Summary
Charcot-Marie-Tooth disease type 2A (CMT2A), the most common inherited peripheral axonal neuropathy, is associated with more than 100 dominant mutations, including R94Q as the most abundant mutation in the Mitofusin2 (MFN2) gene. CMT2A is characterized by progressive motor and sensory loss, color-vision defects, and progressive loss of visual acuity. We used a well-established transgenic mouse model of CMT2A with R94Q mutation on MFN2 gene (MFN2R94Q) to investigate the functional and morphological changes in retina. We documented extensive vision loss due to photoreceptor degeneration, retinal ganglion cell and their axonal loss, retinal secondary neuronal and synaptic alternation, and Müller cell gliosis in the retina of MFN2R94Q mice. Imbalanced MFN1/MFN2 ratio and dysregulated mitochondrial fusion/fission result in retinal degeneration via P62/LC3B-mediated mitophagy/autophagy in MFN2R94Q mice. Finally, transgenic MFN1 augmentation (MFN2R94Q:MFN1) rescued vision and retinal morphology to wild-type level via restoring homeostasis in mitochondrial MFN1/MFN2 ratio, fusion/fission cycle, and PINK1-dependent, Parkin-independent mitophagy.
Subject areas: Biological sciences, Neuroscience, Molecular neuroscience, Sensory neuroscience
Graphical abstract

Highlights
-
•
CMT2A is commonly associated with R94Q mutation on Mitofusin2 (MFN2) gene
-
•
MFN2R94Q mutation induces extensive vision loss due to retinal cell degeneration
-
•
Mitofusin1 augmentation restored MFN1/MFN2 ratio and PINK1-mediated mitophagy
-
•
MFN1 augmentation in MFN2R94Q mice rescued retinal morphology and preserved vision
Biological sciences; Neuroscience; Molecular neuroscience; Sensory neuroscience
Introduction
Charcot-Marie-Tooth (CMT) disease is one of the most common inherited peripheral neuropathies, affecting approximately 126,000 individuals in the United States and 2.6 million people worldwide.1,2 The characteristic clinical ophthalmic manifestations of CMT are optic neuropathy, impaired oculomotility, pupillary abnormalities, retinitis pigmentosa and premature presbyopia, and color-vision defects.3,4 Based on the autosomal dominant forms, this hereditary motor and sensory neuropathy is broadly classified into two subgroups, demyelinating (CMT1) and axonal (CMT2).5,6,7 However, while CMT2 is clinically and genetically heterogeneous, only CMT2A is directly associated with Mitofusin2 (MFN2) mutations.8,9,10 In particular, CMT2A is the most common form of axonal CMT.11 Among several other dominant mutations in MFN2 gene, R94Q is the most abundant mutation that manifests into CMT2A.9 Clinically, CMT2A is characterized by progressive muscle atrophy, sensory loss due to degeneration of long peripheral axons,12 including retinal neuropathies such as central scotoma, color-vision defects, and bilateral, symmetric progressive loss of visual acuity from optic atrophy (OPA).13,14
MFN1 and MFN2 are the two outer mitochondrial membrane fusion regulatory dynamin-related GTPases that play a pivotal role in mitochondrial dynamics, quality control, and distribution, as well as function.15,16,17,18 Despite sharing a high degree of structural motif homology, MFN1 and MFN2 are functionally heterogeneous.19,20,21 MFN1 is predominantly associated with mitochondrial fusion and interacts with OPA1 to regulate the inner mitochondrial membrane (IMM) pro-fusion.22,23,24 MFN2 facilitates inter-mitochondrial and mitochondria-endoplasmic reticulum (ER) tethering25 and interacts with mitochondrial trafficking machinery motors to mediate axonal transport of mitochondria26 in neurons.25,26 Furthermore, MFN2 is also known to have direct effects on the mitochondrial function.27
The intrinsic differences in the cooperativity of MFN1 and MFN2 with GTPases critically regulate mitochondrial fusion and play a key role in balancing the mitochondrial dynamics.24,28 The fine-tuned balance in MFN1/MFN2 ratio is required to maintain the steady-state cyclicity and homeostasis of mitochondrial fusion and fission.29,30 MFN2R94Q mutation results in dysregulated mitochondrial function, distribution, and dynamics.26,31
Retina is one of the most energy-demanding tissues in the body,32 which makes it exquisitely vulnerable to energy insufficiency as well as mitochondrial dysfunction. During and after visual information transduction, to support the exceedingly high energy demand of photoreceptors (PRs) and retinal ganglion cells (RGCs), high mitochondrial activity is required.32,33,34,35,36 Despite some clinical reports mentioning the possible association of optic atrophy with progressive loss of visual acuity and color-vision defects in CMT2A patients,2,8,13,14,37,38 insights on retinal pathological modifications are still lacking. A recent study demonstrated RGC atrophy, axonal degeneration, and impaired visual acuity in a transgenic mouse model of CMT2A disease (MFN2R94Q) and MFN1 augmentation (MFN2R94Q:MFN1)-mediated phenotypic rescue.31 However, a detailed investigation of retinal function and histopathological remodeling underlying vision impairment as well as MFN1 augmentation-mediated rescue is still lacking.
Transgenic mutant MFN2 (MFN2R94Q) mice were generated by introducing Flag-tagged human mutant MFN2 (MFN2R94Q) gene under the neuronal-specific Thy1.2 promoter, which is ubiquitously present in multiple retinal cells, including RGCs, PRs, bipolar cells, horizontal cells, and Müller glia.39 Hence, we hypothesized that, in addition to RGC atrophy and their axonal degeneration, R94Q mutation-mediated vision impairment may also be caused by imbalanced MFN1/MFN2 ratio and impaired mitochondrial fission-fusion in other retinal neurons including PRs, bipolar cells, and RGCs.
In this study, we investigated R94Q mutation-induced retinal functional and histopathological alterations and MFN1 augmentation-mediated rescue using the same mouse models (non-transgenic [nTg], MFN2WT, MFN2R94Q, and MFN2R94Q:MFN1 mice of C57BL/6J background) as used by Zhou et al.31 Interestingly, we found that imbalanced MFN1/MFN2 ratio and impaired mitochondrial fusion-fission lead to degeneration of PRs and bipolar cells as well as RGCs and their axons via P62/LC3B-mediated mitophagy/autophagy in MFN2R94Q mice. Importantly, MFN1 augmentation rescues vision and retinal morphology via restoring MFN1/MFN2 ratio and PINK1-dependent, Parkin-independent mitophagy.
Results
Presence and distribution of flag-tagged human MFN2R94Q and MFN1 in retinas of MFN2R94Q and MFN2R94Q:MFN1 mice
To examine the presence and distribution of Flag-tagged human MFN2R94Q and MFN1 transgene in the retinas of MFN2R94Q and MFN2R94Q:MFN1 mice, Flag immunostaining and immunoblotting were performed (Figures 1A–1D). Immunofluorescence staining confirmed the presence of Flag in the retina of both MFN2R94Q and MFN2R94Q:MFN1 mice (Figures 1A–1C). Flag-positive staining was detected specifically in the RGC layer and inner nuclear layer (INL) of MFN2R94Q (Figure 1B) and MFN2R94Q:MFN1 mice retina (Figure 1C). However, the expression of Flag in other retinal cells including PRs, Müller glia, amacrine cells, etc., cannot be ruled out as previous studies have shown the presence of Thy1.2 gene in all these retinal cell types.39 No Flag immunoreactivity was detected in these cell types may be because of its too low levels. Immunoblots further confirmed and demonstrated the presence of Flag in the retinas of 14-month-old MFN2R94Q and MFN2R94Q:MFN1 mice at the expected molecular weight (86 kDa), similar to corresponding brain samples, which were used as positive controls for validation (Figure 1D). In MFN2R94Q:MFN1 retina, two bands for Flag were detected (Figure 1D), which clearly demonstrates that both Flag-tagged human MFN2R94Q and MFN1 are expressed but run at slightly different sizes. However, two separate bands of Flag-tagged MFN2R94Q and MFN1 were not visible in MFN2R94Q:MFN1 brain sample may be because of separation issues (Figure S1).
Figure 1.
Presence and expression of Flag, MFN1, and MFN2 in retina of nTg, MFN2R94Q, and MFN2R94Q:MFN1 mice
(A–C) Immunofluorescence showing presence and expression of Flag in the inner nuclear layer (INL) and RGC layer of MFN2R94Q and MFN2R94Q:MFN1 mouse retina, but no Flag was present in non-transgenic (nTg) retina.
(D) Immunoblot of Flag in brain and retina of nTg, MFN2R94Q, and MFN2R94Q:MFN1 mice.
(E–G) Immunofluorescence showing localization and expression of MFN1 and MFN2 in the retinas of nTg, MFN2R94Q, and MFN2R94Q:MFN1 mice. Significantly increased MFN1 (green) and decreased MFN2 (red) were observed in MFN2R94Q:MFN1 mice, compared to MFN2R94Q mice, which demonstrated significantly decreased MFN1 and increased MFN2 in the retina.
(H) Representative immunoblots and corresponding densitometric analysis of MFN1 and MFN2 in the retinas of nTg, MFN2R94Q, and MFN2R94Q:MFN1 mice. Scale bar, 25 μm. IS, inner segment; INL, inner nuclear layer; ONL, outer nuclear layer; IPL, inner plexiform layer; OPL, outer plexiform layer; RGC, Retinal ganglion cell. Data represented as mean ± SEM (n = 3). One-way ANOVA followed by Tukey’s multiple comparisons test. Significance of difference fromnTg controls, ∗p ≤ 0.05, ∗∗∗p ≤ 0.001,∗∗∗∗p ≤ 0.0001; Significance of difference from MFN2R94Qmice, ##p ≤ 0.01, ###p ≤ 0.001, ####p ≤ 0.0001.
Distribution and expression of MFN1 and MFN2 in the retinas of MFN2R94Q and MFN2R94Q:MFN1 mice
To examine the alterations in mitochondrial MFN1/MFN2 ratio in the retina of MFN2R94Q and MFN2R94Q:MFN1 mice, the expression of MFN1 and MFN2 was evaluated (Figures 1E–1H). Immunofluorescence showed ubiquitous expression and distribution of both MFN1 and MFN2 in nTg wild-type retina, more pronounced in outer and inner plexiform layers (OPL and IPL) and RGC layer (Figure 1E). In MFN2R94Q retina, MFN1 expression was significantly reduced compared to nTg retina (Figures 1E and 1F and S2A), while MFN2 expression was significantly increased (Figures 1E and 1F). MFN2R94Q:MFN1 retina showed significantly increased expression of MFN1 and MFN2, more concentrated in RGC layer, compared to nTg (Figures 1E and 1G) and MFN2R94Q retinas (Figures 1F and 1G). Furthermore, in MFN2WT retina, the expression of MFN2 significantly increased in OPL, INL, IPL, and RGC layer compared to nTg controls (Figure S3A). Immunoblots and corresponding densitometric analysis also demonstrated significantly reduced expression of MFN1 in MFN2R94Q mice retina compared to MFN2R94Q:MFN1 (p ≤ 0.0001; n = 3; Figures 1H and S4) and nTg mice (p ≤ 0.05; n = 3; Figures 1H and S4) while the expression of MFN2 significantly increased in the retina of MFN2R94Q mice compared to MFN2R94Q:MFN1 (p ≤ 0.001; n = 3; Figures 1H and S4) and nTg mice (p ≤ 0.0001; n = 3; Figures 1H and S4). Contrarily, MFN2R94Q:MFN1 retina showed significantly increased expression of MFN1 (p ≤ 0.001; Figures 1E, 1G, and 1H and S4) and MFN2 (p ≤ 0.0001; Figures 1E, 1G, and 1H and S4) compared to nTg controls. However, in a recent study, no such differences in MFN1 levels between MFN2R94Q and nTg control as well as MFN2 levels between MFN2R94Q and MFN2R94Q:MFN1 mice were observed in the brain and spinal cord at 8 months of age.31
MFN1 augmentation rescued visual function
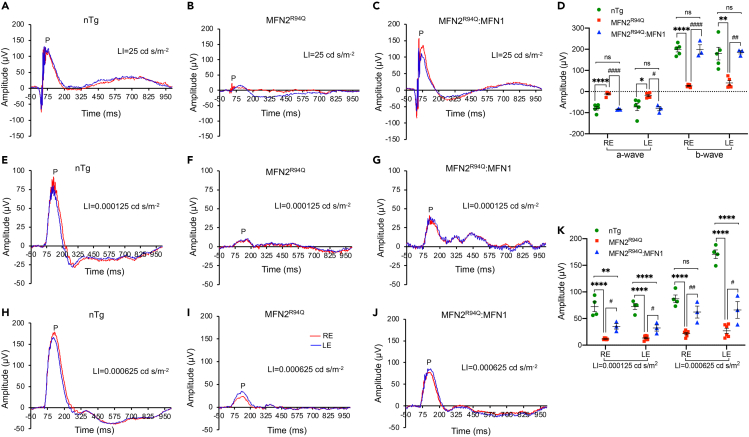
To assess the effects of R94Q mutation and MFN1 augmentation on retinal responses to light stimuli, electroretinography (ERG; Figures 2A–2D) and positive scotopic threshold response (pSTR; Figures 2E–2K) were performed in all the experimental mice. In MFN2R94Q mice, both a- and b-wave amplitudes were nearly not recordable (Figure 2B). In contrast, MFN2R94Q:MFN1 mice demonstrated significantly better ERG for both a- and b-waves than MFN2R94Q mice (Figures 2C and 2D) (Right eye: a- & b-wave: p ≤ 0.0001; Left eye: a-wave: p ≤ 0.05 & b-wave: p ≤ 0.01; n = 3–6, Figures 2A–2D). In fact, statistical analysis demonstrated that both a- and b-wave amplitudes in MFN2R94Q:MFN1 mice were similar to nTg controls. Furthermore, we also assessed pSTR to determine RGC function (Figures 2E–2K). Compared to MFN2R94Q, MFN2R94Q:MFN1 mice showed significantly rescued b-wave positive (P) amplitudes (light stimuli = 0.000125 cd s/m2, right & left eye: p ≤ 0.05, Figures 2F, 2G, and 2K; light stimuli = 0.000625 cd s/m2, right eye: p ≤ 0.01 & left eye: p ≤ 0.05; n = 3–6, Figures 2H–2J and K) that only reached about half of the pSTR values of nTg controls (light stimuli = 0.000125 cd s/m2, right eye: p ≤ 0.01 & left eye: p ≤ 0.0001; light stimuli = 0.000625 cd s/m2, left eye: p ≤ 0.0001; n = 3–6, Figure 2K).
Figure 2.
Visual function assessment and histological evaluation of nTg, MFN2R94Q, and MFN2R94Q:MFN1 mice retina
(A–C) Representative graphs of electroretinography (ERG) from nTg (A) and transgenic mice (B and C). MFN2R94Q mice showed nearly diminished ERG, while MFN2R94Q:MFN1 mice exhibited significantly higher ERG response compared to MFN2R94Q mice.
(D) Dot plots showing higher amplitudes of a- and b-waves from the retina of MFN2R94Q:MFN1 mice compared to MFN2R94Q mice.
(E–K) Retinal ganglion cell (RGC) function of nTg (E and H), MFN2R94Q (F and I), and MFN2R94Q:MFN1 (G and J) mice were evaluated at two light stimuli (0.000125 cd s/m−2 and 0.00625 cd s/m−2) by measuring positive scotopic threshold response (pSTR) of RGCs. We observed significantly increased positive (P) amplitudes in MFN2R94Q:MFN1 mice (almost half of the ERG values of nTg controls), compared to MFN2R94Q mice, which showed significantly decreased positive (P) amplitudes. (K) Dot plots showing average P amplitudes in nTg, MFN2R94Q, and MFN2R94Q:MFN1 mice retina. RE, right eye; LE, left eye; LI, light intensity. Data represented as mean ± SEM (n = 3–6). One-way ANOVA followed by Tukey’s multiple comparisons test. Significance of difference from nTg controls, ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗∗p ≤ 0.0001; Significance of difference from MFN2R94Qmice, #p ≤ 0.05, ##p ≤ 0.01, ####p ≤ 0.0001; ns: non-significant.
MFN1 augmentation rescued PRs from degeneration due to R94Q mutation
ERG demonstrated severely impaired visual function in MFN2R94Q mice. Next, retinal histologic changes were studied to correlate with visual function. Retinal sections were stained with cresyl violet to measure outer nuclear layer (ONL) thickness and number of PR (Figures 3A–3C). Comparison of ONL thickness and number of PR showed significantly reduced ONL thickness (Figure 3D) and PR counts (Figure 3E) in MFN2R94Q mice, compared to MFN2R94Q:MFN1 (Figures 3D and 3E). In addition, the OPL became narrower and had cells from INL according to cresyl violet staining. However, no difference in ONL thickness and PR counts was observed between MFN2R94Q:MFN1 and nTg mice, and the OPL was similar to nTg retina, indicating MFN1 augmentation rescued PRs from degeneration. In fact, the degeneration rate of PRs was slow in MFN2R94Q mice as there were still 8–9 layers of ONL remaining (Figure 3B) at 14 months of age. The ONL thickness did not correlate with almost diminished ERG, indicating that these remaining PRs were not functioning properly.
Figure 3.
Histological evaluation of nTg, MFN2R94Q, and MFN2R94Q:MFN1 mice retina
(A–C) Cresyl violet (CV)-staining revealed significantly decreased number of PRs and the thickness of the outer nuclear layer (ONL) in the retina of MFN2R94Q mice (7–10 layers), compared to nTg (14 layers) and MFN2R94Q:MFN1 (14 layers) mice.
(D) Representative dot plots showed significantly reduced ONL thickness in nasal, central, and temporal regions of MFN2R94Q mice retina compared to nTg and MFN2R94Q:MFN1 retina, while there was no difference between nTg and MFN2R94Q:MFN1.
(E) Representative dot plots showed significantly reduced number of PRs in nasal, central, and temporal regions of MFN2R94Q retina compared to nTg and MFN2R94Q:MFN1 retina. Scale bar, 50 μm. OS, outer segment; IS, inner segment; INL, inner nuclear layer; ONL, outer nuclear layer; IPL, inner plexiform layer; OPL, outer plexiform layer; RGC, Retinal ganglion cell. Data represented as mean ± SEM (n = 4–6). One-way ANOVA followed by Tukey’s multiple comparisons test. Significance of difference from nTg controls, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, ∗∗∗∗p ≤ 0.0001; Significance of difference from MFN2R94Qmice, ##p ≤ 0.01, ###p ≤ 0.001, ####p ≤ 0.0001; ns: non-significant.
To further investigate the effects of R94Q mutation-induced imbalance in MFN1/MFN2 ratio on PRs, immunofluorescence of recoverin (PR marker) was performed (Figures 4A–4C). Recoverin immunolabelling demonstrated reduced ONL thickness along with disorganized inner and outer segments (IS and OS) as well as diminished staining in the OPL (synapses between PRs and INL) in the retina of MFN2R94Q mice, which again may explain the diminished ERG response in these mice. MFN1 augmentation rescued ONL and segments to nTg levels. To evaluate the changes in rods and cones, retinal sections were stained with rod- (rhodopsin) and cone-specific (cone-arrestin) markers (Figures 4D–4F). In nTg retina, cone-arrestin staining revealed the whole profile of cone including cell body, organized IS and OS, long axon, and large pedicle in OPL (Figure 4D). Rod-specific antibody rhodopsin was confined to the rod outer segments (Figure 4D). However, abnormal cone morphology was seen in MFN2R94Q retina (Figures 4E and S2A). The number of cones was significantly reduced compared to nTg mice (nTg: 19.69 ± 0.237/100 μm; MFN2R94Q: 10.69 ± 0.263/100 μm; MFN2R94Q:MFN1: 19.24 ± 0.267/100 μm, p ≤ 0.0001, n = 8, Figure 4G), and axons were shorter with smaller cone pedicles (Figures 4C and S2A). There was no obvious change in rhodopsin staining among all the experimental mice. MFN1 augmentation rescued cones from degenerative changes; cone morphology and density/number were comparable to nTg retina (Figures 4D–4F and S2A). Furthermore, no changes were observed in the number and morphology of cones in MFN2WT mice retina compared to nTg controls (Figure S3B).
Figure 4.
MFN1 augmentation rescued photoreceptors in MFN2R94Q:MFN1 mice retina
(A–C) Recoverin immunostaining revealed disorganized and degenerative PRs with disorganized segments MFN2R94Q mice. In contrast, MFN2R94Q:MFN1 retina showed normal PRs with organized segments, similar to nTg retina.
(D–F) Double immunolabeling of cone-arrestin (green) and rhodopsin (red) revealed degenerative, short, and thin cones in the retina of MFN2R94Q mice while, contrarily, MFN2R94Q:MFN1 mice demonstrated MFN1 augmentation-induced preservation of cones with normal morphology, similar to nTg controls. There is no obvious change on rhodopsin staining.
(G) Representative dot plots showed significantly decreased number of cones in the retina of MFN2R94Q mice, while MFN1 augmentation rescued cones in MFN2R94Q:MFN1 mice.
(H) Representative immunoblots and corresponding densitometric analysis further confirmed significantly increased expression of cone-arrestin in the retina of MFN2R94Q:MFN1 mice (similar to nTg controls), compared to MFN2R94Q mice retina. Scale bar, 25 μm. INL, inner nuclear layer; ONL, outer nuclear layer; IPL, inner plexiform layer; OPL, outer plexiform layer; RGC, Retinal ganglion cell. Data represented as mean ± SEM (n = 3–8). One-way ANOVA followed by Tukey’s multiple comparisons test. Significance of difference from nTg controls, ∗∗∗p ≤ 0.001, ∗∗∗∗p ≤ 0.0001; Significance of difference from MFN2R94Qmice, ###p ≤ 0.001, ####p ≤ 0.0001; ns: non-significant.
Similarly, immunoblots and corresponding densitometric analysis showed significantly decreased expression of cone-arrestin in MFN2R94Q retina compared to nTg (MFN2R94Q vs nTg & MFN2R94Q vs MFN2R94Q:MFN1: p ≤ 0.001, n = 3, Figures 4H and S5). MFN1 augmentation restored cone-arrestin expression levels similar to nTg controls (Figures 4H and S5).
MFN1 augmentation rescued RGCs and their axons from degeneration
Next, to evaluate the effect of R94Q mutation on RGCs and their axons, immunostaining of Brn3a (RGC marker) and neurofilament RT97 (axonal neurofilament marker) was performed on retinal sections from all three groups of mice (Figures 5A–5C). RGC counts (Brn3a+RGCs: 3.96 ± 0.114/100 μm) and their axons were greatly reduced in MFN2R94Q mice compared to nTg retina (9.62 ± 0.127/100 μm) (Figures 5A, 5B, and 5D). Importantly, MFN1 augmentation rescued both RGCs (7.43 ± 0.167/100 μm) and their axons to the level as in nTg mice (Figures 5C and 5D, S2B). No changes were observed in RGC counts and their axons in MFN2WT compared to nTg mice retina (Figure S3C). Immunoblots and corresponding densitometric analysis also demonstrated significantly decreased expression of Brn3a in the retina of MFN2R94Q mice (MFN2R94Q vs Tg & MFN2R94Q vs MFN2R94Q:MFN1: p ≤ 0.001, n = 3, Figures 5E and S6), while MFN1 augmentation led Brn3a expression to nTg level and confirmed the immunofluorescence findings (Figures 5E and S6).
Figure 5.
Increased MFN1 expression rescued RGCs in MFN2R94Q:MFN1 mice retina
(A–C) Double immunolabeling of Brn3a (green: RGC marker) and RT97 (red: Neurofilament marker; RGC axons and horizontal cell axons) in retinas of nTg, MFN2R94Q, and MFN2R94Q:MFN1 mice demonstrated reduced RGCs and their axons, and horizontal axons in MFN2R94Q retina, compared with nTg retina.
(D) Representative dot plots showed significantly decreased number of Brn3a+ RGCs in MFN2R94Q mice, while on the other hand, MFN1 augmentation rescued and preserved RGCs in MFN2R94Q:MFN1 mice, similar to nTg controls.
(E) Representative immunoblots and corresponding densitometric analysis also confirmed significantly increased expression of Brn3a in the retina of MFN2R94Q:MFN1 mice (similar to nTg controls), compared to MFN2R94Q mice retina. Scale bar, 25 μm. INL, inner nuclear layer; ONL, outer nuclear layer; IPL, inner plexiform layer; OPL, outer plexiform layer; RGC, Retinal ganglion cell. Data represented as mean ± SEM (n = 3–8). One-way ANOVA followed by Tukey’s multiple comparisons test. Significance of difference from nTg controls, ∗∗∗p ≤ 0.001, ∗∗∗∗p ≤ 0.0001; Significance of difference from MFN2R94Qmice, ###p ≤ 0.001, ####p ≤ 0.0001; ns: non-significant.
MFN1 augmentation prevented retinal secondary neurons and connections from pathological modification
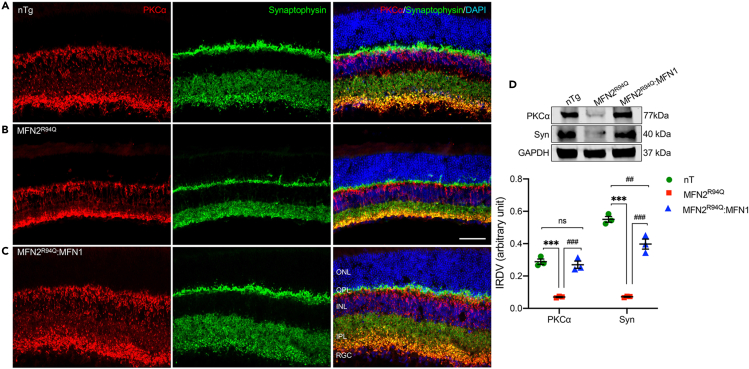
To evaluate whether MFN1 augmentation rescued retinal secondary neurons and connections, immunostaining of retinal secondary neurons and synapse markers was performed on retinal sections from all three groups. Horizontal cells have intimate relation with PRs and make connections with both rod and cone PRs. Their dendrites establish contact with cone terminals, and axonal arborizations connect with rod spherules. To examine whether horizontal cells were also affected in MFN2R94Q mice, immunofluorescence staining of neurofilament RT97 that labels horizontal cell axons was performed. In nTg retina, horizontal cell axons in the OPL were revealed as a dense band (Figure 5A), while the staining was dramatically reduced in MFN2R94Q retina (Figure 5B). Again, MFN1 augmentation restored the axonal band in the OPL (Figure 5C) of MFN2R94Q:MFN1 retina. Rod bipolar cells connect with rod spherules by a large, candelabra-like dendritic arbor at the end of a single dendritic trunk and RGC layer as large axon terminal end-bulbs with lateral varicosities in rodent retina. Immunofluorescence staining using PKCα (rod-bipolar cell marker) and synaptophysin (labels synapses in the OPL and IPL) was performed on retinal sections. In MFN2R94Q retina, the densities of both dendritic arbors at OPL and axonal terminals at IPL were reduced, compared with nTg retina (Figure 6A vs B). In addition, dendritic extensions from bipolar cells into ONL were also observed. Previous studies have shown the pathological modifications in bipolar cells as an immediate consequence of PR loss.40,41,42 The dendritic extensions of bipolar cells into ONL indicated input signals from ONL were lacking. In MFN2R94Q retina, although ONL was present, its function was impaired due to imbalance in MFN1/MFN2 ratio (MFN1≪≪MFN2). This might also explain the diminished ERG response in MFN2R94Q mice. Immunofluorescence staining of synaptophysin showed dense synaptic density in OPL and INL of nTg retina, while in MFN2R94Q retina, synaptic density in both OPL and INL was obviously reduced compared with nTg retina. MFN1 augmentation restored synaptic densities in both OPL and IPL (Figure 6C). Furthermore, no changes were observed in synaptophysin immunoreactivity in MFN2WT compared to nTg mice retina (Figure S3D). Immunoblots and corresponding densitometric analysis results further validated our immunofluorescence findings (PKCα: nTg vs. MFN2R94Q & MFN2R94Q vs. MFN2R94Q:MFN1: p ≤ 0.001; Syn: nTg vs. MFN2R94Q & MFN2R94Q vs. MFN2R94Q:MFN1: p ≤ 0.001, n = 3, Figures 6D and S7).
Figure 6.
MFN1 augmentation rescued retinal secondary neurons and retinal connections in MFN2R94Q:MFN1 mice retina
(A–C) Double immunolabeling of PKCα (red: marker of rod bipolar cells) and Synaptophysin (green: marker of retinal connections) in retina of nTg (A), MFN2R94Q (B), and MFN2R94Q:MFN1 (C) mice demonstrated MFN1 augmentation-mediated preservation of rod bipolar cells, their dendritic arborization, and synapses of retinal connections, while in MFN2R94Q retina, decreased immunoreactivity of PKCα (red) and Synaptophysin (green) clearly indicated decreased synaptic density (OPL and IPL) and arborization (OPL and IPL), compared to MFN2R94Q:MFN1 and nTg mice.
(D) Representative immunoblots and corresponding densitometric analysis further affirmed significantly increased expression of PKCα and Synaptophysin in the retina of MFN2R94Q:MFN1 mice (similar to nTg controls) compared to MFN2R94Q mice. Scale bar, 25 μm. INL, inner nuclear layer; ONL, outer nuclear layer; IPL, inner plexiform layer; OPL, outer plexiform layer; RGC, Retinal ganglion cell. Data represented as mean ± SEM (n = 3). One-way ANOVA followed by Tukey’s multiple comparisons test. Significance of difference from nTg controls, ∗∗∗p ≤ 0.001; Significance of difference from MFN2R94Qmice, ##p ≤ 0.01, ###p ≤ 0.001; ns: non-significant.
MFN1 augmentation prevented Müller cell gliosis
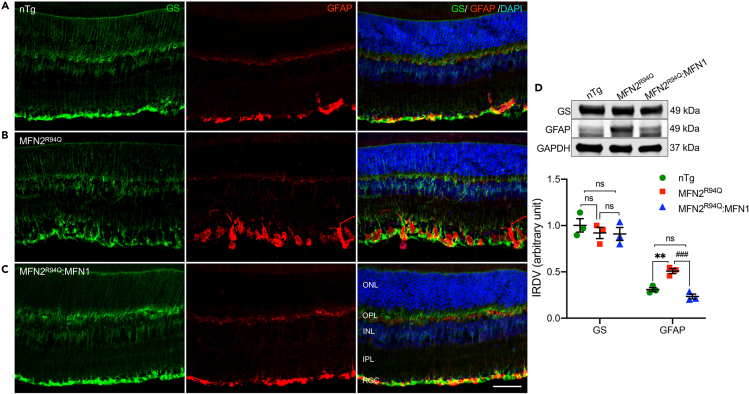
Müller glial cells span the entire thickness of retina and are the major supporting cells with two radially running bands forming inner and outer limiting membranes. Glutamine synthetase (GS) is a well-established marker of Müller cells while increased glial fibrillary acidic protein (GFAP) immunoreactivity is reliably considered a marker for reactive Müller cells. GS staining demonstrated a thicker inner limiting membrane and disorganized morphology of Müller glia while increased GFAP immunoreactivity showed pronounced Müller cell gliosis in MFN2R94Q retina compared to nTg retina (Figures 7A and 7B). However, both GS and GFAP staining in MFN2R94Q:MFN1 were comparable to nTg retina (Figures 7A and C), indicating MFN1 augmentation prevented gliosis in Müller cells. Furthermore, immunoblots and corresponding densitometric analysis also showed significantly increased expression of GFAP but no change in GS expression in MFN2R94Q mice, compared to nTg and MFN2R94Q:MFN1 mice retinas (nTg vs. MFN2R94Q: p ≤ 0.01 & MFN2R94Q vs. MFN2R94Q:MFN1: p ≤ 0.001, n = 3, Figures 7D and S8).
Figure 7.
MFN1 augmentation suppressed Müller cell gliosis in retina of MFN2R94Q:MFN1 mice
(A–C) Double immunolabeling of glutamine synthetase (GS) (green: marker of Müller cells) and GFAP (red: marker of activated Müller cells) in the retina of nTg (A), MFN2R94Q (B), and MFN2R94Q:MFN1 (C) mice showed MFN1 augmentation normalizes Müller cell morphology and reduced Müller cell gliosis, clearly indicated by reduced GFAP immunoreactivity in retina of MFN2R94Q:MFN1 mice (similar to nTg controls), compared to MFN2R94Q mice. No change was observed in GS immunoreactivity.
(D) Representative immunoblots and corresponding densitometric analysis showing significantly decreased expression of GFAP in the retina of MFN2R94Q:MFN1 mice (similar to nTg controls), compared to MFN2R94Q mice. No change was observed in GS expression. Scale bar, 25 μm. INL, inner nuclear layer; ONL, outer nuclear layer; IPL, inner plexiform layer; OPL, outer plexiform layer; RGC, Retinal ganglion cell. Data represented as mean ± SEM (n = 3). One-way ANOVA followed by Tukey’s multiple comparisons test. Significance of difference from nTg controls, ∗∗p ≤ 0.01; Significance of difference from MFN2R94Qmice, ###p ≤ 0.001; ns: non-significant.
MFN1 augmentation rescued mitochondria via restoring mitochondrial fission-fusion balance
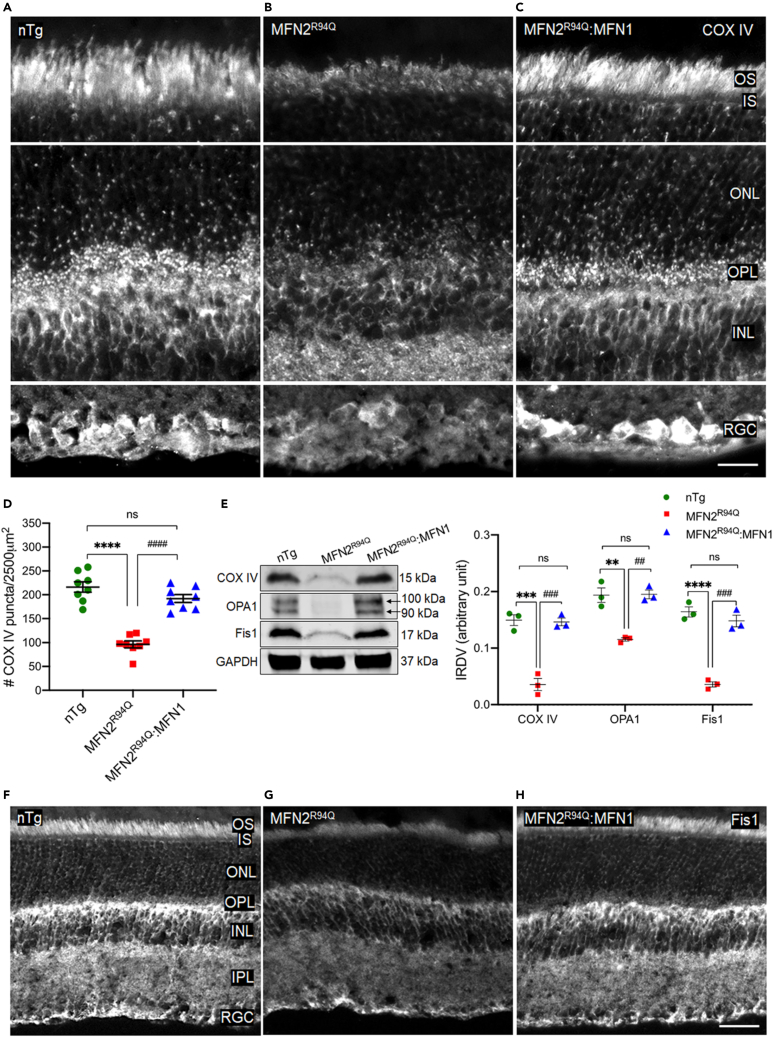
To investigate whether MFN1 augmentation rescued and preserved mitochondria from degeneration in MFN2R94Q:MFN1, retinal sections were stained with mitochondrial marker cytochrome c oxidase or complex IV (COXIV).43 Very weak immunoreactivity and decreased puncta were observed in MFN2R94Q retina compared to nTg mice (Figures 8A and 8B). Decreased mitochondrial counts (very weak immunoreactivity and decreased puncta) in the retina of MFN2R94Q mice suggest mitochondrial degeneration because of the imbalanced MFN1/MFN2 ratio (Figure 8D). In contrast, MFN1 augmentation rescued mitochondria from degeneration, as revealed by increased puncta and intense immunoreactivity (similar to nTg controls; Figures 8A, 8C, and 8D). Immunoblots and corresponding densitometric analysis results also showed significantly decreased expression of COXIV in the retina of MFN2R94Q mice compared to nTg control, while MFN2R94Q:MFN1 retina showed COXIV expression similar to nTg controls (nTg vs MFN2R94Q vs MFN2R94Q:MFN1: p ≤ 0.001, n = 3, Figures 8E and S9) and further validated our immunofluorescence findings.
Figure 8.
MFN1 augmentation rescued degenerative mitochondrial population via restoring mitochondrial fission-fusion balance in retina of MFN2R94Q:MFN1 mice
(A–C) COXIV immunolabeling (mitochondrial marker) in the retina of nTg (A), MFN2R94Q (B), and MFN2R94Q:MFN1 (C) mice showed significantly decreased COXIV immunoreactivity (decreased puncta and very weak cytoplasmic staining) in IS, ONL, OPL, INL, and RGC layers of MFN2R94Q retina compared to nTg controls, while increased puncta and intense cytoplasmic staining were observed in MFN2R94Q:MFN1 retina (similar to nTg controls). Scale bar, 10μm.
(D) Quantification of COXIV puncta.
(E) Representative immunoblots and corresponding densitometric analysis showing significantly increased expression of COXIV, OPA1 (mitochondrial fusion protein), and Fis1 (mitochondrial fission protein) in the retina of MFN2R94Q:MFN1 (similar to nTg controls), compared to MFN2R94Q mice.
(F–H) Immunofluorescence staining of Fis1 (white: marker of mitochondrial fission) in the retina of nTg (F), MFN2R94Q (G), and MFN2R94Q:MFN1 (H) mice showing significantly increased Fis1 immunoreactivity in the retina of MFN2R94Q:MFN1 mice (like nTg controls) compared to MFN2R94Q mice. Scale bar, 25 μm. OS, Outer segment; IS, inner segment; INL, inner nuclear layer; ONL, outer nuclear layer; IPL, inner plexiform layer; OPL, outer plexiform layer; RGC, Retinal ganglion cell. Data represented as mean ± SEM (n = 3–8). One-way ANOVA followed by Tukey’s multiple comparisons test. Significance of difference from nTg controls, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, ∗∗∗∗p ≤ 0.0001; Significance of difference from MFN2R94Qmice, ##p ≤ 0.01, ###p ≤ 0.001, ####p ≤ 0.0001; ns: non-significant.
These results suggest that R94Q mutation in MFN2 led to imbalanced MFN1/MFN2 ratio, which may impair mitochondrial fission-fusion homeostasis in MFN2R94Q retina. In fact, the complex interplay of multiple fission proteins (Fis1 and Drp1) and fusion-regulatory large GTPases (MFN1, MFN2, and OPA1) controls the fine-tuned balance of mitochondrial fission-fusion cycle. Hence, to examine the impaired MFN1/MFN2 ratio-induced fission-fusion imbalance-related changes, the expression of Fis1 and OPA1 was studied in the retina of all the experimental groups. Highly reduced Fis1 immunoreactivity was observed throughout the retina of MFN2R94Q mice, compared to nTg controls (Figures 8F and 8 G). Furthermore, immunoblots and corresponding densitometric analysis demonstrated significantly decreased expression of Fis1 and OPA1 in the retina of MFN2R94Q mice compared to nTg controls (OPA1: nTg vs MFN2R94Q vs MFN2R94Q:MFN1: p ≤ 0.01, Fis1: nTg vs MFN2R94Q: p ≤ 0.0001, MFN2R94Q vs MFN2R94Q:MFN1: p ≤ 0.001, n = 3, Figures 8E and S9). However, expression patterns and immunoreactivity similar to nTg levels were restored after MFN1 augmentation (Figures 8F and 8H). Altogether, these data indicate that impaired MFN1/MFN2 ratio as well as decreased Fis1 and OPA1 in MFN2R94Q mice attenuated mitochondrial fission-fusion and caused mitochondrial degeneration, which may eventually result in retinal degeneration due to severe energy deficit in high energy-demanding retinal cells.
MFN1 augmentation rescued retinal morphology and vision via protecting mitochondria from P62/LC3B-mediated mitophagy and restoring conventional PINK1-dependent, Parkin-independent mitophagy in MFN2R94Q:MFN1 retina
Mitochondrial fission followed by selective re-fusion removes dysfunctional mitochondria through mitophagy and maintains the mitochondrial homeostasis.44,45 Activation of phosphatase and tensin homolog (PTEN)-induced putative kinase protein 1 (PINK1)/Parkin mitophagy pathway is well known to protect cells from death and apoptosis by eliminating damaged mitochondria.46 Hence, the alterations in mitophagy pathways could be a potential explanation for mitochondrial degeneration and rescue in MFN2R94Q and MFN2R94Q:MFN1 retinas, respectively. Therefore, the expression of key molecular markers (Pink1, Parkin, P62, and LC3B) of mitophagy/autophagy was evaluated. Furthermore, as no changes in the number and morphology of cone PRs and RGCs were observed in MFN2WT compared to nTg mice retina, we did not include this group while investigating PINK1-dependent, Parkin-independent mitophagy. Immunofluorescence staining showed intense P62 immunoreactivity as revealed by increased puncta and intense cytoplasmic staining in all the retinal layers of MFN2R94Q mice retina compared to nTg mice (Figures 9A and 9B), while, contrarily, MFN2R94Q:MFN1 retina showed P62 immunoreactivity (very weak puncta and cytoplasmic staining) similar to nTg controls (Figures 9A and 9C). These results revealed that MFN1 augmentation rescued mitochondria from P62-dependent mitophagy in the retina of MFN2R94Q:MFN1 mice. Furthermore, very weak immunoreactivity for LC3B (a key marker for autophagy) in the retina of MFN2R94Q:MFN1 mice also suggested that MFN1 augmentation rescued retinal morphology via protecting mitochondria from P62/LC3B-mediated mitophagy (Figures 9D–9F). Immunoblots and corresponding densitometric analysis strongly supported our immunofluorescence findings and further confirmed significantly increased expression of P62 and LC3B in the retina of MFN2R94Q mice, compared to nTg control (P62: nTg vs MFN2R94Q: p ≤ 0.001, MFN2R94Q vs MFN2R94Q:MFN1: p ≤ 0.01; LC3B: nTg vs MFN2R94Q: p ≤ 0.01, MFN2R94Q vs MFN2R94Q:MFN1: p ≤ 0.05, n = 3, Figures 9G and S10). Furthermore, immunoblots and corresponding densitometric analysis also showed significantly increased expression of Pink1 but no change in Parkin expression in the retina of MFN2R94Q:MFN1 mice (like nTg controls), compared to MFN2R94Q mice (Pink1: nTg vs MFN2R94Q vs MFN2R94Q:MFN1: p ≤ 0.01, n = 3, Figures 9G and S10), and suggested restoration of conventional Pink1-dependent, Parkin-independent mitophagy in MFN2R94Q:MFN1 mice following MFN1 augmentation.
Figure 9.
MFN1 augmentation rescued mitochondrial homeostasis and restored conventional mitophagy in MFN2R94Q retina
(A–C) P62 immunolabeling (marker of mitophagy and autophagy) in the IS, ONL, OPL, INL, and RGC of nTg (A), MFN2R94Q (B), and MFN2R94Q:MFN1 (C) mice retina showed significantly decreased P62 immunoreactivity in IS and ONL as well as in the INL and RGC layers of MFN2R94Q:MFN1 mice (similar to nTg controls), while MFN2R94Q retina showed increased P62 immunoreactivity (increased white puncta and intense cytoplasmic staining) in these layers.
(D–F) Furthermore, very weak LC3B (key marker of autophagy) immunoreactivity was observed in the OS, IS, INL, and RGC layers of MFN2R94Q:MFN1 retina compared to MFN2R94Q retina.
(G) Representative immunoblots and corresponding densitometric analysis showed significantly increased Pink1 but reduced P62 and LC3B in the retina of MFN2R94Q:MFN1 mice (like nTg controls), compared to MFN2R94Q mice, while no change in Parkin was observed among the groups. Scale bar, 10 μm. OS, outer segment; IN, inner segment; INL, inner nuclear layer; ONL, outer nuclear layer; IPL, inner plexiform layer; OPL, outer plexiform layer; RGC, Retinal ganglion cell. Data represented as mean ± SEM (n = 3–8). One-way ANOVA followed by Tukey’s multiple comparisons test. Significance of difference from nTg controls, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001; Significance of difference from MFN2R94Qmice, #p ≤ 0.05, ##p ≤ 0.01, ####p ≤ 0.0001; ns: non-significant.
Discussion
In this study, we investigated retinal morphological changes correlated with visual function in a mouse model of CMT2A disease. For the first time, we demonstrated vision impairment associated with significant pathological modifications of PRs, retinal secondary neurons, synapses, and Müller glia as well as RGCs and their axons as consequences of R94Q mutation. Importantly, MFN1 augmentation restored visual function by rescuing retinal morphology.
Transgenic MFN2R94Q mice were generated by introducing Flag-tagged human MFN2 gene with R94Q mutation under the neuronal-specific Thy1.2 promoter, which is present in almost all the retinal cells.39 Here, we reported degenerative changes in PRs as revealed by the significantly reduced number of PRs and abnormal cone morphology and retinal connections. Correlated with retinal morphological changes, the amplitudes of both ERG a- and b-waves were almost diminished (Figures 2A–2D). Furthermore, pSTR also showed dramatically reduced b-wave-positive amplitudes in MFN2R94Q retina (Figures 2E–2K). In addition to the functional impairment, extensive loss of RGCs and their axons was also documented. Furthermore, secondary retinal neurons such as rod bipolar cells and horizontal cells also underwent degenerative changes. Retinal connections (both in OPL and IPL) revealed by synaptophysin became thinner and reduced in density. The activation of Müller cells, typically seen in injury or disease-associated gliosis was also observed. Overall, characteristic retinal histopathological remodeling and impaired visual function were observed in MFN2R94Q mice. In a recent study, Zhou et al. documented atrophied RGCs and their axonal degeneration in 8-month-old MFN2R94Q mice31 and MFN1 augmentation-mediated rescue. Similarly, in our current study, these mice demonstrated a significant loss of RGCs and their axonal degeneration at the age of 14 months. MFN1 overexpression rescued RGCs and their axons in MFN2R94Q:MFN1 mice. However, pSTR showed that b-wave-positive amplitude was significantly better than MFN2R94Q mice but not restored to the level of nTg control mice. The transmission of visual information from PRs to a higher visual center in the brain is precisely regulated by fine-tuned coordination and complex molecular interactions between specific rods/cones-bipolar cells and bipolar cells-RGCs. Furthermore, the complementary pairing of appropriate PRs and bipolar cells, as well as their conjugation with downstream specific subpopulations of RGCs, processes and translates visual information including color cues from PRs to the visual center in the brain.47,48,49,50,51,52 Hence, concomitant degeneration of PRs and RGCs along with their axonal loss explicitly explains the vision loss including the color vision impairment in MFN2R94Q mice.
Due to exceedingly high energy demands and metabolic activity, both PRs and RGCs are vulnerable to energy deficit as well as mitochondrial dysfunction.53,54 In fact, due to their vulnerability to mitochondrial dysfunction, PRs (especially cones) and RGCs are also highly susceptible to mutations affecting mitochondrial function, dynamics, and clearance.34,55 Furthermore, while processing visual information transduction, metabolically active PRs and RGCs require large amount of energy to maintain their action and resting potentials, respectively.33,35,36 To meet this high energy demand and metabolic activity, these retinal neurons possess large number of mitochondria.32,35 Moreover, among PRs, cones have the highest mitochondrial population to support the energy expenditure required during the processing of the colored visual information.32,56 Furthermore, a recent study also demonstrated significantly increased diurnal energy consumption in cones compared to rods.33 Hence, mitochondrial energy homeostasis as one of the key factors governs PR and RGC function.
Mitochondrial dynamics is maintained by a fine-tuned balance between fusion and fission.57,58 Furthermore, a vicious cycle of fission and selective re-fusion maintains the functionality of mitochondrial network by selective removal of dysfunctional mitochondria.28,59 Among the key regulators of mitochondrial fusion-fission, we found significantly decreased MFN1, OPA1, and Fis1 but increased MFN2 in MFN2R94Q mice, while MFN1 augmentation restored normal expression of these regulators in MFN2R94Q:MFN1 mice. Interestingly, we also found significantly decreased expression of COXIV in ONL, OPL, and RGC layers, suggesting mitochondrial degeneration. On the other hand, MFN1 gene supplementation rescued mitochondria from degeneration in the retina of MFN2R94Q:MFN1 mice. Because of the imbalance in MFN1/MFN2 (MFN1≪≪MFN2) ratio, it is highly likely that impaired selective mitochondrial re-fusion is the major cause of retinal degeneration in MFN2R94Q mice. However, no such differences in MFN1 and MFN2 levels were observed, and no alterations to mitophagy were reported in the brain and spinal cord of these mouse models of CMT at 8 months of age.31 These differences may only be restricted to the retina, which could behave and respond differently to R94Q mutation in MFN2 gene and MFN1 augmentation compared to brain/spinal cord, because of the tissue- and cell-type differences across retina vs brain/spinal cord. Furthermore, no phenotypic changes were observed in MNF2WT compared to wild-type nTg mice retina, suggesting that the degenerative changes observed in MFN2R94Q mice were due to R94Q mutation but not because of MFN2 overexpression.
Mitophagy maintains mitochondrial homeostasis. This autophagic process involves a complex interplay of interconnected regulatory mechanisms and pathways to balance mitochondrial biogenesis and selective removal of dysfunctional mitochondria.44,58 PINK1-dependent and Parkin-(in)dependent as well as receptor-mediated pathways are the two well-established major molecular pathways of the mitophagy.58 Furthermore, under receptor-mediated pathways, mitophagy can typically be regulated by three critically essential components, viz, 1) outer mitochondrial membrane (OMM) proteins, 2) inner mitochondrial membrane (IMM) proteins, and 3) autophagy receptors. MFN1 regulates mitochondrial fusion through interacting with OPA1, while MFN2, other than tethering inter-mitochondrial interactions to mediate axonal transport of mitochondria, also induces mitophagy.28,44,58 Furthermore, P62 (SQSTM1), an autophagy receptor has also been reported to mediate mitophagy.44 Our findings clearly indicated that MFN1 augmentation restored the levels of MFN1, OPA1, and Fis1 in MFN2R94Q:MFN1 mice retina, similar to nTg controls. These findings may further suggest the recuperation of MFN1/MFN2 (MFN1≫≫MFN2) ratio and MFN1-OPA1 interactions, and Fis1 levels may cumulatively offer balanced mitochondrial fusion-fission across all the retinal cell types in MFN2R94Q:MFN1 mice. Interestingly, significantly increased PINK1 and no change in Parkin further indicated the restoration of normal PINK1-dependent, Parkin-independent mitophagy in the retina of MFN2R94Q:MFN1 mice. On the other hand, significantly reduced levels of MFN1, OPA1, and Fis1 in MFN2R94Q retina suggested the imbalance in MFN1/MFN2 (MFN1≪≪MFN2) ratio and mitochondrial fusion-fission. Significantly increased P62 and LC3B levels may also suggest that exceedingly high P62/LC3B-mediated mitophagy/autophagy in the retina induced rod/cone degeneration and RGC loss in MFN2R94Q retina. Fis1 is a pro-fission protein while OPA1 is a pro-fusion protein, and reduction in both of these proteins as well as impaired MFN1/MFN2 ratio would affect mitochondrial dynamics by simply reducing overall mitochondrial number/mass. Given the established role of MFN2 in regulating mitochondrial dynamics and some previously published results showing that MFN1 complementation can reverse aberrant mitochondrial dynamics in the context of the R94Q MFN2 mutation,19,60 it is highly likely that mitochondrial dynamics are aberrantly altered in addition to the overall loss of mitochondria in MFN2R94Q retina via P62/LC3B-mediated mitophagy/autophagy.
Patients with CMT2A demonstrated optic atrophy, low visual acuity, color-vision defects, and moderate-to-severe vision loss.61 Clinical examinations documented significantly reduced thickness of retinal nerve fiber layer (RNFL) and ganglion cell layer complex in these patients. Some studies also demonstrated significant optic nerve damage with mild-to-moderate impaired visual function in these patients.37,61 MFN2R94Q mice, a well-representative model of CMT2A disease, show impaired vision similar to CMT2A patients.31 In our present study, we observed vision loss correlated with retinal degeneration via P62/LC3B-mediated mitophagy/autophagy as the consequence of R94Q mutation-induced imbalance in MFN1/MFN2 (MFN1≪≪MFN2) ratio and mitochondrial fusion-fission in MFN2R94Q retina. However, biochemical, molecular, and pathophysiological differences between mice and humans could also make humans more vulnerable to MFN2R94Q-induced degeneration of cones, RGCs, and their axons.
Taken together, our findings suggest that R94Q mutation-induced imbalance in MFN1/MFN2 ratio and impaired mitochondrial fusion-fission lead to retinal degeneration in MFN2R94Q mice retina via P62/LC3B-mediated mitophagy as well as P62/LC3B-mediated autophagy. Contrarily, MFN1 augmentation rescues vision and retinal morphology via restoring fine-tuned homeostasis in mitochondrial fusion-fission (MFN1/MFN2 ratio) and PINK1-dependent, Parkin-independent mitophagy in the retina of MFN2R94Q:MFN1 mice. However, further research is required to delineate the mechanistic insights of altered cell-specific mitochondrial fusion-fission to better understand their contributory role in vision loss during disease progression. Clinical characteristics of CMT2A oculopathy include optic atrophy, color-vision defects, and progressive loss of vision, which may be a manifestation of an imbalanced MFN1/MFN2 ratio and dysregulated mitochondrial fusion/fission due to the most abundant R94Q mutation in MFN2. MFN1 predominantly regulates mitochondrial fusion, and a balanced MFN1/MFN2 ratio is necessary to maintain mitochondrial fusion/fission homeostasis. The present study demonstrated the degenerative retinal phenotype leading to severe vision loss in MFN2R94Q mice and that MFN1 augmentation preserves vision by rescuing retinal morphology in MFN2R94Q:MFN1 mice. Hence, for future therapeutic interventions at the central nervous system (CNS) for CMT2A, attention should also be paid to ocular changes. Because MFN2R94Q mice recapitulate almost all the pathophysiological characteristics of human patients with CMT2A disease, creating pre-clinical mito-mouse rescue model using mitochondrial targeting sequence adenoassociated virus (MTS-AAV)-mediated62 MFN1 delivery in MFN2R94Q mice may provide a strong foundation to extrapolate this pre-clinical therapeutic intervention to clinical trials using highly efficient intra-ocular/subretinal gene delivery approach to treat eyes, in addition to CNS, in these patients.
Limitations of this study
This study has important clinical relevance as the findings elucidate the retinal pathophysiological mechanisms of CMT2A, the most common inherited peripheral axonal neuropathy, which currently lacks effective clinical treatments. The retinal degeneration associated with CMT2A is relatively understudied, and the findings of this study provide a novel edge, with potential clinical importance. Furthermore, the reversal of MFN2R94Q-associated degenerative phenotypes with MFN1 augmentation provides mechanistic insights and suggests a potential future treatment option for CMT2A. However, this study has some limitations. The lack of the MFN2WT mouse is an important missing control while investigating the PINK1-dependent, Parkin-independent mitophagy. Another limitation is the lack of gain- and loss-of-function in vitro studies with labeled mitochondria to assess the direct on-target effects of MFN2R94Q and MFN1 augmentation on mitochondrial fusion-fission cycle and dynamics. Furthermore, progressive degenerative changes over time or whether R94Q mutation affects the development of different retinal cell types in MFN2R94Q mice were not assessed. A future comprehensive study analyzing the degenerative phenotype over time is needed to conclude the progressive loss of different retinal cell types.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Goat anti-Brn-3a | Santa Cruz Biotechnology | Cat# sc-31984; RRID: AB_2167511 |
| Rabbit anti-cone arrestin | Millipore | Cat# AB15282; RRID: AB_1163387 |
| Rabbit anti-COXIV | Abcam | Cat# AB16056; RRID: AB_443304 |
| Rabbit anti-COXIV | Proteintech | Cat# 11242-1-AP; RRID: AB_2085278 |
| Mouse anti-flag | Sigma | Cat# F3165; RRID: AB_259529 |
| Rabbit anti-Fis1 | Proteintech | Cat# 10956-1-AP; RRID: AB_2102532 |
| Mouse anti-Glutamine synthetase | Millipore | Cat# MAB302; RRID: AB_2110656 |
| Rabbit anti-Glial fibrillary acidic protein | Dako-Agilent | Cat# Z0334; RRID:AB_10013382 |
| Mouse anti-GAPDH | Millipore | Cat# G8795; RRID: AB_1078991 |
| Rabbit anti-GAPDH | Cell Signaling Technology | Cat# 5174; RRID: AB_10622025 |
| Rabbit anti-LC3B | Novus Biologicals | Cat# NB100-2220; RRID: AB_10003146 |
| Mouse anti-mitofusin 1 | Proteintech | Cat# 13798-1AP; RRID: AB_2266318 |
| Rabbit anti-mitofusin 2 | Proteintech | Cat# 12186-1-AP; RRID: AB_2266320 |
| Mouse anti-mitofusin 2 | Abcam | Cat# AB56889; RRID: AB_2142629 |
| Mouse anti-neurofilament (RT97) | Millipore | Cat# CBL212; RRID: AB_93408 |
| Rabbit anti-OPA1 | Proteintech | Cat# 27733-1-AP; RRID: AB_2810292 |
| Mouse anti-P62/SQSTM1 | Novus Biologicals | Cat# H00008878-M01; RRID: AB_548364 |
| Rabbit anti-Parkin | Proteintech | Cat# 14060-1-AP; RRID: AB_2878005 |
| Rabbit anti-Parkin | Proteintech | Cat# 14060-1-AP; RRID: AB_2878005 |
| Rabbit anti-protein kinase C-α | Sigma | Cat# P4334; RRID: NA |
| Rabbit anti-recoverin | Millipore | Cat# AB5585; RRID: AB_2253622 |
| Mouse anti-rhodopsin | Millipore | Cat# MAB5356; RRID: AB_2178961 |
| Mouse anti-synaptophysin | Millipore | Cat# MAB368; RRID: AB_94947 |
| Experimental models: Organisms/strains | ||
| C57BL/6J (non-transgenic-nTg) mice | Robert H Baloh | NA |
| Thy1.2-Flag-MFN2WT(MFN2WT) mice | Robert H Baloh | NA |
| Thy1.2-Flag-MFN2R94Q(MFN2R94Q) line 44 mice | Robert H Baloh | NA |
| Prp-MFN1 MFN2R94Q (MFN2R94Q:MFN1) mice | Robert H Baloh | NA |
| Software and algorithms | ||
| GraphPad Prism 9 | GraphPad | https://www.graphpad.com |
| ImageJ | NIH | https://imagej.nih.gov/ij/ |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Shaomei Wang (Shaomei.Wang@cshs.org).
Materials availability
This study did not generate new unique reagents.
Animal models
Fourteen-month-old male C57BL/6J (non-transgenic-nTg), Thy1.2-Flag-MFN2WT (MFN2WT), Thy1.2-Flag-MFN2R94Q (MFN2R94Q) line 44, and Prp-MFN1 MFN2R94Q (MFN2R94Q:MFN1) mice of C57BL/6J background used in this study are a generous gift from Dr. Baloh and are the same in origin as used by Zhou et al.31 Thy1.2-Flag-MFN2WT (MFN2WT) and Thy1.2-Flag-MFN2R94Q (MFN2R94Q) mice express Flag-tagged human wild-type MFN2 (MFN2WT) or mutant (MFN2R94Q) gene under the neuronal-specific Thy1.2 promoter respectively. Double transgenic Prp-MFN1 MFN2R94Q (MFN2R94Q:MFN1) mice express Flag-tagged human mutant (MFN2R94Q) and wild-type MFN1 genes under Thy1.2 and wild-type human prion protein (PrP) promoters respectively. All mice were housed and maintained at the Cedars-Sinai Medical Center Department of Comparative Medicine vivarium. All animal experiments were approved by the Cedars-Sinai Medical Center’s Institutional Animal Care and Use Committee and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Method details
Visual function test
Visual functions test of photoreceptors (rod and cones) and RGCs were evaluated by performing electroretinography (ERG) and positive Scotopic threshold response (pSTR) respectively according to previously published protocols.63,64 Briefly, mice were dark-adapted for 14–16 h before ERG and pSTR recordings. Bilateral ERG recording was performed simultaneously from both eyes by using Espion Visual Electrophysiology System (Diagnosys LLC, Lowell, MA). The ERG responses were recorded by stimulating the retina with light intensities 25 cd s/m2 for the mixed (rod and cone) response, and 0.000125 cd s/m2 and 0.000625 cd s/m2 for the positive scotopic threshold response (pSTR). For each light intensity level, a series of 20–30 ERG responses for each stimulus per mouse were recorded, and the average responses were used as the response amplitude.
Histologic analysis, immunofluorescence, and confocal microscopy
Histological assessment and immunofluorescence were performed as described in our previous publications.64,65,66 Briefly, the mice were euthanized, eyes were enucleated, and a small cut was made at the center of the cornea. Eyeballs were then fixed in 4% paraformaldehyde in PBS for 1 h at room temperature, transferred to 10%, 20%, and 30% sucrose (1 h for each concentration at room temperature) and kept in 30% sucrose overnight at 4°C. On the next day, eyeballs were embedded in optimal cutting temperature (OCT) compound (Sakura Finetek, Torrance, CA) and stored at −80°C. Frozen transverse 10-μm-thick retinal sections were collected in five series with 4 sections per slide. One set of slides was stained with cresyl violet (0.4% cresyl violet acetate, Sigma Aldrich, St. Lois, MO) to evaluate retinal lamination.
Immunofluorescence staining was performed as described in our previous publications.64,65,66 Primary antibodies used were as follows: anti-Brn3a (1:200, sc-31984, Santa Cruz Biotechnology Inc.), anti-cone arrestin (1:1,000, AB15282, Millipore Sigma), anti-COXIV (1:200, ab16056, Abcam Inc.), anti-Flag (1:500, F3165, Sigma-Aldrich), anti-Fis1 (1:200, 10956-1-AP, Proteintech), anti-glutamine synthetase (GS, 1:1000, MAB302, Millipore, Billerica, MA), anti-Glial Fibrillary Acidic Protein (GFAP, 1:500, Z0334, Dako-Agilent Technologies Inc.), anti-LC3B (1:2,00, NB100-2220, Novus Biologicals), anti-MFN1 (1:200, 13,798-1AP, Proteintech), anti-MFN2 (1:200, ab56889, Abcam Inc.), protein kinase C-α (PKC- α, 1:8,000, P4334, Sigma), anti-recoverin (1:2,000, AB5585, Millipore Sigma), anti-rhodopsin (1:100, MAB5356, Millipore Sigma), anti-neurofilament antibody (RT97, 1:1,000, CBL212, Millipore Sigma), anti-Synaptophysin (1:2,000, MAB368, Millipore Sigma), and anti-P62/SQSTM1 (1:1000, H00008878-M01, Novus Biologicals). Alexa Fluor 488, Alexa Fluor 594 or Cy5 secondary antibodies (1:500; Life Technologies) were used to visualize sections along with nuclear counterstain (DAPI, 49,69-diamidino-2-phenylindole; Vector Laboratories, Burlingame, CA). For antibody specificity, controls included omitting the primary or secondary antibodies, which leads to negative staining. Images were captured using confocal microscope (Zeiss LSM710, Oberkochen, Germany) and saved as TIFF files. For each antigen, images were captured at same magnification using same laser power (exposure), gain and offset.
Image analysis
Measurements of outer nuclear layer (ONL) thickness, number of PRs and cones, Brn3a+ RGC counts, and COXIV and P62/SQSTM1 puncta counts were performed using ImageJ software (ImageJ 1.53 bundled with 64-bit Java, NIH, Bethesda, MD) as described elsewhere.67,68 Images were taken from retinal sections from all the experimental groups (4 images/retinal section, 4 sections/retina) using confocal microscope (Zeiss LSM710, Oberkochen, Germany) and saved as TIFF files. ONL thickness was measured at 2–4 points/image. An average of ONL thickness was compared among the three groups of mice. The number of PRs, cones and Brn3a+ RGCs were counted using ‘threshold’ and ‘analyze particles’ functions. Puncta were quantified by selecting the region of interest (ROI: 2500 μm2) and using ITCN plugins in ImageJ. Differences in immunoreactivity of MFN2 in both MFN2WT and nTg retina were assessed as fluorescent intensities, as mentioned elsewhere.67,68,69 In brief, for fluorescent intensity measurements, ROI for each protein was selected by using ImageJ freehand tool. Fluorescent intensities of ROIs were measured after normalizing to the background. The fluorescent intensities were averaged (4 sections per retina and 4 retina per group; each section was 40 μm apart) to determine the signal density. The fluorescent intensity values were presented as arbitrary units. The processing and analysis were kept consistent between images. Statistical analysis was performed on averaged fluorescent intensities values (mean ± SEM). Image analysis was performed blindly and separately by 2 observers.
Western blotting analysis
Mouse retina and brain tissue were lysed with RIPA buffer and protein was quantified using BCA protein assay (Pierce). Equal amount of protein (50 μg) from each group was resolved on 4–20% precast polyacrylamide gels (catalog 4,561,094, Bio-Rad) and transferred to nitrocellulose blots. Primary antibodies for Western blot included anti-Flag (1:1000, F3165, Millipore Sigma), anti-Brn3a (1:500, sc-31984, Santa Cruz Biotechnology Inc.), anti-cone arrestin (1:1,000, AB15282, Millipore Sigma), anti-COXIV (1:10,000, 11242-1-AP, Proteintech), anti-Fis1 (1:1000, 10956-1-AP, Proteintech), anti-GS (1:500, MAB302, Millipore Sigma), anti-GFAP (1:1000, G3893, Sigma), anti-LC3B (1:1000, NB100-2220, Novus Biologicals), anti-MFN1 (1:1000, 13,798-1AP, Proteintech), anti-MFN2 (1:5000, 12186-1-AP, Proteintech), PKC- α (1:10,000, P4334, Sigma), anti-Synaptophysin (1:1,000, Millipore MAB368), anti-OPA1 (1:1000, 27733-1-AP, Proteintech), anti-P62/SQSTM1 (1:1000, H00008878-M01, Novus Biologicals), anti-Pink1 (1:1000, 23274-1-AP, Proteintech), anti-Parkin (1:1000, 14060-1-AP, Proteintech), and anti-GAPDH (1:1000, catalog G8795, Millipore Sigma and catalog #5174, Cell Signaling Technology). Signals were detected using fluorescent secondary antibodies and the Odyssey imager from LI-COR. Densitometric analysis was performed using Image Studio Acquisition Software and integrated relative density values (IRDV; in arbitrary units) were determined by normalizing with GAPDH.
Quantification and statistical analysis
All statistical analysis was performed by using GraphPad Prism software (version 9.3). Data presented as mean ± SEM. Results were analyzed by Unpaired 2-tailed Student’s t test for 2-group comparisons and 1-way ANOVA coupled with a Tukey’s multiple comparisons test for more than 2-group. p value <0.05 was considered significant.
Acknowledgments
Funding for this study was supported by the Board of Governors Regenerative Medicine Institute at Cedars-Sinai Medical Center.
Author contributions
SS & SW designed the experiments. RHB provided all the experimental animals. HX & BL performed ERG & pSTR. SS, YZ, HX, & JC performed sample collection. SS & JC performed cryo-sectioning and CV staining. SS performed immunofluorescence, western blot, and data analysis. SW provided funding support. SS & SW drafted the manuscript. YZ reviewed the draft and provided valuable comments. SS & SW edited the final manuscript for submission. All authors have read and agreed to the published version of the manuscript.
Declaration of interests
The authors declare no conflict of interest.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: February 25, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.106270.
Supplemental information
9G
3-9
Data and code availability
-
•
This study did not generate or utilize any dataset or code.
-
•
The data that support the findings of this study are shown in this manuscript and in Supplemental Information.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Charcot-Marie-Tooth Disease Fact Sheet, National Institute of Neurological Disorders and Stroke. 2018, https://www.ninds.nih.gov/health-information/disorders/charcot-marie-tooth-disease.
- 2.Guerriero S., D’Oria F., Rossetti G., Favale R.A., Zoccolella S., Alessio G., Petruzzella V. CMT2A harboring mitofusin 2 mutation with optic nerve atrophy and normal visual acuity. Int. Med. Case Rep. J. 2020;13:41–45. doi: 10.2147/IMCRJ.S237620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oporto J.I., Velasco R., Mori A., Olivares C. Ocular manifestations of charcot-marie-tooth disease: a short review. Int. J. Ophthalmol. 2019;3:3. [Google Scholar]
- 4.Zayit-Soudry S., Mimouni M. In: Encyclopedia of Ophthalmology, U. Schmidt-Erfurth and. Kohnen T., editor. Springer Berlin Heidelberg; 2018. Charcot-marie-tooth disease, retinal degeneration; pp. 376–377. [DOI] [Google Scholar]
- 5.Morena J., Gupta A., Hoyle J.C. Charcot-marie-tooth: from molecules to therapy. Int. J. Mol. Sci. 2019;20:3419. doi: 10.3390/ijms20143419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saporta A.S.D., Sottile S.L., Miller L.J., Feely S.M.E., Siskind C.E., Shy M.E. Charcot marie tooth (CMT) subtypes and genetic testing strategies. Ann. Neurol. 2011;69:22–33. doi: 10.1002/ana.22166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vallat J.-M. Dominantly inherited peripheral neuropathies. J. Neuropathol. Exp. Neurol. 2003;62:699–714. doi: 10.1093/jnen/62.7.699. [DOI] [PubMed] [Google Scholar]
- 8.Bombelli F., Stojkovic T., Dubourg O., Echaniz-Laguna A., Tardieu S., Larcher K., Amati-Bonneau P., Latour P., Vignal O., et al. Charcot-marie-tooth disease type 2A: from typical to rare phenotypic and genotypic features. JAMA Neurol. 2014;71:1036–1042. doi: 10.1001/jamaneurol.2014.629. [DOI] [PubMed] [Google Scholar]
- 9.Feely S.M.E., Laura M., Siskind C.E., Sottile S., Davis M., Gibbons V.S., Reilly M.M., Shy M.E. MFN2 mutations cause severe phenotypes in most patients with CMT2A. Neurology. 2011;76:1690–1696. doi: 10.1212/WNL.0b013e31821a441e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Züchner S., Mersiyanova I.V., Muglia M., Bissar-Tadmouri N., Rochelle J., Dadali E.L., Zappia M., Nelis E., Patitucci A., Senderek J., et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat. Genet. 2004;36:449–451. doi: 10.1038/ng1341. [DOI] [PubMed] [Google Scholar]
- 11.Pipis M., Feely S.M.E., Polke J.M., Skorupinska M., Perez L., Shy R.R., Laura M., Morrow J.M., Moroni I., Pisciotta C., et al. Natural history of Charcot-Marie-Tooth disease type 2A: a large international multicentre study. Brain. 2020;143:3589–3602. doi: 10.1093/brain/awaa323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patzkó A., Shy M.E. Update on charcot-marie-tooth disease. Curr. Neurol. Neurosci. Rep. 2011;11:78–88. doi: 10.1007/s11910-010-0158-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kersten H.M., Roxburgh R.H., Danesh-Meyer H.V. Ophthalmic manifestations of inherited neurodegenerative disorders. Nat. Rev. Neurol. 2014;10:349–362. doi: 10.1038/nrneurol.2014.79. [DOI] [PubMed] [Google Scholar]
- 14.Züchner S., De Jonghe P., Jordanova A., Claeys K.G., Guergueltcheva V., Cherninkova S., Hamilton S.R., Van Stavern G., Krajewski K.M., Stajich J., et al. Axonal neuropathy with optic atrophy is caused by mutations in mitofusin 2. Ann. Neurol. 2006;59:276–281. doi: 10.1002/ana.20797. [DOI] [PubMed] [Google Scholar]
- 15.Cerveny K.L., Tamura Y., Zhang Z., Jensen R.E., Sesaki H. Regulation of mitochondrial fusion and division. Trends Cell Biol. 2007;17:563–569. doi: 10.1016/j.tcb.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Chen H., Detmer S.A., Ewald A.J., Griffin E.E., Fraser S.E., Chan D.C. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mishra P., Chan D.C. Metabolic regulation of mitochondrial dynamics. J. Cell Biol. 2016;212:379–387. doi: 10.1083/jcb.201511036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wai T., Langer T. Mitochondrial dynamics and metabolic regulation. Trends Endocrinol. Metabol. 2016;27:105–117. doi: 10.1016/j.tem.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Detmer S.A., Chan D.C. Functions and dysfunctions of mitochondrial dynamics. Nat. Rev. Mol. Cell Biol. 2007;8:870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- 20.Eura Y., Ishihara N., Yokota S., Mihara K. Two mitofusin proteins, mammalian homologues of FZO, with distinct functions are both required for mitochondrial fusion. J. Biochem. 2003;134:333–344. doi: 10.1093/jb/mvg150. [DOI] [PubMed] [Google Scholar]
- 21.Filadi R., Pendin D., Pizzo P. Mitofusin 2: from functions to disease. Cell Death Dis. 2018;9:330. doi: 10.1038/s41419-017-0023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belenguer P., Pellegrini L. The dynamin GTPase OPA1: more than mitochondria? Biochim. Biophys. Acta. 2013;1833:176–183. doi: 10.1016/j.bbamcr.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Olichon A., Guillou E., Delettre C., Landes T., Arnauné-Pelloquin L., Emorine L.J., Mils V., Daloyau M., Hamel C., Amati-Bonneau P., et al. Mitochondrial dynamics and disease. Biochim. Biophys. Acta. 2006;1763:500–509. doi: 10.1016/j.bbamcr.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Yu R., Lendahl U., Nistér M., Zhao J. Regulation of mammalian mitochondrial dynamics: opportunities and challenges. Front. Endocrinol. 2020;11:374. doi: 10.3389/fendo.2020.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Brito O.M., Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 26.Misko A., Jiang S., Wegorzewska I., Milbrandt J., Baloh R.H. Mitofusin 2 is necessary for transport of axonal mitochondria and interacts with the Miro/Milton complex. J. Neurosci. 2010;30:4232–4240. doi: 10.1523/JNEUROSCI.6248-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guillet V., Gueguen N., Verny C., Ferre M., Homedan C., Loiseau D., Procaccio V., Amati-Bonneau P., Bonneau D., Reynier P., Chevrollier A. Adenine nucleotide translocase is involved in a mitochondrial coupling defect in MFN2-related Charcot–Marie–Tooth type 2A disease. Neurogenetics. 2010;11:127–133. doi: 10.1007/s10048-009-0207-z. [DOI] [PubMed] [Google Scholar]
- 28.Chen H., Chan D.C. Mitochondrial dynamics-fusion, fission, movement, and mitophagy-in neurodegenerative diseases. Hum. Mol. Genet. 2009;18:R169–R176. doi: 10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adebayo M., Singh S., Singh A.P., Dasgupta S. Mitochondrial fusion and fission: the fine-tune balance for cellular homeostasis. Faseb. J. 2021;35 doi: 10.1096/fj.202100067R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X., Su B., Lee H.g., Li X., Perry G., Smith M.A., Zhu X. Impaired balance of mitochondrial fission and fusion in alzheimer’s disease. J. Neurosci. 2009;29:9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Y., Carmona S., Muhammad A.K.M.G., Bell S., Landeros J., Vazquez M., Ho R., Franco A., Lu B., Dorn G.W., et al. Restoring mitofusin balance prevents axonal degeneration in a Charcot-Marie-Tooth type 2A model. J. Clin. Invest. 2019;129:1756–1771. doi: 10.1172/JCI124194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan W.W., Wubben T.J., Besirli C.G. Photoreceptor metabolic reprogramming: current understanding and therapeutic implications. Commun. Biol. 2021;4:245. doi: 10.1038/s42003-021-01765-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ingram N.T., Fain G.L., Sampath A.P. Elevated energy requirement of cone photoreceptors. Proc. Natl. Acad. Sci. USA. 2020;117:19599–19603. doi: 10.1073/pnas.2001776117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiyama T., Chen C.-K., Wang S.W., Pan P., Ju Z., Wang J., Takada S., Klein W.H., Mao C.-A. Essential roles of mitochondrial biogenesis regulator Nrf1 in retinal development and homeostasis. Mol. Neurodegener. 2018;13:56. doi: 10.1186/s13024-018-0287-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu H., Prokosch V. Energy metabolism in the inner retina in health and glaucoma. Int. J. Mol. Sci. 2021;22:3689. doi: 10.3390/ijms22073689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okawa H., Sampath A.P., Laughlin S.B., Fain G.L. ATP consumption by mammalian rod photoreceptors in darkness and in light. Curr. Biol. 2008;18:1917–1921. doi: 10.1016/j.cub.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaplan A.T., Yalcin S.O., Sager S.G., Türkyılmaz A., İnan R. Evaluation of optical coherence tomography findings and visual evoked potentials in Charcot-Marie-Tooth disease. Springer. 2022;43:333–341. doi: 10.21203/rs.3.rs-1251257/v1. [DOI] [PubMed] [Google Scholar]
- 38.Leonardi L., Marcotulli C., Storti E., Tessa A., Serrao M., Parisi V., Santorelli F.M., Pierelli F., Casali C. Acute optic neuropathy associated with a novel MFN2 mutation. J. Neurol. 2015;262:1678–1680. doi: 10.1007/s00415-015-7756-x. [DOI] [PubMed] [Google Scholar]
- 39.Chintalapudi S.R., Djenderedjian L., Stiemke A.B., Steinle J.J., Jablonski M.M., Morales-Tirado V.M. Isolation and molecular profiling of primary mouse retinal ganglion cells: comparison of phenotypes from healthy and glaucomatous retinas. Front. Aging Neurosci. 2016;8:93. doi: 10.3389/fnagi.2016.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cuenca N., Fernández-Sánchez L., McGill T.J., Lu B., Wang S., Lund R., Huhn S., Capela A. Phagocytosis of photoreceptor outer segments by transplanted human neural stem cells as a neuroprotective mechanism in retinal degeneration. Invest. Ophthalmol. Vis. Sci. 2013;54:6745–6756. doi: 10.1167/iovs.13-12860. [DOI] [PubMed] [Google Scholar]
- 41.Lu B., Morgans C.W., Girman S., Lund R., Wang S. Retinal morphological and functional changes in an animal model of retinitis pigmentosa. Vis. Neurosci. 2013;30:77–89. doi: 10.1017/S0952523813000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strettoi E., Pignatelli V. Modifications of retinal neurons in a mouse model of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA. 2000;97:11020–11025. doi: 10.1073/pnas.190291097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holmes D.L., Vogt D.T., Lagunoff M. A CRISPR-Cas9 screen identifies mitochondrial translation as an essential process in latent KSHV infection of human endothelial cells. Proc. Natl. Acad. Sci. USA. 2020;117:28384–28392. doi: 10.1073/pnas.2011645117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lou G., Palikaras K., Lautrup S., Scheibye-Knudsen M., Tavernarakis N., Fang E.F. Mitophagy and neuroprotection. Trends Mol. Med. 2020;26:8–20. doi: 10.1016/j.molmed.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 45.Twig G., Elorza A., Molina A.J.A., Mohamed H., Wikstrom J.D., Walzer G., Stiles L., Haigh S.E., Katz S., Las G., et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang Z., Ren S., Jiang Y., Wang T. PINK1 and Parkin cooperatively protect neurons against constitutively active TRP channel-induced retinal degeneration in Drosophila. Cell Death Dis. 2016;7:e2179. doi: 10.1038/cddis.2016.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crook J.D., Davenport C.M., Peterson B.B., Packer O.S., Detwiler P.B., Dacey D.M. Parallel on and off cone bipolar inputs establish spatially coextensive receptive field structure of blue-yellow ganglion cells in primate retina. J. Neurosci. 2009;29:8372–8387. doi: 10.1523/JNEUROSCI.1218-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim U.S., Mahroo O.A., Mollon J.D., Yu-Wai-Man P. Retinal ganglion cells—diversity of cell types and clinical relevance. Front. Neurol. 2021;12:661938. doi: 10.3389/fneur.2021.661938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kling A., Field G.D., Brainard D.H., Chichilnisky E.J. Probing computation in the primate visual system at single-cone resolution. Annu. Rev. Neurosci. 2019;42:169–186. doi: 10.1146/annurev-neuro-070918-050233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kolb H., Marshak D. The midget pathways of the primate retina. Doc. Ophthalmol. 2003;106:67–81. doi: 10.1023/a:1022469002511. [DOI] [PubMed] [Google Scholar]
- 51.Patterson S.S., Neitz M., Neitz J. Reconciling color vision models with midget ganglion cell receptive fields. Front. Neurosci. 2019;13:865. doi: 10.3389/fnins.2019.00865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sabesan R., Schmidt B.P., Tuten W.S., Roorda A. The elementary representation of spatial and color vision in the human retina. Sci. Adv. 2016;2 doi: 10.1126/sciadv.1600797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ito Y.A., Di Polo A. Mitochondrial dynamics, transport, and quality control: a bottleneck for retinal ganglion cell viability in optic neuropathies. Mitochondrion. 2017;36:186–192. doi: 10.1016/j.mito.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 54.Narayan D.S., Chidlow G., Wood J.P.M., Casson R.J. Investigations into bioenergetic neuroprotection of cone photoreceptors: relevance to retinitis pigmentosa. Front. Neurosci. 2019;13:1234. doi: 10.3389/fnins.2019.01234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muench N.A., Patel S., Maes M.E., Donahue R.J., Ikeda A., Nickells R.W. The influence of mitochondrial dynamics and function on retinal ganglion cell susceptibility in optic nerve disease. Cells. 2021;10:1593. doi: 10.3390/cells10071593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giarmarco M.M., Brock D.C., Robbings B.M., Cleghorn W.M., Tsantilas K.A., Kuch K.C., Ge W., Rutter K.M., Parker E.D., Hurley J.B., Brockerhoff S.E. Daily mitochondrial dynamics in cone photoreceptors. Proc. Natl. Acad. Sci. USA. 2020;117:28816–28827. doi: 10.1073/pnas.2007827117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berman S.B., Pineda F.J., Hardwick J.M. Mitochondrial fission and fusion dynamics: the long and short of it. Cell Death Differ. 2008;15:1147–1152. doi: 10.1038/cdd.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giacomello M., Pyakurel A., Glytsou C., Scorrano L. The cell biology of mitochondrial membrane dynamics. Nat. Rev. Mol. Cell Biol. 2020;21:204–224. doi: 10.1038/s41580-020-0210-7. [DOI] [PubMed] [Google Scholar]
- 59.Westermann B. Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 2010;11:872–884. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- 60.Zaman M., Shutt T.E. The role of impaired mitochondrial dynamics in MFN2-mediated pathology. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.858286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Botsford B., Vuong L.N., Hedges T.R., Mendoza-Santiesteban C.E. Characterization of Charcot-Marie-Tooth optic neuropathy. J. Neurol. 2017;264:2431–2435. doi: 10.1007/s00415-017-8645-2. [DOI] [PubMed] [Google Scholar]
- 62.Liu Y., Eastwood J.D., Alba D.E., Velmurugan S., Sun N., Porciatti V., Lee R.K., Hauswirth W.W., Guy J., Yu H. Gene therapy restores mitochondrial function and protects retinal ganglion cells in optic neuropathy induced by a mito-targeted mutant ND1 gene. Gene Ther. 2022;29:368–378. doi: 10.1038/s41434-022-00333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gamm D.M., Wang S., Lu B., Girman S., Holmes T., Bischoff N., Shearer R.L., Sauvé Y., Capowski E., Svendsen C.N., Lund R.D. Protection of visual functions by human neural progenitors in a rat model of retinal disease. PLoS One. 2007;2:e338. doi: 10.1371/journal.pone.0000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsai Y., Lu B., Bakondi B., Girman S., Sahabian A., Sareen D., Svendsen C.N., Wang S. Human iPSC-derived neural progenitors preserve vision in an AMD-like model. Stem Cell. 2015;33:2537–2549. doi: 10.1002/stem.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu B., Morgans C.W., Girman S., Luo J., Zhao J., Du H., Lim S., Ding S., Svendsen C., Zhang K., Wang S. Neural stem cells derived by small molecules preserve vision. Transl. Vis. Sci. Technol. 2013;2:1. doi: 10.1167/tvst.2.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang S., Lu B., Girman S., Duan J., McFarland T., Zhang Q.s., Grompe M., Adamus G., Appukuttan B., Lund R. Non-invasive stem cell therapy in a rat model for retinal degeneration and vascular pathology. PLoS One. 2010;5 doi: 10.1371/journal.pone.0009200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shahin S., Banerjee S., Swarup V., Singh S.P., Chaturvedi C.M. From the cover: 2.45-GHz microwave radiation impairs hippocampal learning and spatial memory: involvement of local stress mechanism-induced suppression of iGluR/ERK/CREB signaling. Toxicol. Sci. 2018;161:349–374. doi: 10.1093/toxsci/kfx221. [DOI] [PubMed] [Google Scholar]
- 68.Shihan M.H., Novo S.G., Le Marchand S.J., Wang Y., Duncan M.K. A simple method for quantitating confocal fluorescent images. Biochem. Biophys. Rep. 2021;25 doi: 10.1016/j.bbrep.2021.100916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shahin S., Xu H., Lu B., Mercado A., Jones M.K., Bakondi B., Wang S. AAV-CRISPR/Cas9 gene editing preserves long-term vision in the P23H rat model of autosomal dominant retinitis pigmentosa. Pharmaceutics. 2022;14:824. doi: 10.3390/pharmaceutics14040824. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
9G
3-9
Data Availability Statement
-
•
This study did not generate or utilize any dataset or code.
-
•
The data that support the findings of this study are shown in this manuscript and in Supplemental Information.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.