Highlights
-
•
We developed a model of polymetastatic progression of colorectal cancer (CRC).
-
•
Data from the multicenter LaIT-SABR study were used in this secondary analysis.
-
•
Oligometastases number and total tumor volume were combined to stratify population.
-
•
We identified 3 risk classes of polymetastatic progression after SABR.
-
•
This model can be used to personalize SABR prescription in oligometastatic CRC.
Keywords: Stereotactic ablative radiotherapy, SABR, SBRT, Colorectal cancer, Oligometastatic disease, Predictive factors
Abstract
Aim
Stereotactic ablative radiotherapy (SABR) showed increasing survival in oligometastatic patients. Few studies actually depicted oligometastatic disease (OMD) evolution and which patient will remain disease-free and which will rapidly develop a polymetastatic disease (PMD) after SABR. Therefore, apart from the number of active metastases, there are no clues on which proven factor should be considered for prescribing local treatment in OMD. The study aims to identify predictive factors of polymetastatic evolution in lung oligometastatic colorectal cancer patients.
Methods
This international Ethical Committee approved trial (Prot. Negrar 2019-ZT) involved 23 Centers and 450 lung oligometastatic patients. Primary end-point was time to the polymetastatic conversion (tPMC). Additionally, oligometastases number and cumulative gross tumor volume (cumGTV) were used as combined predictive factors of tPMC. Oligometastases number was stratified as 1, 2–3, and 4–5; cumGTV was dichotomized to the value of 10 cc.
Results
The median tPMC in the overall population was 26 months. Population was classified in the following tPMC risk classes: low-risk (1–3 oligometastases and cumGTV ≤ 10 cc) with median tPMC of 35.1 months; intermediate-risk (1–3 oligometastases and cumGTV > 10 cc), with median tPMC of 13.9 months, and high-risk (4–5 oligometastases, any cumGTV) with median tPMC of 9.4 months (p = 0.000).
Conclusion
The present study identified predictive factors of polymetastatic evolution after SABR in lung oligometastatic colorectal cancer. The results demonstrated that the sole metastases number is not sufficient to define the OMD since patients defined oligometastatic from a numerical point of view might rapidly progress to PMD when the cumulative tumor volume is high. A tailored approach in SABR prescription should be pursued considering the expected disease evolution after SABR, with the aim to avoid unnecessary treatment and toxicity in those at high risk of polymetastatic spread, and maximize local treatment in those with a favorable disease evolution.
1. Introduction
The oligometastatic disease (OMD) is an intermediate state of metastatic disease characterized by a low metastatic burden amenable of local treatment, aiming to delay disease progression [1], systemic treatment change or extension [2], and finally improve overall survival (OS) or eventually cure [3]. The OMD is usually defined as having a maximum number of 3–5 simultaneous metastases [4]. Apart from the number of metastases that is actually-one of the main criteria for metastasis-directed therapies (MDT) prescription (i.e.: stereotactic ablative radiotherapy (SABR)), the OMD have a biological background that consider the inter-metastases and primary tumor cross-talk, as well as the metastasis-to-metastasis spread [5], [6]. In this complex scenario, the aim of local treatment is reducing the likelihood of metastases to spread themselves co-operating with systemic drugs in controlling the onset of new metastatic foci.
Instead of being an all-or-nothing phenomenon, recent evidence suggest that the OMD is a complex clinical entity. In fact, in the clinical practice, OMD with apparently similar characteristics at presentation might have different clinical behavior [7]. Therefore, there is a clinical need for defining a model of disease progression useful to predict which patient might benefit from local treatments, maximizing its use in those patients clinically favorable or unable to receive systemic therapies and minimizing its use when a rapid metastatic spread is expected. In this context, the clinical benefit of MDT might be evaluated by the onset of the polymetastatic disease. The polymetastatic conversion permits to identify a change in the clinical behavior of the OMD to a state amenable of salvage systemic treatment only and with a limited role of local treatments [8]. Previously, in the LaIT-SABR study, a multicenter study on oligometastatic colorectal cancer, were identified several predictive factors of polymetastatic conversion [7]. Not only lesion number but also their dimension was identified as predictive factors, however their interplay in predicting the development of a polymetastatic disease was not explored.
The present is a secondary analysis of the LaIT-SABR study. The aim of the present study is to identify predictive factors of polymetastatic evolution in lung oligometastatic colorectal cancer patients to tailor SABR prescription.
2. Material and methods
2.1. Retrospective analysis overview
The present study is a subsequent analysis based on the previously published data of the LaIT-SABR study [7]. In the LaIT-SABR study [7] we analyzed the correlation between several biological, clinical, and dosimetrical factors and polymetastatic conversion (factors analyzed: BED, lesion diameter, mutational status of the primary tumor, previous systemic treatment, median SUVmax value, time to the OMD, primary tumor type, lesion site, and number of treated lung lesions). Among all the above reported variables evaluated by Cox regression multivariate analysis, only lesion diameter and lesion number (stratified as 1 versus 2–3 versus 4–5 metastases) were significantly associated with polymetastatic conversion. Therefore, only these two significantly correlated factors were chosen for the present analysis with the purpose of defining a model of polymetastatic conversion after SABR.
The present study was conducted on a multi-institutional, international, large retrospective database from 23 participating Centers including patients with lung oligometastases from colorectal cancer treated with SABR over the decade 2009–2019. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethical Committee (Prot. Negrar 2019-ZT).
Patients’ data were anonymized and collected through an electronic CRF. Anonymized data were subsequently transferred for statistical analyses. The data in the database were validated to ensure the accuracy of the data entered. Required fields were specified to prevent missing data. The inclusion criteria for the present analysis were having received a SABR with a minimum BED of 100 Gy10 to all the treated metastases, and availability of, volume and survival data.
2.2. Study end-point, follow-up and statistics
The primary end-point was to evaluate the role of the oligometastases number and cumulative metastases volume in predicting the conversion to the polymetastatic disease. The time to the polymetastatic disease (tPMC) was defined as the time from the SABR start for the oligometastatic disease to the occurrence of >5 new metastases. Cumulative metastases volume (cumGTV) was the sum of the active treated metastases in cc, and the number of treated metastases was stratified to 1, 2–3, and 4–5, as previously documented [7].
The follow-up up consisted of CT scan or FDG-PET CT each 3 months for the first two years of follow-up and each 6 months thereafter. The choose of the specific imaging technique during follow-up was at the discretion of each participating Center.
The univariate analyses for survival endpoints were performed with the Kaplan-Meier method. The log-rank test was applied to determine differences between the corresponding curves. Prognostic factors were evaluated for survival end-points as previously explained [7]. BED was calculated using an α/β ratio of 10 Gy. Cutoff Finder tool (http://molpath.charite.de/cutoff) was used to determine the optimal cumGTV cut-off defined as the value with the most significant split (log-rank test) (8). The Statistical analysis was performed using STATA V15 software (STATA Corp, College Station, TX). A p-value < 0.05 indicated a significant association.
3. Results
3.1. Patients’ characteristics
The initial population consisted of 620 patients accounting for 1090 metastases. After data checking, 170 patients were excluded from the study due to the missing of required data, errors in data entry, or duplicate patients. The final population consisted of 450 patients and 705 metastases. The median age was 71 years (40–84). One-hundred six (23.5 %) patients had synchronous metastases, while 344 (76.5 %) metachronous oligometastases. Patients’ characteristics are reported in Table 1. Metastases number was distributed as follows: 1 oligometastasis 301 (67 %) patients, 2–3 oligometastases 120 (26 %) patients, and 4–5 oligometastases 29 (5.8 %) patients. The median cumGTV was 4.6 cc (0.2–255.8). The identified cumGTV cut-off for stratification analysis for single oligometastases cases was 10 cc. Treatment characteristics are reported in Table 2.
Table 1.
Patients’ characteristics (n = 450) (%).
| Mean age (range) (years) | 71 (40–84) |
|---|---|
| Sex | |
| Male | 156 (34) |
| Female | 294 (66) |
| Primary site | |
| Colon | 272 (60) |
| Rectum | 178 (40) |
| Initial treatment | |
| Surgery | 371 (82) |
| RCHT + surgery | 68 (15) |
| Systemic therapy | 11 (3) |
| Adjuvant chemotherapy | |
| Yes | 114 (25) |
| No | 324 (72) |
| Unknown | 12 (3) |
| Histology | |
| Adenocarcinoma | 429 (95) |
| Mucinous carcinoma | 21 (5) |
| Initial stage | |
| Stage I | 15 (3) |
| Stage II | 94 (21) |
| Stage III | 186 (41) |
| Stage IV | 127 (28.5) |
| Unknown | 28 (6.5) |
| Tumor mutations (%) | |
| EGFR | 3.3 |
| KRAS | 22.6 |
| NRAS | 3 |
| BRAF | 1.76 |
| MSI | 0.44 |
| Median time to OMD (range) | 23.7 (0–126) |
| Systemic treatment before SABR | |
| Chemotherapy | 249 (55.5) |
| TKI | 4 (0.9) |
| Antiangiogenetic | 32 (7) |
| Target therapy/immunotherapy | 6 (1.3) |
| No | 118 (26.3) |
| Unknown | 41 (9) |
| Type of oligometastases | |
| Synchronous | 106 (23.5) |
| Metachronous | 344 (76.5) |
| RCHT: radiochemotherapy; OMD: oligometastatic disease: SBRT: stereotactic body radiotherapy; TKI: tyrosine-kinases inhibitor | |
Table 2.
Treatment characteristics (n = 705) (%).
| Median lesion diameter (mm) (range) | 14 (5–45) |
| Total treated lesions | |
| 1 | 301 (42.5) |
| 2 | 180 (25.5) |
| 3 | 90 (13) |
| 4 | 44 (6) |
| 5 | 90 (13) |
| Median SUVmax (range) | 4.9 (1–28) |
| Median total dose (Gy) (range) | 48 (23–70) |
| Median dose per fraction (Gy) (range) | 12 (5–30) |
| Median number of fractions (range) | 3 (1–10) |
| Median BED (range) | 125 (100–180) |
| Median GTV volume (cc) (per lesion) | 3.07 (0.1–178) |
| Median cumulative GTV (cc) | 4.6 (0.2–255.8) |
| Mean PTV volume (cc) | 13.2 (1.2–113) |
| Lesion site | |
| Central | 204 (29) |
| Peripheral | 501 (71) |
| BED: biological effective dose; GTV: gross tumor volume; PTV: planning target volume | |
3.2. Time to polymetastatic conversion
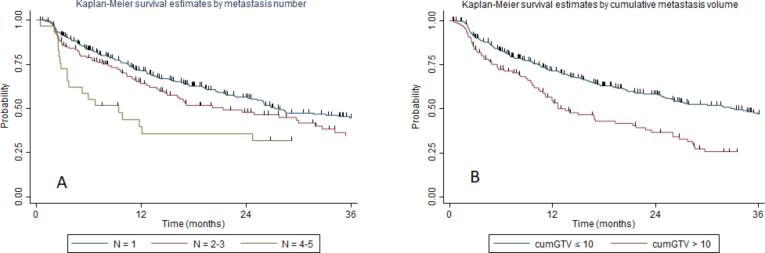
The median tPMC in the overall population was 26 months (range 8.9–92.2 months). The median tPMC stratified for the number of oligometastases was: 27.7, 21.3, and 9.4 months for patients having 1, 2–3, and 4–5 metastases, with a 2-year tPMC of 56.5 %, 47.7 %, and 39.2 %, respectively (p = 0.005, Fig. 1a). The median tPMC stratified for cumGTV was 33.1, and 13.5 months for cumGTV ≤ 10 cc or >10 cc, with a 2-year tPMC of 57.9 %, and 37.5 %, respectively (p = 0.00, Fig. 1b).
Fig. 1.
Kaplan-Meier curve showing time to polymetastatic conversion stratified to: A) number of treated metastases; B) cumulative tumor volume.
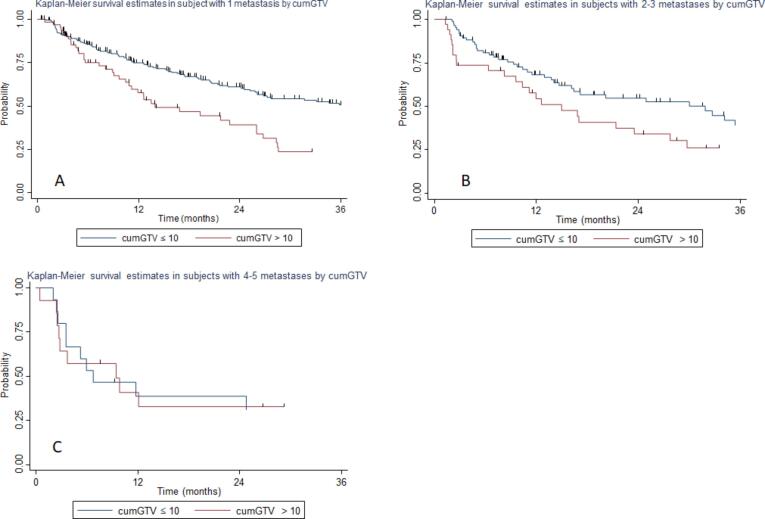
By combining these 2 factors we identified 6 groups (see Table 3) and we analyzed the effect of different tumor volumes for each oligometastases strata. The median tPMC in the population with 1 metastasis was 36.1 and 13.9 months for patients having cumGTV ≤ 10 cc or > 10 cc, respectively, with a 2-year tPMC of 60.5 % and 41.3 %, respectively (p = 0.00, Fig. 2a). The median tPMC in the population with 2–3 oligometastases was 31.9 and 14.9 months for patients having cumGTV ≤ 10 cc or > 10 cc, respectively, with a 2-year tPMC of 54.1 % and 33.65 %, respectively (p = 0.058, Fig. 2b). The median tPMC in the population with 4–5 oligometastases was 6.7 and 9.4 months for patients having cumGTV ≤ 10 cc or >10 cc, respectively, with a 2-year tPMC of 30.3 % and 32.5 %, respectively (p = 0.85, Fig. 2c).
Table 3.
Analysis of time to polymetastatic conversion.
| Covariates | Median tPMC (months) | P | Covariates | Median tPMC (months) | P |
|---|---|---|---|---|---|
| Number of oligometastases | Group 1: 1 oligometastasis and cumGTV < 10 cc | 36.1 | 0.00 | ||
| 1 | 27.7 | 0.005 | Group 2: 1 oligometastasis and cumGTV > 10 cc | 13.9 | |
| 2–3 | 21.3 | Group 3: 2–3 oligometastases and cumGTV < 10 cc | 31.9 | 0.058 | |
| 4–5 | 9.1 | Group 4: 2–3 oligometastases and cumGTV > 10 cc | 14.9 | ||
| Group 5: 4–5 oligometastases and cumGTV < 10 cc | 6.7 | 0.85 | |||
| cumGTV | Group 6: 4–5 oligometastase and cumGTV > 10 cc | 9.4 | |||
| <10 cc | 33.1 | 0.00 | |||
| >10 cc | 13.5 | ||||
| tPMC: time ti polymetastatic conversion; cumGTV: cumulative gross tumor volume | |||||
Fig. 2.
Kaplan-Meier curve showing time to polymetastatic conversion of: A) patients with a single metastasis stratified to cumulative tumor volume (cumGTV); B) patients with 2–3 metastases stratified to cumGTV; C) patients with 4–5 metastases stratified to cumGTV.
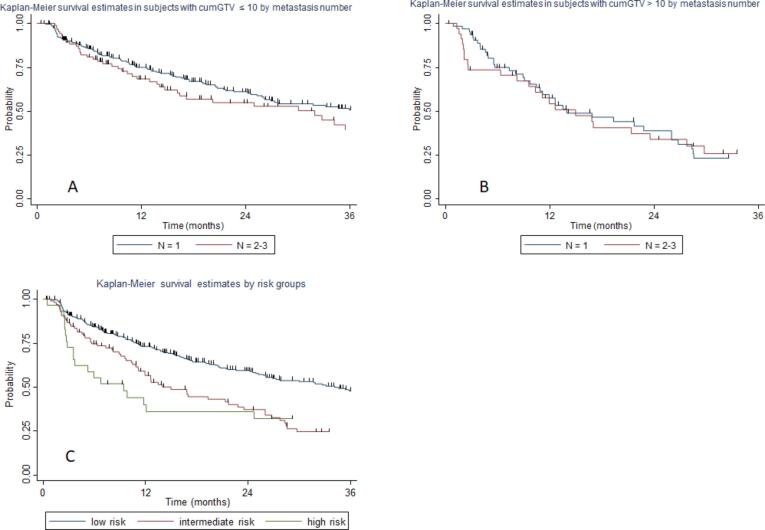
Therefore, we cross-compared group 1 (1 metastasis and cumGTV ≤ 10 cc) with group 3 (2–3 metastases and cum GTV ≤ 10 cc). The median tPMC was 36.1 and 31.9 months for group 1 and 3 respectively, with a 2-year tPMC of 60.5 % and 54.1 %, respectively (p = 0.12, Fig. 3a). Thereafter, we cross-compared group 2 (1 metastasis and cumGTV > 10 cc) with group 4 (2–3 metastases and cumGTV > 10 cc). The median tPMC was 13.9 and 14.9 months for group 2 and 4 respectively, with a 2-year tPMC of 41.3 % and 33.6 %, respectively (p = 0.89, Fig. 3b).
Fig. 3.
Kaplan-Meier curve showing time to polymetastatic conversion of: A) patients with cumulative tumor volume (cumGTV) ≤ 10 cc stratified to number of oligometastases (1 versus 2–3); B) patients with cumGTV > 10 cc stratified to number of oligometastases (1 versus 2–3); C) risk classes stratification of polymetastatic conversion.
3.3. Risk class stratification
By combining the previous groups, we identified the following risk classes of polymetastatic conversion, defined as follows: low-risk (patients with 1–3 metastases and cumGTV ≤ 10 cc), intermediate-risk (patients with 1–3 metastases and cumGTV > 10 cc), and high-risk (4–5 metastases and any cumGTV). The median tPMC was 34.1, 13.9, and 9.4 months for low, intermediate, and high-risk, respectively. The 2-year tPMC according to risk classification was 58.9 %, 38.4 %, and 35.3 % for low, intermediate, and high risk, respectively (p = 0.000, Fig. 3c) (Table 4).
Table 4.
Risk class model (n = 450).
| Characteristics | Median tPMC | 2-year tPMC | |
|---|---|---|---|
| Low risk (323) | 1–3 oligometastases and cumGTV < 10 cc | 34.1 | 58.9 % |
| Intermediate risk (98) | 1–3 oligometatases and cumGTV > 10 cc | 13.9 | 38.4 % |
| High-risk (29) | 4–5 oligometastases and any cumGTV | 9.4 | 35.3 % |
| tPMC: time ti polymetastatic conversion; cumGTV: cumulative gross tumor volume | |||
4. Discussion
The actual criteria to define the oligometastatic disease are mainly based on the number of metastases and technical feasibility for SABR delivery, however as suggested by a recent ESTRO-ASTRO consensus on oligometastatic disease definition “there is no biological evidence supporting the maximal number of metastases, or the maximal lesion size, that can be treated to provide clinical benefit” [4]. In fact, several evidence demonstrated that despite being useful in clinical practice, lesion number is not fully representative of the complex scenario of the OMD. Patients with the same oligometastases number might both rapidly progress to the polymetastatic disease or maintain relatively long progression-free intervals [9], [10]. This fact might suggest that other factors are involved in the disease progression.
Previously in the LaIT-SABR study [7], we demonstrated that also tumor dimension might influence the development of the polymetastatic disease, other than metastases number. In particular, patients with metastases diameter >20 mm and with more than three metastases had a significantly short tPMC. However, how these two factors interact with each other had not been explored. The present study identified predictive risk groups of polymetastatic progression in oligometastatic colorectal cancer.
The benefit of the addition of SABR to systemic treatment in the oligometastatic CRC is still a matter of debate. For example, the PulMICC trial explored the role of surgical metastasectomy in a series of CRC patients with lung oligometastases [11]. The study did not reach the expected accrual. Anyway, the study reported no survival benefit from the addition of surgery to the decisional workflow, questioning whether local treatment should be considered in this disease. On the other hand, the CLOCC trial included patients with liver metastases from CRC treated with MDT plus systemic therapy [12]. The results showed that MDT addition significantly increased survival. Interestingly, the study included also patients with advanced liver disease, underlying in this way the need for identifying more accurate selection criteria for local treatment.
The majority of trials seem to demonstrate a global advantage when MDT is included in the management of OMD. For example, in the randomized phase II trial SABR-COMET [3], it was shown a significant survival advantage by the addition of SABR to systemic treatment in a series of 99 oligometastatic patients affected by different primary tumors (mainly NCLC, breast, prostate, and CRC) with a median survival of 50 versus 28 months [3] and a survival improvement of 13.6 % at 8 years of follow-up [13]. This trial is considered a milestone in the OMD treatment with SABR, however, it is a basket trial including different primary tumors that are commonly treated with different systemic treatments and consequently different survivals. By comparison, the phase III clinical trial SINDAS randomized untreated EGFR-mutated NSCLC patients to first-line tyrosine kinase inhibitor (TKI) or TKI plus SABR to all sites of disease. The preliminary results demonstrated a significant survival advantage favoring the experimental arm (median survival 25.5 months versus 17.4 months; HR 0.68, p = <0.001) [14]. On the other side, the phase II trial by Gomez et al. [15] randomized NSCLC patients (80 % of which harbored no mutation) with oligoresidual disease after primary chemotherapy to consolidative local therapy or observation. The results reported a significant OS improvement (secondary end-point) in the experimental arm (median 41.2 months versus 17 months). The median OS in the study by Klement et al. [16], which included oligometastatic CRC patients treated with SABR, was 27.9 months. Therefore, considering that different primary tumor has different disease progression timing and pattern, and different systemic treatment options (which may modify the disease course) it is reasonable that also disease-specific criteria should be considered for OMD definition and treatment.
In this context, Greco et al. demonstrated the possibility to stratify patients according to different factors other than metastases number [9]. Specifically, a combination of FDG-PET SUVmax and tumor volume could identify different patients’ subgroups with different prognoses in a population of all-comer oligometastatic patients. In a study on oligometastatic prostate cancer patients, disease-free interval, site of metastases, and type of systemic therapy were significant predictors of polymetastatic conversion [17]. In the present study, a combined score of metastases number and total tumor volume allowed to identify-three risk classes. The low-risk population seems the most suitable for SABR, with a median tPMC of 34.1 months, while conversely, the most unfavorable subgroup seems the high-risk with 4–5 metastases and a median tPMC of 9.4 months. In this latter, local treatment could probably not significantly affect disease course and priority should be given to systemic treatment. On the other side, low-risk patients seem to benefit the most from SABR and they should be considered for local treatment, especially in patients not suitable for polychemotherapy or chemotherapy at all. In the intermediate-risk patients, SABR alone could not be sufficient and should eventually be considered alongside chemotherapy.
Metastases volume has a direct connection to the metastatic potential. In fact, growing metastases progressively secret pro-angiogenetic factors that increase vascular alteration and enhance permeability and anergic endothelial cells within the tumor. The poor vascular function recruit macrophages and neutrophils that further increase vascular permeability by the secretion of additional pro-angiogenic factors [6]. Larger lesions became also hypoxic and this can stimulate tumor invasiveness [6]. Furthermore, growing metastases can release specific cytokines through the plasma or in platelets or microvesicles and can contribute to forming pre-metastatic niches which increase the probability of metastases to spread themselves [5], [6]. This biological background might justify our clinical finding and that the use of SABR in the early phase of the oligometastatic disease might shift the metastatogenic equilibrium by reducing the probability of intra-metastatic spread [18].
This study is not without limitations. First of all, the analysis is based on a retrospective data collection, secondly, a proportion of patients was treated with SABR alone and this could have negatively impacted polymetastatic spread. Points of strength are the large sample size, the homogeneity of patients’ selection, and the use of high radiation doses. In conclusion, we developed a predictive model of polymetastatic disease development considering metastases number and total tumor volume. Different patient subgroups retain different clinical behavior and should be treated with a tailored approach.
5. Conclusion
The present is the largest series of colorectal oligometastatic patients and the first to identify a predictive model of polymetastatic spread in the specific setting of colorectal cancer. This model could be used in the clinical practice to improve SABR prescription and as a base stratification for future studies aiming to prospectively evaluate the role of SABR in oligometastatic colorectal cancer.
Funding
None.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Ost P., Reynders D., Decaestecker K., Fonteyne V., Lumen N., De Bruycker A., et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36(5):446–453. doi: 10.1200/JCO.2017.75.4853. [DOI] [PubMed] [Google Scholar]
- 2.Nicosia L., Figlia V., Ricottone N., Cuccia F., Mazzola R., Giaj-Levra N., et al. Stereotactic body radiotherapy (SBRT) and concomitant systemic therapy in oligoprogressive breast cancer patients [published online ahead of print, 2022 May 5] Clin Exp Metastasis. 2022;39(4):581–588. doi: 10.1007/s10585-022-10167-6. [DOI] [PubMed] [Google Scholar]
- 3.Palma D.A., Olson R., Harrow S., Gaede S., Louie A.V., Haasbeek C., et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol. 2020;38(25):2830–2838. doi: 10.1200/JCO.20.00818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lievens Y., Guckenberger M., Gomez D., Hoyer M., Iyengar P., Kindts I., et al. Defining oligometastatic disease from a radiation oncology perspective: An ESTRO-ASTRO consensus document. Radiother Oncol. 2020;148:157–166. doi: 10.1016/j.radonc.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y., Cao X. Characteristics and significance of the pre-metastatic niche. Cancer Cell. 2016;30(5):668–681. doi: 10.1016/j.ccell.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Cedervall J, Dimberg A, Olsson AK. Tumor-induced local and systemic impact on blood vessel function. Mediators Inflamm. 2015;2015:418290. doi: 10.1155/2015/418290. [DOI] [PMC free article] [PubMed]
- 7.Nicosia L., Franceschini D., Perrone-Congedi F., Casamassima F., Gerardi M.A., Rigo M., et al. A multicenter LArge retrospectIve daTabase on the personalization of stereotactic ABlative radiotherapy use in lung metastases from colon-rectal cancer: The LaIT-SABR study. Radiother Oncol. 2022;166:92–99. doi: 10.1016/j.radonc.2021.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Budczies J, Klauschen F, Sinn BV, Győrffy B, Schmitt WD, Darb-Esfahani S, Denkert C. Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS One. 2012;7(12):e51862. doi: 10.1371/journal.pone.0051862. Epub 2012 Dec 14. [DOI] [PMC free article] [PubMed]
- 9.Greco C., Pares O., Pimentel N., Louro V., Morales J., Nunes B., et al. Phenotype-oriented ablation of oligometastatic cancer with single dose radiation therapy. Int J Radiat Oncol Biol Phys. 2019;104(3):593–603. doi: 10.1016/j.ijrobp.2019.02.033. [DOI] [PubMed] [Google Scholar]
- 10.Nicosia L., Cuccia F., Mazzola R., Ricchetti F., Figlia V., Giaj-Levra N., et al. Disease course of lung oligometastatic colorectal cancer treated with stereotactic body radiotherapy. Strahlenther Onkol. 2020;196(9):813–820. doi: 10.1007/s00066-020-01627-7. [DOI] [PubMed] [Google Scholar]
- 11.Milosevic M., Edwards J., Tsang D., Dunning J., Shackcloth M., Batchelor T., et al. Pulmonary Metastasectomy in Colorectal Cancer: updated analysis of 93 randomized patients - control survival is much better than previously assumed. Colorectal Dis. 2020;22(10):1314–1324. doi: 10.1111/codi.15113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruers T., Van Coevorden F., Punt C.J.A., Pierie J.-P., Borel-Rinkes I., Ledermann J.A., et al. Local treatment of unresectable colorectal liver metastases: results of a randomized phase II trial. J Natl Cancer Inst. 2017;109(9) doi: 10.1093/jnci/djx015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrow S., Palma D.A., Olson R., Gaede S., Louie A.V., Haasbeek C., et al. Stereotactic radiation for the comprehensive treatment of oligometastases (SABR-COMET) – extended long-term outcomes [published online ahead of print, 2022 May 25] Int J Radiat Oncol Biol Phys. 2022;114(4):611–616. doi: 10.1016/j.ijrobp.2022.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Wang X., Zeng M. First-line tyrosine kinase inhibitor with or without aggressive upfront local radiation therapy in patients with EGFRm oligometastatic non-small cell lung cancer: Interim results of a randomized phase III, open-label clinical trial (SINDAS) ( NCT02893332) J Clin Oncol. 2020;38(15_suppl):9508. [Google Scholar]
- 15.Gomez D.R., Tang C., Zhang J., Blumenschein G.R., Hernandez M., Lee J.J., et al. Local consolidative therapy Vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol. 2019;37(18):1558–1565. doi: 10.1200/JCO.19.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klement R.J., Abbasi-Senger N., Adebahr S., Alheid H., Allgaeuer M., Becker G., et al. The impact of local control on overall survival after stereotactic body radiotherapy for liver and lung metastases from colorectal cancer: a combined analysis of 388 patients with 500 metastases. BMC Cancer. 2019;19(1) doi: 10.1186/s12885-019-5362-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franzese C., Perrino M., Marzo M.A., Badalamenti M., Baldaccini D., D’Agostino G., et al. Oligoprogressive castration-resistant prostate cancer treated with metastases-directed stereotactic body radiation therapy: predictive factors for patients' selection. Clin Exp Metastasis. 2022;39(3):449–457. doi: 10.1007/s10585-022-10158-7. [DOI] [PubMed] [Google Scholar]
- 18.Nicosia L., Cuccia F., Mazzola R., Figlia V., Giaj-Levra N., Ricchetti F., et al. Stereotactic body radiotherapy (SBRT) can delay polymetastatic conversion in patients affected by liver oligometastases. J Cancer Res Clin Oncol. 2020;146(9):2351–2358. doi: 10.1007/s00432-020-03223-9. [DOI] [PubMed] [Google Scholar]