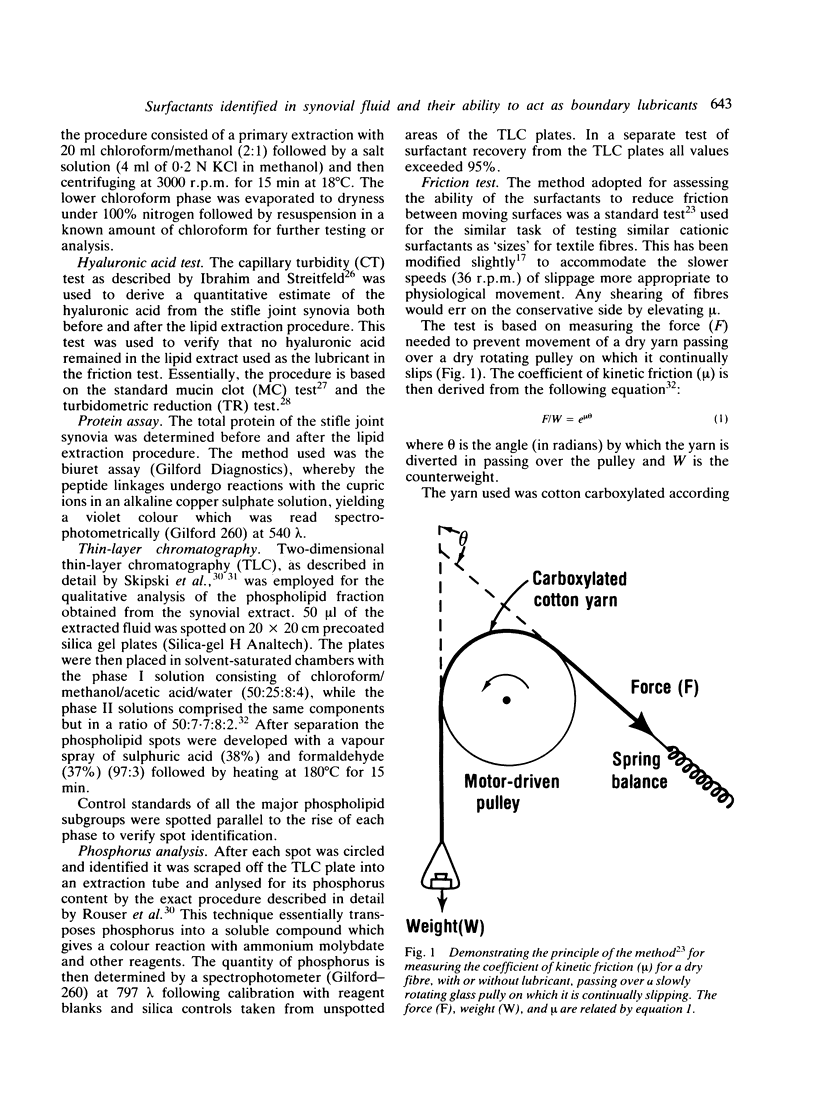
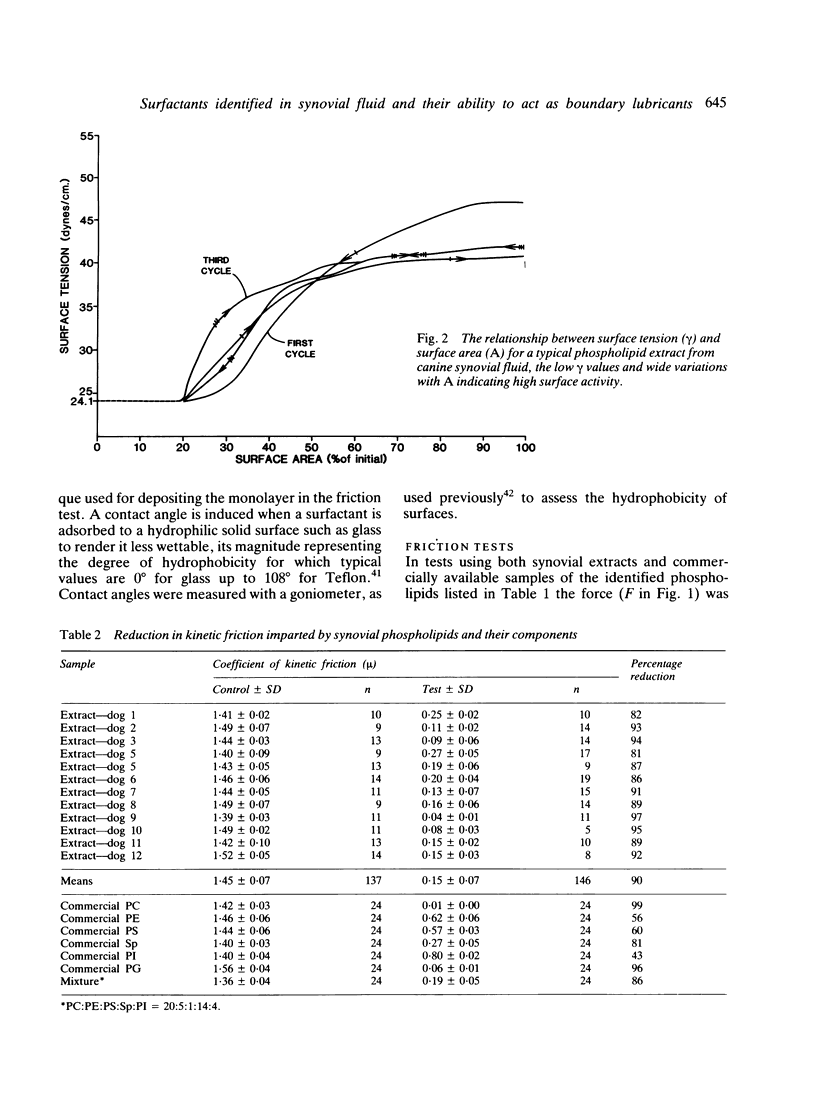
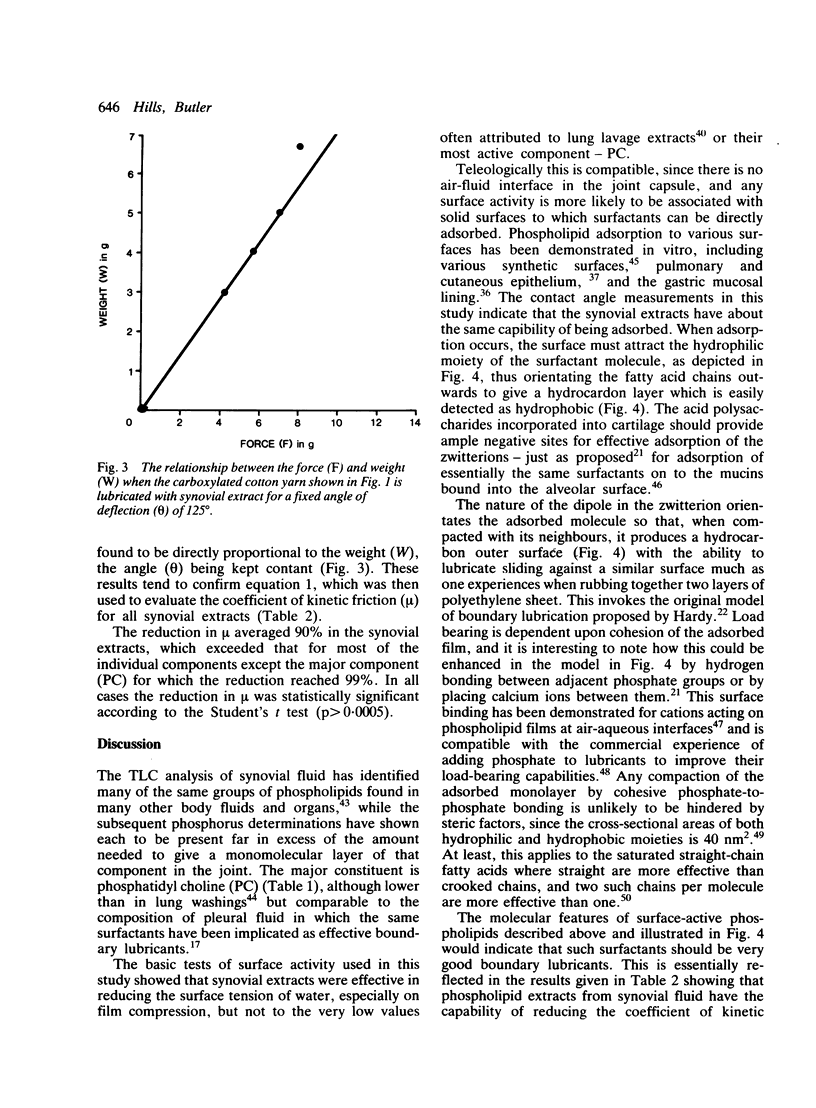
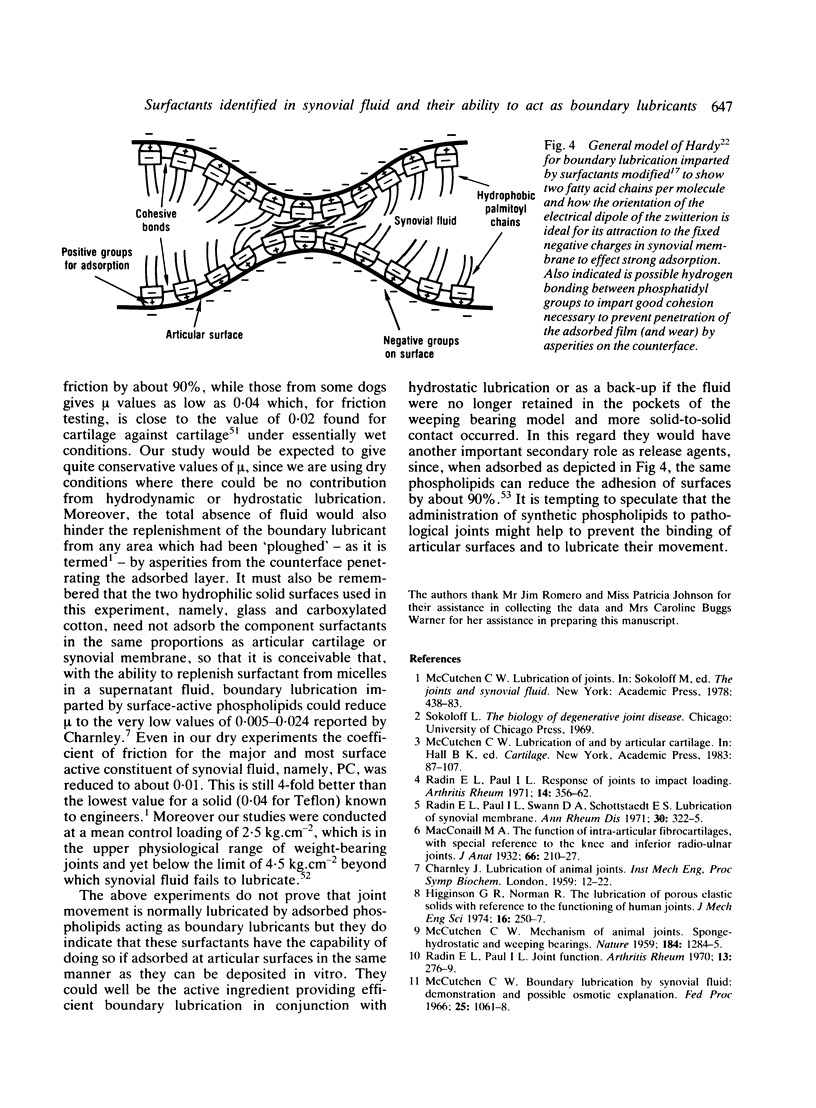
Abstract
Thin-layer chromatography has been used to identify phospholipids extracted from canine synovial fluid, the major component (45%) being phosphatidyl choline (PC). The extracts and their components have been shown to be surface active in reducing the surface tension of water and to be readily adsorbed to hydrophilic solids, whose surfaces then become hydrophobic. These adsorbed monolayers of synovial surfactant were then found to be excellent boundary lubricants in vitro, reducing the coefficient of kinetic friction (mu) in the dry state and under physiological loading by up to 97% for extracts and 99% for PC alone, reaching mu = 0.01. Surface-active phospholipid is put forward as the possible active ingredient in joint lubrication and shown to be consistent with previous biochemical studies to elucidate its identity. The model essentially follows the classical Hardy model for boundary lubrication imparted by surfactants. It is discussed in relation to a new approach in providing artificial lubrication and facilitating tissue release in patients with arthritis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CURTAIN C. C. The nature of the protein in the hyaluronic complex of bovine synovial fluid. Biochem J. 1955 Dec;61(4):688–697. doi: 10.1042/bj0610688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., ASCOLI I., LEES M., MEATH J. A., LeBARON N. Preparation of lipide extracts from brain tissue. J Biol Chem. 1951 Aug;191(2):833–841. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Frosolono M. F., Charms B. L., Pawlowski R., Slivka S. Isolation, characterization, and surface chemistry of a surface-active fraction from dog lung. J Lipid Res. 1970 Sep;11(5):439–457. [PubMed] [Google Scholar]
- Gershfeld N. L. Selective phospholipid adsorption and atherosclerosis. Science. 1979 May 4;204(4392):506–508. doi: 10.1126/science.581915. [DOI] [PubMed] [Google Scholar]
- Hills B. A. Analysis of eustachian surfactant and its function as a release agent. Arch Otolaryngol. 1984 Jan;110(1):3–9. doi: 10.1001/archotol.1984.00800270007003. [DOI] [PubMed] [Google Scholar]
- Hills B. A., Butler B. D., Barrow R. E. Boundary lubrication imparted by pleural surfactants and their identification. J Appl Physiol Respir Environ Exerc Physiol. 1982 Aug;53(2):463–469. doi: 10.1152/jappl.1982.53.2.463. [DOI] [PubMed] [Google Scholar]
- Hills B. A., Butler B. D., Lichtenberger L. M. Gastric mucosal barrier: hydrophobic lining to the lumen of the stomach. Am J Physiol. 1983 May;244(5):G561–G568. doi: 10.1152/ajpgi.1983.244.5.G561. [DOI] [PubMed] [Google Scholar]
- Hills B. A. Contact-angle hysteresis induced by pulmonary surfactants. J Appl Physiol Respir Environ Exerc Physiol. 1983 Feb;54(2):420–426. doi: 10.1152/jappl.1983.54.2.420. [DOI] [PubMed] [Google Scholar]
- Hills B. A. Water repellency induced by pulmonary surfactants. J Physiol. 1982 Apr;325:175–186. doi: 10.1113/jphysiol.1982.sp014143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills B. A. What is the true role of surfactant in the lung? Thorax. 1981 Jan;36(1):1–4. doi: 10.1136/thx.36.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim A. N., Streitfeld M. M. The microassay of hyaluronic acid concentration and hyaluronidase activity by capillary turbidity (CT) and capillary turbidity reduction (CTR) tests. Anal Biochem. 1973 Dec;56(2):428–434. doi: 10.1016/0003-2697(73)90208-x. [DOI] [PubMed] [Google Scholar]
- Linn F. C. Lubrication of animal joints. II. The mechanism. J Biomech. 1968 Aug;1(3):193–205. doi: 10.1016/0021-9290(68)90004-3. [DOI] [PubMed] [Google Scholar]
- Linn F. C., Radin E. L. Lubrication of animal joints. 3. The effect of certain chemical alterations of the cartilage and lubricant. Arthritis Rheum. 1968 Oct;11(5):674–682. doi: 10.1002/art.1780110510. [DOI] [PubMed] [Google Scholar]
- Macconaill M. A. The Function of Intra-Articular Fibrocartilages, with Special Reference to the Knee and Inferior Radio-Ulnar Joints. J Anat. 1932 Jan;66(Pt 2):210–227. [PMC free article] [PubMed] [Google Scholar]
- McClean D. Studies on diffusing factors: 2. Methods of assay of hyaluronidase and their correlation with skin diffusing activity. Biochem J. 1943 Jul;37(2):169–177. doi: 10.1042/bj0370169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcutchen C. W. Boundary lubrication by synovial fluid: demonstration and possible osmotic explanation. Fed Proc. 1966 May-Jun;25(3):1061–1068. [PubMed] [Google Scholar]
- Meban C. An electron microscopic study of the acid mucosubstance lining the alveoli of hamster lung. Histochem J. 1972 Jan;4(1):1–8. doi: 10.1007/BF01005264. [DOI] [PubMed] [Google Scholar]
- Montfoort A., van Golde L. M., van Deenen L. L. Molecular species of lecithins from various animal tissues. Biochim Biophys Acta. 1971 Mar 16;231(2):335–342. doi: 10.1016/0005-2760(71)90147-0. [DOI] [PubMed] [Google Scholar]
- Radin E. L., Paul I. L. Joint function. Arthritis Rheum. 1970 May-Jun;13(3):276–279. doi: 10.1002/art.1780130309. [DOI] [PubMed] [Google Scholar]
- Radin E. L., Paul I. L. Response of joints to impact loading. I. In vitro wear. Arthritis Rheum. 1971 May-Jun;14(3):356–362. doi: 10.1002/art.1780140306. [DOI] [PubMed] [Google Scholar]
- Radin E. L., Paul I. L., Swann D. A., Schottstaedt E. S. Lubrication of synovial membrane. Ann Rheum Dis. 1971 May;30(3):322–325. doi: 10.1136/ard.30.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin E. L., Swann D. A., Weisser P. A. Separation of a hyaluronate-free lubricating fraction from synovial fluid. Nature. 1970 Oct 24;228(5269):377–378. doi: 10.1038/228377a0. [DOI] [PubMed] [Google Scholar]
- Rouser G., Siakotos A. N., Fleischer S. Quantitative analysis of phospholipids by thin-layer chromatography and phosphorus analysis of spots. Lipids. 1966 Jan;1(1):85–86. doi: 10.1007/BF02668129. [DOI] [PubMed] [Google Scholar]
- SHAH D. O., SCHULMAN J. H. BINDING OF METAL IONS TO MONOLAYERS OF LECITHINS, PLASMALOGEN, CARDIOLIPIN, AND DICETYL PHOSPHATE. J Lipid Res. 1965 Jul;6:341–349. [PubMed] [Google Scholar]
- SKIPSKI V. P., PETERSON R. F., SANDERS J., BARCLAY M. THIN-LAYER CHROMATOGRAPHY OF PHOSPHOLIPIDS USING SILICA GEL WITHOUT CALCIUM SULFATE BINDER. J Lipid Res. 1963 Apr;4:227–228. [PubMed] [Google Scholar]
- Silpananta P., Dunstone J. R., Ogston A. G. Protein associated with hyaluronic acid in ox synovial fluid. Aust J Biol Sci. 1969 Aug;22(4):1031–1037. doi: 10.1071/bi9691031. [DOI] [PubMed] [Google Scholar]
- Swann D. A., Hendren R. B., Radin E. L., Sotman S. L., Duda E. A. The lubricating activity of synovial fluid glycoproteins. Arthritis Rheum. 1981 Jan;24(1):22–30. doi: 10.1002/art.1780240104. [DOI] [PubMed] [Google Scholar]


