Summary
The flip-excision switch (FLEX) system with an adeno-associated viral (AAV) vector allows expression of transgenes in specific cell populations having Cre recombinase. A significant issue with this system is non-specific expression of transgenes in tissues after vector injection. We show here that Cre-independent recombination events in the AAV genome carrying the FLEX sequence occur mainly during the production of viral vectors in packaging cells, which results in transgene expression in off-target populations. Introduction of a relatively longer nucleotide sequence between two recognition sites at the unilateral side of the transgene cassette, termed a unilateral spacer sequence (USS), is useful to suppress the recombination in the viral genome, leading to the protection of non-specific transgene expression with enhanced gene expression selectivity. Our FLEX/USS system offers a powerful strategy for highly specific Cre-dependent transgene expression, aiming at various applications for structural and functional analyses of target cell populations.
Keywords: adeno-associated viral vector, Cre-loxP recombination, flip-excision switch system, gene expression selectivity, transgene expression, unilateral spacer sequence
Graphical abstract

Highlights
-
•
Cre-independent AAV/FLEX system recombination occurs during vector production
-
•
The USS suppresses Cre-independent recombination in the viral genome
-
•
USS introduction prevents gene expression in off-target cells in tissues
-
•
The FLEX/USS system enables highly selective gene expression through Cre recombination
Motivation
There is evidence for non-specific expression of transgenes in off-target cell populations after injection of AAV vectors carrying the FLEX system into tissues, but understanding of the molecular mechanism that produces this non-specific transgene expression has been limited. Here, we investigated the possibility that Cre-independent recombination events are predominantly generated in viral genomes with the FLEX sequence during vector production in cultured cells. We then aimed to develop a viral vector technology with the USS to protect non-specific transgene expression, enhancing the selectivity of Cre-dependent gene expression in target cell populations.
A significant issue with the FLEX system is non-specific transgene expression after AAV injection. Here, Matsushita et al. find that USS insertion into the FLEX construct suppresses Cre-independent recombination and resultant transgene expression in off targets. Our FLEX/USS system provides a useful strategy for highly specific Cre-dependent transgene expression.
Introduction
Cre-loxP-mediated recombination is derived from bacteriophage P11,2 and has been used for research in a variety of experimental biology studies.3,4,5 Cre recombinase catalyzes site-specific recombination between two sites of loxP, which is a 34-bp DNA sequence containing an 8-bp asymmetrical spacer flanked by two 13-bp inverted repeats.6,7,8 An application of the Cre-loxP system has been developed to express genes of interest in specific cell populations, termed the flip-excision switch (FLEX) system.9,10,11 In this system, a gene cassette in an inverted orientation is flanked by a pair of double recognition sites, which consist of loxP and lox2272 sites with an ∼50-bp spacer, in the opposite direction with an alternate order at both ends of the cassette. These recognition sites differ by 2 nt in the asymmetrical spacer and are exclusively recombined by Cre recombinase. In the first reaction, the recombinase catalyzes reversion of the gene cassette through recombination between either of the double recognition sites. In the second reaction, it catalyzes deletion of the intervening sequence between the same two recognition sites and produces the reversed cassette with different recognition sites at both ends.
Adeno-associated viral (AAV) vectors are widely used to apply the FLEX system, in particular in the research field of neuroscience, aiming at transgene expression in specific cell populations dependent on Cre recombinase by using transgenic animals expressing the recombinase under the control of cell-type-specific promoters12,13,14,15,16 or viral vectors expressing the recombinase in specific neural circuits.17,18,19,20,21 However, there is evidence for non-specific transgene expression in off-target cell populations after treatment of AAV vectors carrying the FLEX system, which appears to arise at least from transcription from the inverted transgene in the viral genome and recombination events through a short homologous sequence within the recognition sites during plasmid amplification in bacterial cells.22 To resolve these issues, AAV vectors using mutated recognition sites with decreased homology were developed to suppress bacterial recombination events, combined with the in-frame initiation codon located upstream of the recognition sites at the 5′ end of the transgene, in which Cre-mediated recombination deletes a gene cassette containing polyadenylation signals flanked by two recognition sites.22 Another possibility that could explain non-specific transgene expression in the FLEX system may be Cre-independent recombination leading to reversion of the inverted transgene during the production of AAV vectors in packaging cells. The recombination events in the viral genome in the cells have not been fully investigated.
Protection of non-specific transgene expression in off targets is an important issue for application of the FLEX system to achieve highly selective gene expression dependent on Cre recombinase. In the present study, we found that recombination events between a pair of nucleotide sequences within the recognition sites occur mainly during the production of AAV vectors with the FLEX sequence in cultured cells, whereas the frequency of recombination is low during the plasmid amplification in bacterial cells. We then found that the introduction of a relatively longer nucleotide sequence (750 bp) between the loxP and the lox2272 sites at the unilateral side of the inverted transgene cassette, termed a unilateral spacer sequence (USS), is useful for the suppression of recombination in the viral genome, resulting in the protection of non-specific transgene expression with enhanced selectivity of Cre-dependent gene expression in brain tissues.
Results
Cre-independent recombination during AAV vector production and non-specific expression of transgene in tissues
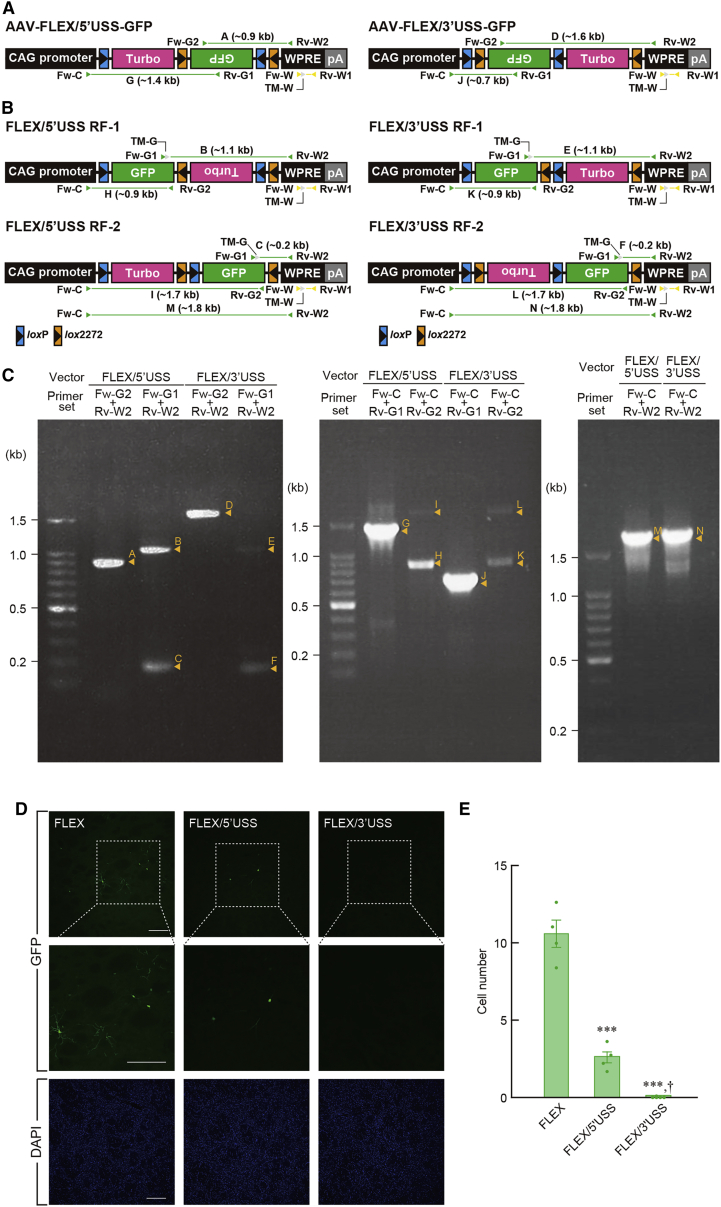
We used an AAV serotype 2 vector encoding the gene cassette for green fluorescent protein (GFP) in the inverted orientation, which is flanked by a pair of double recognition sites between the cytomegalovirus early enhancer/chicken β-actin (CAG) promoter23 and the woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) connected to the human growth factor (hGH) gene polyadenylation signal, termed the AAV-FLEX-GFP vector (see Figures 1A and S1). HEK293T cells were transfected with the plasmids for vector production, and vector particles were collected from the supernatant of cell lysates. To test whether vector particles contain recombinant forms (RFs) carrying the viral genome with the reversed gene cassette, we measured the total and recombinant vector titers by using quantitative polymerase chain reaction (PCR) with the following primer sets (Figures 1A and 1B). The quantitative PCR with the primer set Fw-W/Rv-W1, which amplifies a part of the WPRE sequence, and the primer set Fw-G1/Rv-W2, which amplifies a part containing the 3′ region of a reversed GFP sequence, provided a total vector titer of 3.16 ± 0.43 × 1012 genome copies/mL and a recombinant vector titer of 3.65 ± 0.48 × 1010 genome copies/mL (n = 4 samples), respectively. The frequency of recombination, defined as the percentage of the recombinant vector titer divided by the total vector titer, was 1.16% ± 0.02% (n = 4 samples).
Figure 1.
Cre-independent recombination events and non-specific transgene expression after vector injection
(A) Genome structure of AAV-FLEX-GFP vector. The GFP gene cassette in the inverted orientation is flanked by two double recognition sites and located between the CAG promoter and the WPRE sequence connected to the hGH polyadenylation signal (pA).
(B) Structures of the RF genomes contained in AAV-FLEX-GFP vector particles. Arrowheads indicate the position and direction of forward primers (Fw), reverse primers (Rv), and TaqMan probes (TM) for PCR and quantitative PCR amplification. DNA fragments with their sizes obtained from PCR amplification are indicated.
(C) Analysis of amplified DNA fragments. PCR amplification was carried out with viral genome of 1.0 × 106 genome copies as a template using the indicated primer sets, and PCR products were subjected to 1% agarose gel electrophoresis.
(D) Schematic illustration of intracranial injection of AAV vector. Anteroposterior coordinates (mm) from bregma are shown, and dots indicate the injection sites in the striatum.
(E) GFP immunohistochemistry with striatal sections. Wild-type rats were unilaterally given the injection of the vector with different titers into the striatum, and sections through the striatum were immunostained with anti-GFP antibody. Sections were also stained with DAPI. Representative images of the staining are shown for each vector titer. Middle images show magnified views of the squares in the upper images.
(F) Titer-dependent increase in the number of GFP+ cells. Data are presented as mean ± SEM (n = 4 animals). Individual data are overlaid. ∗p < 0.05 and ∗∗∗p < 0.001 compared with the lowest titer (Bonferroni test). Scale bars: 1 mm (D) and 200 μm (E). See also Figures S1 and S2 and Table S1.
To characterize the structures of the viral genomes of the RFs in detail, we carried out PCR amplification of vector particles with different primer sets (Figures 1A and 1B) and detected PCR products using 1% agarose gel electrophoresis (Figure 1C). The Fw-G2/Rv-W2 primer set generated a DNA band of ∼0.9 kb, or fragment A in the original viral genome. The primer set Fw-G1/Rv-W2 provided two kinds of DNA band of ∼0.3 and ∼0.2 kb (fragments B and C, respectively) in the RF genomes. The Fw-C/Rv-G1 primer set gave a DNA band of ∼0.7 kb (fragment D) in the original genome, and the Fw-C/Rv-G2 primer set produced two DNA bands of ∼0.9 and ∼1.1 kb (fragments E and F, respectively) in the RFs. Nucleotide sequence analysis of fragments B and E indicated the primary structure of RF-1, which was composed of the reversal of the inverted GFP sequence flanked by a single loxP site at the 5′ end and by lox2272-loxP-lox2272 sites at the 3′ end (Figure S2A), and the analysis of fragments C and F showed the structure of RF-2, which contained the reversed GFP sequence flanked by loxP-lox2272-loxP sites at the 5′ end and by a single lox2272 site at the 3′ end (Figure S2A). The structures of RF-1 and RF-2 coincided with those of the recombinants formed through the first recombination in the FLEX system, suggesting that these RFs seem to be produced, in the absence of Cre recombinase, through recombination events between a pair of nucleotide sequences within the loxP or lox2272 site (see Figure S2B). In addition, PCR amplification with the Fw-C/Rv-W2 primer set, which amplifies a DNA fragment from the 3′ portion of the CAG promoter to the 5′ portion of WPRE, displayed only a single band of ∼1.2 kb (fragment G) and did not detect any smaller bands corresponding to DNA fragments deduced from deletion between a pair of recognition sites in the same orientation (Figure 1C), suggesting that the second recombination between the two recognition sites rarely occurs in the vector particles.
There is a possibility that the RFs with the reversion of the inverted gene cassette are contained in the transfer plasmid pAAV-FLEX-GFP DNA preparation. To search for the presence of the recombinants, we carried out quantitative PCR for the titration of the vector particles and measured the concentrations of total and recombinant plasmids. The recombination frequency in the plasmid preparation, defined as the percentage of the recombinant plasmid concentration divided by the total plasmid concentration, was (7.14 ± 0.96) × 10−3% (see Table S1), indicating a low frequency of recombination events during the plasmid amplification in bacterial cells. The value corresponded to a ratio of 5.8 × 10−3, relative to the recombination frequency in the AAV-FLEX-GFP vector. The difference in the frequency between vector and plasmid preparations suggests that the majority of Cre-independent recombination in the viral genome carrying the FLEX sequence occurs during AAV vector production in packaging cells.
Next, to examine whether the RFs in vector particles cause non-specific transgene expression in the brain tissues, we performed an intracranial injection of the vector with different total titers (0.3 × 1012 to 2.4 × 1012 genome copies/mL) into the striatum of Long Evans rats (see Figure 1D). Sections through the striatum were stained by immunohistochemistry with anti-GFP antibody. Representative images of immunostaining are shown in Figure 1E. The number of GFP+ cells in regions of interest (ROIs; 1.0 × 1.0 mm) in the striatum was counted and the average cell number was calculated. The cell number increased gradually, along with the increasing titers (Figure 1F; one-way ANOVA, F3,12 = 19.621, p < 0.001). The number at the higher titers of 1.2 × 1012 and 2.4 × 1012 genome copies/mL (6.72 ± 0.70 and 10.97 ± 1.48 cells, respectively, n = 4 animals) indicated significant increases compared with that at the lowest titer of 0.3 × 1012 genome copies/mL (1.94 ± 0.42 cells, n = 4 animals) (Bonferroni test; p < 0.05 and p < 0.001, respectively). These data show that the RFs in vector particles result in titer-dependent, non-specific transgene expression after AAV vector treatment of the wild-type brains.
Suppression of Cre-independent recombination and non-specific transgene expression by the FLEX system with the USS
To perform the selective transgene expression based on the FLEX system, we need to suppress Cre-independent recombination in the viral genome and non-specific transgene expression in the tissues after vector injection. In our preliminary experiments, we generated a modified AAV vector carrying the FLEX system, in which the gene encoding TurboFP63524 was introduced between the loxP and the lox2272 sites at the 5′ end of the inverted GFP cassette to develop a strategy to distinguish Cre+ and Cre− populations in cells transduced by the AAV vector. We aimed to label Cre− cells with TurboFP635 expression and to induce GFP expression in Cre+ cells through Cre-dependent recombination. During the experiments, we observed that the introduction of the TurboFP635 gene between double recognition sites suppressed non-specific GFP expression following vector injection into the wild-type rat brains. Based on these observations, we hypothesized that the spacing between double recognition sites with a relatively longer sequence may be able to prevent recombination events in the AAV genome with the FLEX sequence. To test this possibility, we introduced a nucleotide sequence of 750 bp containing the TurboFP635 gene between the loxP and the lox2272 sites at either the 5′ or the 3′ end as a USS, resulting in the AAV-FLEX/5′USS-GFP or AAV-FLEX/3′USS-GFP vector (see Figures 2A and S3 and S4). AAV vectors were produced and vector particles were collected. Total and recombinant vector titers were measured using quantitative PCR similar to the case of the AAV-FLEX-GFP vector (see Figures 2A and 2B). The frequency of recombination showed a significant difference among the three vectors (Table S2; one-way ANOVA, F2,9 = 92.177, p < 0.001), and the frequency was markedly reduced in the AAV-FLEX/5′USS-GFP (0.15% ± 0.04%, n = 4 samples) and AAV- FLEX/3′USS-GFP (0.02% ± 0.01%, n = 4 samples) vectors compared with the AAV-FLEX-GFP vector (1.22% ± 0.11%, n = 4 samples) (Bonferroni test, p < 0.001). In particular, the extent of reduction was greater in the AAV-FLEX/3′USS-GFP vector than the AAV-FLEX/5′USS-GFP vector.
Figure 2.
Suppression of Cre-independent, non-specific transgene expression by using the USS
(A) Genome structures of the AAV-FLEX/5′USS-GFP and AAV-FLEX/3′USS-GFP vectors. The TurboFP635 sequence as the USS was introduced between the loxP and the lox2272 sites at either the 5′ or the 3′ end of the inverted GFP gene cassette in the AAV-FLEX-GFP vector, resulting in AAV-FLEX/5′USS-GFP and AAV-FLEX/3′USS-GFP vectors. pA, hGH polyadenylation signal.
(B) Genome structures of RFs in AAV-FLEX/5′USS-GFP and AAV-FLEX/3′USS-GFP vector particles. Arrowheads indicate the position and direction of forward primers (Fw), reverse primers (Rv), and TaqMan probes (TM) for PCR and quantitative PCR amplification. DNA fragments with their sizes obtained from PCR amplification are presented.
(C) Analysis of amplified DNA fragments. PCR amplification was carried out with 1.0 × 106 viral genome copies as a template by using the indicated primer sets, and PCR products were subjected to 1% agarose gel electrophoresis.
(D) GFP immunohistochemistry with striatal sections. Wild-type rats received injections of three kinds of vectors into the striatum, and striatal sections were used for GFP immunohistochemistry, together with DAPI staining. Representative images of the staining are shown for each vector. Middle images are magnified views of the squares in the upper images.
(E) Introduction of the USS into the vector markedly reduced the number of GFP+ cells. Data are presented as mean ± SEM (n = 4 animals). Individual data are overlaid. ∗∗∗p < 0.001 compared with the AAV-FLEX-GFP vector, †p < 0.05 compared with the AAV-FLEX/5′USS-GFP vector (Bonferroni test). Scale bars: 200 μm (D). See also Figures S3–S7 and Tables S1 and S2.
We characterized viral genome structures of the RFs in the AAV-FLEX/5′USS-GFP and AAV-FLEX/3′USS-GFP vectors using PCR amplification with the same primer sets as those used for the AAV-FLEX-GFP vector (see Figures 2A and 2B). PCR products were detected by 1% agarose gel electrophoresis (Figure 2C). First, the structures of the RFs in AAV-FLEX/5′USS-GFP particles were analyzed. The Fw-G2/Rv-W2 primer set gave a DNA band of ∼0.9 kb (fragment A) in the original viral genome, and the Fw-G1/Rv-W2 primer set generated two DNA bands of ∼1.1 and ∼0.2 kb (fragments B and C, respectively) in the RF genomes. The Fw-C/Rv-G1 primer set gave a DNA band of ∼1.4 kb (fragment G) in the original genome, and the Fw-C/Rv-G2 primer set produced two DNA bands of ∼0.9 and ∼1.7 kb (fragments H and I, respectively) in the RFs. Nucleotide sequence determination of fragments B and H indicated the primary structure of RF-1, which consisted of a single loxP site, a reversed GFP sequence, a single lox2272 site, an inverted TurboFP635 sequence, and loxP-lox2272 sites, and the analysis of fragments C and I showed the structure of RF-2, which contained a single loxP site, a TurboFP635 sequence, lox2272-loxP sites, a reversed GFP sequence, and a single lox2272 site (Figure S5). Second, the RF structures in the AAV-FLEX/3′USS-GFP particles were analyzed. The Fw-G2/Rv-W2 primer set gave a DNA band of ∼1.6 kb (fragment D) in the original genome, and the Fw-G1/Rv-W2 primer set generated two DNA bands of ∼1.1 and ∼0.2 kb (fragments E and F, respectively) in the RFs. The Fw-C/Rv-G1 primer set gave a DNA band of ∼0.7 kb (fragment J) in the original genome, and the Fw-C/Rv-G2 primer set produced two DNA bands of ∼0.9 and ∼1.7 kb (fragments K and L, respectively) in the RFs. Nucleotide sequencing of fragments E and K showed the primary structure of RF-1, which consisted of a single loxP site, a reversed GFP sequence, lox2272-loxP sites, a TurboFP635 sequence, and a single lox2272 site, and the sequencing of fragments F and L displayed the structure of RF-2, which included loxP-lox2272 sites, an inverted Turbo FP635 sequence, a single loxP site, a reversed GFP sequence, and a single lox2272 site (Figure S6). In addition, PCR amplification with the Fw-C/Rv-W2 primer set produced only a single major band of ∼1.8 kb (fragments M and N for AAV-FLEX/5′USS-GFP and AAV-FLEX/3′USS-GFP, respectively), showing no smaller bands equivalent to DNA fragments deduced from recombination between a pair of recognition sites in the same orientation (Figure 2C). These results suggest that RF-1 and RF-2 in the two kinds of vectors, similar to the case of the AAV-FLEX-GFP vector, are generated via recombination events between nucleotide sequences within either of the double recognition sites (see Figure S7) and that the deletion between recognition sites in the same direction does not efficiently happen in the two vectors. However, the frequency of recombination in AAV-FLEX/5′USS-GFP and AAV-FLEX/3′USS-GFP particles had largely declined compared with that in the AAV-FLEX-GFP vector (Table S2), suggesting that the presence of the USS between the loxP and the lox2272 sites in the FLEX system interferes with Cre-independent recombination events in vector preparations.
We examined the presence of RFs with the reversion of the inverted GFP cassette in the transfer plasmids pAAV-FLEX/5′USS-GFP and pAAV-FLEX/3′USS-GFP with quantitative PCR. The recombination frequencies were profoundly decreased in the AAV-FLEX/5′USS-GFP ([6.24 ± 1.09] × 10−4%, n = 4 samples) and AAV- FLEX/3′USS-GFP ([1.99 ± 0.08] × 10−4%, n = 4 samples) plasmids compared with the AAV-FLEX-GFP plasmid ([7.14 ± 0.96] × 10−3%, n = 4 samples) (Bonferroni test, p < 0.001) (Table S1). The data suggest that the insertion of the USS between the recognition sites appears to decrease the frequency of recombination events during plasmid amplification in bacterial cells. However, the amplitude of the recombination frequency in the plasmid preparations was much lower than the values in the corresponding vector particles, showing the ratios of 4.1 × 10−3 and 1.0 × 10−2, relative to the recombination frequency in the AAV-FLEX/5′USS-GFP and AAV-FLEX/3′USS-GFP vectors, respectively (compare with Table S2). These data support that Cre-independent recombination events are predominantly generated in the viral genome during the production of AAV vectors in packaging cells, as observed in the case of the AAV-FLEX-GFP vector, suggesting that the USS insertion is effective for the prevention of recombination events during the vector production.
We then investigated whether the decreased recombination frequency from using the USS indeed protects non-specific expression of transgenes in the wild-type brains. The AAV-FLEX-GFP, AAV-FLEX/5′USS-GFP, and AAV-FLEX/3′USS-GFP vectors with equivalent total titers (2.4 × 1012 genome copies/mL) were injected into the striatum of the wild-type rats, and striatal sections were stained by GFP immunohistochemistry. Representative images of immunostaining are shown in Figure 2D. The number of GFP+ cells showed a significant difference among the three kinds of vectors (Figure 2E; one-way ANOVA, F2,9 = 98.965, p < 0.001). The cell numbers for the AAV-FLEX/5′USS-GFP (2.63 ± 0.41 cells, n = 4 animals) and AAV-FLEX/3′USS-GFP (0.03 ± 0.03 cells, n = 4 animals) vectors were significantly reduced compared with that for the AAV-FLEX-GFP vector (10.59 ± 0.87 cells, n = 4 animals) (Bonferroni test, p < 0.001), and the number for AAV-FLEX/3′USS-GFP was even lower than that for AAV-FLEX/5′USS-GFP (Bonferroni test, p < 0.05). These data demonstrate that the use of USS in the FLEX system has a suppressive effect on non-specific transgene expression in wild-type brains and that 3′USS is more efficient than 5′USS at protecting non-specific gene expression.
Highly selective Cre-dependent transgene expression by the FLEX/USS system
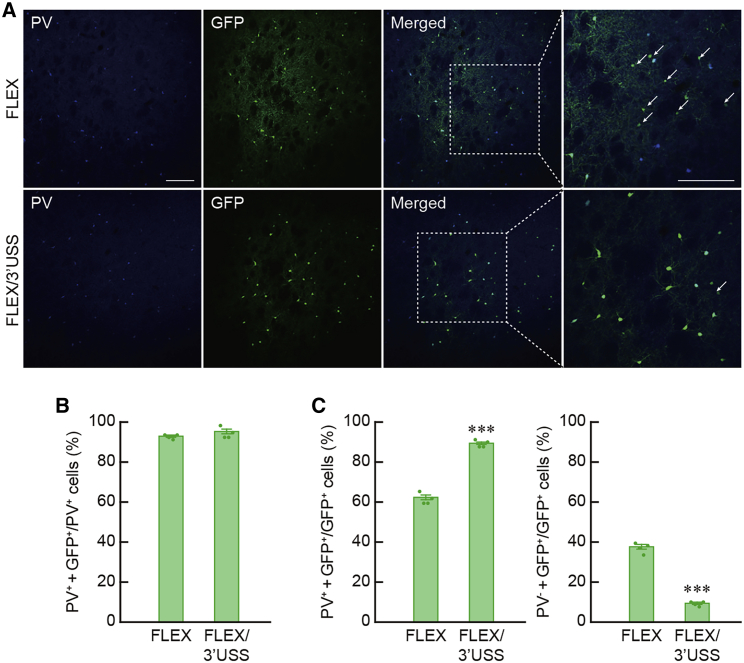
To ascertain whether the selectivity of Cre-dependent transgene expression is improved by the FLEX system with the USS, we examined the efficacy of USS introduction in the selectivity of Cre-dependent transgene expression in the brain tissues. In this experiment, we compared the efficacy of the AAV-FLEX/3′USS-GFP vector to that of the AAV-FLEX-GFP vector, because this vector showed a greater effect on the protection of non-specific transgene expression compared with the AAV-FLEX/5′USS-GFP vector. The AAV-FLEX-GFP and AAV-FLEX/3′USS-GFP vectors (2.4 × 1012 genome copies/mL) were injected into the striatum of knockin transgenic rats expressing Cre under the control of the gene encoding parvalbumin (PV).25 Double immunohistochemistry for PV and Cre indicated that Cre transgene was expressed in all PV-containing neurons in the striatum of PV-Cre-knockin rats (data not shown). Sections through the striatum were used for double immunohistochemistry for PV and GFP, and typical images of immunostaining are shown in Figure 3A. The percentages of PV+ + GFP+ cells in the total PV+ cells were 93.53% ± 0.53% for the AAV-FLEX-GFP vector (n = 4 animals) and 95.00% ± 1.24% for the AAV-FLEX/3′USS-GFP vector (n = 4 animals) (Figure 3B; Student’s t test, t6 = 1.092, p = 0.317), indicating the efficient expression of GFP in PV+ neurons in both vector-injected rats. In contrast, the percentage of PV+ + GFP+ cells in the total GFP+ cells was significantly increased in the AAV-FLEX/3′USS-GFP vector (89.97% ± 0.69%) compared with the AAV-FLEX-GFP vector (62.34% ± 1.24%), whereas the percentage of PV− + GFP+ cells in the total GFP+ cells was markedly decreased in the AAV-FLEX/3′USS-GFP vector (10.03% ± 0.69%) compared with the AAV-FLEX-GFP vector (37.66% ± 1.24%) (Figure 3C; Student’s t test, t6 = 19.435, p < 0.001), indicating the improved selectivity of Cre-dependent transgene expression by the FLEX system with the 3′USS.
Figure 3.
Improved selectivity of Cre-dependent transgene expression through the FLEX/USS system
(A) Double immunohistochemistry for PV and GFP with striatal sections. PV-Cre-knockin rats were given an injection of AAV-FLEX-GFP or AAV-FLEX/3′USS-GFP vector unilaterally into the striatum, and sections through the striatum were used for double immunohistochemistry with anti-PV and anti-GFP antibodies. Typical images of double immunostaining are presented for each vector. Photos on the right are magnified views of the squares in the merged photos. Arrows indicate PV−/GFP+ cells.
(B) High frequency of Cre-dependent expression by the FLEX/USS system. The percentage of PV+/GFP+ cells in the total PV+ cells was calculated.
(C) Improved selectivity of Cre-dependent expression by using the USS. The percentages of PV+/GFP+ or PV−/GFP+ cells in the total GFP+ cells were calculated. Data are presented as mean ± SEM (n = 4 animals). Individual data are overlaid. ∗∗∗p < 0.001 compared with AAV-FLEX-GFP vector (Student’s t test). Scale bars: 200 μm (A).
Influence of transgene size on Cre-independent recombination and non-specific expression in off-target cells
The reduced frequency of Cre-independent recombination may be due to the longer size of transgenes between two loxP sites in the AAV-FLEX/5′-USS-GFP vector or two lox2272 sites in the AAV-FLEX/3′USS-GFP vector, but not the spacing between the loxP and the lox2272 sites at the unilateral side. To address this possibility, we constructed a transfer plasmid termed pAAV-FLEX-Turbo/GFP, in which the TurboFP635 sequence was introduced between the double-floxed site at the 5′ side and the inverted GFP gene cassette (see Figure 4A). In this construct, the transgene size between both the two loxP sites and the two lox2272 sites became longer, with no USS insertion between the recognition sites. We prepared the AAV-FLEX-Turbo/GFP vector, together with the AAV-FLEX-GFP vector as the control, and measured total and recombinant vector titers by quantitative PCR (Figures 4A and 4B). For the recombinant vector titer, we used the primer set Fw-C/Rv-G3, which amplifies a part containing the 5′ region of a reversed sequence. The recombination frequency showed no significant difference between AAV-FLEX-Turbo/GFP (0.76% ± 0.02%, n = 4 samples) and AAV-FLEX-GFP (0.69% ± 0.02%, n = 4 samples) vectors (Table S3; Student’s t test, t6 = 2.406, p = 0.053). In addition, we examined non-specific expression of the transgene after the injection of the vectors into the striatum of the wild-type rats. Sections were stained by immunohistochemistry for GFP, and representative images are shown in Figure 4C. The cell number for the AAV-FLEX-Turbo/GFP vector (13.22 ± 0.84 cells, n = 4 animals) was similar to that for the AAV-FLEX-GFP vector (10.22 ± 1.28 cells, n = 4 animals) (Student’s t test, t6 = 1.959, p = 0.098) (Figure 4D). Furthermore, we examined the impact of transgene size on the selectivity of Cre-dependent gene expression in the brain tissues. The two kinds of vectors were injected into the striatum of PV-Cre-knockin rats. Sections were used for double immunohistochemistry for PV and GFP, and typical images of immunostaining are shown in Figure 4E. The percentages of PV+ + GFP+ cells in the PV+ cells were 95.50% ± 1.53% for AAV-FLEX-GFP vector (n = 4 animals) and 95.55% ± 1.20% for AAV-FLEX-Turbo/GFP vector (n = 4 animals) (Figure 4F; Student’s t test, t6 = 0.024, p = 0.982), indicating a similarity of the transgene expression frequency in PV+ neurons between both vector-injected rats. The percentage of PV+ + GFP+ cells in the total GFP+ cells was similar between AAV-FLEX-GFP (72.47% ± 2.02%) and AAV-FLEX-Turbo/GFP (68.59% ± 1.63%), and the percentage of PV− + GFP+ cells in the GFP+ cells was also similar between the two vectors (27.53% ± 2.02% for AAV-FLEX-GFP and 31.41% ± 1.63% for AAV-FLEX-Turbo/GFP, n = 4 animals) (Figure 4G; Student’s t test, t6 = 1.496, p = 0.185). These results exclude the possibility that the longer transgene size between the recognition sites reduces the recombination frequency and non-specific transgene expression in off-target cells, supporting the importance of USS spacing between the loxP and the lox2272 sites for the suppression of recombination events.
Figure 4.
Impact of transgene size on Cre-independent recombination and non-specific transgene expression
(A) Genome structure of the AAV-FLEX-Turbo/GFP vector. The TurboFP635 sequence was introduced between the double-floxed site at the 5′ side and the inverted GFP gene cassette in the AAV-FLEX-GFP vector. pA, hGH polyadenylation signal.
(B) Genome structure of RFs in AAV-FLEX-Turbo/GFP vector particles. Arrowheads indicate the position and direction of forward primers (Fw) and reverse primers (Rv) for quantitative PCR amplification. DNA fragments obtained from PCR amplification are presented.
(C) GFP immunohistochemistry with striatal sections. Wild-type rats received an intrastriatal injection of two kinds of vectors, and sections were used for GFP immunostaining with DAPI staining. Representative images of the staining are shown for each vector. Middle images are magnified views of the squares in the left images.
(D) No effect of transgene size lengthening on the frequency of non-specific GFP expression. Data are presented as mean ± SEM (n = 4 animals). Individual data are overlaid.
(E) Double immunohistochemistry for PV and GFP with striatal sections. PV-Cre-knockin rats were given an intrastriatal injection of the two vectors, and sections were used for double immunohistochemistry with anti-PV and anti-GFP antibodies. Typical images of double immunostaining are presented for each vector. Right photos are magnified views of the squares in the merged photos. Arrows indicate PV−/GFP+ cells.
(F) Frequency of Cre-dependent transgene expression by the two vectors. The percentage of PV+/GFP+ cells in the total PV+ cells was calculated.
(G) Selectivity of Cre-dependent expression by the vectors. The percentage of PV+/GFP+ or PV−/GFP+ cells in the total GFP+ cells was calculated. Data are presented as mean ± SEM (n = 4 animals). Individual data are overlaid. Scale bars: 200 μm (C and E). See also Table S3.
Characterization of USS and its length in Cre-independent, non-specific transgene expression
It is important to determine whether specific sequences of TurboFP635 are involved in the suppression of Cre-independent recombination and non-specific transgene expression in the tissues. To test this issue, the TurboFP635 sequence in the AAV-FLEX/3′USS-GFP vector was substituted by a sequence of 750 bp containing the luciferase (Luc) gene derived from a marine copepod,26 with a homology of ∼32% between the TurboFP635 and the Luc genes, resulting in AAV-FLEX/3′USS(Luc)-GFP (Figure 5A). We prepared the AAV-FLEX/3′USS(Luc)-GFP vector, together with the AAV-FLEX-GFP vector as the control, and measured vector titers by quantitative PCR with the same primer sets as used for the characterization of the AAV-FLEX/3′USS vector preparation (see Figures 5A and 5B). The recombination frequency in AAV-FLEX/3′USS(Luc)-GFP vector (0.08% ± 0.01%, n = 4 samples) showed a significant reduction compared with that in AAV-FLEX-GFP vector (1.03% ± 0.16%, n = 4 samples) (Table S4; Student’s t test, t6 = 6.083, p < 0.001). In addition, we examined non-specific expression of the GFP transgene after the injection of the two vectors into the striatum of the wild-type rats. Sections were stained by immunohistochemistry for GFP (Figure 5C). The cell number for AAV-FLEX/3′USS(Luc)-GFP (0.13 ± 0.09 cells, n = 4 animals) was significantly decreased relative to that for the AAV-FLEX-GFP vector (9.56 ± 1.04 cells, n = 4 animals) (Student’s t test, t6 = 9.029, p < 0.001) (Figure 5D). These results show that the use of Luc gene as the 3′USS can repress the recombination frequency in vector particles and non-specific transgene expression in the wild-type tissues and suggest that specific sequences of TurboFP635 are not required for the suppression of Cre-independent recombination, confirming the significance of spacing between the recognition sites for the protection of the recombination events.
Figure 5.
Influence of USS on Cre-independent expression of the transgene
(A) Genome structure of the AAV-FLEX/3′USS(Luc)-GFP vector. The TurboFP635 sequence in the AAV-FLEX/3′USS-GFP vector was replaced by a sequence containing the Luc gene cassette.
(B) Genome structure of RFs contained in AAV-FLEX/3′USS(Luc)-GFP vector particles. Arrowheads indicate the position and direction of forward primers (Fw), reverse primers (Rv), and TaqMan probes (TM) for quantitative PCR. DNA fragments obtained from PCR amplification are indicated.
(C) GFP immunohistochemistry with striatal sections. Wild-type rats received an intrastriatal injection of the AAV-FLEX-GFP or AAV-FLEX/3′USS(Luc)-GFP vector, and sections were used for GFP immunostaining with DAPI staining. Representative images of the staining are shown for each vector. Middle images are magnified views of the squares in the left images.
(D) Introduction of the USS(Luc) into the vector markedly decreased the number of GFP+ cells. Data are presented as mean ± SEM (n = 4 animals). Individual data are overlaid. ∗∗∗p < 0.001 compared with the AAV-FLEX-GFP vector (Student’s t test). Scale bars: 200 μm (C). See also Table S4.
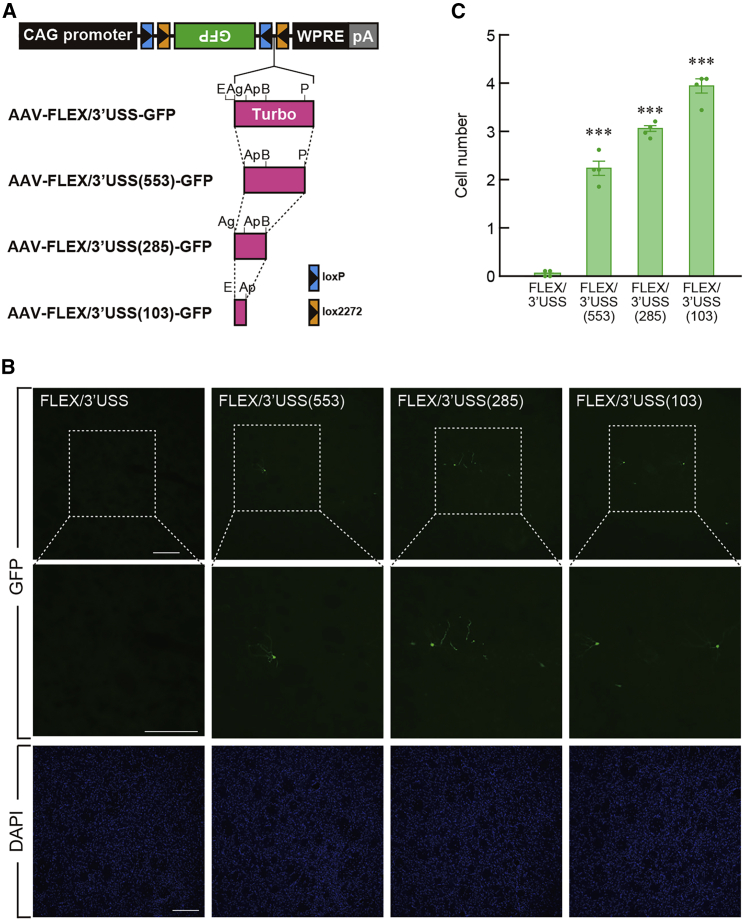
Another issue is to check the impact of USS length on non-specific transgene expression in off-target cells. AAV-FLEX/3′USS-GFP vectors were generated that contained different sizes of the USS (750 bp for the controls and 553/285/103 bp for the shortened versions) (see Figure 6A). The recombination frequency indicated a gradual increase along with the shortening of the USS (Table S5; one-way ANOVA, F3,12 = 49.130, p < 0.001). The frequency for AAV-FLEX/3′USS(553)-GFP (0.65% ± 0.07%, n = 4 samples), AAV-FLEX/3′USS(285)-GFP (0.69% ± 0.06%, n = 4 samples), or AAV-FLEX/3′USS(103)-GFP (0.78% ± 0.04%, n = 4 samples) showed a significant elevation compared with that for the AAV-FLEX/3′USS-GFP vector (0.03% ± 0.00%, n = 4 samples) (Table S5, Bonferroni test, p < 0.001). In addition, these vectors were similarly injected into the striatum of wild-type rats, and sections were immunostained for GFP (Figure 6B). The number of GFP+ cells indicated a gradual increase throughout the shortening of the USS (Figure 6C; one-way ANOVA, F3,12 = 206.512, p < 0.001). The cell number for AAV-FLEX/3′USS(553)-GFP (2.25 ± 0.15 cells, n = 4 animals), AAV-FLEX/3′USS(285)-GFP (3.06 ± 0.08 cells, n = 4 animals), or AAV- FLEX/3′USS(103)-GFP (3.94 ± 0.15 cells, n = 4 animals) was significantly larger than that for the AAV-FLEX/3′USS-GFP vector (0.06 ± 0.04 cells, n = 4 animals) (Bonferroni test, p < 0.001). These data highlight that the distance between the recognition sites at the unilateral side is important for the protection of non-specific transgene expression and that 3′-USS at the size of 750 bp is at least necessary for intense protection.
Figure 6.
Effect of USS length on Cre-independent expression of the transgene
(A) Genome structure of AAV-FLEX/3′USS-GFP vectors with different sizes of the USS. The TurboFP635 sequence in the AAV-FLEX/3′USS-GFP vector was replaced by its shorter fragments of 553, 285, or 103 bp. Restriction enzyme site abbreviations: Ag, AgeI; Ap, ApaLI; B, Bsu36I; E, Eco47III; and P, PshAI. pA, hGH polyadenylation signal.
(B) GFP immunohistochemistry with striatal sections. Wild-type rats were given an intrastriatal injection of four kinds of vectors, and sections were immunostained for GFP and stained with DAPI. Typical immunostaining images are indicated for each vector. Middle images are magnified views of the squares in the upper images.
(C) Shortening of 3′USS gradually increased the GFP+ cell number. Data are presented as mean ± SEM (n = 4 animals). Individual data are overlaid. ∗∗∗p < 0.001 compared with AAV-FLEX/3′USS-GFP vector (Bonferroni test). Scale bars: 200 μm (B). See also Table S5.
The FLEX/USS system prevents non-specific expression of other transgenes with increased selectivity of gene expression
To search for whether the FLEX/USS system is applicable for the selective expression of other transgenes, we converted the inverted transgene in the gene cassette of the AAV-FLEX-GFP or AAV-FLEX/3′USS-GFP vector from GFP to a variant of channelrhodopsin-2 fused to Venus (ChRWR/Venus),27 a mutant form of the human muscarinic M4 acetylcholine receptor connected to 2A-GFP (hM4Di/2A/GFP),19 or a mutant form of red fluorescent protein (RFP; mCherry)28 (Figure 7A). The CAG promoter in these vectors was changed to the shorter promoter of the gene encoding human elongation factor-1α (EF-1α),29 and 11 in-frame methionine codons in the TurboFP635 sequence were mutated to the termination codon TAG to prevent the synthesis of TurboFP635 protein or its fragments. First, we checked non-specific transgene expression in the wild-type brain tissues. AAV vectors with equivalent titers between the corresponding transgenes (2.1 × 1012 genome copies/mL for ChRWR/Venus, 2.4 × 1012 genome copies/mL for hMD4i/2A/GFP, and 2.2 × 1012 genome copies/mL for mCherry) were injected into the rat striatum. Sections were immunostained with anti-GFP or anti-RFP antibody, and typical images of the staining are presented in Figure 7B. In the ChRWR/Venus transgene, the number of immunopositive cells for the AAV-FLEX/3′USS-ChRWR/Venus vector (0.47 ± 0.19 cells, n = 4 animals) showed a significant reduction compared with that for the AAV-FLEX-ChRWR/Venus vector (6.28 ± 0.89 cells, n = 4 animals) (Figure 7C; Student’s t test, t6 = 6.390, p < 0.001). For the hM4Di/2A/GFP transgene, the immunopositive cell number for the AAV-FLEX/3′USS-hM4Di/2A/GFP vector (1.14 ± 0.26 cells, n = 4 animals) was significantly decreased compared with that for the AAV-FLEX-hM4Di/2A/GFP vector (5.13 ± 0.71 cells, n = 4 animals) (Figure 7C; Student’s t test, t6 = 5.236, p < 0.01). For the mCherry gene, the number of positive cells for the AAV-FLEX/3′USS-mCherry vector (1.05 ± 0.32 cells, n = 4 animals) was also significantly lower than that for the AAV-FLEX-mCherry vector (7.32 ± 0.51 cells, n = 4 animals) (Figure 7C; Student’s t test, t6 = 10.491, p < 0.001). These data indicate that the FLEX system with the 3′USS can suppress non-specific expression of other transgenes in wild-type brains.
Figure 7.
Silencing Cre-independent expression of other transgenes with enhanced gene expression selectivity by using the USS
(A) Genome structure of AAV-FLEX/3′USS vectors for expression of ChRWR/Venus, hM4Di/2A/GFP, and mCherry transgenes. The mTurbo sequence was used as the 3′USS. pA, hGH polyadenylation signal.
(B) Immunostaining with anti-GFP or anti-RFP antibody with striatal sections. Wild-type rats received an intrastriatal injection of the AAV-FLEX and AAV-FLEX/3′USS vectors encoding three kinds of transgenes with equivalent titers between the corresponding vectors, and sections were stained for GFP or RFP. Typical immunostaining images are presented for each vector. Right, magnified views of the squares in the left photos.
(C) Introduction of the 3′USS into the vectors suppresses the number of cells expressing the transgenes. Data are presented as mean ± SEM (n = 4 animals). Individual data are overlaid. ∗∗p < 0.01, ∗∗∗p < 0.001 compared with the corresponding AAV-FLEX vectors (Student’s t test).
(D) Double immunohistochemistry for PV and ChRWR/Venus with striatal sections. PV-Cre-knockin rats received an intrastriatal injection of AAV-FLEX-ChRWR/Venus or AAV-FLEX/3′USS-ChRWR/Venus vector, and sections were used for double immunohistochemistry with anti-PV and anti-GFP antibodies. Typical images of double immunostaining are shown for each vector. Right, magnified views of the squares in the merged photos. Arrows indicate PV−/ChRWR/Venus+ cells.
(E) High efficiency of Cre-dependent expression by the FLEX/USS system. The percentage of PV+/ChRWR/Venus+ cells in the total PV+ cells was calculated.
(F) Enhanced selectivity of Cre-dependent expression by the FLEX/USS system. The percentage of PV+/ChRWR/Venus+ or PV−/ChRWR/Venus+ cells in the total ChRWR/Venus+ cells was calculated. Data are presented as mean ± SEM (n = 4 animals). Individual data are overlaid. ∗∗∗p < 0.001 compared with AAV-FLEX-ChRWR/Venus vector (Student’s t test). Scale bars: 200 μm (B and D).
To assess the selectivity of Cre-dependent expression of other transgenes using the FLEX/USS system, we studied the expression patterns of the ChRWR/Venus transgene via injection with the AAV-FLEX-ChRWR/Venus and AAV-FLEX/3′USS-ChRWR/Venus vectors (2.1 × 1012 genome copies/mL) into the striatum of PV-Cre-knockin rats. Sections were stained by double immunohistochemistry with anti-PV and anti-GFP antibodies, and typical images of immunostaining are presented in Figure 7D. The percentage of PV+ + ChRWR/Venus+ cells in the total PV+ cells was 78.45% ± 1.57% for AAV-FLEX-ChRWR/Venus vector (n = 4 animals) and 79.22% ± 1.38% for AAV-FLEX/3′USS-ChRWR/Venus vector (n = 4 animals) (Figure 7E; Student’s t test, t6 = 0.370, p = 0.724), indicating the efficient expression of ChRWR/Venus in PV+ neurons in both vector-injected rats. In contrast, the percentage of PV+ + ChRWR/Venus+ cells in the total ChRWR/Venus+ cells was significantly increased for the AAV-FLEX/3′USS-ChRWR/Venus vector (90.25% ± 0.33%) compared with the AAV-FLEX-ChRWR/Venus vector (60.45% ± 0.89%), whereas the percentage of PV− + ChRWR/Venus+ cells in the total ChRWR/Venus+ cells was markedly decreased for the AAV-FLEX/3′USS-ChRWR/Venus vector (9.75% ± 0.33%) relative to the AAV-FLEX-ChRWR/Venus vector (39.55% ± 0.89%) (Figure 7F; Student’s t test, t6 = 31.257, p < 0.001). These results support the enhanced selectivity of Cre-dependent transgene expression by the FLEX system with the 3′USS.
Discussion
In the present study, we observed that the majority of Cre-independent recombination events in the AAV genome with the FLEX sequence occurred during vector production in cultured cells and appeared to be generated through recombination between a pair of short nucleotide sequences within the loxP or lox2272 sites. In contrast, the extent of the recombination in the plasmid preparations was lower, with ratios ranging from 4.1 × 10−3 to 1.0 × 10−2, relative to the recombination frequency in viral vectors. The frequency of Cre-independent recombination was markedly suppressed by introduction of the USS between the two recognition sites at the unilateral side of the transgene, and the suppressive effect was greater at the 3′ end than at the 5′ end, which resulted in protection of non-specific transgene expression, improving the selectivity of Cre-dependent expression in target cell populations. Therefore, our FLEX/USS system provides a useful strategy for highly selective Cre-dependent transgene expression with no influence on the efficiency of Cre-mediated recombination.
Recombination events seemed to occur between a pair of short nucleotide sequences within the loxP or lox2272 sites in the AAV genome carrying the FLEX sequence. When the plasmid constructs for AAV vector production are transfected into packaging cells, AAV DNA replication is initiated by cellular DNA polymerase together with Rep78 and Rep68 proteins provided from the replication/encapsulation gene plasmid, leading to the formation of double-stranded DNA replicative intermediates in monomer and dimer forms, from which single-stranded viral genomes with plus and minus polarities are synthesized and packaged into vector particles.30 During viral DNA replication, recombination based on a short sequence homology may cause the reversion of the gene cassette in the AAV genome with the FLEX sequence. One possible mechanism that explains this reaction may involve homologous recombination, which mediates DNA repair of double-strand breaks.31 Although homologous recombination is generally considered to require long homologous sequences,32,33 recent studies of gene editing with a single-strand template suggest that recombination is also mediated via the homology of relatively shorter sequences.34,35 In addition, short homologous sequences of 5–25 bp are known to be implicated in microhomology-mediated end joining, which is another repair mechanism for double-strand breaks.36,37 Double-strand breaks occur naturally during cell division when DNA replication forks encounter a blocking lesion, leading to fork stall and collapse.38 Inverted repeats that potentially form secondary structures such as hairpin and cruciform structures appear to promote the generation of double-strand breaks at the site of repeats in eukaryotic cells.39 The inverted repeats at the loxP and lox2272 sites may produce secondary structures that interfere with the progression of the replication forks and then produce double-strand breaks within these recognition sites, thereby resulting in the reversion of the transgene in the AAV genome through short sequence homology-based recombination.
Introduction of the USS between the loxP and the lox2272 sites at the 5′ or 3′ end of the inverted transgene in the FLEX system effectively suppressed Cre-independent recombination in the viral genome and non-specific transgene expression in brain tissues. However, shortening of the USS between the two recognition sites increased the frequency of non-specific transgene expression, indicating the importance of the distance between the recognition sites for protection of non-specific gene expression. The size of 750 bp for the 3′USS was at least required for this protection. As mentioned earlier, secondary structures formed by inverted repeats in the two recognition sites may be involved in short sequence homology-based recombination in the AAV genome carrying the FLEX system. The introduction of the USS between the recognition sites is hypothesized to shift the secondary structures by distancing two pairs of inverted repeats at the loxP and lox2272 sites and contribute to the suppressing effects against double-strand break formation and subsequent short sequence homology-based recombination. However, this hypothesis cannot explain the results showing that introduction of the USS into the 3′ end of the inverted cassette was more effective compared with that into the 5′ end of the cassette. Investigation of the molecular mechanism is needed to explain spacing effects on Cre-independent recombination events, including the stronger effects of the 3′USS relative to the 5′USS on the suppression of recombination.
Injection of the AAV-FLEX/3′USS-GFP vector into the brains of PV-Cre-knockin rats resulted in efficient expression of GFP in PV+ neurons and suppressed non-specific GFP expression in PV− cells, resulting in the improved selectivity of Cre-dependent transgene expression. In contrast, the number of PV− + GFP+ cells in PV-Cre rats (2.53 ± 0.28 cells, n = 4 animals) was increased compared with that of GFP+ cells obtained from the experiments in which the same vector was injected into the wild-type brains (Figure 2E; 0.03 ± 0.03 cells, n = 4 animals), showing a significant difference between the two values (Student’s t test, t6 = 8.800, p < 0.001). We did not observe any Cre+ signals in PV− neurons in the knockin rat striatum in our double immunohistochemistry. However, in these rats there may be a small number of cells expressing a low level of Cre protein that was not detectable in our experimental conditions, and these cells may express GFP through site-specific recombination.
We successfully achieved the protection of Cre-independent recombination in the AAV genome and non-specific expression of the transgene in off targets, enhancing the selectivity of Cre-dependent gene expression by introducing the USS between the loxP and the lox2272 sites at either the 5′ or the 3′ end of the inverted transgene, in particular at the 3′ end with more efficient protection. This FLEX system with the USS was also applicable to the selective expression of different types of transgenes. Our FLEX/USS system will provide a powerful tool for highly specific Cre-dependent transgene expression for the labeling of target cell populations and functional imaging, as well as the manipulation of the activity of these populations in the future.
Limitations of the study
AAV vectors have a size capacity for transgene constructs suitable for the packaging of viral genomes.40 Inserting the USS (750 bp) into the transfer plasmid may shorten the size of transgene constructs. Alternatively, the USS insertion may decrease the titer during the vector production when researchers use a relatively longer size of construct. By decreasing the vector titer used for the experiments with AAV vectors carrying the FLEX system, researchers may be able to suppress the frequency of non-specific transgene expression (see Figure 1F). However, decreasing the titer at the same time leads to the reduction of transgene expression levels in target cell populations. Compared with a general FLEX system, our FLEX/USS strategy is effective at protecting Cre-independent recombination in a viral genome and transgene expression in off-target populations with higher titers of the vector.
Our FLEX/USS strategy was used for the protection of Cre-independent transgene expression in off-target cells after tissue injection with the AAV serotype 2 vector. In addition, another site-specific recombination system is mediated by the yeast Flp recombinase.41,42 The application of our USS for the improvement of selectivity of transgene expression through the FLEX system with other AAV serotypes or other recombinase should be investigated in the future.
STAR★Methods
Key resources table
| REAGENT OR RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Goat polyclonal anti-GFP | Frontier Science | Cat# GFW-Go-Af1480; RRID:AB 2571574 |
| Rabbit polyclonal anti-PV | Frontier Science | Cat# PV-Rb-Af750; RRID:AB 2209751 |
| Rabbit polyclonal anti-RFP | Rockland | Cat# 600-401-379; RRID:AB 945213 |
| Mouse monoclonal anti-Cre | Merck | Cat# MAB3120; RRID:AB 2085748 |
| Donkey anti-goat IgG (H + L) cross-absorbed secondary antibody, Alexa Fluor 488 | Life technologies | Cat# A-11055; RRID:AB 150129 |
| Donkey anti-rabbit IgG (H + L) secondary antibody, Dylight 405 | Jackson ImmunoResearch Labs | Cat# 711-475-152; RRID:AB 2340616 |
| Donkey anti-mouse IgG (H + L) secondary antibody, Cy3 | Jackson ImmunoResearch Labs | Cat# 711-165-150; RRID: AB 2307443 |
| Bacterial and viral strains | ||
| DH5α competent cells | Takara Bio Inc. | Cat# 9057 |
| AAV-FLEX-GFP | This paper | N/A |
| AAV-FLEX/5′USS-GFP | This paper | N/A |
| AAV-FLEX/3′USS-GFP | This paper | N/A |
| AAV-FLEX-Turbo/GFP | This paper | N/A |
| AAV-FLEX/3′USS(Luc)-GFP | This paper | N/A |
| AAV-FLEX/3′USS(553)-GFP | This paper | N/A |
| AAV-FLEX/3′USS(285)-GFP | This paper | N/A |
| AAV-FLEX/3′USS(103)-GFP | This paper | N/A |
| AAV-FLEX/3′USS-ChRWR/Venus | This paper | N/A |
| AAV-FLEX/3′USS-hM4Di/2A/GFP | This paper | N/A |
| AAV-FLEX/3′USS-mCherry | This paper | N/A |
| Critical commercial assays | ||
| AAV Helper-Free System | Agilent Technologies | Cat# 240071 |
| Gibson Assembly Cloning Kit | New England Biolabs | Cat# E5510S |
| TaqMan Universal PCR Master Mix | Thermo Fisher Scientific | Cat# 4304437 |
| PowerUp SYBR Green Master Mix | Thermo Fisher Scientific | Cat# A25742 |
| NucleoBond Xtra Maxi EF Kit | Macherey-Nagel | Cat# 740426 |
| BigDye Terminator v3.1 Cycle Sequencing Kit | Thermo Fisher Scientific | Cat# 4337455 |
| Experimental models: Cell lines | ||
| HEK293T cell line | American Type Culture Collection | Cat# CRL-11268 |
| Experimental models: Organisms/strains | ||
| Rat: Long Evans | Charles River Laboratories Japan | N/A |
| Rat: PV-Cre knockin | NBRP-rat in Japan | N/A |
| Oligonucleotides | ||
| Fw-C: 5′ CTGCTAACCATGTTCATGCCT-3′ | Thermo Fisher Scientific | N/A |
| Fw-G1: 5′ CGCGATCACATGGTCCTG-3′ | Integrated DNA Technologies | N/A |
| Fw-G2: 5′ GAACTCCAGCAGGACCATGTG-3′ | Thermo Fisher Scientific | N/A |
| Fw-W: 5′ CCGTTGTCAGGCAACGTG-3′ | Thermo Fisher Scientific | N/A |
| Rv-G1: 5′ CCGACCACATGAAGCAGCACG-3′ | Thermo Fisher Scientific | N/A |
| Rv-G2: 5′ TTACTTGTACAGCTCGTCCATG-3′ | Thermo Fisher Scientific | N/A |
| Rv-G3: 5′ CGTCGCCGTCCAGCTCGACCAG-3′ | Thermo Fisher Scientific | N/A |
| Rv-W1: 5′ AGCTGACAGGTGGTGGCAAT-3′ | Thermo Fisher Scientific | N/A |
| Rv-W2: 5′GCAACATAGTTAAGAATACCAGTCAATC-3′ | Integrated DNA Technologies | N/A |
| TM-G: 5′-FAM ATCACTCTCGGCATGGACGAGC MGB-3′ | Integrated DNA Technologies | N/A |
| TM-W: 5′-FAM TGCTGACGCAACCCCCACTGGT MGB-3′ | Thermo Fisher Scientific | N/A |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Kazuto Kobayashi (kazuto@fmu.ac.jp).
Materials availability
Viral vectors and plasmids used in this study are available by direct distribution after completion of a standard MTA. PV-Cre-knockin rats are deposited to the National BioResource Project for the Rat (NBRP-rat) in Japan (http://www.anim.med.kyoto-u.ac.jp/nbr/Default. aspx).
Experimental model and subject details
Animal care and handling procedures were conducted in accordance with the guidelines established by the Laboratory Animal Research Center of Fukushima Medical University. All procedures were approved by the Fukushima Medical University Institutional Animal Care and Use Committee. Rats were maintained at 22 ± 2°C and 60% humidity in a 12-hr light/12-hr dark cycle, and food and water were continuously available. Long Evans rats (Charles River Laboratories) and PV-Cre-knockin rats25 were used for the intracranial surgery. Both male and female rats at the age of 8–12 weeks were used for the experiments.
HEK293T cells (American Type Culture Collection No. CRL-11268) were stored in frozen aliquots, and thawed and propagated in 10-cm dishes before use in viral production. Cells were cultured in Dulbecco’s modified Eagle’s medium (Sigma-Aldrich) containing 10% fetal bovine serum (Invitrogen) and supplemented with 2 mM glutaMax supplement (Gibco), and penicillin-streptomycin of 100 units/mL (Gibco) at 37°C with 5% CO2, and used for transfection.
Method details
Plasmid construction
The transfer plasmid pAAV-FLEX-GFP containing the gene cassette encoding GFP (Clontech) in the inverted orientation, which is flanked by two double recognition sites between the CAG promoter23 and WPRE sequence connected to hGH gene polyadenylation signal, was constructed using a Gibson Assembly Cloning Kit (New England Biolabs) (see Figure 1A). The gene cassette (750 bp) encoding TurboFP635 (Evrogen) was inserted between the loxP and lox2272 sites either at the 5′- or 3′-end of the inverted GFP sequence, resulting in the plasmids pAAV-FLEX/5′USS-GFP and pAAV-FLEX/3′USS-GFP (see Figure 2A). The TurboFP635 gene cassette was inserted between the double-floxed site at the 5′-side and inverted GFP sequence in pAAV-FLEX/3′USS-GFP, resulting in pAAV-FLEX-Turbo/GFP (see Figure 4A). The TurboFP635 sequence in pAAV-FLEX/3′USS-GFP was substituted by the sequence containing the Luc gene cassette26 to generate pAAV-FLEX/3′USS(Luc)-GFP (see Figure 5A). The TurboFP635 sequence in the same plasmid was replaced by a 553-bp ApaLI/PshAI, 285-bp Eco47III/Bsu36I, and 103-bp AgeI/ApaLI DNA fragments of the cassette, resulting in pAAV-FLEX/3′USS(553)-GFP, pAAV-FLEX/3′USS(285)-GFP and pAAV-FLEX/3′USS (103)-GFP, respectively (see Figure 6A). In the plasmid pAAV-FLEX/3′USS-GFP, the CAG promoter was substituted by the promoter of the gene encoding human EF1α,29 11 in-frame methionine codons in the TurboFP635 sequence were mutated to the termination codon TAG (termed mTurbo), and then the inverted GFP sequence was converted to the gene cassettes for ChRWR/Venus,27 hM4Di/2A/GFP,19 and mCherry,28 designed the plasmids pAAV-EF1α-FLEX/3′USS-ChRWR/Venus, pAAV-EF1α-FLEX/3′USS-hM4Di/2A/GFP, and pAAV-EF1α-FLEX/3′USS-mCherry, respectively (see Figure 7A).
Plasmid preparation
E. coli DH5α strain containing a mutation in the RecA1 gene (Takara Bio Inc.) was used for DNA preparations. E. coli competent cells were transformed with appropriate amounts of plasmids. The colonies were picked up and cultured in a shaker at 160 rpm in 3 mL of LB containing 50 μg/mL ampicillin at 37°C for 8 h. Then, the medium was scaled up to 500 mL and continued to be cultured for 16 h. Plasmids were extracted and purified with a NucleoBond Xtra Maxi EF Kit (Macherey-Nagel).
Viral vector preparation
AAV vector serotype 2 was prepared based on AAV Helper-Free System (Agilent Technologies) as described.19 HEK293T cells (American Type Culture Collection) were transfected with the transfer gene plasmid, together with the replication/encapsulation gene and adeno-helper gene plasmids through the calcium phosphate precipitation method. The crude viral lysate was purified with two rounds of CsCl gradient ultracentrifugation at 100,000×g and 4°C for 23 h using 13.5-mL Quick-Seal tubes and a NVT65 rotor (Beckman Coulter). Each tube was placed gently and 500–750 μL of white turbid bands were collected. The solution was applied to three rounds of dialysis against 1 liter of PBS by using a Slide-A-Lyzer dialysis cassette (MWCO10,000; Thermo Fisher Scientific). The dialyzed vector solution was finally concentrated by centrifugation through a Vivaspin Turbo filter (Sartorius). The viral genome titer for AAV vectors except for AAV-FLEX-Turbo/GFP was determined by quantitative PCR using the TaqMan System (Thermo Fisher Scientific). Total vector titer was measured with the Fw-W/Rv-W1 primer set, which amplifies a part of WPRE, and the probe TM-W, and recombinant vector titer was measured with the Fw-G1/Rv-W2 primer set, which amplifies the sequence containing the 3′ part of GFP transgene, and the probe TM-G. The viral genome titer for the AAV-FLEX-Turbo/GFP vector was determined by quantitative PCR with the SYBR Green reaction system (Thermo Fisher Scientific). Total vector titer was measured with the Fw-W/Rv-W1 primer set, which amplifies the same part of the WPRE sequence, and the recombinant vector titer was measured with the Fw-C/Rv-G3 primer set, which amplifies the sequence containing the 5′ part of GFP transgene.
PCR amplification
PCR assays were carried out in the reaction mixture (10 μL) containing 1.5 units rTaq DNA polymerase, 10 pmol each of forward and reverse primer pair, 2.5 nmol each dNTP mixture in PCR buffer (10 nmol Tris-HCl; pH 8.3, 50 nmol KCl, and 1.5 nmol MgCl2) (Takara Bio Inc.). The amplifications were performed with 25 cycles of denaturation at 96°C for 10 s, annealing at 58°C for 20 s, and extension at 72°C for 75 s. The PCR products were analyzed by electrophoresis in 1% agarose gels containing ethidium bromide with 100-bp ladder marker (Takara Bio Inc.). The DNA fragments were excised from the gel and eluted into 10 mM Tris-HCl buffer (pH8.0) containing 1 mM EDTA by using QIAGEN II Gel Extraction Kit (QIAGEN).
Nucleotide sequence analysis
Dye-terminator sequencing was carried out in the reaction mixture (10 μL) containing a 3 pmol specific primer by using a BigDye Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific). The chain terminations were performed with 25 cycles of denaturation at 96°C for 10 s, annealing at 50°C for 5 s, and extension and termination at 60°C for 3 min. The extension products were collected by high-speed centrifugation after ethanol addition, dissolved in the Hi-Di formamide (Thermo Fisher Scientific), and then analyzed by the capillary electrophoresis instrument (3500 Genetic Analyzer with a data collection software; Thermo Fisher Scientific). The nucleotide sequences were determined by using sequencing analysis software (Thermo Fisher Scientific).
Intracranial surgery
The surgery was conducted under isoflurane (4% induction and 1.5% maintenance) anesthesia, and AAV vectors were introduced into the dorsal striatum (1.0 μL/site, 4 sites) through a glass microinjection capillary connected to a microinfusion pump (ESP-32; Eicom). The anteroposterior, mediolateral, and dorsoventral coordinates (mm) from bregma and dura were 1.5/3.0/3.0 (site 1), 1.5/3.0/3.5 (site 2), 0.5/3.0/3.0 (site 3), and 0.5/3.0/3.5 (site 4) according to an atlas of the rat brain.43 Injection was performed at a constant flow rate of 0.1 μL/min.
Immunohistochemistry
Rats were anesthetized with 4% isoflurane and perfused transcardially with PBS, followed by fixation with 4% paraformaldehyde in 0.1 M phosphate buffer (pH7.4). Fixed brains were cut into sections (30-μm thick) through a coronal plane with a cryostat. For immunofluorescence histochemistry, sections were incubated with anti-GFP antibody (goat, 1 μg/mL; Frontier Science), anti-PV antibody (rabbit, 1 μg/mL; Frontier Science), and anti-RFP (rabbit, 1 μg/mL; Rockland), and then with species-specific secondary antibodies conjugated to DyLight 405 (Jackson ImmunoResearch Labs), Alexa 488 (Life technologies), or Cy3 (Jackson ImmunoResearch Labs). The sections were stained with 0.3 μM 4′,6-diamidino-2-phenylindole (DAPI; Thermo Fisher Scientific). Fluorescence images were obtained with a confocal laser-scanning microscope (Nikon A1) equipped with proper filter cube specifications.
Cell counts
Eight sections through the dorsal striatum along with the anteroposterior coordinates (mm) between 1.68 and 0.48 from bregma were prepared from each rat, and the number of immunopositive cells in ROIs (1.0 × 1.0 mm) in the striatum was counted. The average of stained cell number per ROI was calculated in individual animals.
Quantification and statistical analysis
Statistical analysis
All data are presented as mean ± SEM. For multiple comparisons, one-way ANOVA was applied followed by post hoc Bonferroni test. An unpaired Student’s t test was also used. All statistical details of the experiments can be found in the results section. Differences were considered statistically significant at p < 0.05.
Acknowledgments
This work was supported by a grant-in-aid for Scientific Research (C) (MO20K06912) from the Japan Society for the Promotion of Science (S.K.); a grant-in-aid for Scientific Research on Transformative Research Areas (A) Adaptive Circuit Census (21H05244) from the Ministry of Education, Science, Sports, and Culture of Japan; and a grant-in-aid from the Japan Agency for Medical Research and Development under grant no. 22dm0207113h0002 (K.K.). The plasmid DNAs encoding the ChRWR, hM4Di, and mCherry genes were kindly gifted from Drs. H. Yawo, B. Roth, and I. Wada, respectively. We are grateful to R. Fukabori, Y. Hashimoto, M. Kikuchi, and Y. Nakazato for their technical support during the animal experiments and to T. Kobayashi for her helpful illustrations.
Author contributions
N.M., S.K., and K.K. conceived the study, designed the experiments, and directed the project. N.M., S.K., K.T., and K.K. designed the vector expression strategy and generated the vector. N.M., Y.M., and T.M. produced and analyzed knockin transgenic rats. N.M., S.K., and K.T. analyzed vector structure, and K.N. and M.S. performed intracranial injections and histological examinations. N.M., S.K., and K.K. wrote the paper. All authors discussed the results and implications and commented on the manuscript at all stages.
Declaration of interests
The authors declare no competing interests.
Published: January 18, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.crmeth.2022.100393.
Supplemental information
Data and code availability
-
•
Original data reported in this paper are deposited at Mendeley Data (https://data.mendeley.com/datasets/b8fzpndz29/draft?a=9303c4c8-9fce-46e1-9d3c-a073d9863b62).
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Sternberg N., Hamilton D. Bacteriophage P1 site-specific recombination. I. Recombination between loxP sites. J. Mol. Biol. 1981;150:467–486. doi: 10.1016/0022-2836(81)90375-2. [DOI] [PubMed] [Google Scholar]
- 2.Abremski K., Hoess R., Sternberg N. Studies on the properties of P1 site-specific recombination: evidence for topologically unlinked products following recombination. Cell. 1983;32:1301–1311. doi: 10.1016/0092-8674(83)90311-2. [DOI] [PubMed] [Google Scholar]
- 3.Sauer B. Functional expression of the cre-lox site-specific recombination system in the yeast Saccharomyces cerevisiae. Mol. Cell Biol. 1987;7:2087–2096. doi: 10.1128/mcb.7.6.2087-2096.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sauer B., Henderson N. Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc. Natl. Acad. Sci. USA. 1988;85:5166–5170. doi: 10.1073/pnas.85.14.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu H., Zou Y.R., Rajewsky K. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell. 1993;73:1155–1164. doi: 10.1016/0092-8674(93)90644-6. [DOI] [PubMed] [Google Scholar]
- 6.Lee G., Saito I. Role of nucleotide sequences of loxP spacer region in Cre-mediated recombination. Gene. 1998;216:55–65. doi: 10.1016/s0378-1119(98)00325-4. [DOI] [PubMed] [Google Scholar]
- 7.Hoess R.H., Ziese M., Sternberg N. P1 site-specific recombination: nucleotide sequence of the recombining sites. Proc. Natl. Acad. Sci. USA. 1982;79:3398–3402. doi: 10.1073/pnas.79.11.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voziyanov Y., Pathania S., Jayaram M. A general model for site-specific recombination by the integrase family recombinases. Nucleic Acids Res. 1999;27:930–941. doi: 10.1093/nar/27.4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schnütgen F., Doerflinger N., Calléja C., Wendling O., Chambon P., Ghyselinck N.B. A directional strategy for monitoring Cre-mediated recombination at the cellular level in the mouse. Nat. Biotechnol. 2003;21:562–565. doi: 10.1038/nbt811. [DOI] [PubMed] [Google Scholar]
- 10.Sohal V.S., Zhang F., Yizhar O., Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saunders A., Johnson C.A., Sabatini B.L. Novel recombinant adeno-associated viruses for Cre activated and inactivated transgene expression in neurons. Front. Neural Circuits. 2012;6:47. doi: 10.3389/fncir.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozorovitskiy Y., Saunders A., Johnson C.A., Lowell B.B., Sabatini B.L. Recurrent network activity drives striatal synaptogenesis. Nature. 2012;485:646–650. doi: 10.1038/nature11052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyamichi K., Shlomai-Fuchs Y., Shu M., Weissbourd B.C., Luo L., Mizrahi A. Dissecting local circuits: parvalbumin interneurons underlie broad feedback control of olfactory bulb output. Neuron. 2013;80:1232–1245. doi: 10.1016/j.neuron.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Connor D.H., Hires S.A., Guo Z.V., Li N., Yu J., Sun Q.Q., Huber D., Svoboda K. Neural coding during active somatosensation revealed using illusory touch. Nat. Neurosci. 2013;16:958–965. doi: 10.1038/nn.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srinivasan R., Lu T.Y., Chai H., Xu J., Huang B.S., Golshani P., Coppola G., Khakh B.S. New transgenic mouse lines for selectively targeting astrocytes and studying calcium signals in astrocyte processes in situ and in vivo. Neuron. 2016;92:1181–1195. doi: 10.1016/j.neuron.2016.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nonomura S., Nishizawa K., Sakai Y., Kawaguchi Y., Kato S., Uchigashima M., Watanabe M., Yamanaka K., Enomoto K., Chiken S., et al. Monitoring and updating of action selection for goal-directed behavior through the striatal direct and indirect pathways. Neuron. 2018;99:1302–1314.e5. doi: 10.1016/j.neuron.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Aelvoet S.A., Pascual-Brazo J., Libbrecht S., Reumers V., Gijsbers R., Van den Haute C., Baekelandt V. Long-term fate mapping using conditional lentiviral vectors reveals a continuous contribution of radial glia-like cells to adult hippocampal neurogenesis in mice. PLoS One. 2015;10 doi: 10.1371/journal.pone.0143772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arguello A.A., Richardson B.D., Hall J.L., Wang R., Hodges M.A., Mitchell M.P., Stuber G.D., Rossi D.J., Fuchs R.A. Role of a lateral orbital frontal cortex-basolateral amygdala circuit in cue-induced cocaine-seeking behavior. Neuropsychopharmacology. 2017;42:727–735. doi: 10.1038/npp.2016.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kato S., Fukabori R., Nishizawa K., Okada K., Yoshioka N., Sugawara M., Maejima Y., Shimomura K., Okamoto M., Eifuku S., Kobayashi K. Action selection and flexible switching controlled by the intralaminar thalamic neurons. Cell Rep. 2018;22:2370–2382. doi: 10.1016/j.cellrep.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 20.Tschida K., Michael V., Takatoh J., Han B.X., Zhao S., Sakurai K., Mooney R., Wang F. A specialized neural circuit gates social vocalizations in the mouse. Neuron. 2019;103:459–472.e4. doi: 10.1016/j.neuron.2019.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woods N.I., Stefanini F., Apodaca-Montano D.L., Tan I.M.C., Biane J.S., Kheirbek M.A. The dentate gyrus classifies cortical representations of learned stimuli. Neuron. 2020;107:173–184.e6. doi: 10.1016/j.neuron.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer K.B., Collins H.K., Callaway E.M. Sources of off-target expression from recombinase-dependent AAV vectors and mitigation with cross-over insensitive ATG-out vectors. Proc. Natl. Acad. Sci. USA. 2019;116:27001–27010. doi: 10.1073/pnas.1915974116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niwa H., Yamamura K., Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 24.Shcherbo D., Merzlyak E.M., Chepurnykh T.V., Fradkov A.F., Ermakova G.V., Solovieva E.A., Lukyanov K.A., Bogdanova E.A., Zaraisky A.G., Lukyanov S., Chudakov D.M. Bright far-red fluorescent protein for whole-body imaging. Nat. Methods. 2007;4:741–746. doi: 10.1038/nmeth1083. [DOI] [PubMed] [Google Scholar]
- 25.Yoshimi K., Oka Y., Miyasaka Y., Kotani Y., Yasumura M., Uno Y., Hattori K., Tanigawa A., Sato M., Oya M., et al. Combi-CRISPR: combination of NHEJ and HDR provides efficient and precise plasmid-based knock-ins in mice and rats. Hum. Genet. 2021;140:277–287. doi: 10.1007/s00439-020-02198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markova S.V., Golz S., Frank L.A., Kalthof B., Vysotski E.S. Cloning and expression of cDNA for a luciferase from the marine copepod Metridia longa. A novel secreted bioluminescent reporter enzyme. J. Biol. Chem. 2004;279:3212–3217. doi: 10.1074/jbc.M309639200. [DOI] [PubMed] [Google Scholar]
- 27.Wang H., Sugiyama Y., Hikima T., Sugano E., Tomita H., Takahashi T., Ishizuka T., Yawo H. Molecular determinants differentiating photocurrent properties of two channelrhodopsins from chlamydomonas. J. Biol. Chem. 2009;284:5685–5696. doi: 10.1074/jbc.M807632200. [DOI] [PubMed] [Google Scholar]
- 28.Shaner N.C., Campbell R.E., Steinbach P.A., Giepmans B.N.G., Palmer A.E., Tsien R.Y. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- 29.Kim D.W., Uetsuki T., Kaziro Y., Yamaguchi N., Sugano S. Use of the human elongation factor 1 alpha promoter as a versatile and efficient expression system. Gene. 1990;91:217–223. doi: 10.1016/0378-1119(90)90091-5. [DOI] [PubMed] [Google Scholar]
- 30.Gonçalves M.A.F.V. Adeno-associated virus: from defective virus to effective vector. Virol. J. 2005;2:43. doi: 10.1186/1743-422X-2-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X., Heyer W.D. Homologous recombination in DNA repair and DNA damage tolerance. Cell Res. 2008;18:99–113. doi: 10.1038/cr.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hockemeyer D., Soldner F., Beard C., Gao Q., Mitalipova M., DeKelver R.C., Katibah G.E., Amora R., Boydston E.A., Zeitler B., et al. Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc finger nucleases. Nat. Biotechnol. 2009;27:851–857. doi: 10.1038/nbt.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sommer D., Peters A., Wirtz T., Mai M., Ackermann J., Thabet Y., Schmidt J., Weighardt H., Wunderlich F.T., Degen J., et al. Efficient genome engineering by targeted homologous recombination in mouse embryos using transcription activator-like effector nucleases. Nat. Commun. 2014;5:3045. doi: 10.1038/ncomms4045. [DOI] [PubMed] [Google Scholar]
- 34.Davis L., Maizels N. Two distinct pathways support gene correction by single-stranded donors at DNA nicks. Cell Rep. 2016;17:1872–1881. doi: 10.1016/j.celrep.2016.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paquet D., Kwart D., Chen A., Sproul A., Jacob S., Teo S., Olsen K.M., Gregg A., Noggle S., Tessier-Lavigne M. Efficient introduction of specific homozygous and heterozygous mutations using CRISPR/Cas9. Nature. 2016;533:125–129. doi: 10.1038/nature17664. [DOI] [PubMed] [Google Scholar]
- 36.McVey M., Lee S.E. MMEJ repair of double-strand breaks (director’s cut): deleted sequences and alternative endings. Trends Genet. 2008;24:529–538. doi: 10.1016/j.tig.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Truong L.N., Li Y., Shi L.Z., Hwang P.Y.H., He J., Wang H., Razavian N., Berns M.W., Wu X. Microhomology-mediated end joining and homologous recombination share the initial end resection step to repair DNA double-strand breaks in mammalian cells. Proc. Natl. Acad. Sci. USA. 2013;110:7720–7725. doi: 10.1073/pnas.1213431110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shrivastav M., De Haro L.P., Nickoloff J.A. Regulation of DNA double-strand break repair pathway choice. Cell Res. 2008;18:134–147. doi: 10.1038/cr.2007.111. [DOI] [PubMed] [Google Scholar]
- 39.Lobachev K.S., Rattray A., Narayanan V. Hairpin- and cruciform-mediated chromosome breakage: causes and consequences in eukaryotic cells. Front. Biosci. 2007;12:4208–4220. doi: 10.2741/2381. [DOI] [PubMed] [Google Scholar]
- 40.Choi J.H., Yu N.K., Baek G.C., Bakes J., Seo D., Nam H.J., Baek S.H., Lim C.S., Lee Y.S., Kaang B.K. Optimization of AAV expression cassettes to improve packaging capacity and transgene expression in neurons. Mol. Brain. 2014;7:17. doi: 10.1186/1756-6606-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schnütgen F., Ghyselinck N.B. Adopting the good reFLEXes when generating conditional alterations in the mouse genome. Transgenic Res. 2007;16:405–413. doi: 10.1007/s11248-007-9089-8. [DOI] [PubMed] [Google Scholar]
- 42.Boniface E.J., Lu J., Victoroff T., Zhu M., Chen W. FlEx-based transgenic reporter lines for visualization of Cre and Flp activity in live zebrafish. Genesis. 2009;47:484–491. doi: 10.1002/dvg.20526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paxinos G., Watson C. Ed 6. Academic Press; 2007. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Original data reported in this paper are deposited at Mendeley Data (https://data.mendeley.com/datasets/b8fzpndz29/draft?a=9303c4c8-9fce-46e1-9d3c-a073d9863b62).
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.