Recently two lymphomas were reported in a chimeric antigen receptor (CAR) T cancer therapy trial (the CARTELL study)1,2 in which the piggyBac DNA transposon was used to deliver an engineered receptor gene to human T cells. The transformed cells were found to contain multiple integrated transposon DNA copies (4–24 per cell) and harbor multiple rearrangements in cellular chromosomal DNA. A variety of factors may have contributed to transformation, including aspects of the vector design and cell processing.1,2 In evaluating possible mechanisms of transformation, it may be useful to consider the properties of transposition that differ from the more familiar retroviral integration and ask whether these could have influenced development of the two lymphomas. For example, transposon DNA integrated in a host chromosome can be a substrate for further rounds of excision and transposition, where each excision event generates a DNA double-strand break at the empty transposon-donor site. Transposases can also cleave genomic DNA directly at chromosomal sequences that, by chance, resemble transposase-binding sites. These mechanisms are described more fully below, and additional experiments are described that could help clarify possible pathways of genotoxicity and potentially mitigate risks.
Use of DNA transposons3 for gene addition has the advantage over retroviral delivery of bypassing the step of vector particle generation and so may be simpler and less expensive to use. However, recently two cases of lymphoma were found in a CAR T trial to treat B cell malignancies following piggybac delivery,1,2 and the transformed cells contained multiple integrated transposons and chromosomal rearrangements. The authors suggested that insertional mutagenesis was not a factor in the two cases, though transformed cells from both adverse events harbored integration in BACH2, a gene known to be a target of insertional mutagenesis that is linked to T cell proliferation during HIV latency;4,5,6 as the authors point out, it would be useful to investigate these events further. In addition, might action of transposons have contributed to the chromosomal rearrangements detected in transformed cells? In evaluating possible mechanisms, it seems useful to consider the distinctive properties of transposase proteins and how they differ from the more familiar retroviral integrases.
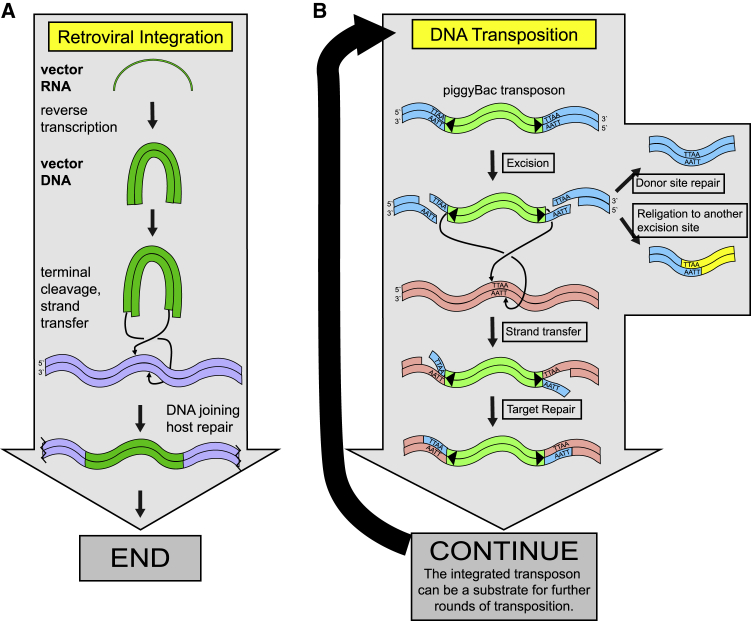
For retroviral transduction (Figure 1A), steps include (1) reverse transcription, which generates a double-stranded linear DNA from the viral RNA genome; (2) terminal cleavage, in which the viral integrase protein removes two nucleotides from each viral DNA 3′ end; (3) strand transfer, in which integrase joins one strand at each viral DNA end to host DNA; and (4) host-enzyme mediated DNA repair, which fills in the resulting DNA gaps at each junction and completes provirus formation. Crucially for what follows, DNA of the integrated provirus is not a substrate for integrase, which cannot cleave in a continuous double helical DNA. The proviral sequence can only be duplicated by further rounds of viral transcription, particle formation, and infection of new cells.7,8
Figure 1.
DNA breaking and joining reactions involved in retroviral DNA integration and piggyBac transposition
(A) After entry of the retroviral core into the cytoplasm of an infected cell, retroviral DNA integration proceeds, starting with reverse transcription of the viral RNAs to make a double-stranded linear DNA copy of the viral RNA genome. The viral-encoded integrase protein then removes two nucleotides from each viral DNA end, and the recessed 3′ hydroxyls attack target DNA. Host-mediated DNA repair completes attachment of the second DNA strand at each end. Viral DNA embedded in continuous duplex DNA is not a substrate for further cleavage by integrase. (B) Transposition directed by piggyBac starts with cleavage of the transposon DNA on each strand at each end by transposase to release the integrated transposon DNA. The excision reaction also yields a DNA double-strand break at the empty target site, which is repaired by nonhomologous DNA end joining or homologous DNA repair. The complex of transposase and transposon DNA then captures a new chromosomal target DNA site and carries out integration. The integrated transposon can then in fact be a substrate for further rounds of excision and transposition. piggyBac transposase favors cleavage and joining at host cell target sites with a 5′-TTAA-3′ sequence. This can be preserved even if two separate excision events were religated in swapped arrangement, providing one marker of involvement of piggyBac transposase. The diagram is adapted from Chen et al.23 Note that a step is omitted in the piggBac reaction, in which a DNA hairpin is initially formed at the transposon end and then opened by hydrolysis. Another potential pathway of DNA rearrangement involving piggyBac transposase would involve cleavage by transposase at chromosomal sites resembling inverted terminal repeat (ITRs; triangles) to yield double-strand DNA breaks. Such events resemble reactions at one side of the integrated transposon during excision. Formation of two double-strand breaks by this mechanism would allow potential religation in swapped arrangement, yielding a chromosomal rearrangement. For these reactions, the 5′-TTAA-3′ sequence is likely dispensable,17,23 so only the ITR sequence, and not the 5′-TTAA-3′ sequence, may be present at junctions.
DNA transposons such as piggyBac move via simple DNA breaking and joining reactions that in fact can continue through multiple cycles in a single cell (Figure 1B). Naturally occurring transposons encode a transposase enzyme responsible for mobilization. For therapeutic gene transfer, the transposase protein is encoded separately from the sequences needed in cis, allowing mobilization of therapeutic genes such as engineered CARs. The transposase protein binds to inverted terminal repeat (ITR) recognition sites at each end of the transposon and typically cleaves both DNA strands at each end to release the transposon DNA. The transposase/DNA complex then captures target DNA at another site and integrates the DNA into the new chromosomal location. The DNA double-strand break at the empty transposon donor site is then resealed by host DNA repair.
In contrast to the integrated retroviral provirus, the integrated transposon DNA is in fact a substrate for transposase.3,9 The newly integrated transposon can be cleaved at each end by transposase protein and the transposon DNA excised (Figure 1B). The transposon DNA can then be integrated again at a new location. Critically, this generates a second DNA double-strand break at the empty target site. In principle, if a continuous source of transposase protein is present, the cycle can continue indefinitely, generating a new DNA double-strand break with each excision.
These ongoing cycles of transposition can seem senseless—why would natural selection create a mechanism in which transposons don’t increase in number but just fecklessly move from place to place? The answer is that transposons commonly do increase in number. The empty excision site can be repaired against a sister chromosome where the transposon is still present by homology-directed repair so that the transposon sequence is restored at the empty donor site. In addition, some DNA transposons jump after passage of a replication fork to a new site ahead of the fork, again resulting in an increase in transposon number.10
For the two leukemia cases in the piggyBac/CAR-19 trial, transformed cells contained relatively high numbers of integrated transposons, estimated at 24 and 4 for the two patients with lymphoma.1,2 This suggests extensive exposure of cells to transposase and transposon DNA, providing an opportunity for multiple rounds of transposition and associated double-strand DNA (dsDNA) break formation. For the first patient, where the most extensive data were available, copy-number changes were “numerous” and included seven gains and two losses of chromosomal regions. The second patient was also stated to have “a marked increase” in structural variants compared with untransduced cells and nonmalignant CAR T cells.
Thus, a connection could be posited between the high copy-number variations in the transformed cells and transposon activity. According to this idea, piggyBac transposons integrated in what became the transformed cells and carried out multiple cycles of transposition. With each excision event, a new DNA double-strand break was generated. Given multiple transposons in each transformed cell, multiple different DNA double-strand breaks might have been present at the same time, potentially allowing religation in swapped arrangements, producing the observed chromosomal structural variants (Figure 1B).
This model makes a specific prediction. For piggyBac, which was used in the CAR T trial, there is an additional nuance in the DNA breaking and joining reactions (Figure 1B). A short sequence is strongly favored at the target DNA site (5′-TTAA-3′). Upon transposon excision, overhanging ends are produced, allowing reannealing and ligation to restore the 5′-TTAA-3′ motif. Given the piggyBac mechanism, it is expected that these same overhangs will usually be present at excision sites so that religation of chromosomal fragments in swapped arrangements would often yield junctions harboring the conserved 5′-TTAA-3′ sequence.
In another mechanism, transposase might bind to sites in chromosomes resembling the normal ITR binding sites at transposon edges and cleave chromosomal DNA directly without involvement of a transposition reaction. Thus, it would be useful to investigate possible ITR-like sequences at rearrangement junctions as well.
In fact, the human genome encodes several DNA transposase-like proteins, and for one, encoded by the recombination activating 1 and 2 (RAG1/RAG2) genes, extensive evidence indicates that dsDNA breaks generated by RAG1/RAG2 protein result in rearrangements important in human cancer.11,12,13 RAG1/RAG2 is well known as the recombinase that drives VDJ recombination.14 This enzyme has transposase activity in vitro, and likely derived evolutionarily from a hAT family DNA transposon.14 Multiple cancers of cell types expressing RAG1/RAG2 show translocations with sequences resembling VDJ recombination signals (e.g., RAG protein-binding sites) at junctions,11,12,13 providing evidence for involvement of dsDNA cleavage by transposase in transformation in humans. Another human gene, encoding a piggyBac-family transposase, piggyBac transposable element derived 5 (PGBD5), has also been proposed to be important in the generation of chromosomal rearrangements and transformation,15,16,17 though here the data are controversial.18,19 In a third example, DNA cleavage by another class of transposons, the L1 retrotransposons, has been suggested to have caused chromothripsis, or “chromosome shattering,” and generation of multiple rearrangements.20
Further experiments could be useful to assess whether such mechanisms contributed to genotoxicity by piggyBac in the CARTELL study. Junction sequences in rearrangements from transformed cells could be interrogated for 5′-TTAA-3′ or nearby ITR-like sequences. It could also be informative to test cell lines exposed to piggyBac transposase to assess whether rearrangements accumulate (e.g., Saha et al.21). One recent paper documented dsDNA break accumulation preferentially in cells in the presence of both transposase and transposon DNA.21 The authors of the CARTELL trial mention that transposase doses were particularly high in their study, and as they suggest, it will be important to compare outcomes with different transposase/transposon doses using these assays and others.1 Such systems could also be used to quantify the proportion of excision and rearrangement events that preserve the 5′-TTAA-3′ flanking sequence or ITR sequences at breakpoints, strengthening these as markers of piggyBac transposon involvement. Lastly, it might be possible to engineer the piggyBac transposase to remove undesirable properties. For example, there are piggyBac transposase derivatives that are capable of excision only and not de novo integration.22 Might it be possible to engineer transposases with the reverse properties, yielding a transposase capable of integration but not excision, so that ongoing cycles of excision and reintegration could be suppressed?
Thus, there is reason to be optimistic that the genotoxic potential of DNA transposons can be subjected to more complete investigation, and undesirable properties potentially modified.
Acknowledgments
Thanks to Laurie Zimmerman for artwork and Joe Fraietta, Andrew Marques, Aradhana Kasimsetty and Scott Sherrill-Mix for comments on the manuscript. This work was supported in part by NIH grants P30AI045008, U01AI125051, R01CA241762, R01HL142791, RO1HL142791, and U19AI149680.
References
- 1.Micklethwaite K.P., Gowrishankar K., Gloss B.S., Li Z., Street J.A., Moezzi L., Mach M.A., Sutrave G., Clancy L.E., Bishop D.C., et al. Investigation of product-derived lymphoma following infusion of piggyBac-modified CD19 chimeric antigen receptor T cells. Blood. 2021;138:1391–1405. doi: 10.1182/blood.2021010858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bishop D.C., Clancy L.E., Simms R., Burgess J., Mathew G., Moezzi L., Street J.A., Sutrave G., Atkins E., McGuire H.M., et al. Development of CAR T-cell lymphoma in 2 of 10 patients effectively treated with piggyBac-modified CD19 CAR T cells. Blood. 2021;138:1504–1509. doi: 10.1182/blood.2021010813. [DOI] [PubMed] [Google Scholar]
- 3.Sandoval-Villegas N., Nurieva W., Amberger M., Ivics Z. Contemporary transposon tools: a review and guide through mechanisms and applications of sleeping beauty, piggyBac and Tol2 for genome engineering. Int. J. Mol. Sci. 2021;22:5084. doi: 10.3390/ijms22105084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ikeda T., Shibata J., Yoshimura K., Koito A., Matsushita S. Recurrent HIV-1 integration at the BACH2 locus in resting CD4+ T cell populations during effective highly active antiretroviral therapy. J. Infect. Dis. 2007;195:716–725. doi: 10.1086/510915. [DOI] [PubMed] [Google Scholar]
- 5.Wagner T.A., McLaughlin S., Garg K., Cheung C.Y.K., Larsen B.B., Styrchak S., Huang H.C., Edlefsen P.T., Mullins J.I., Frenkel L.M. HIV latency. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science. 2014;345:570–573. doi: 10.1126/science.1256304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maldarelli F., Wu X., Su L., Simonetti F.R., Shao W., Hill S., Spindler J., Ferris A.L., Mellors J.W., Kearney M.F., et al. HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science. 2014;345:179–183. doi: 10.1126/science.1254194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coffin J.M., Hughes S.H., Varmus H.E. In: Retroviruses. Coffin J.M., Hughes S.H., Varmus H.E., editors. Cold Spring Harbor; 1997. The interactions of retroviruses and their hosts. [PubMed] [Google Scholar]
- 8.Bushman F.D. Cold Spring Harbor Laboratory Press, Cold Spring Harbor; NY: 2001. Lateral DNA Transfer: Mechanisms and Consequences. [Google Scholar]
- 9.Li M.A., Pettitt S.J., Eckert S., Ning Z., Rice S., Cadiñanos J., Yusa K., Conte N., Bradley A. The piggyBac transposon displays local and distant reintegration preferences and can cause mutations at noncanonical integration sites. Mol. Cel. Biol. 2013;33:1317–1330. doi: 10.1128/MCB.00670-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig N.L. ASM Press; Washington, DC: 2015. Mobile DNA III. [Google Scholar]
- 11.Zhang M., Swanson P.C. V(D)J recombinase binding and cleavage of cryptic recombination signal sequences identified from lymphoid malignancies. J. Biol. Chem. 2008;283:6717–6727. doi: 10.1074/jbc.M710301200. [DOI] [PubMed] [Google Scholar]
- 12.Papaemmanuil E., Rapado I., Li Y., Potter N.E., Wedge D.C., Tubio J., Alexandrov L.B., Van Loo P., Cooke S.L., Marshall J., et al. RAG-mediated recombination is the predominant driver of oncogenic rearrangement in ETV6-RUNX1 acute lymphoblastic leukemia. Nat. Genet. 2014;46:116–125. doi: 10.1038/ng.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halper-Stromberg E., Steranka J., Giraldo-Castillo N., Fuller T., Desiderio S., Burns K.H. Fine mapping of V(D)J recombinase mediated rearrangements in human lymphoid malignancies. BMC Genomics. 2013;14:565. doi: 10.1186/1471-2164-14-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiom K., Melek M., Gellert M. DNA transposition by the RAG1 and RAG2 proteins: a possible source of oncogenic translocations. Cell. 1998;94:463–470. doi: 10.1016/s0092-8674(00)81587-1. [DOI] [PubMed] [Google Scholar]
- 15.Henssen A.G., Henaff E., Jiang E., Eisenberg A.R., Carson J.R., Villasante C.M., Ray M., Still E., Burns M., Gandara J., et al. Genomic DNA transposition induced by human PGBD5. Elife. 2015;4:e10565. doi: 10.7554/eLife.10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henssen A.G., Koche R., Zhuang J., Jiang E., Reed C., Eisenberg A., Still E., MacArthur I.C., Rodríguez-Fos E., Gonzalez S., et al. PGBD5 promotes site-specific oncogenic mutations in human tumors. Nat. Genet. 2017;49:1005–1014. doi: 10.1038/ng.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henssen A.G., Jiang E., Zhuang J., Pinello L., Socci N.D., Koche R., Gonen M., Villasante C.M., Armstrong S.A., Bauer D.E., et al. Forward genetic screen of human transposase genomic rearrangements. BMC Genomics. 2016;17:548. doi: 10.1186/s12864-016-2877-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beckermann T.M., Luo W., Wilson C.M., Veach R.A., Wilson M.H. Cognate restriction of transposition by piggyBac-like proteins. Nucleic Acids Res. 2021;49:8135–8144. doi: 10.1093/nar/gkab578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helou L., Beauclair L., Dardente H., Piégu B., Tsakou-Ngouafo L., Lecomte T., Kentsis A., Pontarotti P., Bigot Y. The piggyBac-derived protein 5 (PGBD5) transposes both the closely and the distantly related piggyBac-like elements Tcr-pble and Ifp2. J. Mol. Biol. 2021;433:166839. doi: 10.1016/j.jmb.2021.166839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nazaryan-Petersen L., Bertelsen B., Bak M., Jønson L., Tommerup N., Hancks D.C., Tümer Z. Germline chromothripsis driven by L1-mediated retrotransposition and alu/alu homologous recombination. Hum. Mutat. 2016;37:385–395. doi: 10.1002/humu.22953. [DOI] [PubMed] [Google Scholar]
- 21.Saha S., Woodard L.E., Charron E.M., Welch R.C., Rooney C.M., Wilson M.H. Evaluating the potential for undesired genomic effects of the piggyBac transposon system in human cells. Nucleic Acids Res. 2015;43:1770–1782. doi: 10.1093/nar/gkv017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X., Burnight E.R., Cooney A.L., Malani N., Brady T., Sander J.D., Staber J., Wheelan S.J., Joung J.K., McCray P.B., Jr., et al. piggyBac transposase tools for genome engineering. Proc. Natl. Acad. Sci. USA. 2013;110:E2279–E2287. doi: 10.1073/pnas.1305987110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Q., Luo W., Veach R.A., Hickman A.B., Wilson M.H., Dyda F. Structural basis of seamless excision and specific targeting by piggyBac transposase. Nat. Commun. 2020;11:3446. doi: 10.1038/s41467-020-17128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]