Abstract
Cytomegalovirus (CMV) pneumonitis infections might present mild or severe illnesses and need sophisticated diagnostic tools, so it remains a diagnostic challenge. We reported five infants diagnosed with CMV pneumonitis who were initially and undiagnosed by the pediatrician in secondary private or public health hospitals with no improvement with standard and escalation of antibiotics treatment for bronchopneumonia as the initial diagnoses. As all cases occurred during the COVID-19 pandemic, they proved negative COVID-19 identified by polymerase chain reaction (PCR) SARS-CoV-2. We diagnosed acquired perinatal pneumonitis CMV in all claims based on clinical criteria, imaging studies, CMV serology, and PCR-CMV urinary tests as diagnostic tools. They showed clinical improvement after two weeks of valganciclovir therapy. Other organs’ involvement was considered to be evaluated, including brain-evoked response audiometry (BERA) and eye examination. The physician should consider the possibility of CMV pneumonitis, who did not respond to standard and escalation of antibiotics treatment after initial diagnoses of bronchopneumonia.
Keywords: CMV pneumonitis, PCR-CMV urinary, Respiratory infection, Case report
Introduction
Cytomegalovirus infection may result from an intrauterine infection known as congenital CMV, perinatal infection due to contact with infected genital secretion during delivery, or postpartum transmission through breast and transfusion with CMV-seropositive blood. 1–4 CMV pneumonitis is infrequent in congenital infection while more common in perinatal infection. After primary infection, CMV remains in the host cells in latent form and further reactivates, particularly in the immunocompromised host. [5] The incubation period of perinatal CMV is from 4 to 12 weeks. Clinical symptoms of pneumonitis CMV were dyspnea, cough, and fever. Difficulties in the definitive diagnosis of CMV pneumonitis are due to its invasive, expensive, and complicated procedure. [5], [6], [7] We presented five infants with CMV pneumonitis who were initially undiagnosed and delayed in diagnosis. The unavailability of molecular diagnostic of urinary PCR-CMV in many hospital settings, and it is also not covered by government assurance (healthcare and social security agency), i.e., in Indonesia, is another problem.
Report of five cases
Case 1
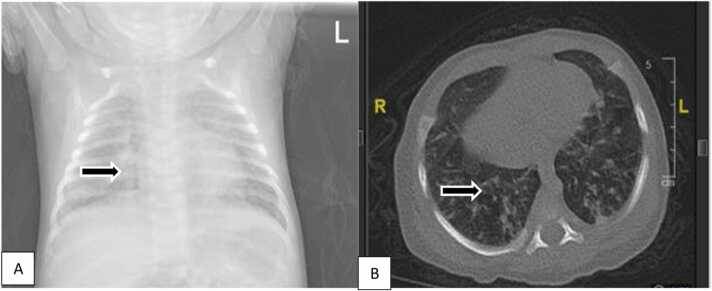
A 5-weeks old, female infant, breastfed, spontaneously delivered term infant with normal birth weight (2.800 g) was referred with dyspnea, cough, and fever for six days on admission, a body weight of 3.850 g. Physical examination showed chest indrawing, crackles, and hypoxemia (88%). Jaundice, purpura, petechiae, microcephaly, liver, or spleen enlargement were not identified. Laboratory examination showed normal hemoglobin (Hb) 11,9, leukocytosis (23.990/µL), thrombocytosis (614.000/uL), increased c-reactive protein (CRP) (0.33 mg/dL), non-reactive anti-HIV, no growth of pathogen from blood culture and PCR SARS-CoV-2 was negative. Chest X-ray and high-resolution computerized tomography (HRCT) (Fig. 1A and B). Head ultrasonography was normal. Since initial hospitalization, no improvement after three weeks of empirical and escalation of antibiotics (ampicillin is escalated to meropenem). CMV serology was reactive for anti-CMV IgM and IgG (14,1 index and 83,9 U/mL, respectively). PCR-CMV urinary test yielded a positive result (1,28 ×104 copies/mL) and then diagnosed as CMV pneumonitis. Valganciclovir 16 mg/kg body weight orally, twice daily, showed dramatic improvement after two weeks and continued up to 6 months. BERA showed normal results, while visual examination showed suspected delayed visual maturation.
Fig. 1.
A. Chest X-ray showed bilateral bronchopneumonia. B. HRCT showed consolidation in the apical segment of the superior lobe, the superior segment of the inferior lobe, a posterodorsal segment of the inferior lobe of the right lung as well as in the anterior segment of the superior lobe, superior lingula, posterodorsal inferior lobe of the left lung; patchy consolidation in the superior-inferior lobe and posterodorsal segments of the inferior lobe of the left lung; Fibrosis in the laterodorsal and posterodorsal segments of the inferior lobes of the lung bilaterally suggestive of bilateral pneumonia with pulmonary interstitial involvement.
Case 2
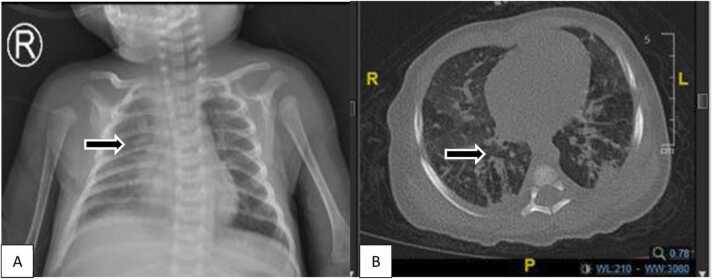
A 3-months old female infant, breastfed, spontaneously delivered, term infant with normal birth weight (2.900 g), presented with dyspnea, cough, and fever for three days, with a body weight of 3.600 g. After no improvement in hospitalization for a week in the secondary public hospital, the patient was referred. At admission, chest indrawing, crackles, and hypoxemia (85%) were noted. Jaundice, purpura, petechiae, microcephaly, and liver or spleen enlargement were not identified. Laboratory examination showed anemia (Hb 8,7 g/dL), leukocytosis (26.560/µL), normal thrombocyte (255.000/uL), normal CRP (0,26 mg/dL), nonreactive anti-HIV, no growth of pathogen from blood culture and PCR SARS-CoV-2 was negative. Head ultrasonography was normal for chest X-ray and HRCT (Fig. 2 A and B). No improvement after four weeks of empirical and escalation of antibiotics (ampicillin is escalated to meropenem) in the previous hospitalization. Investigation of IgM and IgG anti-CMV (173,8 index and 9,8 U/mL, respectively) were reactive. PCR-CMV urinary test was positive (1.65 ×105 copies/mL), and CMV pneumonitis was diagnosed. Improvement was noted with oral valganciclovir 16 mg/kg body weight twice daily. After one week of valganciclovir, they patiently showed improvement, and the drugs continued for up to 6 months. BERA and visual examination showed normal results.
Fig. 2.
A. Chest X-ray showed bilateral bronchopneumonia. B HRCT showed ground glass opacity in the apical, anterior, and posterior segments of the superior lobe, medial segment, lateral lobe medius, superior segment, anterobasal, laterodorsal, posterodorsal, and mediobasal inferior lobe of the right and left lung. Interlobular septal thickening in the apical, anterior, and posterior segments of the superior lobe, laterodorsal segment, posterodorsal inferior lobe of the right lung; anterior segment, apicoposterior superior lobe, laterodorsal segment, anterobasal, posterodorsal inferior lobe of the left lung.
Case 3
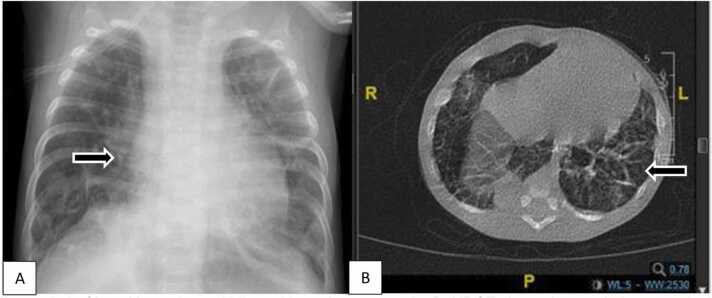
A 1-months old female infant, breastfed, spontaneously delivered, preterm infant 36 weeks with normal birth weight (2.750 g), presented with dyspnea, cough, and fever for five days, body weight 3.140 g. The patient has been hospitalized for two weeks in a secondary public hospital showing no improvement. At admission, chest indrawing, crackles, and hypoxemia (87%) were noted. Jaundice, purpura and petechiae, microcephaly, liver, or spleen enlargement were not identified. Laboratory examination showed normal Hb (13,3 gr/dL), leukocytosis (20.560/µL), thrombocytopenia (76.000/µL), increased CRP (0,54 mg/dL), nonreactive anti-HIV, no growth of pathogen from blood culture, and PCR SARS-CoV-2 was negative. Head ultrasonography was normal for chest X-ray and HRCT (Fig. 3 A & B). No improvement was shown after four weeks of antibiotics escalation (ampicillin is escalated to meropenem). Investigation of serology CMV was reactive for IgM and IgG anti-CMV (30,0 index and 0,73 U/mL, respectively). PCR-CMV urinary was positive (3,88 ×103 copies/mL), and CMV pneumonitis was then diagnosed. Improvement was noted with oral valganciclovir 16 mg/kg body weight, twice daily, and showed improvement after two weeks. The drug was only taken for four weeks. BERA and visual examination were planned in an outpatient clinic, but the parent refused to do the procedure on follow-up.
Fig. 3.
A. Chest X-ray showed bilateral bronchopneumonia. B. HRCT showed ground glass opacity in the apical, anterior, and posterior segments of the superior lobe, medial segment, lateral lobe medius, superior segment, anterobasal, laterodorsal, posterodorsal, and mediobasal inferior lobe of the right and left lung. Consolidation and fibrosis within interstitial lung involvement suggest pneumonia with interstitial participation.
Case 4
A 3-months old male infant, breastfed, spontaneously delivered, term infant with low birth weight (1.900 g), presented with dyspnea, cough, and fever for seven days, body weight 3.200 g. The patient has been hospitalized for three weeks in a secondary private hospital and referred with CMV diagnoses due to insufficient ganciclovir or valganciclovir. Chest radiography and HRCT are shown in Fig. 4 A & B. Serology CMV examinations were reactive for IgM and IgG anti-CMV (12,08 index and 7,64 U/mL, respectively), and PCR-CMV urinary test was positive (2.45 ×104 copies/mL). At admission, deep chest indrawing, crackles, and hypoxemia (84%) were noted, and the patient was admitted to the high-care unit. Jaundice, purpura and petechiae, microcephaly, liver, or spleen enlargement were not identified. Laboratory examinations showed normal Hb (10,8 gr/dL), normal leukocyte (12.290/uL), thrombocytosis (502.000/uL), normal CRP (0,14 mg. dL), nonreactive anti-HIV, no growth of pathogen from blood culture, and PCR SARS-CoV-2 was negative. Head ultrasonography was normal. After two weeks of oral valganciclovir 16 mg/kg body weight, two times daily, and antibiotics with its escalation, improvement was not noted. Investigation of immunoglobulin profile resulting low value of IgA (20 mg/dL), IgG (115 mg/dL), IgM (35 mg/dL) but normal IgE (9,2 IU/mL). The low value of CD4 absolute (705 cell/uL), CD8 absolute (747 cell/uL), ratio CD4:CD8 (0.4). The patient was diagnosed with CMV pneumonitis and primary immunodeficiency (PID). Oral valganciclovir continued, and the suggestion was to give immunoglobulin intravenously. Unfortunately, the parents brought the baby home against medical advice.
Fig. 4.
A. Chest X-ray showed bilateral bronchopneumonia. B. HRCT showed ground glass opacity in the apical lobe superior, anterobasal, posterodorsal inferior lobe of the right lung, superior lobe apicoposterior, lingula superior, lingula inferior, superior, posterodorsal inferior lobe of the left lung; Multifocal consolidation in the posterior segment of the superior lobe, superior, inferior lobe of the right lung, anterobasal segment of inferior lobe of left lung e.c. Suggestive of pneumonia. Fibrosis in the apical segment, superior posterior lobe, posterodorsal inferior lobe of the right lung, apicoposterior, superior anterior lobe, laterodorsal, posterodorsal inferior lobe of the left lung.
Case 5
A 10-months old female infant, breastfed, spontaneously delivered, term infant with low birth weight (1900 g) and normal head circumference, presented with dyspnea, cough, and fever for one month, BW 5000 g. The patient has been hospitalized for three weeks in a secondary general hospital. The patient has a history of repeated hospitalizations and was referred to our hospital due to cardiac co-morbidity (heart failure, aorta pulmonary (A-P) window diagnosed by echocardiography since 2 months. Chest indrawing, crackles, and hypoxemia (80%) were identified at admission. Jaundice, purpura, petechiae, liver, and spleen enlargement were not placed except for microcephaly. Laboratory examinations showed anemia with Hb (8,6 gr/dL), leukocytosis (16.220/µL), normal thrombocyte (446.000/uL), nonreactive anti-HIV, no growth of pathogen from blood culture, and PCR SARS-CoV-2 was negative. Head ultrasonography was normal. Chest X-ray and HRCT are shown in Fig. 5 A & B. No improvement after four weeks of antibiotics escalation, and investigation of serology CMV was reactive for IgM and IgG anti-CMV (30,0 index and 0,73 U/mL, respectively). PCR-CMV urinary was positive (5,92 ×104 copies/mL), and CMV pneumonitis was then diagnosed. Improvement was noted with oral valganciclovir 16 mg/kg body weight twice daily. BERA and eye examination showed normal results. Unfortunately, the patient died at 19 months due to cardiorespiratory problems.
Fig. 5.
A. Chest X-ray showed bilateral bronchopneumonia and cardiomegaly. B. HRCT showed consolidation with air bronchogram (+) in the posterior and anterior segments of the superior lobe and the entire inferior lobe of the right lung; throughout the superior and inferior lobes of the left lung accompanied by ground-glass opacities and surrounding fibrosis, Appears emphysema in the anterior segment of the superior lobe of the right lung.
Discussion
Clinical manifestations of CMV pneumonitis include cough, chest indrawing, fever, myalgia, or lymphadenopathy. [1], [2], [3] All of our cases presented to the emergency ward with dyspnea, cough, fever, chest retraction, and desaturation after being hospitalized in a secondary public and private hospital. During the COVID-19 pandemic, all patients with those symptoms were investigated for swab PCR-SARS-CoV-2, and their result was negative. During hospitalization, they noted no response to empiric and antibiotics escalation after three to four weeks. All our cases were diagnosed with probable acquired perinatal CMV because there were no identified signs of congenital CMV, such as microcephaly, petechiae or purpura, jaundice, hepatosplenomegaly, abnormality of neurologic examination (hypotonia, seizure, poor sucking reflex), anemia, thrombocytopenia, elevated liver enzyme, neuroimaging study (calcification or cysts periventricular), sensory hearing loss unilaterally or bilaterally and eye examination (chorioretinitis or retinal hemorrhage). [1], [2], [3], [6], [8].
CMV screening in pregnancy has not been normally carried out in our country; all patient mothers in these cases did not have CMV examinations before, during, or after pregnancy. The risk factor of acquired perinatal infections can be reached by three routes, contact with the virus in maternal genital tract secretions during delivery, ingestion of breast milk containing the virus, and transfusions of CMV seropositive blood. [5], [9], [10], [11], [12] All of our cases had breast milk spontaneously delivered, and there was not a history of blood transfusion, assuming that spontaneously paying may likely be a risk factor for patients.
The gold standard in diagnosing CMV pneumonitis is intranuclear or intracytoplasmic CMV inclusion bodies through histopathological examination of lung biopsy. Other findings are diffuse alveolar damage (DAD) similar to hyaline membrane disease, swollen pneumocytes, intra-alveolar exudate, alveolar wall edema, combined features of DAD, inflammation, or interstitial fibrosis, and features of inflammation or interstitial fibrosis. [2], [4], [7] Lung biopsy examinations are complex, invasive, and expensive, so they are not normally performed in daily clinical practice. This procedure was not done in our cases.
Several clinicians established CMV pneumonitis based on combining clinical, radiological, and virological findings. The radiological features of HRCT are ground-glass appearance and opacity (80%), interstitial infiltrates (50%), and pulmonary nodules (30%). [6] The virological findings could be identified from non-bronchoscopy bronchoalveolar lavage (NBBAL) specimens for viral culture and CMV DNA examination or PCR-CMV examination from saliva, blood, or urine specimens. [4] Blood PCR-CMV examinations have poor predictive value because the presence of a virus in the blood could be a latent infection. PCR-CMV examination of airways and urinary specimens is better for describing local viral replication and higher significance value. [6], [7], [8] All infants had positive PCR-CMV urinary. Ground-glass opacity and interstitial infiltrate were noted based on HRCT.
Several case reports described how to diagnose CMV pneumonitis without a lung biopsy or taking BAL specimens. Diagnosing CMV pneumonitis is challenging incredibly for general pediatricians. [6] The case report study compared the PCR-CMV results from NBBAL specimens and plasma and found that the viral load of the NBBAL specimen was higher than the plasma specimen. The viral load of NBBAL was more predictive and had higher sensitivity and specificity for diagnosing CMV pneumonitis. [6], [9].
A retrospective study of 186 pneumonia children in which PCR-CMV samples were taken from the urine and airway showed no significant difference in detecting CMV pneumonitis using both models. There were only 6.9% and 7.1%, respectively. Suggesting that urine is a suitable sample. The accuracy of PCR-CMV urinary examination was higher (82.32%) than serology detection for IgM examination (67.78%). Therefore, PCR-CMV urinary examination can be effective and non-invasive in diagnosing CMV pneumonitis. [8].
The management of CMV infection is oral valganciclovir 16 mg/kg/dose, twice daily for six months, or intravenous ganciclovir 6 mg/kg/dose twice daily for six weeks. [10], [11], [12] All of our cases were treated with oral valganciclovir 16 mg/kg/dose twice daily for six months and are likely to show improvement. In all cases, significant respiratory problems improved after two weeks days of taking valganciclovir.
We need help with were unavailability of screening programs for congenital CMV (CMV) after birth and diagnosis of CMV infection due to molecular diagnostic of PCR-CMV urinary uncovered by the Indonesia government assurance (healthcare and social security agency). Also, that investigation is unavailable in our hospital, which further referred to a private laboratory. So, the limitation of this report is that we did not investigate the CMV serology and HIV status of mothers nor reexamine PCR-CMV urinary after valganciclovir because of limited funding uncovered by our national assurance.
Conclusion
The diagnosis of CMV pneumonitis is challenging. The gold standard of its diagnosis is not feasible due to invasive, expensive, and complex procedures. The evidence of PCR-CMV urinary virus detection could be an alternative tool. Every clinician should consider CMV pneumonitis as a differential diagnosis of pneumonia that does not improve after standard antibiotic treatment.
Ethics statements
The parent’s consent was required for publication.
Funding
No funding.
CRediT authorship contribution statement
Heda Melinda Nataprawira: Conceptualization, Writing the first draft of manuscript, Methodology, Software, Validation. Ria Resti Sukur: Data curation, Writing – original draft preparation. Harry Galuh Nugraha: Visualization imaging. Djatnika Setiabudi: Supervision, Software, Writing – original draft preparation. All authors agree on the final manuscript.
Acknowledgments
We thank the patient’s parents/guardian for giving written consent for their child for publication, and the name of their children were anonymous. We would also like to thank Diah Asri Wulandari and Sri Sudarwati (pediatrician), who have ever been examined the patients.
Conflict of interest
Not to declare.
References
- 1.Gantt S., Bitnun A., Renaud C., Kakkar F., Vaudry W. Diagnosis and management of infants with congenital cytomegalovirus infection. Paediatr Child Health. 2017;22(2):72–74. doi: 10.1093/pch/pxx002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinez-Gomez E., Perez-Carpena P., Flook M., Lopez-Escamez J.A. A Systematic review on the association of acquired human cytomegalovirus infection with hearing loss. J Clin Med. 2020;9(12) doi: 10.3390/jcm9124011. 1–14.10.3390/jcm9124011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brito L.F., Brune W., Stahl F.R. Cytomegalovirus (CMV) pneumonitis: Cell tropism, inflammation, and immunity. Int J Mol Sci. 2019;20(16):1–14. doi: 10.3390/ijms20163865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bardanzellu F., Fanos V., Reali A. Human breast milk-acquired cytomegalovirus infection: Certainties, doubts, and perspectives. Curr. Pedia Rev. 2018;15(1):30–41. doi: 10.2174/1573396315666181126105812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Styczynski Jan. Who is the patient at risk of CMV recurrence: A review of the current scientific evidence focusing on hematopoietic cell transplantation. Infect Dis Ther. 2018;7:1–16. doi: 10.1007/s40121-017-0180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Govender K., Jeena P., Parboosing R. Clinical utility of bronchoalveolar lavage cytomegalovirus viral loads in diagnosing cytomegalovirus pneumonitis in infants. J Med Virol. 2017;89:1080–1087. doi: 10.1002/jmv.24730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuadrado M.M., Ahmed A., Carpenter B., Brown J.S. Cytomegalovirus pneumonitis is complicated by a central peribronchial pattern of organizing pneumonia. Respir Med Case Rep. 2017;20:184–187. doi: 10.1016/j.rmcr.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Z., Zhang P., Tang S., He X., Zhang R., Wang X., et al. Urine real-time polymerase chain reaction detection for children virus pneumonia with acute human cytomegalovirus infection. BMC Infect Dis. 2014;14(245):1–9. doi: 10.1186/1471-2334-14-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Restrepo-Gualteros S.M., Gutierrez M.J., Villamil-Osorio M., Arroyo M.A., Nino G. Challenges and clinical implications of the diagnosis of cytomegalovirus lung infection in children. Curr Infect Dis Rep. 2019;21(7):1–9. doi: 10.1186/1471-2334-14-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gopal N., Sharma J., Agarwal V., Mathur P., Jadoun L. Congenital CMV infection with CMV pneumonitis. 2020;6(11):5255–5258. doi: 〈10.24327/23956429.ijcmpr202009895〉.
- 11.Lee-Yoshimoto M., Goishi K., Torii Y., Ito Y., Ono H., Mori T., et al. Congenital cytomegalovirus pneumonitis and treatment response evaluation using viral load during ganciclovir therapy: A case report. Jpn J Infect Dis. 2018;71(4):309–311. doi: 10.7883/token.JJID.2017.577. [DOI] [PubMed] [Google Scholar]
- 12.Al-Eyadhy A.A., Hasan G., Bassrawi R., Al-Jelaify M., Temsah M.H., Alhaboob A., et al. Cytomegalovirus associated severe pneumonia, multi-organ failure, and Ganciclovir associated arrhythmia in an immunocompetent child. J Infect Chemother. 2017;23(12):844–847. doi: 10.1016/j.jiac.2017.08.003. [DOI] [PubMed] [Google Scholar]