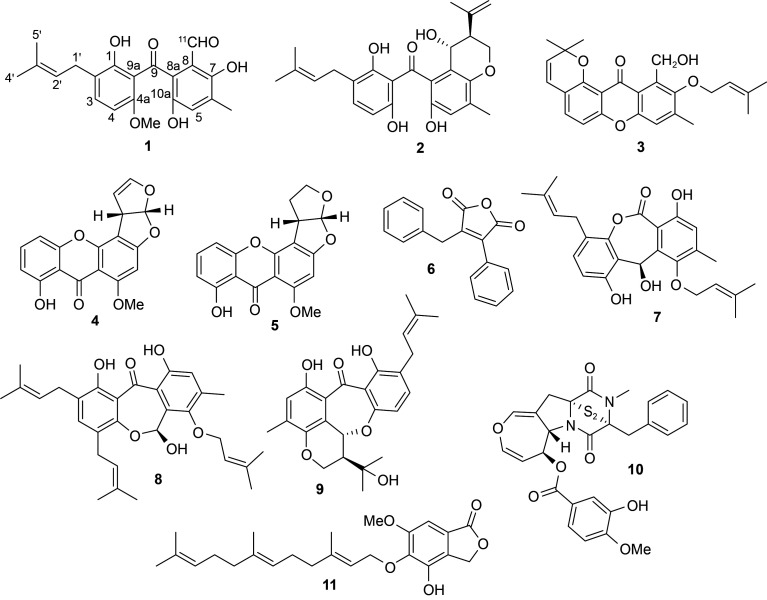
北部湾藴育着丰富且亟待研究的海洋(微)生物资源,是活性天然产物的重要来源。本研究从涠洲岛珊瑚共附生构巢裸胞壳菌GXIMD 02509中分离获得一个多硫代二酮哌嗪生物碱和系列芳香聚酮类化合物,含一个新化合物4a-O-methoxyarugosin H (1)。我们通过采用多种波、光谱学技术及对比文献方法鉴定了化合物的化学结构。化合物1-5、7和10对786-O、SW1990和SW480等3株肿瘤细胞增殖具抑制活性,半抑制浓度(IC50)值为4.3–33.4 μmol/L。化合物emestrin J (10)具有一个二硫桥键,还能够显著抑制786-O细胞克隆及迁移,诱导786-O细胞凋亡并阻滞细胞分裂在G2/M期,是一个潜在具抗肿瘤活性的先导化合物。
Keywords: 巢裸胞壳菌, 海洋真菌, 芳香聚酮, 多硫代二酮哌嗪, 细胞毒
Marine microorganisms, especially marine fungi, have historically proven their value as a prolific source for structurally novel and pharmacologically active secondary metabolites (Deshmukh et al., 2018; Carroll et al., 2022). The corals constitute a dominant part of reefs with the highest biodiversity, and harbor highly diverse and abundant microbial symbionts in their tissue, skeleton, and mucus layer, with species-specific core members that are spatially partitioned across coral microhabitats (Wang WQ et al., 2022). The coral-associated fungi were very recently found to be vital producers of structurally diverse compounds, terpenes, alkaloids, peptides, aromatics, lactones, and steroids. They demonstrate a wide range of bioactivity such as anticancer, antimicrobial, and antifouling activity (Chen et al., 2022). The genetically powerful genus Emericella (Ascomycota), which has marine and terrestrial sources, includes over 30 species and is distributed worldwide. It is considered a rich source of diverse secondary metabolites with antimicrobial activity or cytotoxicity (Alburae et al., 2020). Notably, Emericella nidulans, the sexual state of a classic biosynthetic strain Aspergillus nidulans, was recently reported as an important source of highly methylated polyketides (Li et al., 2019) and isoindolone-containing meroterpenoids (Zhou et al., 2016) with unusual skeletons.
The Beibu Gulf is a semi-enclosed gulf in the northwest of the South China Sea, and harbors tremendous underexplored biodiversity in terms of both marine organisms and microorganisms; these are rich in diversified secondary metabolites (Huang et al., 2022). In continuation of our efforts to discover interesting lead compounds from Beibu Gulf coral-derived marine fungi, a plethora of structurally novel secondary metabolites with remarkable biological activity have been recently obtained, including anti-tumor ascochlorins (Guo et al., 2021; Luo et al., 2021) and cytochalasans (Luo et al., 2020), anti-osteoclastogenic chlorinated polyketides (Zhang et al., 2022), phenolic derivatives (Lu et al., 2022), and cyclopiazonic acid alkaloids (Wang JM et al., 2022). In this study, a fungus Emericella nidulans GXIMD 02509 endemic to Weizhou coral reefs attracted our attention owing to the intriguing high-performance liquid chromatography (HPLC)-ultraviolet (UV) profiles of its extract. Subsequent chemical investigation led to the isolation of nine diverse aromatic polyketides, an epipolythiodioxopiperazine alkaloid, and a farnesylated phthalide derivative (Fig. 1). Several of these compounds showed cytotoxicity against three human cancer-cell lines (786-O, SW1990, and SW480). Here, the process of isolation and structural determination, as well as the cytotoxicity results, are described in detail.
Fig. 1. Chemical structures of compounds 1‒11.
Compound 1 was isolated as a bright-yellow solid and was deduced with the molecular formula C21H22O6 based on the high resolution-electrospray ionization-mass spectroscopy (HR-ESI-MS) data [M+H]+ ion peak at m/z 371.1499 (calcd for C21H23O6, 371.1495). The UV spectrum revealed the presence of benzene chromophores with absorption bands at 203, 255, and 320 nm. The 1H nuclear magnetic resonance (NMR) (Table 1) and heteronuclear single quantum coherence (HSQC) data for 1 displayed a series of signals, including: two hydroxyl groups attributable to 1-OH (δ H 10.93, s) and 7-OH (δ H 11.38, s); one aldehyde group, H-11 (δ H 9.98, s); four aromatic or olefinic protons, H-3 (δ H 7.12, d, J=8.5 Hz), H-4 (δ H 6.12, d, J=8.5 Hz), H-5 (δ H 7.16, s), and H-2' (δ H 5.25, t, J=7.0 Hz); one methylene, H-1' (δ H 3.28, d, J=7.0 Hz); three singlet methyls, 6-Me (δ H 2.24, s), H3-4' (δ H 1.72, s), and H3-5' (δ H 2.24, s); and one methoxyl, 4a-OMe (δ H 4.03, s). Aside from these ten corresponding hydrogen-bearing carbons, ten aromatic or olefinic (four oxygenated) carbons and a carbonyl (δ C 198.3) remained in the 13C NMR spectrum.
Table 1.
1H (500 MHz) and 13C (125 MHz) NMR spectroscopic data for 4a-O-methoxyarugosin H (1) (CDCl3)
| Position | δ C, type | δ H (J (Hz)) | HMBC |
|---|---|---|---|
| 1 | 160.4, C | ||
| 2 | 125.2, C | ||
| 3 | 134.9, CH | 7.12, d (8.5) | 1, 1' |
| 4 | 105.5, CH | 6.12, d (8.5) | 2, 4a, 9a |
| 4a | 170.5, C | ||
| 4a-OMe | 53.2, CH3 | 4.03, s | 4a |
| 5 | 128.0, CH | 7.16, s | 6, 6-Me, 7, 10a |
| 6 | 139.8, C | ||
| 6-Me | 15.2, CH3 | 2.24, s | 5, 6, 7 |
| 7 | 154.8, C | ||
| 8 | 113.7, C | ||
| 8a | 125.3, C | ||
| 9 | 198.3, C | ||
| 9a | 103.5, C | ||
| 10a | 140.2, C | ||
| 11 | 194.1, CH | 9.98, s | 7, 8, 8a |
| 1' | 27.8, CH2 | 3.28, d (7.0) | 1, 2, 3, 2', 3' |
| 2' | 121.5, CH | 5.25, t (7.0) | 2, 1', 4', 5' |
| 3' | 133.7, C | ||
| 4' | 25.9, CH3 | 1.72, s | 2', 3', 5' |
| 5' | 17.9, CH3 | 2.24, s | 2', 3', 4' |
| 1-OH | 10.93, s | 1, 2, 9a | |
| 7-OH | 11.38, s | 6, 7, 8 |
NMR: nuclear magnetic resonance; HMBC: heteronuclear multiple bond correlation.
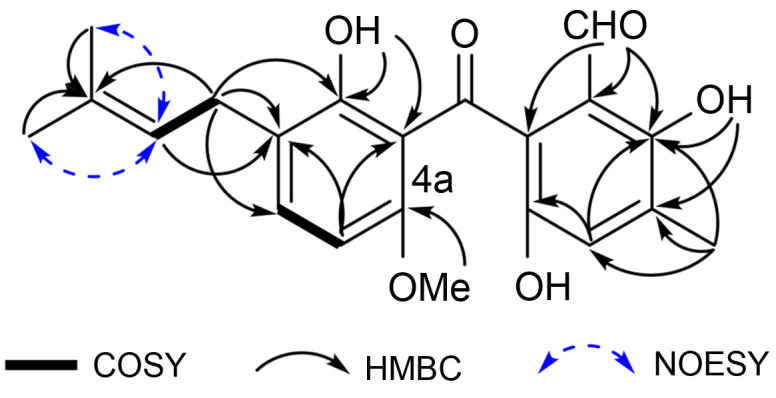
This information revealed that structurally, 1 was closely related to arugosin H, which was also obtained from the marine-derived fungus Emericella nidulans var. acristata (Kraljet al., 2006). The main difference was the appearance of a methoxyl group (δ H/C 4.03/53.2) at C-4a (δ C 170.5) in 1 instead of the hydroxyl group that appears in arugosin H. This deduction was verified by the heteronuclear multiple bond correlation (HMBC) correlation from 4a-OCH 3 to C-4a (Fig. 2). Based on these findings, we determined that the structure of 1 was a methyl derivative of arugosin H, and accordingly assigned it a trival name: 4a-O-methoxyarugosin H (Figs. S1–S9). Compound 1 was probably an artifact produced during the isolation procedure when methanol was used as the main solvent (Capon, 2020).
Fig. 2. Key 1H-1H COSY, HMBC, and NOESY correlations of 4a-O-methoxyarugosin H (1). COSY: correlation spectroscopy; HMBC: heteronuclear multiple bond correlation; NOESY: nuclear overhauser effect spectroscopy.
We were able to pinpoint known compounds by comparing the physicochemical data of known compounds 2–11 (supplementary information) with data from the literature. We identified pre-shamixanthone (2) (Wu et al., 2015a), cycloisoemericellin (3) (Kawahara et al., 1988), sterigmatocystin (4) (Zhu and Lin, 2007), dihydrosterigmatocystin (5) (Zhu and Lin, 2007), dehydromicroperfuranone (6) (Kralj et al., 2006; Roux et al., 2020), varioxiranol I (7) (Wu et al., 2015b), arugosin G (8) (Kralj et al., 2006), arugosin C (9) (Kawahara et al., 1988; El-Kashef et al., 2021), emestrin J (10) (Li et al., 2016), and farnesylemefuranone D (11) (Chi et al., 2020). Interestingly, emestrin J (10) harbors with an uncommon disulfide moiety, which was biosynthesized by a peptide cyclization pathway along with additional ring-expansion and macrocyclization (Li et al., 2016).
During the course of our search for anti-tumor lead compounds from marine natural products (Zhouet al., 2019; Luo et al., 2021), all obtained compounds were evaluated for cytotoxicity against three human cancer cell lines, i.e., 786-O (human renal carcinoma cell), SW1990 (human pancreatic cancer cell), and SW480 (human colorectal cancer cell), along with the normal human liver cell line LO2 (Table 2). Among them, compounds 1-5, 7, and 10 showed inhibitory activity against these cell lines, with the half maximal inhibitory concentration (IC50) values ranging from 4.3 to 33.4 μmol/L. Notably, emestrin J (10) exhibited the strongest activity against these cancer cell lines, especially for 786-O (4.3 μmol/L), and was at least as potent as the positive control, cisplatin. Interestingly, two xanthone derivatives (4 and 5) displayed antiproliferative activity, with IC50 values of 18.3 and 24.7 μmol/L against 786-O, and 19.6 and >40 μmol/L against SW1990 cells, respectively. This revealed that the Δ 16 double bond in 4 probably promoted cytotoxic activity.
Table 2.
Cytotoxicity of compounds 1-11
| Compound | IC50 (μmol/L) | |||
|---|---|---|---|---|
| 786-O | SW1990 | SW480 | LO2 | |
| 1 | – | – | 32.6±1.2 | – |
| 2 | – | 33.2±5.1 | – | – |
| 3 | – | 24.7±2.2 | 30.2±4.8 | – |
| 4 | 18.3±1.9 | 19.6±2.8 | – | – |
| 5 | 24.7±1.8 | – | – | – |
| 6 | – | – | – | – |
| 7 | 33.4±1.8 | 30.4±5.1 | 27.2±6.5 | – |
| 8 | – | – | – | – |
| 9 | – | – | – | – |
| 10 | 4.3±0.7 | 14.1±1.8 | 4.9±0.3 | 11.1±2.4 |
| 11 | – | – | – | – |
| Cisplatin | 2.5±0.6 | 33.8±4.7 | 26.7±4.6 | 5.2±0.6 |
All data shown above are mean±SD of three independent experiments. IC50: half maximal inhibitory concentration; SD: standard deviation. "‒": >40 μmol/L.
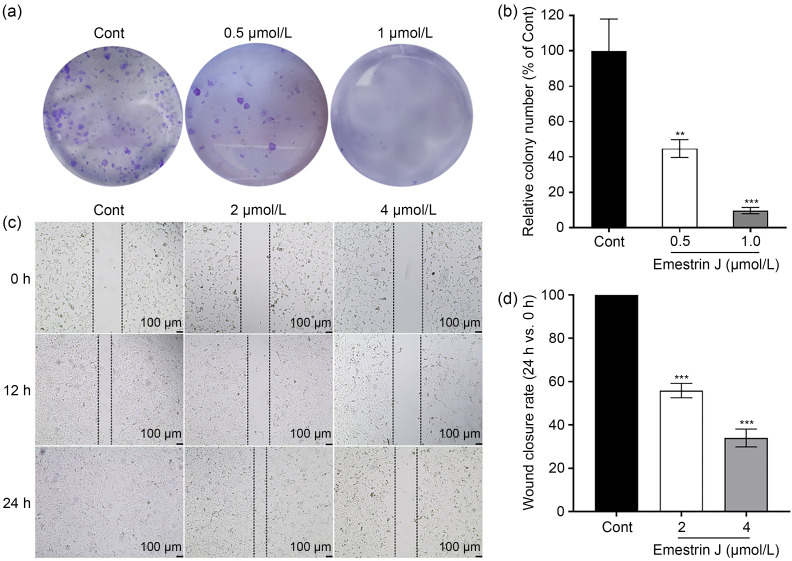
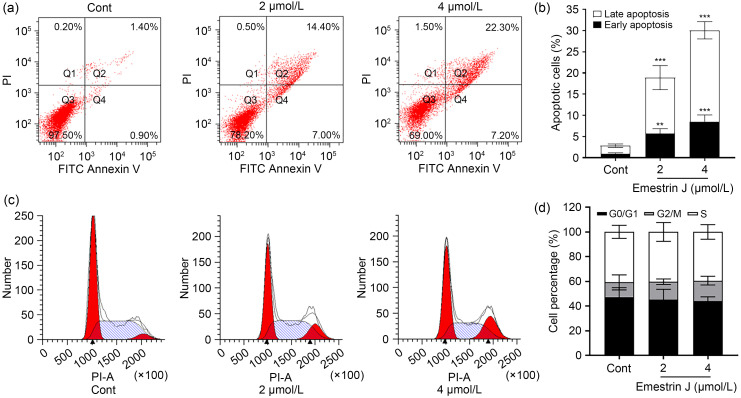
To further evaluate the potential anti-tumor activity of 10, we next investigated its activity against 786-O cells in cell colony and scratch wound assays. Consistent with the above-mentioned antiproliferative activity, compound 10 significantly reduced cell colony formation of 786-O cells at concentrations of 0.5 and 1.0 μmol/L (Figs. 3a and 3b). Also, compared with the vehicle group, compound 10 significantly suppressed migration of 786-O cells in a time- and dose‐dependent manner (Figs. 3c and 3d). To explore whether the antiproliferative activity of 10 was related to apoptosis, we further evaluated the compound for its effect on cell apoptosis and cell-cycle arrest in 786-O cells. The results were analyzed by flow cytometry and showed that the total apoptotic cells (early and late) induced by 10 at 24 h rose by 21.4% (2 μmol/L) and 29.5% (4 μmol/L), suggesting that 10 induced 786-O cell apoptosis in a dose-dependent manner (Figs. 4a and 4b). Meanwhile, the cell-cycle distribution results revealed that 10 primarily blocked the cell cycle during the G2/M phase, resulting in an inability of cells to proliferate (Figs. 4c and 4d). Therefore, it was clear that 10 could suppress the proliferation, colony formation, and migration of 786-O cells, and induce apoptosis, acting as a potential anti-tumor compound.
Fig. 3. Suppressive effects of emestrin J (10) on colony formation and migration of 786-O cellsin vitro. Representative wells (a) and quantitative results (b) of the colony-formation assay. Representative images (c) and quantitative results (d) of the scratch wound assay. 786-O cells were treated with vehicle (DMSO, Cont) or 10, as indicated. All data shown above are mean±SD of three independent experiments. ** P<0.01, *** P<0.001 vs. Cont. DMSO: dimethylsulfoxide; Cont: control; SD: standard deviation.
Fig. 4. Effects of emestrin J (10) on cell apoptosis and cell-cycle arrest in 786-O cells. Emestrin J (10) induced apoptosis of 786-O cells (a, b) and arrested the cell cycle (c, d) in the G2/M phase. 786-O cells were treated with vehicle (DMSO, Cont) or 10 (2 and 4 μmol/L) for 24 h, as indicated. All data shown above are mean±SD of three independent experiments. ** P<0.01, *** P<0.001 vs. Cont. DMSO: dimethylsulfoxide; Cont: control; SD: standard deviation; FITC: fluorescein isothiocyanate; PI: propidium iodide; PI-A: PI-area.
In conclusion, nine aromatic polyketides, including a new one, 4a-O-methoxyarugosin H (1), along with an epipolythiodioxopiperazine alkaloid, emestrin J (10), and a farnesylated phthalide derivative, were obtained from the Beibu Gulf coral-associated fungus Emericella nidulans GXIMD 02509. We determined their structures by spectral data analysis, as well as comparison with reported data. Several of the compounds exhibit cytotoxicity against three human cancer cell lines, 786-O, SW1990, and SW480. The most potent one, emestrin J (10), has an uncommon disulfide bond and suppresses proliferation, colony formation, and migration of 786-O cells, as well as inducing apoptosis. Our findings provide a basis for further development and utilization of emestrin derivatives as sources of potential anti-tumor chemotherapy agents.
Materials and methods
Detailed methods are provided in the electronic supplementary materials of this paper.
Supplementary information
Acknowledgments
This research was supported by the Guangxi Natural Science Foundation (Nos. 2020GXNSFBA159001, 2020GXNSFGA297002, and 2021GXNSFAA220052), the National Natural Science Foundation of China (Nos. U20A20101 and 22007019), the Special Fund for Bagui Scholars of Guangxi (Yonghong LIU), and the Scientific Research Foundation of Guangxi University of Chinese Medicine (No. 2022C038), China.
Author contributions
Xiaowei LUO and Yonghong LIU conceived the research and designed experiments. Miaoping LIN, Zhenzhou TANG, Jiaxi WANG, Humu LU, Chenwei WANG, Yanting ZHANG, Xinming LIU, and Chenghai GAO performed the experiments and analysis. Xiaowei LUO, Miaoping LIN, and Zhenzhou TANG interpreted the data and wrote the paper. All authors have read and approved the final manuscript, and therefore, have full access to all the data in the study and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines
Miaoping LIN, Zhenzhou TANG, Jiaxi WANG, Humu LU, Chenwei WANG, Yanting ZHANG, Xinming LIU, Chenghai GAO, Yonghong LIU, and Xiaowei LUO declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- Alburae NA, Mohammed AE, Alorfi HS, et al. , 2020. Nidulantes of Aspergillus (formerly Emericella): a treasure trove of chemical diversity and biological activities. Metabolites, 10(2): 73. 10.3390/metabo10020073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capon RJ, 2020. Extracting value: mechanistic insights into the formation of natural product artifacts‒case studies in marine natural products. Nat Prod Rep, 37(1): 55-79. 10.1039/C9NP00013E [DOI] [PubMed] [Google Scholar]
- Carroll AR, Copp BR, Davis RA, et al. , 2022. Marine natural products. Nat Prod Rep, 39(6): 1122-1171. 10.1039/D1NP00076D [DOI] [PubMed] [Google Scholar]
- Chen Y, Pang XY, He YC, et al. , 2022. Secondary metabolites from coral-associated fungi: source, chemistry and bioactivities. J Fungi, 8(10): 1043. 10.3390/jof8101043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi LP, Li XM, Wan YP, et al. , 2020. Ophiobolin sesterterpenoids and farnesylated phthalide derivatives from the deep sea cold-seep-derived fungus Aspergillus insuetus SD-512. J Nat Prod, 83(12): 3652-3660. 10.1021/acs.jnatprod.0c00860 [DOI] [PubMed] [Google Scholar]
- Deshmukh SK, Gupta MK, Prakash V, et al. , 2018. Mangrove-associated fungi: a novel source of potential anticancer compounds. J Fungi, 4(3): 101. 10.3390/jof4030101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Kashef DH, Youssef FS, Reimche I, et al. , 2021. Polyketides from the marine-derived fungus Aspergillus falconensis: in silico and in vitro cytotoxicity studies. Bioorg Med Chem, 29: 115883. 10.1016/j.bmc.2020.115883 [DOI] [PubMed] [Google Scholar]
- Guo L, Luo XW, Yang P, et al. , 2021. Ilicicolin A exerts antitumor effect in castration-resistant prostate cancer via suppressing EZH2 signaling pathway. Front Pharmacol, 12: 723729. 10.3389/fphar.2021.723729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang BY, Peng S, Liu SF, et al. , 2022. Isolation, screening, and active metabolites identification of anti-Vibrio fungal strains derived from the Beibu Gulf coral. Front Microbiol, 13: 930981. 10.3389/fmicb.2022.930981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara N, Nozawa K, Nakajima S, et al. , 1988. Studies on fungal products. Part 15. Isolation and structure determination of arugosin E from Aspergillus silvaticus and cycloisoemericellin from Emericella striata. J Chem Soc Perkin Trans, 1(4): 907-911. 10.1039/P19880000907 [DOI] [Google Scholar]
- Kralj A, Kehraus S, Krick A, et al. , 2006. Arugosins G and H: prenylated polyketides from the marine-derived fungus Emericella nidulans var. acristata. J Nat Prod, 69(7): 995-1000. 10.1021/np050454f [DOI] [PubMed] [Google Scholar]
- Li Q, Chen CM, He Y, et al. , 2019. Emeriones A‒C: three highly methylated polyketides with bicyclo[4. 2.0]octene and 3, 6-dioxabicyclo[3.1.0]hexane functionalities from Emericella nidulans. Org Lett, 21(13): 5091-5095. 10.1021/acs.orglett.9b01680 [DOI] [PubMed] [Google Scholar]
- Li Y, Yue Q, Krausert NM, et al. , 2016. Emestrins: anti-cryptococcus epipolythiodioxopiperazines from Podospora australis . J Nat Prod, 79(9): 2357-2363. 10.1021/acs.jnatprod.6b00498 [DOI] [PubMed] [Google Scholar]
- Lu HM, Tan YH, Zhang YT, et al. , 2022. Osteoclastogenesis inhibitory phenolic derivatives produced by the Beibu Gulf coral-associated fungus Acremonium sclerotigenum GXIMD 02501. Fitoterapia, 159: 105201. 10.1016/j.fitote.2022.105201 [DOI] [PubMed] [Google Scholar]
- Luo XW, Gao CH, Lu HM, et al. , 2020. HPLC-DAD-guided isolation of diversified chaetoglobosins from the coral-associated fungus Chaetomium globosum C2F17. Molecules, 25(5): 1237. 10.3390/molecules25051237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo XW, Cai GD, Guo YF, et al. , 2021. Exploring marine-derived ascochlorins as novel human dihydroorotate dehydrogenase inhibitors for treatment of triple-negative breast cancer. J Med Chem, 64(18): 13918-13932. 10.1021/acs.jmedchem.1c01402 [DOI] [PubMed] [Google Scholar]
- Roux I, Woodcraft C, Hu JY, et al. , 2020. CRISPR-mediated activation of biosynthetic gene clusters for bioactive molecule discovery in filamentous fungi. ACS Synth Biol, 9(7): 1843-1854. 10.1021/acssynbio.0c00197 [DOI] [PubMed] [Google Scholar]
- Wang JM, Li ZC, Zhang YT, et al. , 2022. A new α-cyclopiazonic acid alkaloid identified from the Weizhou Island coral-derived fungus Aspergillus flavus GXIMD 02503. J Ocean Univ China, 21(5): 1307-1312. 10.1007/s11802-022-4959-5 [DOI] [Google Scholar]
- Wang WQ, Tang KH, Wang PX, et al. , 2022. The coral pathogen Vibrio coralliilyticus kills non-pathogenic holobiont competitors by triggering prophage induction. Nat Ecol Evol, 6(8): 1132-1144. 10.1038/s41559-022-01795-y [DOI] [PubMed] [Google Scholar]
- Wu Q, Wu CM, Long HL, et al. , 2015a. Varioxiranols A‒G and 19-O-methyl-22-methoxypre-shamixanthone, PKS and hybrid PKS-derived metabolites from a sponge-associated Emericella variecolor fungus. J Nat Prod, 78(10): 2461-2470. 10.1021/acs.jnatprod.5b00578 [DOI] [PubMed] [Google Scholar]
- Wu Q, Long HL, Liu D, et al. , 2015b. Varioxiranols I‒L, new lactones from a sponge-associated Emericella variecolor fungus. J Asian Nat Prod Res, 17(12): 1137-1145. 10.1080/10286020.2015.1119127 [DOI] [PubMed] [Google Scholar]
- Zhang YT, Li ZC, Huang BY, et al. , 2022. Anti-osteoclastogenic and antibacterial effects of chlorinated polyketides from the Beibu Gulf coral-derived fungus Aspergillus unguis GXIMD 02505. Mar Drugs, 20(3): 178. 10.3390/md20030178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HB, Sun XH, Li N, et al. , 2016. Isoindolone-containing meroperpenoids from the endophytic fungus Emericella nidulans HDN12-249. Org Lett, 18(18): 4670-4673. 10.1021/acs.orglett.6b02297 [DOI] [PubMed] [Google Scholar]
- Zhou XF, Liang Z, Li KL, et al. , 2019. Exploring the natural piericidins as anti-renal cell carcinoma agents targeting peroxiredoxin 1. J Med Chem, 62(15): 7058-7069. 10.1021/acs.jmedchem.9b00598 [DOI] [PubMed] [Google Scholar]
- Zhu F, Lin YC, 2007. Three xanthones from a marine-derived mangrove endophytic fungus. Chem Nat Compd, 43(2): 132-135. 10.1007/s10600-007-0062-9 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.