Abstract
Background
There is a growing demand for total joint arthroplasty (TJA) surgery. The applications of machine learning (ML), mathematical optimization, and computer simulation have the potential to improve efficiency of TJA care delivery through outcome prediction and surgical scheduling optimization, easing the burden on health-care systems. The purpose of this study was to evaluate strategies using advances in analytics and computational modeling that may improve planning and the overall efficiency of TJA care.
Methods
A systematic review including MEDLINE, Embase, and IEEE Xplore databases was completed from inception to October 3, 2022, for identification of studies generating ML models for TJA length of stay, duration of surgery, and hospital readmission prediction. A scoping review of optimization strategies in elective surgical scheduling was also conducted.
Results
Twenty studies were included for evaluating ML predictions and 17 in the scoping review of scheduling optimization. Among studies generating linear or logistic control models alongside ML models, only 1 found a control model to outperform its ML counterpart. Furthermore, neural networks performed superior to or at the same level as conventional ML models in all but 1 study. Implementation of mathematical and simulation strategies improved the optimization efficiency when compared to traditional scheduling methods at the operational level.
Conclusions
High-performing predictive ML-based models have been developed for TJA, as have mathematical strategies for elective surgical scheduling optimization. By leveraging artificial intelligence for outcome prediction and surgical optimization, there exist greater opportunities for improved resource utilization and cost-savings in TJA than when using traditional modeling and scheduling methods.
Keywords: Artificial intelligence, Predictive modeling, Surgical scheduling, Optimization, Total knee arthroplasty, Total hip arthroplasty
Introduction
Total joint arthroplasty (TJA) procedures, including total knee arthroplasty (TKA) and total hip arthroplasty (THA), are the most commonly performed surgical procedures in North America [[1], [2], [3]]. Given their success in restoring function and improving quality of life, combined with an aging population and increasing demand, the rate at which TJA procedures are performed will continue to rise [4,5]. By 2030, the number of TKAs and THAs performed annually in the United States are projected to reach over 1.26 million and 635,000, respectively [6]. With an average cost of $16,000 to $60,000 USD, the financial burden of these procedures will place significant strain on health-care institutions [7,8].
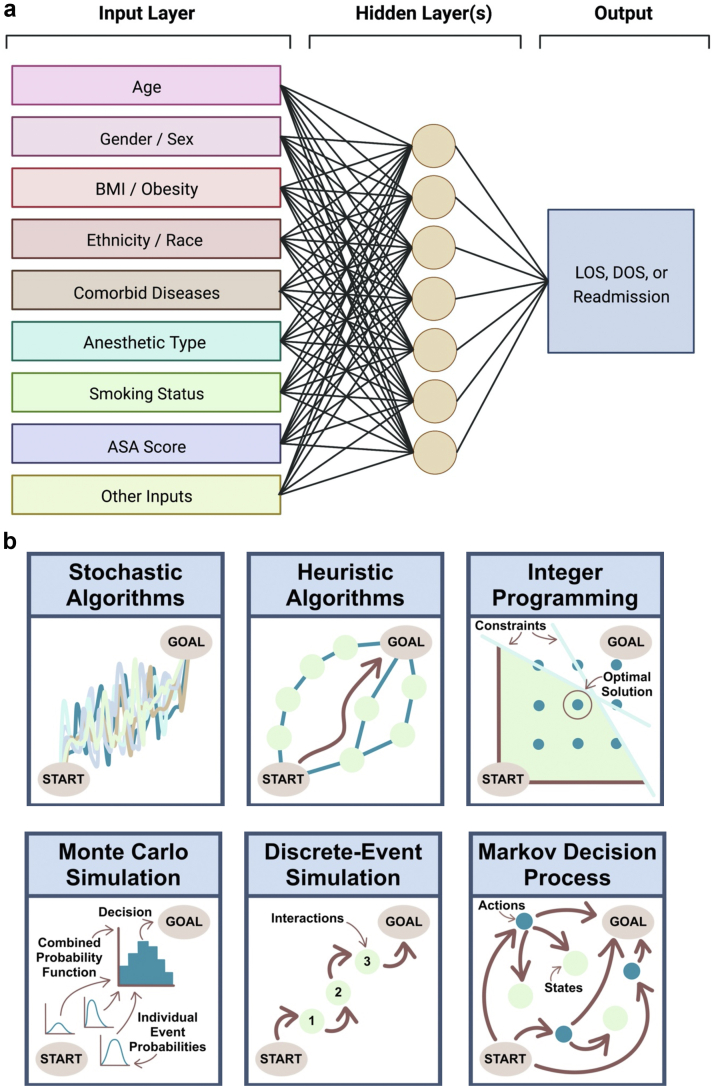
In order to keep up with the growing demand for TJA procedures, significant investments must be made in strategies to improve the efficiency and cost-effectiveness of care [9]. The growing volume of patient data combined with the utilization of new technologies such as artificial intelligence (AI) provides opportunities to improve the delivery of care in orthopaedic surgery [[9], [10], [11], [12]]. In particular, machine learning (ML), a subset of AI, has caught the attention of orthopaedic surgeons and health-care institutions due to its potential for generating accurate patient-specific predictive models by recognizing linear and nonlinear relationships from large data sources [[13], [14], [15], [16]]. Deep learning (DL) models, typically in the form of neural networks, are a subset of ML models theoretically capable of generating highly accurate predictions when trained with appropriate and sufficient data (Fig. 1a). Using conventional ML and DL models, perioperative outcomes associated with increased costs can be anticipated, and measures can be taken to minimize their burden. Such outcomes include length of stay (LOS), duration of surgery (DOS), and unplanned hospital readmissions [[17], [18], [19], [20]]. Following the generation of accurate predictions, in order to implement or realize any predicted gains, optimization of available resources must also be performed. The optimization of surgical schedules is typically considered a nondeterministic polynomial time-hard problem, with various optimization strategies available (Fig. 1b) [17]. Applying the principles of operations research in health care is facilitated by advances and wider-spread availability of high-performance computing and growing amounts of curated digital data.
Figure 1.
Diagrammatic representation of (a) neural network models, demonstrating the transformation of a combination of input features in hidden layers to yield outcome predictions, and (b) types of optimization strategies. ASA, American Society of Anesthesiologists; BMI, body mass index.
Numerous studies have recently emerged assessing the ability of ML models to predict perioperative resource-utilization-related outcomes surrounding TJA; however, the success of different algorithms, data sets, and comparison to traditional statistical methods have not been systematically evaluated. Furthermore, the theoretical optimization of elective surgical scheduling across surgical specialties has been heavily investigated in the engineering literature. However, many of these principles have not been translated into the orthopaedic literature to guide surgeons and key stakeholders as they evaluate and implement these methods of efficiency optimization in real-life situations. The purpose of this study was to systematically evaluate the literature investigating the impact of ML and optimization strategies on the planning, scheduling, and overall efficiency of TJA care.
Material and methods
This systematic review was registered in the National Institute for Health Research PROSPERO (International Prospective Register of Systematic Reviews) database (ID #: CRD42022377977). The review process was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 2020 guidelines [18].
Search strategy
The MEDLINE, Embase, and IEEE Xplore databases were searched using the following combination of search terms: ((machine learning) OR (artificial intelligence) OR (deep learning) OR ML OR AI OR prediction OR (neural network) OR (branch and cut) OR (Monte∗Carlo) OR simulation OR heuristic OR stochastic OR (ant colony) OR (meta∗heuristics) OR optimization OR (operations research)) AND (scheduling OR planning OR theatre OR theater OR (patient admission scheduling) OR (length of∗ stay) OR (duration of surgery) OR (surg∗ time) OR (surgical duration) OR (operative time) OR (operating time) OR (outpatient) OR DOS OR LOS) AND (TKA OR THA OR TKR OR THR OR (total knee arthroplasty) OR (total hip arthroplasty) OR (total knee replacement) OR (total hip replacement) OR (joint replacement) OR (joint arthroplasty)). All studies from inception to October 4, 2022, were retrieved, and duplicate manuscripts removed. Manual searching of the reference lists of included studies was also conducted.
Inclusion and exclusion criteria
Abstract and title screening of search results was performed by 3 independent reviewers (B.E., J.R.L., and R.K.). Full texts were screened for study inclusion according to the inclusion and exclusion criteria by all 3 reviewers. Conflicts were resolved by consensus among all reviewers throughout the screening process. Relevant data were extracted and recorded on a predetermined data-collection form by 2 independent reviewers (B.E. and R.K.). Studies of all languages were included and translated into English as required. Abstracts and reviews were excluded.
Artificial intelligence prediction
This section of the review was performed systematically. Studies were included if they used any type of ML model to predict 1 of 3 outcomes following TJA; LOS, DOS, or postoperative readmission. Studies evaluating patients undergoing primary or revision TKA, THA, partial knee arthroplasty, or hip resurfacing were included. Studies were not excluded based on varying definitions of DOS, namely whether it was total patient time in the room, surgical time, or included room turnover. No studies were excluded based on data set size or type.
Mathematical optimization
Due to the size, complexity, and depth of the existing literature, many of the concepts and articles were beyond the scope of this review. Therefore, this section of the review was performed in the form of a narrative review. Studies were included if they used computational modeling or mathematical optimization in an attempt to improve the efficiency of elective surgical scheduling. These articles were recorded regardless of surgery specialty as operations research for health care is traditionally specialty-agnostic and aims to optimize the schedule of the entire institution; however, these strategies can be applied to any elective surgery scheduling problem.
Data extraction
Artificial intelligence prediction
General study data collected included first author name, publication year and country, data source, and procedure. Model data were collected for control and ML models, where control models were considered those using average times, multivariable linear or logistic regression. ML models were considered as any algorithms having undergone training without explicit manual programming. Model data collected included outcome(s) of interest (LOS, DOS, and hospital readmission), algorithm type(s), total population size, training size, validation size and method, testing size, input features, and feature importance. For articles generating multiple models, the most important features from the best-performing model were recorded. Metrics for scoring model performance were collected. For the purpose of model comparison, mean squared error (MSE) was used as the gold standard for regression-type models, followed by root MSE, and mean absolute error; area under the curve (AUC) was used as the gold standard for classification-type models, followed by accuracy, and F1 score.
Mathematical optimization
Collected data included the study optimization problem goal, overall strategy, and the main findings. Surgical scheduling is typically broken down into 3 levels with corresponding definitions: (1) strategic level, planning and operating room (OR) allocation to specialties/surgeons over the period of a year or longer; (2) tactical level, cyclic regular seasonal or weekly OR schedules; and (3) operational level, scheduling cases by date, time, and specific resources required. The decision level at which the optimization problem was targeted to solve was recorded. The optimization strategy was categorized into the use of a heuristic algorithm, stochastic algorithm, integer programming, or simulations (including Markov decision process) to find the optimized scheduling solution (Table 1).
Table 1.
Categories of optimization strategies and their associated benefits and limitations.
| Optimization strategy | Definition | Benefits | Limitations |
|---|---|---|---|
| Heuristic algorithms | Specific rules-based functions to provide an approximate solution | Less computational resources and time to solve | Solution may not be optimal or complete |
| Stochastic algorithms | Optimization that uses random inputs, random objective functions, or random constraints | Accommodates for imprecise measurements in data, estimates the average model performance | Random elements prevent problem from finding optimal solution |
| Integer programming | Mathematical optimization to solve problems, can be linear or mixed (if some variables are not integers). Overarching term including exact algorithm solutions and heuristic and stochastic algorithms | Used to find optimal optimization problem solutions | Searching for optimal solutions may require high computational power, and random inputs after solution implementation may differ from optimal solution |
| Monte Carlo simulation | Use of random sampling with a range of assigned probabilities to model or find best outcome from systems with uncertainty (such as scheduling) | To solve mathematical problems too complicated to solve analytically | Requires many samples for good approximation, high computational power requirement |
| Discrete-event simulation | Used to model systems over time. Systems are broken down into a sequence of steps where entities compete for limited resources and can develop queues | Evaluate the impact of changes in practice prior to implementation | Complex decision-making processes and real-world constraints can be hard to model, require significant amount of data |
| Markov decision process | Mathematical framework for modeling decision-making, combining random inputs with chosen inputs | Evaluate the impact of changes in practice prior to implementation, less computational power required than other simulations | Complex decision-making processes and real-world constraints can be hard to model, require significant amount of data |
Results
Search results and study characteristics
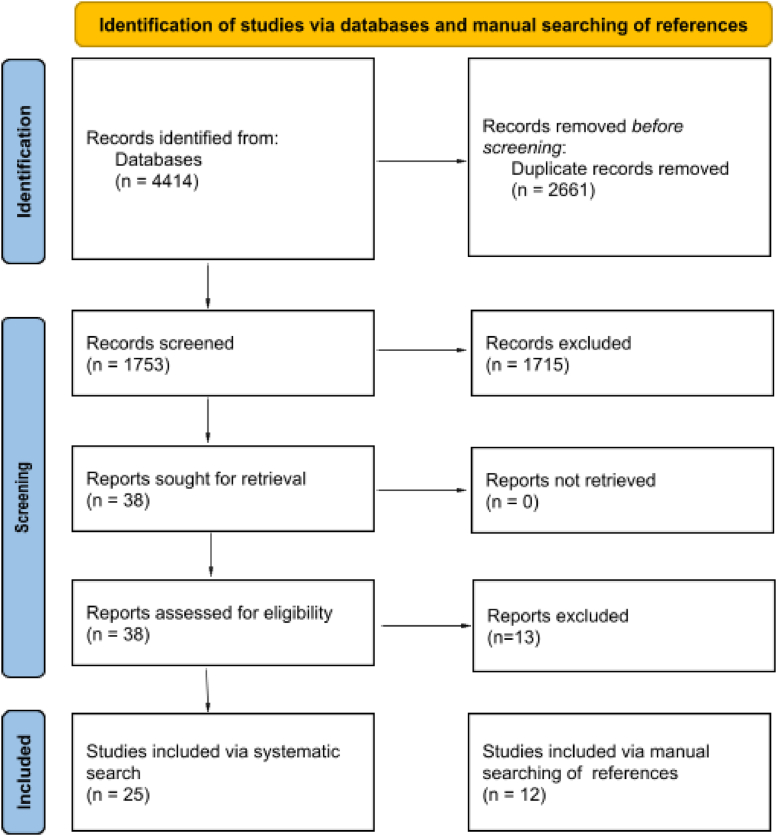
Following removal of duplicates, the search revealed a total of 1753 unique articles via MEDLINE, Embase, and IEEE Xplore databases (Fig. 2). Upon abstract and title screening, 1715 articles were excluded. Eighteen additional articles were excluded following full-text review, leaving 25 eligible articles for inclusion [[19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43]]. An additional 12 articles were retrieved via manual searching of references and included in the scoping review of mathematical optimization [[44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55]].
Figure 2.
PRISMA flow diagram of the search strategy.
Study characteristics
Across the 20 included studies reporting results of AI models, 16 developed predictive models for LOS, 3 for DOS, and 2 for 90-day hospital readmission (Table 2). Models were built using various data sources, ranging from single institutional data sets to national (ie, the New York State Department of Health’s Statewide Planning and Research Cooperative System [SPARCS], National Inpatient Sample [NIS], and Orthopedic Minimal Data Set Episode of Care [OrthoMiDaS OME] databases) and multinational databases (ie, the American College of Surgeons National Surgical Quality Improvement Program [NSQIP] database), with data set sizes ranging from 525 to 424,443 patients (Table 2). Despite the variability in data sets, there was significant similarity in the features used to generate the models. The 10 most frequently utilized input features for model development were age (100%), gender/sex (100%), body mass index/obesity (70%), ethnicity/race (60%), diabetes (55%), hypertension (50%), anaesthetic type (45%), smoking status (45%), cardiovascular disease (45%), and American Society of Anesthesiologists score (45%) (Supplementary Table S1). Fifteen different types of ML algorithms were developed (Supplementary Table S2). These included Bayesian, K-nearest neighbor, support vector machine, stochastic gradient descent, random forest classifier, decision tree, gradient boosted decision tree, XGBoost, AdaBoost, CatBoost, RUSBoost, ridge regression, lasso regression, elastic net regression, and artificial neural network (ANN) models. Ten of the 20 studies (50.0%) compared the developed ML models to control models, including mean regressor, multivariate linear regression, and logistic regression models (Supplementary Table S2).
Table 2.
Characteristics of the included predictive modeling studies.
| Study, year | Country | Procedure | Total patients | Data source | Outcome(s) of interest | Number of input features |
|---|---|---|---|---|---|---|
| Navarro et al., 2018 | USA, UK | TKA | 141,446 | SPARCS | LOS | 8 |
| Ramkumar et al., 2019 | USA, UK | TKA | 170,766 | NIS, OrthoMiDaS OME | LOS | 15 |
| Ramkumar et al., 2019 | USA, UK | THA | 79,226 | NIS, OrthoMiDaS OME | LOS | 15 |
| Ramkumar et al., 2019 | USA, UK | THA | 122,334 | SPARCS | LOS | 8 |
| Lee et al., 2019 | USA | Both | 525 | Local (USA) | 90-d readmission | 33 |
| Gabriel et al., 2019 | USA | THA | 960 | Local (USA) | LOS | 9 |
| Wei et al., 2021 | USA | TKA | 25,115 | NSQIP | LOS | 11 |
| Han et al., 2021 | China | TKA | 1298 | Local (China) | LOS | 27 |
| Kugelman et al., 2021 | USA | THA | 1409 | Local (USA) | LOS | 15 |
| Yeo et al., 2022 | USA | TKA | 10,021 | Local (USA) | DOS | 15 |
| Klemt et al., 2022 | USA | rTKA | 2588 | Local (USA) | LOS | 26 |
| Lopez et al., 2022 | USA | Both | 424,443 | NSQIP | LOS | 20 |
| Abbas et al., 2022 | Canada | TKA | 302,300 | NSQIP | LOS, DOS | 29 |
| Motesharei et al., 2022 | France | TKA | 1061 | Local (USA) | DOS | 17 |
| Zalikha et al., 2022 | USA | TKA | 305,577 | NIS | LOS | 15 |
| Johannesdottir et al., 2022 | Denmark | Both | 9512 | Local (Denmark) | LOS | 22 |
| Klemt et al., 2022 | USA | TKA | 10,021 | Local (USA) | 90-d readmission | 24 |
| Li et al., 2022 | China | TKA | 1590 | Local (Singapore) | LOS | 14 |
| Kugelman et al., 2022 | USA | TKA | 899 | Local (USA) | LOS | 15 |
| Trunfio et al., 2022 | Italy | THA | 2515 | Local (Italy) | LOS | 15 |
NIS, National Inpatient Sample; NSQIP, National Surgical Quality Improvement Program; OrthoMiDaS OME, Orthopedic Minimal Data Set Episode of Care; rTKA, revision total knee arthroplasty; SPARCS, New York State Department of Health’s Statewide Planning and Research Cooperative System.
Length of stay
Six studies developed predictive models for THA LOS, 9 for TKA LOS, and 1 for revision TKA LOS [[19], [20], [21],[24], [25], [26], [27], [28],[31], [32], [33],[35], [36], [37],56]. One study generated a combined model for TKA and THA LOS [38]. The average number of input features was 16.7 (range: 8 to 29) (Table 3). Among those studies reporting AUC values, model performance ranged from 0.64 to 0.87. The best-performing model for THA LOS prediction was the Bayesian model generated by Ramkumar et al. using 8 input features, with an AUC of 0.87 [22]. For TKA LOS, the ANN developed by Ramkumar et al. using 15 input features yielded the highest reported AUC of 0.83 [20]. The ANN developed by Klemt et al. using 26 input features for the prediction of revision TKA LOS had an AUC of 0.87, outperforming the support vector machine and elastic net regression models developed with the same data [31]. Within no single study did a control model outperform its equivalent ML model in the prediction of LOS.
Table 3.
Metrics describing the performance of the best-performing machine learning and control algorithms of the included studies.
| Stud, year | Procedure | Outcome | Training size | Validation size | Testing size | Best control algorithm | Control metric, value | Best ML algorithm | ML metric, value |
|---|---|---|---|---|---|---|---|---|---|
| Navarro et al., 2018 | TKA | LOS | 106,085 | - | 35,361 | - | - | Bayesian | AUC, 0.78 |
| Ramkumar et al., 2019 | TKA | LOS | 150,074 | 16,675 | 4017 | - | - | ANN | AUC, 0.83 |
| Ramkumar et al., 2019 | THA | LOS | 68,810 | 7645 | 2771 | - | - | ANN | AUC, 0.80 |
| Ramkumar et al., 2019 | THA | LOS | 91,751 | - | 30,583 | - | - | Bayesian | AUC, 0.87 |
| Lee et al., 2019 | Both | 90-d readmission | 473 | 52 | - | Logistic regression | Accuracy, 0.93 | RUSBoost | Accuracy, 0.87 |
| Gabriel et al., 2019 | THA | LOS | 644 | 316 | - | Logistic regression | AUC, 0.75 | Ridge regression | AUC, 0.76 |
| Wei et al., 2021 | TKA | LOS | 15,069 | - | 10,046 | Logistic regression | AUC, 0.80 | ANN | AUC, 0.80 |
| Han et al., 2021 | TKA | LOS | 1038 | 260 | - | Logistic regression | AUC, 0.70 | RFC | AUC, 0.77 |
| Kugelman et al., 2021 | THA | LOS | 902 | 225 | 282 | - | - | XGBoost | AUC, 0.82 |
| Yeo et al., 2022 | TKA | DOS | 6413 | 1603 | 2005 | - | - | ANN | AUC, 0.82 |
| Klemt et al., 2022 | rTKA | LOS | 1656 | 414 | 518 | - | - | ANN | AUC, 0.87 |
| Lopez et al., 2022 | TKA | LOS | 216,960 | - | 54,420 | - | - | ANN | AUC, 0.80 |
| THA | LOS | 122,442 | - | 30,611 | - | - | ANN | AUC, 0.81 | |
| Abbas et al., 2022 | TKA | DOS | 182,000 | 57,841 | 62,459 | Linear regression | MSE, 0.99 | ANN | MSE, 0.89 |
| TKA | LOS | 182,000 | 57,841 | 62,459 | Linear regression | MSE, 0.79 | ANN | MSE, 0.69 | |
| Motesharei et al., 2022 | TKA | DOS | 708 | 177 | 176 | Linear regression | R2, 0.71 | CatBoost | R2, 0.76 |
| Zalikha et al., 2022 | TKA | LOS | 195,556 | 48,906 | 61,115 | - | - | SVM | AUC, 0.68 |
| Johannesdottir et al., 2022 | Both | LOS | 8561 | 951 | - | Logistic regression | AUC, 0.70 | RFC | AUC, 0.71 |
| Klemt et al., 2022 | TKA | 90-day readmission | 6413 | 1603 | 2005 | Logistic regression | - | ANN | AUC, 0.85 |
| Li et al., 2022 | TKA | LOS | - | - | - | Logistic regression | AUC, 0.64 | XGBoost | AUC, 0.74 |
| Kugelman et al., 2022 | TKA | LOS | 575 | 144 | 180 | - | - | XGBoost | AUC, 0.69 |
| Trunfio et al., 2022 | THA | LOS | 2012 | - | 503 | Linear regression | RMSE, 3.84 | GBDT | RMSE, 3.84 |
GBDT, gradient-boosted decision tree; rTKA, revision total hip arthroplasty; R2, coefficient of determination; RFC, random forest classifier; RMSE, root mean square error; SVM, support vector machines.
Among studies that generated ANNs and conventional ML models, only 1 found a conventional ML model to outperform its neural network counterpart: The random forest classifier model generated by Han et al. using 27 input features for the prediction of LOS showed significantly superior performance to their ANN (Table 3) [26]. Using a decision curve, this model was also found to yield superior clinical usefulness when compared to the ANN model [45]. All other studies demonstrated ANN models performed superior to or at the same level as their conventional ML counterparts (including k-nearest neighbor, support vector machine, stochastic gradient descent, random forest classifier, XGBoost, etc.) [25,29,33,35].
Duration of surgery
Three studies developed ML models for the prediction of DOS, all of which were for TKA [30,33,34]. The CatBoost model of Motesharei et al. demonstrated an R2 value of 0.76, outperforming their control multivariate linear regression model with an R2 value of 0.71, as well as the random forest classifier and gradient-boosted decision tree models generated in this study (Table 3) [34]. Similarly, the ANN developed by Abbas et al. demonstrated a MSE of 0.89, outperforming their control multivariate linear regression model with an MSE of 0.99, as well as every other ML model (Bayesian, K-nearest neighbor, stochastic gradient descent, random forest classifier, decision tree, XGBoost, AdaBoost, and elastic net regression) [33]. Finally, with an AUC value of 0.82, the ANN model of Yeo et al. yielded the best performance when compared to their k-nearest neighbor and random forest classifier models [30].
Hospital readmission
Two studies developed ML models for the prediction of hospital readmissions [23,29]. In addition to a control logistic regression model, Klemt et al. developed K-nearest neighbor, support vector machine, elastic net regression, and ANN models for the prediction of 90-day readmissions following TKA using 24 input variables (Supplementary Table S2) [29]. The ANN was the best-performing model, with an associated AUC of 0.85 (Table 3). Using 33 input variables, Lee et al. developed a RUSBoost model for the prediction of 90-day readmissions following both TKA and THA [23]. This model demonstrated an accuracy of 87%, compared to an accuracy of 93% produced by the control logistic regression model. The recall rate of this control model, however, was significantly lower than that of the ML model, thus resulting in the ML model yielding an overall more reliable output.
Optimization
There has been a wide variety of optimization goals for the scheduling problems ranging from maximizing overall OR utilization to optimizing all processes of OR, recovery, and ward bed utilization (Table 4). Few studies have been conducted specifically targeting schedule optimization at the tactical level [39,43,46,49]. Adan et al. and Cardoen et al. utilized mixed integer programming to generate an optimized assignment of weekly OR schedules to maximize hospital bed capacity [39,46]. Adan et al. also used stochastic LOS times from a distribution of surgical groupings to improve the model by accounting for random variation [46].
Table 4.
Characteristics and summary findings of the included optimization studies.
| Study | Decision level | Schedule optimization strategy | Optimization goal | Main findings |
|---|---|---|---|---|
| Denton et al., 2007 | Operational level | Stochastic, heuristics | Minimize cost | Heuristic to sequence surgeons in order of increasing DOS variance and use of stochastic modelling to hedge against uncertain DOS times improves OR utilization. |
| Hans et al., 2008 | Tactical level Operational level |
Heuristics, Monte Carlo simulation | Minimize overtime | Clustering surgeries with a similar DOS and variability leads to reduced overtime and slack compared to base surgical plans generated by specialists. |
| Adan et al., 2009 | Tactical level | MIP, stochastic | Minimize OR, ICU and ward bed overutilization and underutilization | Using MIP, can generate improved master surgical schedules by considering a stochastic LOS. |
| Lamiri et al., 2009 | Operational level | Monte Carlo simulation, MIP, multiple heuristics | Minimize cost and overtime | Compared multiple optimization techniques. Combination of Monte Carlo simulation and MIP performed best and with least data. |
| Fei et al., 2009 | Tactical level | Heuristics | Maximize OR utilization, minimize cost | Using a column-generation-based heuristic, cases are assigned to optimized ORs for the week, using an open scheduling strategy. |
| Cardoen et al., 2009 | Tactical level Operational level |
MIP | Maximize bed utilization | To determine the amount of OR time assigned to surgeons for outpatient surgery. |
| Marques et al., 2012 | Operational level | Integer linear programming | Maximize OR utilization | Improvement in total OR utilization with reduction in length of surgical wait lists. |
| Lehtonen et al., 2013 | Operational level | Discrete-event simulation | Maximize OR utilization | Improved DOS categorization and higher levels of schedule granularity (30 min vs 60 min) improve utilization. |
| M’Hallah et al., 2014 | Operational level | Discrete-event simulation | Maximize OR utilization, minimize overtime | Cases grouped by mean DOS and OR utilization simulated. Recommends transfer of the last case in a busy room to a free one, group patient waitlists, and reduce workload by 10% or cancel last cases if planned overtime in schedule. |
| Van Huele et al., 2014 | Tactical level Operational level |
MIP | Minimize overtime | Evaluated the effect of certain surgeon constraints (surgeon availability, number of OR days/week, and consecutive surgeon hours and days) on performance of elective OR schedule. |
| Astaraky et al., 2015 | Operational level | Heuristics, stochastic, Markov decision process | Minimize patient wait, overtime, and ward capacity | Improved surgical planning using combined model with stochastics over heuristics alone. Provides different schedules depending on hospital resource availability. |
| Baesler et al., 2015 | Operational level | Heuristics, discrete-event simulation | Minimize total OR time | Account for surgery-grouping-specific preoperative, postoperative, setup, and recovery times. Combined heuristics with simulation to search for an optimal schedule. |
| Silva et al., 2015 | Operational level | Heuristics, integer linear programming | Maximize OR utilization | Assign surgeries to maximize the OR utilization while matching surgeries to anaesthetist skills. |
| Wang et al., 2015 | Operational level | Heuristics | Optimize number of ORs and PACU beds | Schedule patient surgeries based on priority where fixed resources are limited. But optimizes ORs and surgery allocation if flexibile. |
| Guido et al., 2017 | Tactical level | Heuristics | Maximize number of surgeries | Assigns available OR time to surgeons while considering hospital objectives, surgery characteristics. |
| Zhang et al., 2019 | Operational level | Stochastic, Markov decision process | Minimize cost | Combined Markov decision process and stochastic optimization lowered cost, shortened wait time, and improved OR and recovery bed utilization compared to stochastic optimization alone. |
| Bai et al., 2022 | Operational level | Heuristic | Minimize OR idle time | Model can reduce total OR time while meeting resource (personnel and hospital) constraints. Connected stages of preop, OR, and recovery optimization. |
ICU, intensive care unit; MIP, mixed integer programming; PACU, postoperative anesthesia care unit.
Compared to using heuristic, or rules-based, optimization strategies alone, accounting for uncertain DOS times with the combination of stochastics rather than using an average time was found to significantly improve model performance [42,44]. However, other strategies such as tight clustering of surgeries with similar DOS have also been proven to be effective when combined with heuristic schedule optimization [40,45,48]. Patient and provider characteristics can also be considered in optimization problems. Silva et al. optimized OR utilization accounting for anaesthetist skill, while Wang et al. optimized scheduling while accounting for patient priority level [52,53].
Using discrete-event simulation, Lehtonen et al. identified that using a schedule with higher granularity improved OR utilization [40]. Similarly, Baesler et al. used discrete-event simulation to also account for preoperative and postoperative times in addition to DOS to optimize surgical scheduling [51]. Other simulation strategies such as Monte Carlo simulation and Markov decision process have been used to simulate the impact of different optimization strategies and determine the ideal number of required hospital resources from historical data prior to the implementation of change (Table 4).
Discussion
Given the rapid projected rise in costs associated with TJA, there exists an imminent need for the development and implementation of novel strategies aimed at improving hospital efficiency and optimizing resource utilization. Generally, the findings of this study support the use of ML for prediction modelling and surgical optimization in TJA. In the prediction of LOS, the ML models evaluated in this study performed superior to or at the same level as matched control models. With only 3 studies generating ML models for the prediction of DOS and 2 for hospital readmissions, further research is required to assess the performance of ML models for the prediction of these outcomes in particular. Preliminary models, however, show promising outputs and encourage further investigation. As for surgical scheduling, a majority of optimization research for surgical scheduling has been targeted at the operational level. This is likely due to the complex and multifactorial nature of the problem, presenting as an ideal target for optimization compared to the distribution of yearly or weekly OR time among specialties. Various optimization strategies have been utilized to improve the efficiency of surgical scheduling, all of which improved the outcome of interest compared to traditional manual scheduling practices.
Potential clinical uses of these algorithms include the automation of surgical scheduling and improved utilization of hospital resources. This may lead to a reduction in cost and increased patient throughput. Targeted education, resources, and monitoring can be provided to those identified to be at high risk of readmission following TJA to reduce readmission events. Custom-bundled hospital compensation based on patient-specific predicted resource requirements may also be developed using more accurate models.
There exist countless variations of ML models that may be generated for the prediction of TJA LOS, DOS, and hospital readmissions. To avoid research waste, algorithms standing out above others must be identified, refined, validated, and ultimately implemented in clinical practice [57]. As a part of the fine-tuning process, input features may be adjusted to improve model performance, with more heavily weighted features in top-performing models being retained in future model iterations. In addition to patient factors, institutional factors, including surgeon, teaching status, geographic region, and location, as well as features derived from medical imaging may also be considered where sufficiently sized data sets are available. When considering the use of DL models vs ML models, the unique advantages and disadvantages of both must be considered. It is recognized that compared to conventional ML models, neural networks have the potential to yield superior results due to their ability to perform more sophisticated transformations of data [14,58]. For this to hold true, ANNs must be built using expansive data sets, requiring considerable investments in time and computational resources [59,60]. Another potential barrier to the utilization of ANNs is that they are less interpretable than typical ML models [61]. This is important as methods to evaluate these models must be developed to ensure bias (eg, based on certain patient characteristics) does not affect access to care if these models are to eventually be used to inform surgical scheduling.
All attempts to optimize scheduling rely on using an average DOS or LOS. Assumptions of DOS have a significant impact on OR underutilization and overtime [62,63]. Despite strategies to account for this by using stochastic methods of randomly sampling from a historical distribution, this is still a major limitation to the current attempts at optimization. A potentially effective strategy to improve scheduling may be to combine patient-specific ML predictions of DOS and downstream resource requirements, by predicting LOS, with optimization research.
A wide range of optimization goals were encountered in the literature depending on the generated mathematical problem. Most models were generated to optimize total OR utilization and minimize overtime and/or idle time, which may be the best metric when trying to maximize the utility of a finite resource. However, one ideal metric cannot be defined, as the solution to each OR optimization problem depends on the specific goals of the institution. Some hospitals may have incentive to maximize the throughput of cases, reducing the size of surgical waitlists. Without appropriate constraints, this could bias the optimization models to select cases with a shorter predicted DOS and impact care for patients with more complex needs. Most research in OR optimization has focused on a daily planning horizon. This focused the problem on direct patient care and not at decisions made involving stakeholders from multiple different specialties. However, optimizing OR utilization at a higher level, based on overall hospital resources and demand, may be important and reveal greater room for improvement.
This present review is not without limitations. The quality of the included studies was not formally assessed due to the lack of a standardized risk-of-bias assessment tool for studies generating ML-based predictive models; however, this tool is currently under development [64]. This can be attributed to the relatively recent rise in popularity of ML in predictive analytics within medicine, resulting in a paucity of defined criteria describing best practices in model generation and evaluation. For example, it remains unclear as to what performance metrics to report and the minimum required sample size to train a robust model [65]. Despite there being no formal assessment of the quality of the included studies, many gross shortcomings are observed. First, the details of the model-generation process, most notably, validation size and/or method, were not described for 12 of the presented ML models. This calls into question the veracity of the results described in these studies [66]. Furthermore, only 2 of the 22 generated models externally validated their models on test sets from sources unique to those used during training and validation. Beyond the limitations associated with the quality of the included studies, an additional shortcoming of this present review lies in the lack of quantitative comparison of the predictive abilities of ML models vs control models. This is due to the variable use of metrics describing the predictive abilities of models generated across studies. Many studies reported AUC; however, this is a classification metric based on varying arbitrary cutoffs (eg, LOS of <2 days or ≥2 days vs <3 days or ≥3 days), which should be avoided for continuous outcomes [67]. Finally, synthesizing optimization research to improve elective surgical scheduling is a challenge as unique solutions may be required to address different goals, institutional constraints, and data sets. As such, optimization results can only be compared to local historical performance or within a limited context.
Conclusions
With TJA costs expected to rise rapidly in association with an increasing demand, there exists an imminent need for the development and implementation of novel strategies aimed at improving hospital efficiency and optimizing resource utilization. With increasing access to big data in addition to technological advances in AI reaching new heights, applications of ML within medicine are becoming increasingly feasible and gaining notable popularity. High-performing ML models have been developed for predictive analytics in TJA, as have mathematical strategies for surgical scheduling optimization. While there remains work to be done in refining these tools, there exists considerable opportunities for improved efficiency in resource utilization surrounding TJA, especially when considering the combined utilization of predictive modelling with optimization strategies.
Conflicts of interest
Dr. J. I. Wolfstadt is in the speakers' bureau of or gave paid presentations for Depuy-Synthes, is a paid consultant for Microport Orthopaedics, is the American Association of Hip and Knee Surgeons Young Arthroplasty Group Vice Chair, a Canadian Arthroplast Society Education Committee member, and is a Canadian Orthopaedic Association Standards Committee member. Dr. J. R. Lex serves on the Resident Advisory Board for PrecisionOS Technologies. The other authors declare no potential conflicts of interest.
For full disclosure statements refer to https://doi.org/10.1016/j.artd.2023.101116.
Consent to publish
All authors have read the final manuscript and consent to publication.
Author contributions
Bahar Entezari: Methodology, Writing–original draft, Writing–review & editing, Visualization. Johnathan R. Lex: Conceptualization, Methodology, Writing–original draft, Writing–review & editing. Robert Koucheki: Methodology, Data curation, Writing–review & editing, Visualization. Aazad Abbas: Methodology, Writing–Review & Editing. Jay Toor: Supervision, Writing–review and editing. Jesse I. Wolfstadt: Supervision, Writing–review and editing. Bheeshma Ravi: Supervising, Writing–review & editing. Cari Whyne: Conceptualization, Supervision, Writing–review & editing.
Data availability
The data collected were from publicly available journal articles.
Appendix A. Supplementary Data
Appendix
Supplementary Table S1.
Fifteen most frequently used input features included among predictive modeling studies.
| Study, year | Input features |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Gender/sex | BMI/obesity | Ethnicity/race | Diabetes | HTN | Anesthetic | Smoking | CVD | ASA score | CCI | Hb/HCT/anemia | Pulmonary disease/COPD | Neoplastic disease | CKD/dialysis | |
| Navarro, 2018 | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Ramkumar, 2019 | ✓ | ✓ | ✓ | ||||||||||||
| Ramkumar, 2019 | ✓ | ✓ | ✓ | ||||||||||||
| Ramkumar, 2019 | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Lee, 2019 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Gabriel 2019 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Wei, 2021 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Han, 2021 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Kugelman, 2021 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Yeo, 2022 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Klemt, 2022 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Lopez, 2022 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Abbas, 2022 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Motesharei, 2022 | ✓ | ✓ | |||||||||||||
| Zalikha, 2022 | ✓ | ✓ | ✓ | ||||||||||||
| Johannesdottir, 2022 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Klemt, 2022 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Li, 2022 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Kugelman, 2022 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Trunfio, 2022 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Total Number of Studies | 20 | 20 | 14 | 12 | 11 | 10 | 9 | 9 | 9 | 9 | 8 | 8 | 8 | 8 | 7 |
ASA, American Society of Anesthesiologists; BMI, body mass index; CCI, Charlson Comorbidity Index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; Hb, hemoglobin; HCT, hematocrit; HTN, hypertension.
Supplementary Table S2.
Control and machine learning algorithms generated among predictive modeling studies.
| Study, year | Control algorithms |
ML algorithms |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean regressor | Linear reg | Logistic reg | Bayesian | KNN | SVM | SDG | RFC | DT | GBDT | XGB | ADB | CB | RUSBt | Ridge reg | Lasso reg | Elastic net reg | ANN | |
| Navarro, 2018 | ✓ | |||||||||||||||||
| Ramkumar, 2019 | ✓ | |||||||||||||||||
| Ramkumar, 2019 | ✓ | |||||||||||||||||
| Ramkumar, 2019 | ✓ | |||||||||||||||||
| Lee, 2019 | ✓ | ✓ | ✓ | |||||||||||||||
| Gabriel 2019 | ✓ | ✓ | ✓ | ✓ | ||||||||||||||
| Wei, 2021 | ✓ | ✓ | ||||||||||||||||
| Han, 2021 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Kugelman, 2021 | ✓ | ✓ | ✓ | ✓ | ||||||||||||||
| Yeo, 2022 | ✓ | ✓ | ✓ | |||||||||||||||
| Klemt, 2022 | ✓ | ✓ | ✓ | |||||||||||||||
| Lopez, 2022 | ✓ | |||||||||||||||||
| Abbas, 2022 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Motesharei, 2022 | ✓ | ✓ | ✓ | ✓ | ||||||||||||||
| Zalikha, 2022 | ✓ | ✓ | ✓ | ✓ | ||||||||||||||
| Johannesdottir, 2022 | ✓ | ✓ | ✓ | ✓ | ||||||||||||||
| Klemt, 2022 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||
| Li, 2022 | ✓ | ✓ | ||||||||||||||||
| Kugelman, 2022 | ✓ | ✓ | ✓ | ✓ | ||||||||||||||
| Trunfio, 2022 | ✓ | ✓ | ✓ | ✓ | ||||||||||||||
ADB, AdaBoost; BMI, body mass index; CB, CatBoost; DT, decision tree; GBDT, gradient boosted decision tree; KNN, k-nearest neighbor; RFC, random forest classifier; reg, regression; RUSB, RUSBoost SDG, stochastic gradient descent; SVM, support vector machine; XGB, XGBoost.
References
- 1.HCUP Fast Stats . Agency for Healthcare Research and Quality; Rockville (MD): 2021. Healthcare cost and utilization project (HCUP) [PubMed] [Google Scholar]
- 2.Kremers H.M., Larson D.R., Crowson C.S., Kremers W.K., Washington R.E., Steiner C.A., et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2014;97:1386–1397. doi: 10.2106/JBJS.N.01141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh J.A., Yu S., Chen L., Cleveland J.D., Singh J.A., Yu S., et al. Rates of total joint replacement in the United States: future projections to 2020 − 2040 using the national inpatient sample. J Rheumatol. 2020;46:1134–1140. doi: 10.3899/jrheum.170990. [DOI] [PubMed] [Google Scholar]
- 4.Inacio M.C.S., Paxton E.W., Graves S.E., Namba R.S., Nemes S. Projected increase in total knee arthroplasty in the United States – an alternative projection model. Osteoarthritis Cartilage. 2017;25:1797–1803. doi: 10.1016/j.joca.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 5.Klug A., Gramlich Y., Rudert M., Drees P., Hoffmann R., Weißenberger M., et al. The projected volume of primary and revision total knee arthroplasty will place an immense burden on future health care systems over the next 30 years. Knee Surg Sports Traumatol Arthrosc. 2021;29:3287–3298. doi: 10.1007/s00167-020-06154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sloan M., Premkumar A., Sheth N.P. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am. 2018;100:1455–1460. doi: 10.2106/JBJS.17.01617. [DOI] [PubMed] [Google Scholar]
- 7.Weeks W.B., Schoellkopf W.J., Ballard D.J., Kaplan G.S., James B., Weinstein J.N. Episode-of-Care characteristics and costs for hip and knee replacement surgery in hospitals belonging to the high value healthcare collaborative compared with similar hospitals in the same health care markets. Med Care. 2017;55:583–589. doi: 10.1097/MLR.0000000000000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.BlueCross, BlueShield . A study of cost variations for knee and hip replacement surgeries in the U.S. 2015. https://www.bcbs.com/the-health-of-america/reports/study-of-cost-variations-knee-and-hip-replacement-surgeries-the-us [accessed 20.11.22] [Google Scholar]
- 9.Batailler C., Shatrov J., Sappey-Marinier E., Servien E., Parratte S., Lustig S. Artificial intelligence in knee arthroplasty: current concept of the available clinical applications. Arthroplasty. 2022;4:17. doi: 10.1186/s42836-022-00119-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang C.C. Explainable artificial intelligence for predictive modeling in healthcare. J Healthc Inform Res. 2022;6:228–239. doi: 10.1007/s41666-022-00114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J.H., Asch S.M. Machine learning and prediction in medicine — beyond the peak of inflated expectations. N Engl J Med. 2017;376:2507–2509. doi: 10.1056/NEJMp1702071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gangal A., Kumar P., Kumari S., Saini A. Prediction models for healthcare using machine learning. IGI Global; Hershey, PA: 2021. pp. 70–91. [Google Scholar]
- 13.Erickson B.J. Basic artificial intelligence techniques: machine learning and deep learning. Radiol Clin North Am. 2021;59:933–940. doi: 10.1016/j.rcl.2021.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Choi R.Y., Coyner A.S., Kalpathy-Cramer J., Chiang M.F., Peter Campbell J. Introduction to machine learning, neural networks, and deep learning. Transl Vis Sci Technol. 2020;9:1–12. doi: 10.1167/tvst.9.2.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bini S.A. Artificial intelligence, machine learning, deep learning, and cognitive computing: what do these terms mean and how will they impact health care? J Arthroplasty. 2018;33:2358–2361. doi: 10.1016/j.arth.2018.02.067. [DOI] [PubMed] [Google Scholar]
- 16.Greener J.G., Kandathil S.M., Moffat L., Jones D.T. A guide to machine learning for biologists. Nat Rev Mol Cell Biol. 2022;23:40–55. doi: 10.1038/s41580-021-00407-0. [DOI] [PubMed] [Google Scholar]
- 17.van Essen J.T., Hans E.W., Hurink J.L., Oversberg A. Minimizing the waiting time for emergency surgery. Oper Res Heal Care. 2012;1:34–44. doi: 10.1016/j.orhc.2012.05.002. [DOI] [Google Scholar]
- 18.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Navarro S.M., Wang E.Y., Haeberle H.S., Mont M.A., Krebs V.E., Patterson B.M., et al. Machine learning and primary total knee arthroplasty: patient forecasting for a patient-specific payment model. J Arthroplasty. 2018;33:3617–3623. doi: 10.1016/j.arth.2018.08.028. [DOI] [PubMed] [Google Scholar]
- 20.Ramkumar P.N., Karnuta J.M., Navarro S.M., Haeberle H.S., Scuderi G.R., Mont M.A., et al. Deep learning preoperatively predicts value metrics for primary total knee arthroplasty: development and validation of an artificial neural network model. J Arthroplasty. 2019;34:2220–2227.e1. doi: 10.1016/j.arth.2019.05.034. [DOI] [PubMed] [Google Scholar]
- 21.Ramkumar P.N., Karnuta J.M., Navarro S.M., Haeberle H.S., Iorio R., Mont M.A., et al. Preoperative prediction of value metrics and a patient-specific payment model for primary total hip arthroplasty: development and validation of a deep learning model. J Arthroplasty. 2019;34:2228–2234.e1. doi: 10.1016/j.arth.2019.04.055. [DOI] [PubMed] [Google Scholar]
- 22.Ramkumar P.N., Navarro S.M., Haeberle H.S., Karnuta J.M., Mont M.A., Iannotti J.P., et al. Development and validation of a machine learning algorithm after primary total hip arthroplasty: applications to length of stay and payment models. J Arthroplasty. 2019;34:632–637. doi: 10.1016/j.arth.2018.12.030. [DOI] [PubMed] [Google Scholar]
- 23.Lee H.K., Jin R., Feng Y., Bain P.A., Goffinet J., Baker C., et al. An analytical framework for TJR readmission prediction and cost-effective intervention. IEEE J Biomed Health Inform. 2019;23:1760–1772. doi: 10.1109/JBHI.2018.2859581. [DOI] [PubMed] [Google Scholar]
- 24.Gabriel R.A., Doan C.N., Jiang X., Vaida F. A predictive model for determining patients not requiring prolonged hospital length of stay after elective primary total hip. Arthroplasty. 2019;129:43–50. doi: 10.1213/ANE.0000000000003798. [DOI] [PubMed] [Google Scholar]
- 25.Wei C., Quan T., Wang K.Y., Gu A., Fassihi S.C., Kahlenberg C.A., et al. Artificial neural network prediction of same-day discharge following primary total knee arthroplasty based on preoperative and intraoperative variables. Bone Joint J. 2021;103-B:1358–1366. doi: 10.1302/0301-620X.103B8.BJJ-2020-1013.R2. [DOI] [PubMed] [Google Scholar]
- 26.Han C., Liu J., Wu Y., Chong Y., Chai X., Weng X. To predict the length of hospital stay after total knee arthroplasty in an orthopedic center in China: the use of machine learning algorithms. Front Surg. 2021;8:1–11. doi: 10.3389/fsurg.2021.606038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kugelman D., Huang S., Teo G., Doran M., Singh V., Buchalter D., et al. A novel machine learning predictive tool assessing outpatient or inpatient designation for medicare patients undergoing total knee arthroplasty. Arthroplast Today. 2022;13:120–124. doi: 10.1016/j.artd.2021.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kugelman D.N., Teo G., Huang S., Doran M.G., Singh V., Long W.J. A novel machine learning predictive tool assessing outpatient or inpatient designation for medicare patients undergoing total hip arthroplasty. Arthroplast Today. 2021;8:194–199. doi: 10.1016/j.artd.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klemt C., Tirumala V., Habibi Y., Buddhiraju A., Chen T.L.W., Kwon Y.M. The utilization of artificial neural networks for the prediction of 90-day unplanned readmissions following total knee arthroplasty. Arch Orthop Trauma Surg. 2022 doi: 10.1007/s00402-022-04566-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Yeo I., Klemt C., Melnic C.M., Pattavina M.H., De Oliveira B.M.C., Kwon Y.M. Predicting surgical operative time in primary total knee arthroplasty utilizing machine learning models. Arch Orthop Trauma Surg. 2022 doi: 10.1007/s00402-022-04588-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Klemt C., Tirumala V., Barghi A., Cohen-Levy W.B., Robinson M.G., Kwon Y.M. Artificial intelligence algorithms accurately predict prolonged length of stay following revision total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2022;30:2556–2564. doi: 10.1007/s00167-022-06894-8. [DOI] [PubMed] [Google Scholar]
- 32.Lopez C.D., Ding J., Trofa D.P., Cooper H.J., Geller J.A., Hickernell T.R. Machine learning model developed to aid in patient selection for outpatient total joint arthroplasty. Arthroplast Today. 2022;13:13–23. doi: 10.1016/j.artd.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abbas A., Mosseri J., Lex J.R., Toor J., Ravi B., Khalil E.B., et al. Machine learning using preoperative patient factors can predict duration of surgery and length of stay for total knee arthroplasty. Int J Med Inform. 2022;158:104670. doi: 10.1016/j.ijmedinf.2021.104670. [DOI] [PubMed] [Google Scholar]
- 34.Motesharei A., Batailler C., De Massari D., Vincent G., Chen A.F., Lustig S. Predicting robotic-assisted total knee arthroplasty operating time benefits of machine-learning and 3D patient-specific data. Bone Jt Open. 2022;3:383–389. doi: 10.1302/2633-1462.35.BJO-2022-0014.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zalikha A.K., El-Othmani M.M., Shah R.P. Predictive capacity of four machine learning models for in-hospital postoperative outcomes following total knee arthroplasty. J Orthop. 2022;31:22–28. doi: 10.1016/j.jor.2022.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trunfio T.A., Borrelli A., Improta G. Is it possible to predict the length of stay of patients undergoing hip-replacement surgery? Int J Environ Res Public Health. 2022;19:1–16. doi: 10.3390/ijerph19106219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li H., Jiao J., Zhang S., Tang H., Qu X., Yue B. Construction and comparison of predictive models for length of stay after total knee arthroplasty: regression model and machine learning analysis based on 1,826 cases in a single Singapore center. J Knee Surg. 2022;35:7–14. doi: 10.1055/s-0040-1710573. [DOI] [PubMed] [Google Scholar]
- 38.Johannesdottir K.B., Kehlet H., Petersen P.B., Aasvang E.K., Sørensen H.B.D., Jørgensen C.C. Machine learning classifiers do not improve prediction of hospitalization > 2 days after fast-track hip and knee arthroplasty compared with a classical statistical risk model. Acta Orthop. 2022;93:117–123. doi: 10.2340/17453674.2021.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cardoen B., Demeulemeester E., Beliën J. Sequencing surgical cases in a day-care environment: an exact branch-and-price approach. Comput Oper Res. 2009;36:2660–2669. doi: 10.1016/j.cor.2008.11.012. [DOI] [Google Scholar]
- 40.Lehtonen J.M., Torkki P., Peltokorpi A., Moilanen T. Increasing operating room productivity by duration categories and a newsvendor model. Int J Health Care Qual Assur. 2013;26:80–92. doi: 10.1108/09526861311297307. [DOI] [PubMed] [Google Scholar]
- 41.Van Huele C., Vanhoucke M. Analysis of the integration of the physician rostering problem and the surgery scheduling problem topical collection on systems-level quality improvement. J Med Syst. 2014;38:43. doi: 10.1007/s10916-014-0043-z. [DOI] [PubMed] [Google Scholar]
- 42.Astaraky D., Patrick J. A simulation based approximate dynamic programming approach to multi-class, multi-resource surgical scheduling. Eur J Oper Res. 2015;245:309–319. doi: 10.1016/j.ejor.2015.02.032. [DOI] [Google Scholar]
- 43.Guido R., Conforti D. A hybrid genetic approach for solving an integrated multi-objective operating room planning and scheduling problem. Comput Oper Res. 2017;87:270–282. doi: 10.1016/j.cor.2016.11.009. [DOI] [Google Scholar]
- 44.Denton B., Viapiano J., Vogl A. Optimization of surgery sequencing and scheduling decisions under uncertainty. Health Care Manag Sci. 2007;10:13–24. doi: 10.1007/s10729-006-9005-4. [DOI] [PubMed] [Google Scholar]
- 45.Hans E., Wullink G., van Houdenhoven M., Kazemier G. Robust surgery loading. Eur J Oper Res. 2008;185:1038–1050. doi: 10.1016/j.ejor.2006.08.022. [DOI] [Google Scholar]
- 46.Adan I., Bekkers J., Dellaert N., Vissers J., Yu X. Patient mix optimisation and stochastic resource requirements: a case study in cardiothoracic surgery planning. Health Care Manag Sci. 2009;12:129–141. doi: 10.1007/s10729-008-9080-9. [DOI] [PubMed] [Google Scholar]
- 47.Lamiri M., Grimaud F., Xie X. Optimization methods for a stochastic surgery planning problem. Int J Prod Econ. 2009;120:400–410. doi: 10.1016/j.ijpe.2008.11.021. [DOI] [Google Scholar]
- 48.M’Hallah R., Al-Roomi A.H. The planning and scheduling of operating rooms: a simulation approach. Comput Ind Eng. 2014;78:235–248. doi: 10.1016/j.cie.2014.07.022. [DOI] [Google Scholar]
- 49.Fei H., Chu C., Meskens N. Solving a tactical operating room planning problem by a column-generation-based heuristic procedure with four criteria. Ann Oper Res. 2009;166:91–108. doi: 10.1007/s10479-008-0413-3. [DOI] [Google Scholar]
- 50.Marques I., Captivo M.E., Pato M.V. An integer programming approach to elective surgery scheduling. OR Spectr. 2012;34:407–427. doi: 10.1007/s00291-011-0279-7. [DOI] [Google Scholar]
- 51.Baesler F., Gatica J., Correa R. Simulation optimisation for operating room scheduling. Int J Simul Model. 2015;14:215–226. doi: 10.2507/IJSIMM14(2)3.287. [DOI] [Google Scholar]
- 52.Silva T.A.O., De Souza M.C., Saldanha R.R., Burke E.K. Surgical scheduling with simultaneous employment of specialised human resources. Eur J Oper Res. 2015;245:719–730. doi: 10.1016/j.ejor.2015.04.008. [DOI] [Google Scholar]
- 53.Wang Y., Tang J., Pan Z., Yan C. Particle swarm optimization-based planning and scheduling for a laminar-flow operating room with downstream resources. Soft Comput. 2015;19:2913–2926. doi: 10.1007/s00500-014-1453-z. [DOI] [Google Scholar]
- 54.Zhang J., Dridi M., El Moudni A. A two-level optimization model for elective surgery scheduling with downstream capacity constraints. Eur J Oper Res. 2019;276:602–613. doi: 10.1016/j.ejor.2019.01.036. [DOI] [Google Scholar]
- 55.Bai X., Zhang W., Luo L., Ma H., Zhu T. Day surgery scheduling and optimization in large public hospitals in China: a three-station job shop scheduling problem. J Healthc Eng. 2022;2022:1149657. doi: 10.1155/2022/1149657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Helm J.M., Swiergosz A.M., Haeberle H.S., Karnuta J.M., Schaffer J.L., Krebs V.E., et al. Machine learning and artificial intelligence: definitions, applications, and future directions. Curr Rev Musculoskelet Med. 2020;13:69–76. doi: 10.1007/s12178-020-09600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andaur Navarro C.L., Damen J.A.A., Takada T., Nijman S.W.J., Dhiman P., Ma J., et al. Risk of bias in studies on prediction models developed using supervised machine learning techniques: systematic review. BMJ. 2021;375:n2281. doi: 10.1136/bmj.n2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olczak J., Pavlopoulos J., Prijs J., Ijpma F.F.A., Doornberg J.N., Lundström C., et al. Presenting artificial intelligence, deep learning, and machine learning studies to clinicians and healthcare stakeholders: an introductory reference with a guideline and a Clinical AI Research (CAIR) checklist proposal. Acta Orthop. 2021;92:513–525. doi: 10.1080/17453674.2021.1918389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sarker I.H. Deep learning: a comprehensive overview on techniques, taxonomy, applications and research directions. SN Comput Sci. 2021;2:420. doi: 10.1007/s42979-021-00815-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghassemi M., Naumann T., Schulam P., Beam A.L., Chen I.Y., Ranganath R. A review of challenges and opportunities in machine learning for health. AMIA Jt Summits Transl Sci Proc. 2020;2020:191–200. [PMC free article] [PubMed] [Google Scholar]
- 61.Meng C., Trinh L., Xu N., Enouen J., Liu Y. Interpretability and fairness evaluation of deep learning models on MIMIC-IV dataset. Sci Rep. 2022;12:7166. doi: 10.1038/s41598-022-11012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guda H., Dawande M., Janakiraman G., Jung K.S. Optimal policy for a stochastic scheduling problem with applications to surgical scheduling. Prod Oper Manag. 2016;25:1194–1202. doi: 10.1111/poms.12538. [DOI] [Google Scholar]
- 63.Ogulata S.N., Erol R. A hierarchical multiple criteria mathematical programming approach for scheduling general surgery operations in large hospitals. J Med Syst. 2003;27:259–270. doi: 10.1023/A:1022575412017. [DOI] [PubMed] [Google Scholar]
- 64.Collins G.S., Dhiman P., Andaur Navarro C.L., Ma J., Hooft L., Reitsma J.B., et al. Protocol for development of a reporting guideline (TRIPOD-AI) and risk of bias tool (PROBAST-AI) for diagnostic and prognostic prediction model studies based on artificial intelligence. BMJ Open. 2021;11:e048008. doi: 10.1136/bmjopen-2020-048008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steyerberg E.W. Overfitting and optimism in prediction models. 2009. pp. 83–100. [DOI] [Google Scholar]
- 66.Ho S.Y., Phua K., Wong L., Bin Goh W.W. Extensions of the external validation for checking learned model interpretability and generalizability. Patterns. 2020;1:100129. doi: 10.1016/j.patter.2020.100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Altman D.G., Royston P. The cost of dichotomising continuous variables. Br Med J. 2006;332:1080. doi: 10.1136/bmj.332.7549.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data collected were from publicly available journal articles.