wölte P., Knieling S., Wohlenberg C., Gieselmann V., Galla H.J., Matzner U. Comparison of five peptide vectors for improved brain delivery of the lysosomal enzyme arylsulfatase A. J. Neurosci. 2014;34:3122–3129. doi: 10.1523/JNEUROSCI.4785-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boado R.J., Hui E.K.W., Lu J.Z., Pardridge W.M. Glycemic control and chronic dosing of rhesus monkeys with a fusion protein of iduronidase and a monoclonal antibody against the human insulin receptor. Drug Metab. Dispos. 2012;40:2021–2025. doi: 10.1124/dmd.112.046375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boado R.J., Ka-Wai Hui E., Zhiqiang Lu J., Pardridge W.M. Insulin receptor antibody-iduronate 2-sulfatase fusion protein: pharmacokinetics, anti-drug antibody, and safety pharmacology in Rhesus monkeys. Biotechnol. Bioeng. 2014;111:2317–2325. doi: 10.1002/bit.25289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ullman J.C., Arguello A., Getz J.A., Bhalla A., Mahon C.S., Wang J., Giese T., Bedard C., Kim D.J., Blumenfeld J.R., et al. Brain delivery and activity of a lysosomal enzyme using a blood-brain barrier transport vehicle in mice. Sci. Transl. Med. 2020;12:eaay1163. doi: 10.1126/scitranslmed.aay1163. [DOI] [PubMed] [Google Scholar]
- 44.Sonoda H., Morimoto H., Yoden E., Koshimura Y., Kinoshita M., Golovina G., Takagi H., Yamamoto R., Minami K., Mizoguchi A., et al. A blood-brain-barrier-penetrating anti-human transferrin receptor antibody fusion protein for neuronopathic mucopolysaccharidosis II. Mol. Ther. 2018;26:1366–1374. doi: 10.1016/j.ymthe.2018.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okuyama T., Eto Y., Sakai N., Minami K., Yamamoto T., Sonoda H., Yamaoka M., Tachibana K., Hirato T., Sato Y. Iduronate-2-Sulfatase with anti-human transferrin receptor antibody for neuropathic mucopolysaccharidosis II: a phase 1/2 trial. Mol. Ther. 2019;27:456–464. doi: 10.1016/j.ymthe.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okuyama T., Eto Y., Sakai N., Nakamura K., Yamamoto T., Yamaoka M., Ikeda T., So S., Tanizawa K., Sonoda H., Sato Y. A phase 2/3 trial of pabinafusp alfa, IDS fused with anti-human transferrin receptor antibody, targeting neurodegeneration in MPS-II. Mol. Ther. 2021;29:671–679. doi: 10.1016/j.ymthe.2020.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamamoto R., Kawashima S. Pharmacological property, mechanism of action and clinical study results of Pabinafusp Alfa (Genetical Recombination) (IZCARGO((R)) I.V. Infusion 10 mg) as the therapeutic for Mucopolysaccharidosis type-II (Hunter syndrome) Nihon Yakurigaku Zasshi. 2022;157:62–75. doi: 10.1254/fpj.21080. [DOI] [PubMed] [Google Scholar]
- 48.Grubb J.H., Vogler C., Levy B., Galvin N., Tan Y., Sly W.S. Chemically modified β-glucuronidase crosses blood–brain barrier and clears neuronal storage in murine mucopolysaccharidosis VII. Proc. Natl. Acad. Sci. USA. 2008;105:2616–2621. doi: 10.1073/pnas.0712147105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huynh H.T., Grubb J.H., Vogler C., Sly W.S. Biochemical evidence for superior correction of neuronal storage by chemically modified enzyme in murine mucopolysaccharidosis VII. Proc. Natl. Acad. Sci. USA. 2012;109:17022–17027. doi: 10.1073/pnas.1214779109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rozaklis T., Beard H., Hassiotis S., Garcia A.R., Tonini M., Luck A., Pan J., Lamsa J.C., Hopwood J.J., Hemsley K.M. Impact of high-dose, chemically modified sulfamidase on pathology in a murine model of MPS IIIA. Exp. Neurol. 2011;230:123–130. doi: 10.1016/j.expneurol.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 51.Gustavsson S., Ohlin Sjöström E., Tjernberg A., Janson J., Westermark U., Andersson T., Makower Å., Arnelöf E., Andersson G., Svartengren J., et al. Intravenous delivery of a chemically modified sulfamidase efficiently reduces heparan sulfate storage and brain pathology in mucopolysaccharidosis IIIA mice. Mol. Genet. Metab. Rep. 2019;21:100510. doi: 10.1016/j.ymgmr.2019.100510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Janson J., Andersson G., Bergquist L., Eriksson M., Folgering J.H.A. Impact of chemical modification of sulfamidase on distribution to brain interstitial fluid and to CSF after an intravenous administration in awake, freely-moving rats. Mol. Genet. Metab. Rep. 2020;22:100554. doi: 10.1016/j.ymgmr.2019.100554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beard H., Luck A.J., Hassiotis S., King B., Trim P.J., Snel M.F., Hopwood J.J., Hemsley K.M. Determination of the role of injection site on the efficacy of intra-CSF enzyme replacement therapy in MPS IIIA mice. Mol. Genet. Metab. 2015;115:33–40. doi: 10.1016/j.ymgme.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Grover A., Crippen-Harmon D., Nave L., Vincelette J., Wait J.C.M., Melton A.C., Lawrence R., Brown J.R., Webster K.A., Yip B.K., et al. Translational studies of intravenous and intracerebroventricular routes of administration for CNS cellular biodistribution for BMN 250, an enzyme replacement therapy for the treatment of Sanfilippo type B. Drug Deliv. Transl. Res. 2020;10:425–439. doi: 10.1007/s13346-019-00683-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marshall N.R., Hassiotis S., King B., Rozaklis T., Trim P.J., Duplock S.K., Winner L.K., Beard H., Snel M.F., Jolly R.D., et al. Delivery of therapeutic protein for prevention of neurodegenerative changes: comparison of different CSF-delivery methods. Exp. Neurol. 2015;263:79–90. doi: 10.1016/j.expneurol.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 56.Vuillemenot B.R., Katz M.L., Coates J.R., Kennedy D., Tiger P., Kanazono S., Lobel P., Sohar I., Xu S., Cahayag R., et al. Intrathecal tripeptidyl-peptidase 1 reduces lysosomal storage in a canine model of late infantile neuronal ceroid lipofuscinosis. Mol. Genet. Metab. 2011;104:325–337. doi: 10.1016/j.ymgme.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 57.Katz M.L., Coates J.R., Sibigtroth C.M., Taylor J.D., Carpentier M., Young W.M., Wininger F.A., Kennedy D., Vuillemenot B.R., O'Neill C.A. Enzyme replacement therapy attenuates disease progression in a canine model of late-infantile neuronal ceroid lipofuscinosis (CLN2 disease) J. Neurosci. Res. 2014;92:1591–1598. doi: 10.1002/jnr.23423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Higuchi T., Shimizu H., Fukuda T., Kawagoe S., Matsumoto J., Shimada Y., Kobayashi H., Ida H., Ohashi T., Morimoto H., et al. Enzyme replacement therapy (ERT) procedure for mucopolysaccharidosis type II (MPS II) by intraventricular administration (IVA) in murine MPS II. Mol. Genet. Metab. 2012;107:122–128. doi: 10.1016/j.ymgme.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 59.Treleaven C.M., Tamsett T., Fidler J.A., Taksir T.V., Cheng S.H., Shihabuddin L.S., Dodge J.C. Comparative analysis of acid sphingomyelinase distribution in the CNS of rats and mice following intracerebroventricular delivery. PLoS One. 2011;6:e16313. doi: 10.1371/journal.pone.0016313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ziegler R.J., Salegio E.A., Dodge J.C., Bringas J., Treleaven C.M., Bercury S.D., Tamsett T.J., Shihabuddin L., Hadaczek P., Fiandaca M., et al. Distribution of acid sphingomyelinase in rodent and non-human primate brain after intracerebroventricular infusion. Exp. Neurol. 2011;231:261–271. doi: 10.1016/j.expneurol.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 61.Dodge J.C., Clarke J., Treleaven C.M., Taksir T.V., Griffiths D.A., Yang W., Fidler J.A., Passini M.A., Karey K.P., Schuchman E.H., et al. Intracerebroventricular infusion of acid sphingomyelinase corrects CNS manifestations in a mouse model of Niemann-Pick A disease. Exp. Neurol. 2009;215:349–357. doi: 10.1016/j.expneurol.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 62.Lee W.C., Tsoi Y.K., Troendle F.J., DeLucia M.W., Ahmed Z., Dicky C.A., Dickson D.W., Eckman C.B. Single-dose intracerebroventricular administration of galactocerebrosidase improves survival in a mouse model of globoid cell leukodystrophy. FASEB J. 2007;21:2520–2527. doi: 10.1096/fj.06-6169com. [DOI] [PubMed] [Google Scholar]
- 63.Belichenko P.V., Dickson P.I., Passage M., Jungles S., Mobley W.C., Kakkis E.D. Penetration, diffusion, and uptake of recombinant human alpha-L-iduronidase after intraventricular injection into the rat brain. Mol. Genet. Metab. 2005;86:141–149. doi: 10.1016/j.ymgme.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 64.Ou L., Herzog T., Koniar B.L., Gunther R., Whitley C.B. High-dose enzyme replacement therapy in murine Hurler syndrome. Mol. Genet. Metab. 2014;111:116–122. doi: 10.1016/j.ymgme.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nestrasil I., Shapiro E., Svatkova A., Dickson P., Chen A., Wakumoto A., Ahmed A., Stehel E., McNeil S., Gravance C., Maher E. Intrathecal enzyme replacement therapy reverses cognitive decline in mucopolysaccharidosis type I. Am. J. Med. Genet. A. 2017;173:780–783. doi: 10.1002/ajmg.a.38073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu J.Y., Nelvagal H.R., Wang L., Birnbaum S.G., Cooper J.D., Hofmann S.L. Intrathecal enzyme replacement therapy improves motor function and survival in a preclinical mouse model of infantile neuronal ceroid lipofuscinosis. Mol. Genet. Metab. 2015;116:98–105. doi: 10.1016/j.ymgme.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 67.Calias P., Papisov M., Pan J., Savioli N., Belov V., Huang Y., Lotterhand J., Alessandrini M., Liu N., Fischman A.J., et al. CNS penetration of intrathecal-lumbar idursulfase in the monkey, dog and mouse: implications for neurological outcomes of lysosomal storage disorder. PLoS One. 2012;7:e30341. doi: 10.1371/journal.pone.0030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen A., Vogler C., McEntee M., Hanson S., Ellinwood N.M., Jens J., Snella E., Passage M., Le S., Guerra C., Dickson P. Glycosaminoglycan storage in neuroanatomical regions of mucopolysaccharidosis I dogs following intrathecal recombinant human iduronidase. APMIS. 2011;119:513–521. doi: 10.1111/j.1600-0463.2011.02760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Crawley A.C., Marshall N., Beard H., Hassiotis S., Walsh V., King B., Hucker N., Fuller M., Jolly R.D., Hopwood J.J., Hemsley K.M. Enzyme replacement reduces neuropathology in MPS IIIA dogs. Neurobiol. Dis. 2011;43:422–434. doi: 10.1016/j.nbd.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 70.Kondagari G.S., King B.M., Thomson P.C., Williamson P., Clements P.R., Fuller M., Hemsley K.M., Hopwood J.J., Taylor R.M. Treatment of canine fucosidosis by intracisternal enzyme infusion. Exp. Neurol. 2011;230:218–226. doi: 10.1016/j.expneurol.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 71.Vite C.H., Wang P., Patel R.T., Walton R.M., Walkley S.U., Sellers R.S., Ellinwood N.M., Cheng A.S., White J.T., O'Neill C.A., Haskins M. Biodistribution and pharmacodynamics of recombinant human alpha-L-iduronidase (rhIDU) in mucopolysaccharidosis type I-affected cats following multiple intrathecal administrations. Mol. Genet. Metab. 2011;103:268–274. doi: 10.1016/j.ymgme.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hemsley K.M., Luck A.J., Crawley A.C., Hassiotis S., Beard H., King B., Rozek T., Rozaklis T., Fuller M., Hopwood J.J. Examination of intravenous and intra-CSF protein delivery for treatment of neurological disease. Eur. J. Neurosci. 2009;29:1197–1214. doi: 10.1111/j.1460-9568.2009.06666.x. [DOI] [PubMed] [Google Scholar]
- 73.Hemsley K.M., Norman E.J., Crawley A.C., Auclair D., King B., Fuller M., Lang D.L., Dean C.J., Jolly R.D., Hopwood J.J. Effect of cisternal sulfamidase delivery in MPS IIIA Huntaway dogs--a proof of principle study. Mol. Genet. Metab. 2009;98:383–392. doi: 10.1016/j.ymgme.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 74.Dickson P., McEntee M., Vogler C., Le S., Levy B., Peinovich M., Hanson S., Passage M., Kakkis E. Intrathecal enzyme replacement therapy: successful treatment of brain disease via the cerebrospinal fluid. Mol. Genet. Metab. 2007;91:61–68. doi: 10.1016/j.ymgme.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hemsley K.M., King B., Hopwood J.J. Injection of recombinant human sulfamidase into the CSF via the cerebellomedullary cistern in MPS IIIA mice. Mol. Genet. Metab. 2007;90:313–328. doi: 10.1016/j.ymgme.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 76.Kakkis E., McEntee M., Vogler C., Le S., Levy B., Belichenko P., Mobley W., Dickson P., Hanson S., Passage M. Intrathecal enzyme replacement therapy reduces lysosomal storage in the brain and meninges of the canine model of MPS I. Mol. Genet. Metab. 2004;83:163–174. doi: 10.1016/j.ymgme.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 77.Tong W., Dwyer C.A., Thacker B.E., Glass C.A., Brown J.R., Hamill K., Moremen K.W., Sarrazin S., Gordts P.L.S.M., Dozier L.E., et al. Guanidinylated neomycin conjugation enhances intranasal enzyme replacement in the brain. Mol. Ther. 2017;25:2743–2752. doi: 10.1016/j.ymthe.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wolf D.A., Hanson L.R., Aronovich E.L., Nan Z., Low W.C., Frey W.H., 2nd, McIvor R.S. Lysosomal enzyme can bypass the blood-brain barrier and reach the CNS following intranasal administration. Mol. Genet. Metab. 2012;106:131–134. doi: 10.1016/j.ymgme.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 79.Haney M.J., Klyachko N.L., Harrison E.B., Zhao Y., Kabanov A.V., Batrakova E.V. TPP1 delivery to lysosomes with extracellular vesicles and their enhanced brain distribution in the animal model of batten disease. Adv. Healthc. Mater. 2019;8:e1801271. doi: 10.1002/adhm.201801271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kelly J.M., Gross A.L., Martin D.R., Byrne M.E. Polyethylene glycol-b-poly(lactic acid) polymersomes as vehicles for enzyme replacement therapy. Nanomedicine. 2017;12:2591–2606. doi: 10.2217/nnm-2017-0221. [DOI] [PubMed] [Google Scholar]
- 81.Papademetriou J., Garnacho C., Serrano D., Bhowmick T., Schuchman E.H., Muro S. Comparative binding, endocytosis, and biodistribution of antibodies and antibody-coated carriers for targeted delivery of lysosomal enzymes to ICAM-1 versus transferrin receptor. J. Inherit. Metab. Dis. 2013;36:467–477. doi: 10.1007/s10545-012-9534-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dawson G. Quantum dots and potential therapy for Krabbe's disease. J. Neurosci. Res. 2016;94:1293–1303. doi: 10.1002/jnr.23805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goldsmith M., Abramovitz L., Braunstein H., Horowitz M., Peer D. Quantitative analysis of recombinant glucocerebrosidase brain delivery via lipid nanoparticles. Nano Futures. 2018;2:045003. doi: 10.1088/2399-1984/aadd34. [DOI] [Google Scholar]
- 84.Del Grosso A., Galliani M., Angella L., Santi M., Tonazzini I., Parlanti G., Signore G., Cecchini M. Brain-targeted enzyme-loaded nanoparticles: a breach through the blood-brain barrier for enzyme replacement therapy in Krabbe disease. Sci. Adv. 2019;5:eaax7462. doi: 10.1126/sciadv.aax7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rigon L., Salvalaio M., Pederzoli F., Legnini E., Duskey J.T., D'Avanzo F., De Filippis C., Ruozi B., Marin O., Vandelli M.A., et al. Targeting brain disease in MPSII: preclinical evaluation of IDS-loaded PLGA nanoparticles. Int. J. Mol. Sci. 2019;20:2014. doi: 10.3390/ijms20082014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schuster T., Mühlstein A., Yaghootfam C., Maksimenko O., Shipulo E., Gelperina S., Kreuter J., Gieselmann V., Matzner U. Potential of surfactant-coated nanoparticles to improve brain delivery of arylsulfatase A. J. Control Release. 2017;253:1–10. doi: 10.1016/j.jconrel.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 87.King B., Marshall N.R., Hassiotis S., Trim P.J., Tucker J., Hattersley K., Snel M.F., Jolly R.D., Hopwood J.J., Hemsley K.M. Slow, continuous enzyme replacement via spinal CSF in dogs with the paediatric-onset neurodegenerative disease, MPS IIIA. J. Inherit. Metab. Dis. 2017;40:443–453. doi: 10.1007/s10545-016-9994-1. [DOI] [PubMed] [Google Scholar]
- 88.King B., Setford M.L., Hassiotis S., Trim P.J., Duplock S., Tucker J.N., Hattersley K., Snel M.F., Hopwood J.J., Hemsley K.M. Low-dose, continual enzyme delivery ameliorates some aspects of established brain disease in a mouse model of a childhood-onset neurodegenerative disorder. Exp. Neurol. 2016;278:11–21. doi: 10.1016/j.expneurol.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 89.Beard H., Hassiotis S., Luck A.J., Rozaklis T., Hopwood J.J., Hemsley K.M. Continual low-dose infusion of sulfamidase is superior to intermittent high-dose delivery in ameliorating neuropathology in the MPS IIIA mouse brain. JIMD Rep. 2016;29:59–68. doi: 10.1007/8904_2015_495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stroobants S., Gerlach D., Matthes F., Hartmann D., Fogh J., Gieselmann V., D'Hooge R., Matzner U. Intracerebroventricular enzyme infusion corrects central nervous system pathology and dysfunction in a mouse model of metachromatic leukodystrophy. Hum. Mol. Genet. 2011;20:2760–2769. doi: 10.1093/hmg/ddr175. [DOI] [PubMed] [Google Scholar]
- 91.Chang M., Cooper J.D., Sleat D.E., Cheng S.H., Dodge J.C., Passini M.A., Lobel P., Davidson B.L. Intraventricular enzyme replacement improves disease phenotypes in a mouse model of late infantile neuronal ceroid lipofuscinosis. Mol. Ther. 2008;16:649–656. doi: 10.1038/mt.2008.9. [DOI] [PubMed] [Google Scholar]
- 92.Muenzer J., Hendriksz C.J., Fan Z., Vijayaraghavan S., Perry V., Santra S., Solanki G.A., Mascelli M.A., Pan L., Wang N., et al. A phase I/II study of intrathecal idursulfase-IT in children with severe mucopolysaccharidosis II. Genet. Med. 2016;18:73–81. doi: 10.1038/gim.2015.36. [DOI] [PubMed] [Google Scholar]
- 93.Sohn Y.B., Lee J., Cho S.Y., Kim S.J., Ko A.R., Nam M.H., Jin D.K. Improvement of CNS defects via continuous intrathecal enzyme replacement by osmotic pump in mucopolysaccharidosis type II mice. Am. J. Med. Genet. A. 2013;161A:1036–1043. doi: 10.1002/ajmg.a.35869. [DOI] [PubMed] [Google Scholar]
- 94.https://www.clinigengroup.com/news/news-container/2021/clinigen-receives-marketing-approval-for-hunterase-idursulfase-beta-icv-in-japan/. (Clinigen Group).
- 95.Mehta A.M., Sonabend A.M., Bruce J.N. Convection-enhanced delivery. Neurotherapeutics. 2017;14:358–371. doi: 10.1007/s13311-017-0520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lonser R.R., Schiffman R., Robison R.A., Butman J.A., Quezado Z., Walker M.L., Morrison P.F., Walbridge S., Murray G.J., Park D.M., et al. Image-guided, direct convective delivery of glucocerebrosidase for neuronopathic Gaucher disease. Neurology. 2007;68:254–261. doi: 10.1212/01.wnl.0000247744.10990.e6. [DOI] [PubMed] [Google Scholar]
- 97.Souweidane M.M., Fraser J.F., Arkin L.M., Sondhi D., Hackett N.R., Kaminsky S.M., Heier L., Kosofsky B.E., Worgall S., Crystal R.G., Kaplitt M.G. Gene therapy for late infantile neuronal ceroid lipofuscinosis: neurosurgical considerations. J. Neurosurg. Pediatr. 2010;6:115–122. doi: 10.3171/2010.4.PEDS09507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shihabuddin L.S., Cheng S.H. Neural stem cell transplantation as a therapeutic approach for treating lysosomal storage diseases. Neurotherapeutics. 2011;8:659–667. doi: 10.1007/s13311-011-0067-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Siddiqi F., Wolfe J.H. Stem cell therapy for the central nervous system in lysosomal storage diseases. Hum. Gene Ther. 2016;27:749–757. doi: 10.1089/hum.2016.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Oliveira Miranda C. Mesenchymal stem cells for lysosomal storage and polyglutamine disorders: possible shared mechanisms. Eur. J. Clin. Invest. 2022;52:e13707. doi: 10.1111/eci.13707. [DOI] [PubMed] [Google Scholar]
- 101.Capotondo A., Milazzo R., Garcia-Manteiga J.M., Cavalca E., Montepeloso A., Garrison B.S., Peviani M., Rossi D.J., Biffi A. Intracerebroventricular delivery of hematopoietic progenitors results in rapid and robust engraftment of microglia-like cells. Sci. Adv. 2017;3:e1701211. doi: 10.1126/sciadv.1701211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Capotondo A., Milazzo R., Politi L.S., Quattrini A., Palini A., Plati T., Merella S., Nonis A., di Serio C., Montini E., et al. Brain conditioning is instrumental for successful microglia reconstitution following hematopoietic stem cell transplantation. Proc. Natl. Acad. Sci. USA. 2012;109:15018–15023. doi: 10.1073/pnas.1205858109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee J.P., Jeyakumar M., Gonzalez R., Takahashi H., Lee P.J., Baek R.C., Clark D., Rose H., Fu G., Clarke J., et al. Stem cells act through multiple mechanisms to benefit mice with neurodegenerative metabolic disease. Nat. Med. 2007;13:439–447. doi: 10.1038/nm1548. [DOI] [PubMed] [Google Scholar]
- 104.Wilkinson F.L., Sergijenko A., Langford-Smith K.J., Malinowska M., Wynn R.F., Bigger B.W. Busulfan conditioning enhances engraftment of hematopoietic donor-derived cells in the brain compared with irradiation. Mol. Ther. 2013;21:868–876. doi: 10.1038/mt.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sailor K.A., Agoranos G., López-Manzaneda S., Tada S., Gillet-Legrand B., Guerinot C., Masson J.B., Vestergaard C.L., Bonner M., Gagnidze K., et al. Hematopoietic stem cell transplantation chemotherapy causes microglia senescence and peripheral macrophage engraftment in the brain. Nat. Med. 2022;28:517–527. doi: 10.1038/s41591-022-01691-9. [DOI] [PubMed] [Google Scholar]
- 106.Zeng L., Jia L., Xu S., Yan Z., Ding S., Xu K. Vascular endothelium changes after conditioning in hematopoietic stem cell transplantation: role of cyclophosphamide and busulfan. Transpl. Proc. 2010;42:2720–2724. doi: 10.1016/j.transproceed.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 107.Vassord C., Lapouméroulie C., Koumaravelou K., Srivastava A., Krishnamoorthy R. Endothelial cells do not express GSTA1: potential relevance to busulfan-mediated endothelial damage during hematopoetic stem cell transplantation. Eur. J. Haematol. 2008;80:299–302. doi: 10.1111/j.1600-0609.2008.01031.x. [DOI] [PubMed] [Google Scholar]
- 108.Ciurea S.O., Andersson B.S. Busulfan in hematopoietic stem cell transplantation. Biol. Blood Marrow Transpl. 2009;15:523–536. doi: 10.1016/j.bbmt.2008.12.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Czechowicz A., Palchaudhuri R., Scheck A., Hu Y., Hoggatt J., Saez B., Pang W.W., Mansour M.K., Tate T.A., Chan Y.Y., et al. Selective hematopoietic stem cell ablation using CD117-antibody-drug-conjugates enables safe and effective transplantation with immunity preservation. Nat. Commun. 2019;10:617. doi: 10.1038/s41467-018-08201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yokoi T., Yokoi K., Akiyama K., Higuchi T., Shimada Y., Kobayashi H., Sato T., Ohteki T., Otsu M., Nakauchi H., et al. Non-myeloablative preconditioning with ACK2 (anti-c-kit antibody) is efficient in bone marrow transplantation for murine models of mucopolysaccharidosis type II. Mol. Genet. Metab. 2016;119:232–238. doi: 10.1016/j.ymgme.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 111.Chhabra A., Ring A.M., Weiskopf K., Schnorr P.J., Gordon S., Le A.C., Kwon H.S., Ring N.G., Volkmer J., Ho P.Y., et al. Hematopoietic stem cell transplantation in immunocompetent hosts without radiation or chemotherapy. Sci. Transl. Med. 2016;8:351ra105. doi: 10.1126/scitranslmed.aae0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Agarwal R., Dvorak C.C., Prohaska S., Long-Boyle J., Kwon H.S., Brown J.W.M., Weinberg K.I., Le A., Guttman-Klein A., Logan A.C., et al. Toxicity-free hematopoietic stem cell engraftment achieved with anti-CD117 monoclonal antibody conditioning. Biol. Blood Marrow Transplant. 2019;25:S92. doi: 10.1016/j.bbmt.2018.12.172. [DOI] [Google Scholar]
- 113.Chen Y., Kornblit B., Hamlin D.K., Sale G.E., Santos E.B., Wilbur D.S., Storer B.E., Storb R., Sandmaier B.M. Durable donor engraftment after radioimmunotherapy using alpha-emitter astatine-211-labeled anti-CD45 antibody for conditioning in allogeneic hematopoietic cell transplantation. Blood. 2012;119:1130–1138. doi: 10.1182/blood-2011-09-380436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Palchaudhuri R., Saez B., Hoggatt J., Schajnovitz A., Sykes D.B., Tate T.A., Czechowicz A., Kfoury Y., Ruchika F., Rossi D.J., et al. Non-genotoxic conditioning for hematopoietic stem cell transplantation using a hematopoietic-cell-specific internalizing immunotoxin. Nat. Biotechnol. 2016;34:738–745. doi: 10.1038/nbt.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.George B.M., Kao K.S., Kwon H.S., Velasco B.J., Poyser J., Chen A., Le A.C., Chhabra A., Burnett C.E., Cajuste D., et al. Antibody conditioning enables MHC-mismatched hematopoietic stem cell transplants and organ graft tolerance. Cell Stem Cell. 2019;25:185–192.e3. doi: 10.1016/j.stem.2019.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Spangenberg E., Severson P.L., Hohsfield L.A., Crapser J., Zhang J., Burton E.A., Zhang Y., Spevak W., Lin J., Phan N.Y., et al. Sustained microglial depletion with CSF1R inhibitor impairs parenchymal plaque development in an Alzheimer's disease model. Nat. Commun. 2019;10:3758. doi: 10.1038/s41467-019-11674-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu Z., Rao Y., Huang Y., Zhou T., Feng R., Xiong S., Yuan T.F., Qin S., Lu Y., Zhou X., et al. Efficient strategies for microglia replacement in the central nervous system. Cell Rep. 2020;33:108443. doi: 10.1016/j.celrep.2020.108443. [DOI] [PubMed] [Google Scholar]
- 118.Gleitz H.F., Liao A.Y., Cook J.R., Rowlston S.F., Forte G.M., D'Souza Z., O'Leary C., Holley R.J., Bigger B.W. Brain-targeted stem cell gene therapy corrects mucopolysaccharidosis type II via multiple mechanisms. EMBO Mol. Med. 2018;10:e8730. doi: 10.15252/emmm.201708730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Biffi A., Capotondo A., Fasano S., del Carro U., Marchesini S., Azuma H., Malaguti M.C., Amadio S., Brambilla R., Grompe M., et al. Gene therapy of metachromatic leukodystrophy reverses neurological damage and deficits in mice. J. Clin. Invest. 2006;116:3070–3082. doi: 10.1172/JCI28873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Visigalli I., Ungari S., Martino S., Park H., Cesani M., Gentner B., Sergi Sergi L., Orlacchio A., Naldini L., Biffi A. The galactocerebrosidase enzyme contributes to the maintenance of a functional hematopoietic stem cell niche. Blood. 2010;116:1857–1866. doi: 10.1182/blood-2009-12-256461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sergijenko A., Langford-Smith A., Liao A.Y., Pickford C.E., McDermott J., Nowinski G., Langford-Smith K.J., Merry C.L.R., Jones S.A., Wraith J.E., et al. Myeloid/Microglial driven autologous hematopoietic stem cell gene therapy corrects a neuronopathic lysosomal disease. Mol. Ther. 2013;21:1938–1949. doi: 10.1038/mt.2013.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Holley R.J., Ellison S.M., Fil D., O'Leary C., McDermott J., Senthivel N., Langford-Smith A.W.W., Wilkinson F.L., D'Souza Z., Parker H., et al. Macrophage enzyme and reduced inflammation drive brain correction of mucopolysaccharidosis IIIB by stem cell gene therapy. Brain. 2018;141:99–116. doi: 10.1093/brain/awx311. [DOI] [PubMed] [Google Scholar]
- 123.Ellison S.M., Liao A., Wood S., Taylor J., Youshani A.S., Rowlston S., Parker H., Armant M., Biffi A., Chan L., et al. Pre-clinical safety and efficacy of lentiviral vector-mediated ex vivo stem cell gene therapy for the treatment of mucopolysaccharidosis IIIA. Mol. Ther. Methods Clin. Dev. 2019;13:399–413. doi: 10.1016/j.omtm.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sorrentino N.C., Cacace V., De Risi M., Maffia V., Strollo S., Tedesco N., Nusco E., Romagnoli N., Ventrella D., Huang Y., et al. Enhancing the therapeutic potential of sulfamidase for the treatment of mucopolysaccharidosis IIIA. Mol. Ther. Methods Clin. Dev. 2019;15:333–342. doi: 10.1016/j.omtm.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fraldi A., Hemsley K., Crawley A., Lombardi A., Lau A., Sutherland L., Auricchio A., Ballabio A., Hopwood J.J. Functional correction of CNS lesions in an MPS-IIIA mouse model by intracerebral AAV-mediated delivery of sulfamidase and SUMF1 genes. Hum. Mol. Genet. 2007;16:2693–2702. doi: 10.1093/hmg/ddm223. [DOI] [PubMed] [Google Scholar]
- 126.Elliger S.S., Elliger C.A., Lang C., Watson G.L. Enhanced secretion and uptake of beta-glucuronidase improves adeno-associated viral-mediated gene therapy of mucopolysaccharidosis type VII mice. Mol. Ther. 2002;5:617–626. doi: 10.1006/mthe.2002.0594. [DOI] [PubMed] [Google Scholar]
- 127.Biffi A., Montini E., Lorioli L., Cesani M., Fumagalli F., Plati T., Baldoli C., Martino S., Calabria A., Canale S., et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341:1233158. doi: 10.1126/science.1233158. [DOI] [PubMed] [Google Scholar]
- 128.Sessa M., Lorioli L., Fumagalli F., Acquati S., Redaelli D., Baldoli C., Canale S., Lopez I.D., Morena F., Calabria A., et al. Lentiviral haemopoietic stem-cell gene therapy in early-onset metachromatic leukodystrophy: an ad-hoc analysis of a non-randomised, open-label, phase 1/2 trial. Lancet. 2016;388:476–487. doi: 10.1016/S0140-6736(16)30374-9. [DOI] [PubMed] [Google Scholar]
- 129.Fumagalli F., Calbi V., Natali Sora M.G., Sessa M., Baldoli C., Rancoita P.M.V., Ciotti F., Sarzana M., Fraschini M., Zambon A.A., et al. Lentiviral hematopoetic stem-cell gene therapy for early-onset metachromatic leukodystrophy: long-term results from a non-randomised, open-label, phase 1/2 trial and expanded access. Lancet. 2022;399:372–383. doi: 10.1016/s0140-6736(21)02017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cartier N., Hacein-Bey-Abina S., Bartholomae C.C., Bougnères P., Schmidt M., Kalle C.V., Fischer A., Cavazzana-Calvo M., Aubourg P. Lentiviral hematopoietic cell gene therapy for X-linked adrenoleukodystrophy. Methods Enzymol. 2012;507:187–198. doi: 10.1016/B978-0-12-386509-0.00010-7. [DOI] [PubMed] [Google Scholar]
- 131.Cartier N., Hacein-Bey-Abina S., Bartholomae C.C., Veres G., Schmidt M., Kutschera I., Vidaud M., Abel U., Dal-Cortivo L., Caccavelli L., et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–823. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- 132.Eichler F., Duncan C., Musolino P.L., Orchard P.J., De Oliveira S., Thrasher A.J., Armant M., Dansereau C., Lund T.C., Miller W.P., et al. Hematopoietic stem-cell gene therapy for cerebral adrenoleukodystrophy. N. Engl. J. Med. 2017;377:1630–1638. doi: 10.1056/NEJMoa1700554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.https://www.ema.europa.eu/en/medicines/human/EPAR/libmeldy. (European Medicines Agency).
- 134.https://www.ema.europa.eu/en/medicines/human/EPAR/skysona. (European Medicines Agency).
- 135.Sakurai K., Iizuka S., Shen J.S., Meng X.L., Mori T., Umezawa A., Ohashi T., Eto Y. Brain transplantation of genetically modified bone marrow stromal cells corrects CNS pathology and cognitive function in MPS VII mice. Gene Ther. 2004;11:1475–1481. doi: 10.1038/sj.gt.3302338. [DOI] [PubMed] [Google Scholar]
- 136.Nan Z., Shekels L., Ryabinin O., Evavold C., Nelson M.S., Khan S.A., Deans R.J., Mays R.W., Low W.C., Gupta P. Intracerebroventricular transplantation of human bone marrow-derived multipotent progenitor cells in an immunodeficient mouse model of mucopolysaccharidosis type I (MPS-I) Cell Transpl. 2012;21:1577–1593. doi: 10.3727/096368912X636894. [DOI] [PubMed] [Google Scholar]
- 137.Lentz T.B., Gray S.J., Samulski R.J. Viral vectors for gene delivery to the central nervous system. Neurobiol. Dis. 2012;48:179–188. doi: 10.1016/j.nbd.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Massaro G., Geard A.F., Liu W., Coombe-Tennant O., Waddington S.N., Baruteau J., Gissen P., Rahim A.A. Gene therapy for lysosomal storage disorders: ongoing studies and clinical development. Biomolecules. 2021;11:611. doi: 10.3390/biom11040611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Foust K.D., Nurre E., Montgomery C.L., Hernandez A., Chan C.M., Kaspar B.K. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hocquemiller M., Giersch L., Audrain M., Parker S., Cartier N. Adeno-associated virus-based gene therapy for CNS diseases. Hum. Gene Ther. 2016;27:478–496. doi: 10.1089/hum.2016.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Donsante A., Boulis N.M. Progress in gene and cell therapies for the neuronal ceroid lipofuscinoses. Expert Opin. Biol. Ther. 2018;18:755–764. doi: 10.1080/14712598.2018.1492544. [DOI] [PubMed] [Google Scholar]
- 142.Keeler A.M., Zieger M., Todeasa S.H., McCall A.L., Gifford J.C., Birsak S., Choudhury S.R., Byrne B.J., Sena-Esteves M., ElMallah M.K. Systemic delivery of AAVB1-GAA clears glycogen and prolongs survival in a mouse model of pompe disease. Hum. Gene Ther. 2019;30:57–68. doi: 10.1089/hum.2018.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fu H., Cataldi M.P., Ware T.A., Zaraspe K., Meadows A.S., Murrey D.A., McCarty D.M. Functional correction of neurological and somatic disorders at later stages of disease in MPS IIIA mice by systemic scAAV9-hSGSH gene delivery. Mol. Ther. Methods Clin. Dev. 2016;3:16036. doi: 10.1038/mtm.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Saraiva J., Nobre R.J., Pereira de Almeida L. Gene therapy for the CNS using AAVs: the impact of systemic delivery by AAV9. J. Control Release. 2016;241:94–109. doi: 10.1016/j.jconrel.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 145.https://investors.abeonatherapeutics.com/press-releases/detail/234/ultragenyx-acquires-global-rights-to-aav-gene-therapy. (Abeona Therapeutics).
- 146.Flanigan K., Truxal K., McBride K.L., McNally K.A., Kunkler K.L., Zumberge N.A., Martin L., Aylward S., Corridore M., Ruiz J., et al. A phase 1/2 clinical trial of systemic gene transfer of scAAV9.U1a.HSGSH for MPS IIIA: safety, tolerability, and preliminary evidence of biopotency. Mol. Genet. Metab. 2018;123:S46. doi: 10.1016/j.ymgme.2017.12.103. [DOI] [Google Scholar]
- 147.https://clinicaltrials.gov/ct2/show/NCT04088734. (ClinicalTrials.gov).
- 148.https://investors.abeonatherapeutics.com/press-releases/detail/174/abeona-therapeutics-announces-positive-interim-data-from. (Abeona Therapeutics).
- 149.Ravindra Kumar S., Miles T.F., Chen X., Brown D., Dobreva T., Huang Q., Ding X., Luo Y., Einarsson P.H., Greenbaum A., et al. Multiplexed Cre-dependent selection yields systemic AAVs for targeting distinct brain cell types. Nat. Methods. 2020;17:541–550. doi: 10.1038/s41592-020-0799-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nonnenmacher M., Wang W., Child M.A., Ren X.Q., Huang C., Ren A.Z., Tocci J., Chen Q., Bittner K., Tyson K., et al. Rapid evolution of blood-brain-barrier-penetrating AAV capsids by RNA-driven biopanning. Mol. Ther. Methods Clin. Dev. 2021;20:366–378. doi: 10.1016/j.omtm.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Deverman B.E., Pravdo P.L., Simpson B.P., Kumar S.R., Chan K.Y., Banerjee A., Wu W.L., Yang B., Huber N., Pasca S.P., Gradinaru V. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat. Biotechnol. 2016;34:204–209. doi: 10.1038/nbt.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chen X., Ravindra Kumar S., Adams C.D., Yang D., Wang T., Wolfe D.A., Arokiaraj C.M., Ngo V., Campos L.J., Griffiths J.A., et al. Engineered AAVs for non-invasive gene delivery to rodent and non-human primate nervous systems. Neuron. 2022;110:2242–2257.e6. doi: 10.1016/j.neuron.2022.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Asokan A., Schaffer D.V., Samulski R.J. The AAV vector toolkit: poised at the clinical crossroads. Mol. Ther. 2012;20:699–708. doi: 10.1038/mt.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hinderer C., Katz N., Buza E.L., Dyer C., Goode T., Bell P., Richman L.K., Wilson J.M. Severe toxicity in nonhuman primates and piglets following high-dose intravenous administration of an adeno-associated virus vector expressing human SMN. Hum. Gene Ther. 2018;29:285–298. doi: 10.1089/hum.2018.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wilson J.M., Flotte T.R. Moving forward after two deaths in a gene therapy trial of myotubular myopathy. Hum. Gene Ther. 2020;31:695–696. doi: 10.1089/hum.2020.182. [DOI] [PubMed] [Google Scholar]
- 156.Colella P., Ronzitti G., Mingozzi F. Emerging issues in AAV-mediated in vivo gene therapy. Mol. Ther. Methods Clin. Dev. 2018;8:87–104. doi: 10.1016/j.omtm.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gray S.J., Woodard K.T., Samulski R.J. Viral vectors and delivery strategies for CNS gene therapy. Ther. Deliv. 2010;1:517–534. doi: 10.4155/tde.10.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Worgall S., Sondhi D., Hackett N.R., Kosofsky B., Kekatpure M.V., Neyzi N., Dyke J.P., Ballon D., Heier L., Greenwald B.M., et al. Treatment of late infantile neuronal ceroid lipofuscinosis by CNS administration of a serotype 2 adeno-associated virus expressing CLN2 cDNA. Hum. Gene Ther. 2008;19:463–474. doi: 10.1089/hum.2008.022. [DOI] [PubMed] [Google Scholar]
- 159.Sondhi D., Kaminsky S.M., Hackett N.R., Pagovich O.E., Rosenberg J.B., De B.P., Chen A., Van de Graaf B., Mezey J.G., Mammen G.W., et al. Slowing late infantile Batten disease by direct brain parenchymal administration of a rh.10 adeno-associated virus expressing CLN2. Sci. Transl. Med. 2020;12:eabb5413. doi: 10.1126/scitranslmed.abb5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tardieu M., Zérah M., Husson B., de Bournonville S., Deiva K., Adamsbaum C., Vincent F., Hocquemiller M., Broissand C., Furlan V., et al. Intracerebral administration of adeno-associated viral vector serotype rh.10 carrying human SGSH and SUMF1 cDNAs in children with mucopolysaccharidosis type IIIA disease: results of a phase I/II trial. Hum. Gene Ther. 2014;25:506–516. doi: 10.1089/hum.2013.238. [DOI] [PubMed] [Google Scholar]
- 161.Tardieu M., Zérah M., Gougeon M.L., Ausseil J., de Bournonville S., Husson B., Zafeiriou D., Parenti G., Bourget P., Poirier B., et al. Intracerebral gene therapy in children with mucopolysaccharidosis type IIIB syndrome: an uncontrolled phase 1/2 clinical trial. Lancet Neurol. 2017;16:712–720. doi: 10.1016/s1474-4422(17)30169-2. [DOI] [PubMed] [Google Scholar]
- 162.Hinderer C., Bell P., Louboutin J.P., Zhu Y., Yu H., Lin G., Choa R., Gurda B.L., Bagel J., O'Donnell P., et al. Neonatal systemic AAV induces tolerance to CNS gene therapy in MPS I dogs and nonhuman primates. Mol. Ther. 2015;23:1298–1307. doi: 10.1038/mt.2015.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Marcó S., Haurigot V., Bosch F. In vivo gene therapy for mucopolysaccharidosis type III (sanfilippo syndrome): a new treatment horizon. Hum. Gene Ther. 2019;30:1211–1221. doi: 10.1089/hum.2019.217. [DOI] [PubMed] [Google Scholar]
- 164.Katz M.L., Johnson G.C., Leach S.B., Williamson B.G., Coates J.R., Whiting R.E.H., Vansteenkiste D.P., Whitney M.S. Extraneuronal pathology in a canine model of CLN2 neuronal ceroid lipofuscinosis after intracerebroventricular gene therapy that delays neurological disease progression. Gene Ther. 2017;24:215–223. doi: 10.1038/gt.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Whiting R.E.H., Jensen C.A., Pearce J.W., Gillespie L.E., Bristow D.E., Katz M.L. Intracerebroventricular gene therapy that delays neurological disease progression is associated with selective preservation of retinal ganglion cells in a canine model of CLN2 disease. Exp. Eye Res. 2016;146:276–282. doi: 10.1016/j.exer.2016.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wolf D.A., Lenander A.W., Nan Z., Belur L.R., Whitley C.B., Gupta P., Low W.C., McIvor R.S. Direct gene transfer to the CNS prevents emergence of neurologic disease in a murine model of mucopolysaccharidosis type I. Neurobiol. Dis. 2011;43:123–133. doi: 10.1016/j.nbd.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.McIntyre C., Derrick-Roberts A.L.K., Byers S., Anson D.S. Correction of murine mucopolysaccharidosis type IIIA central nervous system pathology by intracerebroventricular lentiviral-mediated gene delivery. J. Gene Med. 2014;16:374–387. doi: 10.1002/jgm.2816. [DOI] [PubMed] [Google Scholar]
- 168.Gray A.L., O'Leary C., Liao A., Agúndez L., Youshani A.S., Gleitz H.F., Parker H., Taylor J.T., Danos O., Hocquemiller M., et al. An improved adeno-associated virus vector for neurological correction of the mouse model of mucopolysaccharidosis IIIA. Hum. Gene Ther. 2019;30:1052–1066. doi: 10.1089/hum.2018.189. [DOI] [PubMed] [Google Scholar]
- 169.Puzzo F., Colella P., Biferi M.G., Bali D., Paulk N.K., Vidal P., Collaud F., Simon-Sola M., Charles S., Hardet R., et al. Rescue of Pompe disease in mice by AAV-mediated liver delivery of secretable acid alpha-glucosidase. Sci. Transl. Med. 2017;9:eaam6375. doi: 10.1126/scitranslmed.aam6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hu J., Lu J.Y., Wong A.M.S., Hynan L.S., Birnbaum S.G., Yilmaz D.S., Streit B.M., Lenartowicz E.M., Thompson T.C.M., Cooper J.D., Hofmann S.L. Intravenous high-dose enzyme replacement therapy with recombinant palmitoyl-protein thioesterase reduces visceral lysosomal storage and modestly prolongs survival in a preclinical mouse model of infantile neuronal ceroid lipofuscinosis. Mol. Genet. Metab. 2012;107:213–221. doi: 10.1016/j.ymgme.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dierenfeld A.D., McEntee M.F., Vogler C.A., Vite C.H., Chen A.H., Passage M., Le S., Shah S., Jens J.K., Snella E.M., et al. Replacing the enzyme alpha-L-iduronidase at birth ameliorates symptoms in the brain and periphery of dogs with mucopolysaccharidosis type I. Sci. Transl. Med. 2010;2:60ra89. doi: 10.1126/scitranslmed.3001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dunder U., Valtonen P., Kelo E., Mononen I. Early initiation of enzyme replacement therapy improves metabolic correction in the brain tissue of aspartylglycosaminuria mice. J. Inherit. Metab. Dis. 2010;33:611–617. doi: 10.1007/s10545-010-9158-7. [DOI] [PubMed] [Google Scholar]
- 173.Allewelt H., Taskindoust M., Troy J., Page K., Wood S., Parikh S., Prasad V.K., Kurtzberg J. Long-term functional outcomes after hematopoietic stem cell transplant for early infantile Krabbe disease. Biol. Blood Marrow Transpl. 2018;24:2233–2238. doi: 10.1016/j.bbmt.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 174.Selvanathan A., Ellaway C., Wilson C., Owens P., Shaw P.J., Bhattacharya K. Effectiveness of early hematopoietic stem cell transplantation in preventing neurocognitive decline in mucopolysaccharidosis type II: a case series. JIMD Rep. 2018;41:81–89. doi: 10.1007/8904_2018_104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Arends M., Wijburg F.A., Wanner C., Vaz F.M., van Kuilenburg A.B.P., Hughes D.A., Biegstraaten M., Mehta A., Hollak C.E.M., Langeveld M. Favourable effect of early versus late start of enzyme replacement therapy on plasma globotriaosylsphingosine levels in men with classical Fabry disease. Mol. Genet. Metab. 2017;121:157–161. doi: 10.1016/j.ymgme.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 176.Barth A.L., de Magalhães T.S.P.C., Reis A.B.R., de Oliveira M.L., Scalco F.B., Cavalcanti N.C., Silva D.S.E., Torres D.A., Costa A.A.P., Bonfim C., et al. Early hematopoietic stem cell transplantation in a patient with severe mucopolysaccharidosis II: a 7 years follow-up. Mol. Genet. Metab. Rep. 2017;12:62–68. doi: 10.1016/j.ymgmr.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Muenzer J. Early initiation of enzyme replacement therapy for the mucopolysaccharidoses. Mol. Genet. Metab. 2014;111:63–72. doi: 10.1016/j.ymgme.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 178.Nguyen Q.H., Witt R.G., Wang B., Eikani C., Shea J., Smith L.K., Boyle G., Cadaoas J., Sper R., MacKenzie J.D., et al. Tolerance induction and microglial engraftment after fetal therapy without conditioning in mice with Mucopolysaccharidosis type VII. Sci. Transl. Med. 2020;12:eaay8980. doi: 10.1126/scitranslmed.aay8980. [DOI] [PubMed] [Google Scholar]
- 179.Vogel C.M., Gallicchio V.S. In utero hematopoietic stem cell transplantation. J. Stem Cell Res. 2021;2:1. doi: 10.52793/jscr.2021.2(2)-s5. [DOI] [Google Scholar]
- 180.Dickson P.I., Ellinwood N.M., Brown J.R., Witt R.G., Le S.Q., Passage M.B., Vera M.U., Crawford B.E. Specific antibody titer alters the effectiveness of intrathecal enzyme replacement therapy in canine mucopolysaccharidosis I. Mol. Genet. Metab. 2012;106:68–72. doi: 10.1016/j.ymgme.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Somanathan S., Breous E., Bell P., Wilson J.M. AAV vectors avoid inflammatory signals necessary to render transduced hepatocyte targets for destructive T cells. Mol. Ther. 2010;18:977–982. doi: 10.1038/mt.2010.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Buchlis G., Podsakoff G.M., Radu A., Hawk S.M., Flake A.W., Mingozzi F., High K.A. Factor IX expression in skeletal muscle of a severe hemophilia B patient 10 years after AAV-mediated gene transfer. Blood. 2012;119:3038–3041. doi: 10.1182/blood-2011-09-382317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nathwani A.C., Reiss U.M., Tuddenham E.G.D., Rosales C., Chowdary P., McIntosh J., Della Peruta M., Lheriteau E., Patel N., Raj D., et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N. Engl. J. Med. 2014;371:1994–2004. doi: 10.1056/NEJMoa1407309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Thomas C.E., Ehrhardt A., Kay M.A. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 2003;4:346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 185.Rossi A., Dupaty L., Aillot L., Zhang L., Gallien C., Hallek M., Odenthal M., Adriouch S., Salvetti A., Büning H. Vector uncoating limits adeno-associated viral vector-mediated transduction of human dendritic cells and vector immunogenicity. Sci. Rep. 2019;9:3631. doi: 10.1038/s41598-019-40071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gao G., Vandenberghe L.H., Alvira M.R., Lu Y., Calcedo R., Zhou X., Wilson J.M. Clades of Adeno-associated viruses are widely disseminated in human tissues. J. Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Boutin S., Monteilhet V., Veron P., Leborgne C., Benveniste O., Montus M.F., Masurier C. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum. Gene Ther. 2010;21:704–712. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- 188.Louis Jeune V., Joergensen J.A., Hajjar R.J., Weber T. Pre-existing anti-adeno-associated virus antibodies as a challenge in AAV gene therapy. Hum. Gene Ther. Methods. 2013;24:59–67. doi: 10.1089/hgtb.2012.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Verdera H.C., Kuranda K., Mingozzi F. AAV vector immunogenicity in humans: a long journey to successful gene transfer. Mol. Ther. 2020;28:723–746. doi: 10.1016/j.ymthe.2019.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gray S.J., Matagne V., Bachaboina L., Yadav S., Ojeda S.R., Samulski R.J. Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol. Ther. 2011;19:1058–1069. doi: 10.1038/mt.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Keam S.J.E. Autotemcel: first approval. Mol. Diagn. Ther. 2021;25:803–809. doi: 10.1007/s40291-021-00555-1. [DOI] [PubMed] [Google Scholar]
- 192.https://investor.bluebirdbio.com/news-releases/news-release-details/bluebird-bio-receives-fda-accelerated-approval-skysonar-gene. (bluebird bio).
- 193.Katsigianni E.I., Petrou P. A systematic review of economic evaluations of enzyme replacement therapy in Lysosomal storage diseases. Cost Eff. Resour. Alloc. 2022;20:51. doi: 10.1186/s12962-022-00369-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gene therapies should be for all. Nat. Med. 2021;27:1311. doi: 10.1038/s41591-021-01481-9. [DOI] [PubMed] [Google Scholar]
- 195.Garrison L.P., Jr., Jiao B., Dabbous O. Gene therapy may not be as expensive as people think: challenges in assessing the value of single and short-term therapies. J. Manag. Care Spec. Pharm. 2021;27:674–681. doi: 10.18553/jmcp.2021.27.5.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rossini L., Durante C., Marzollo A., Biffi A. New indications for hematopoietic stem cell gene therapy in lysosomal storage disorders. Front. Oncol. 2022;12:885639. doi: 10.3389/fonc.2022.885639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Macauley S.L. Combination therapies for lysosomal storage diseases: a complex answer to a simple problem. Pediatr. Endocrinol. Rev. 2016;13:639–648. https://pubmed.ncbi.nlm.nih.gov/27491211. [PMC free article] [PubMed] [Google Scholar]