Highlights
-
•
Baseline inflammatory markers evaluated in large rectal cancer multicentric cohort.
-
•
Neutrophil/lymphocyte ratio, Systemic index of inflammation predicted pCR.
-
•
Monocyte/lymphocyte ratio, Hemo-eosinophil inflammation index predicted OS and DFS.
Keywords: Rectal cancer, Neoadjuvant radiotherapy, Inflammatory markers, Prognostic factors, Predictive factors
Abstract
Background
Patients (pts) affected with locally advanced rectal cancer (LARC) may respond differently to neoadjuvant chemoradiotherapy (nCRT). The identification of reliable biomarkers able to predict oncological outcomes could help in the development of risk-adapted treatment strategies. It has been suggested that inflammation parameters may have a role in predicting tumor response to nCRT and survival outcomes and in rectal cancer, but no definitive conclusion can be drawn at present. The aim of the current study is to evaluate the role of baseline inflammatory markers as prognostic and predictive factors in a large multicentric Italian cohort of LARC pts.
Methods
Patients diagnosed with LARC from January 2002 to December 2019 in 9 Italian centers were retrospectively collected. Patients underwent long-course RT with chemotherapy based on fluoropyrimidine ± oxaliplatin followed by surgery. Inflammatory markers were retrieved based on a pre-treatment blood sample including HEI (hemo-eosinophils inflammation index), SII (systemic index of inflammation), NLR (neutrophil-to-lymphocyte ratio), PLR (platelet-to-lymphocyte ratio) and MLR (monocyte-to-lymphocyte ratio). Outcomes of interest were pathological complete response (pCR), disease-free survival (DFS), and overall survival (OS).
Results
808 pts were analyzed. pCR rate was 22 %, 5yOS and 5yDFS were 84.0% and 63.1% respectively. Multivariate analysis identified that a NLR cut-off value >1.2 and SII cut-off value >500 could predict pCR (p = 0.05 and 0.009 respectively). In addition to age, extramesorectal nodes and RT dose, MLR >0.18 (p = 0.03) and HEI = 3 (p = 0.05) were independent prognostic factors for DFS. Finally, age, RT dose, MLR with a cut-off >0.35 (p = 0.028) and HEI = 3 (p = 0.045) were independent predictors of OS.
Conclusions
Higher values of baseline composite inflammatory markers can serve as predictors of lower pCR rates and worse survival outcomes in LARC patients undergoing nCRT. More reliable data from prospective studies could lead to the integration of these inexpensive and easy-to-derive tools into clinical practice.
1. Introduction
Currently, the standard treatment for locally advanced rectal cancer (LARC) consists of neoadjuvant radiotherapy (nRT) or chemoradiotherapy (nCRT) followed by radical surgery [1], [2], [3]. It is still a challenge to predict tumor response and patients’ outcomes after treatment. The best-known prognostic factor is the disease stage, provided by the American Joint Committee on Cancer, but patients with similar disease presentations may have a different prognosis; thus, it is fundamental to identify reliable markers for clinical outcomes that can inform the development of risk-adapted therapeutic and follow-up strategies. Several prognostic and predictive biomarkers for LARC were investigated recently such as genomic profiling, and radiomics analysis [4], [5]. However, these tools are still expensive and mainly require an interpretation of the tumor's biological behaviour.
In contrast, inflammatory indices are easy to acquire and inexpensive biomarkers, and several studies have investigated the role of inflammatory markers as prognostic and predictive factors in different types of cancer. In a highly complex and plastic environment in which cancer, stromal, and inflammatory cells interact, inflammation promotes mutagenesis, proliferation, and cell metastatization by generating cytokines, reactive oxygen species (ROS), nitrogen and tumor necrosis factor (TNF)-α, all of which are involved in DNA damage [6]. Virchow first reported on the association between inflammation and tumor biology in 1863, and this topic has gained interest in the scientific community over the past decade [7], [8].
A higher systemic inflammatory status reflected by pre-treatment laboratory parameters has been found to predict poor oncological outcomes in rectal cancer [9]. Anaemia is frequent in cancer patients and could contribute to tumor cells resistance to radiotherapy (RT) and chemotherapy (CT), while high baseline eosinophils levels can predict poor survival outcomes [10], [11]. Indeed, neutrophils production increases in inflammatory situations; they are known to promote tumor initiation and growth and help metastatic spread [12]. Platelet count is often increased in cancer patients and platelet activity facilitates tumor cells growth and extravasation [13]. In contrast, lymphocytes are recognized to be involved in contrasting tumor progression [14]. Zhang et al. studied the prognostic value of inflammatory markers in a large cohort of LARC patients, identifying neutrophil-to-lymphocyte ratio (NLR) as the most effective marker, being an independent predictor of disease-free survival (DFS) and overall survival (OS) [15].
Other studies investigating the predictive potential of these parameters in patients with rectal cancer undergoing preoperative CRT have yielded heterogeneous results [16], [17], [18].
The optimal cut-off values of these laboratory markers have yet to be defined and their validation and integration into clinical practice is pending. The aim of the present study is to evaluate the prognostic and predictive role of several baseline combined inflammatory markers in a large Italian retrospective multicentric cohort of LARC patients treated with nCRT.
2. Materials and methods
2.1. Population and procedures
This is an observational, retrospective, and multicentric study conducted on consecutive patients undergoing nCRT for LARC in 9 Italian Radiation Oncology centers.
The protocol was approved by the Ethics Committee of Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy (Protocol ID 4874). Clinical data were retrospectively collected by each participating center into electronic databases. All patients provided written informed consent to the treatment. We considered patients treated between January 2008 and December 2019 at the Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome and between January 2002 and December 2019 at other eight centers (Centro di Riferimento Oncologico, Aviano; Policlinico S. Orsola Malpighi, Bologna; Policlinico SS. Annunziata, Chieti; IRCCS Ospedale Policlinico S. Martino, Genova; Ospedale Civile ASL TO4, Ivrea; Ospedale S. Maria Goretti, Latina; A.O. San Gerardo, Monza; Ospedale S. Maria della Misericordia, Rovigo).
Patients aged ≥18 years undergoing nCRT for LARC were considered for inclusion in our study. RT performed for non-curative purposes, the presence of metastasis at diagnosis, and a follow-up time of below two years in the absence of events were considered exclusion criteria. All patients underwent long-course nCRT with CT based on fluoropyrimidine ± oxaliplatin. The radiation treatment was delivered by conformational RT technique (3D-CRT) or intensity modulated RT (IMRT) up to a total prescription dose to the primary tumor of 56 Gy delivered with conventional fractionation.
Approximately 8 weeks after the end of nCRT, patients were re-evaluated by digital rectal examination and pelvic magnetic resonance imaging (MRI) and subsequently underwent surgery; in the case of major clinical response (mCR) or complete clinical response (cCR) a conservative approach (watch-and-wait or local excision) was allowed. Adjuvant CT was an option, depending on clinical and/or pathological disease risk factors. After the end of primary treatments, patients were evaluated every 3–6 months during the first 2 years and every 6–12 months during the following 3 years.
2.2. Data collection
We collected data related to demographic variables, clinical stage (TNM 7th edition), tumor markers, treatments related data, pathological stage and tumor response, patients’ status during follow-up, blood tests including complete blood count with leukocyte formula at diagnosis (within 1 week from the start of nCRT), from which the inflammatory markers were calculated (Table S1, Supplementary Material).
2.3. Statistical analysis
Endpoints of the study were OS, DFS and pCR rate. Survival outcomes were defined as the time elapsed from cancer diagnosis (for OS) or the date of surgery or the end of nCRT alternatively (for DFS) to the date of the event. In the absence of the event, the date of the last follow-up examination was considered. Death, local recurrence (recurrence of disease in the pelvis) and distant recurrence (recurrence of disease in any other location) were considered as events. Pathologic complete response (pCR) was defined as the absence of tumor cells in the resected specimen (ypT0N0 or ypT0Nx). Surgical Interval was defined as the time elapsed from the end of nCRT to the date of surgery.
Descriptive analysis was performed calculating mean, standard deviation, median, minimum, maximum, 1st and 3rd quartiles to better describe quantitative items. Associations between endpoints and baseline variables was assessed through a logistic regression model when considering a binary outcome (i.e. pCR) and by the Cox regression model when dealing with survival times. Associations with inflammatory parameters was firstly assessed leaving variables as a continuum, subsequently we looked for the best cut-off. The best cut-off was selected as the value which maximizes the differences between the two survival curves measured by the log-rank test. Covariates screened by univariate analysis were included into multivariate models in case of a p-value ≤ 0.10. When considering multivariable analysis, a stepwise forward approach was used to detect the most significantly factors independently associated with the outcome. Estimates (hazard ratios, HR; odds ratios, OR) are presented with 95 % confidence intervals (95 % CI). We considered a p-value ≤ 0.05 to be significant. DFS and OS were also evaluated using the Kaplan-Meier method. All statistical analyses were performed using IBM SPSS Statistics version 28.0.
3. Results
3.1. Patients baseline and treatment characteristics
Among 1262 patients who met the inclusion criteria, 454 were excluded due to incomplete information on laboratory parameters; thus, 808 patients were eligible for analysis. Patients’ demographics, tumor and treatment characteristics are summarized in Table 1 and baseline inflammatory parameters are showed in Table 2.
Table 1.
Patients and treatment characteristics.
| N (%) | |
|---|---|
| PATIENTS, total number | 808 (100) |
| GENDER Male Female |
493 (61.0) 315 (39.0) |
| AGE, years Median (range) ≥65 |
64 (26–88) 403 (49.9) |
| CEA, ng/ml Median (range) ≥5 unknown |
3.1 (0.1–316) 156 (19.3) 297 (36.7) |
| cT 1 2 3 4 unknown |
1 (0.1) 57 (7.1) 557 (68.9) 168 (20.8) 25 (3.1) |
| cN 0 1 2 3 unknown |
155 (19.2) 367 (45.4) 276 (34.2) 1 (0.1) 9 (1.1) |
| EXTRAMESORECTAL NODES No Yes unknown |
572 (70.8) 165 (20.4) 71 (8.8) |
| CONCOMITANT CT single agent double agent unknown |
595 (73.6) 201 (24.9) 12 (1.5) |
| RT DOSE, Gy Median (range) ≥55 |
55 (30.8–56) 488 (60.4) |
| SURGICAL INTERVAL, weeks Median (range) ≥12 unknown |
11 (2–41) 320 (39.6) 40 (5.0) |
| pT 0 1 2 3 4 unknown |
178 (22.0) 53 (6.6) 192 (23.8) 251 (31.1) 16 (2.0) 118 (14.6) |
| pN 0 1 2 3 unknown |
515 (63.7) 127 (15.7) 24 (3.0) 1 (0.1) 141 (17.5) |
| pCR yes no unknown |
178 (22) 534 (66) 98 (12) |
CEA: Carcinoembryonic Antigen; CT: chemotherapy; Gy: Gray; pCR: pathologic complete response; RT: radiotherapy.
Table 2.
Baseline inflammatory parameters.
| Mean (SD) | Median (range) (IQR) | |
|---|---|---|
| NLR | 2.82 (1.58) | 2.47 (0.42–14.1) (1.87–3.31) |
| PLR | 164.39 (94.40) | 140.45 (19.9–1113.21) (108.03–191.88) |
| MLR | 0.28 (0.16) | 0.25 (0.01–1.46) (0.19–0.33) |
| SII | 722.99 (502.42) | 602.28 (52.32–5748.12) (416.45–905.85) |
| HEI (n, %) 0 1 2 3 |
57 (7.0) 328 (40.6) 315 (39.0) 108 (13.4) |
HEI: hemo-eosinophils inflammation index; IQR: interquartile range; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; MLR: monocyte-to-lymphocyte ratio; SD: standard deviation; SII: systemic index of inflammation.
Median age was 64 years (range 26–88), 61 % of patients were male. Most of the patients had clinical T3 stage (68.9 %) and positive lymph nodes (79.7 %). The median CEA level at baseline was 3.1 ng/ml (range 0.1–316). Single-agent concomitant CT was administered in 73.6 % of patients, while double-agent CT with the addition of oxaliplatin to fluoropyrimidine was prescribed in 24.9 % of cases. The median radiation dose delivered was 55 Gy (range 30.8–56). A total of 39 patients (4.8 %) showing major or complete clinical response after nCRT were managed conservatively.
3.2. Outcomes
At a median follow-up time of 53.5 months (range 6–198) the local recurrences, distant recurrences, deaths for the entire cohort were 107 (13.2 %), 162 (20.0 %), and 166 (20.5 %), respectively. pCR rate was 22 %; five-year OS and DFS (5yOS and 5yDFS) estimates were 84.0 % and 63.1 % respectively.
3.3. Univariate and multivariate analysis
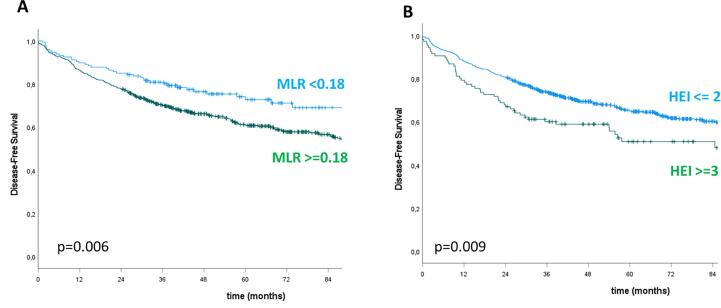
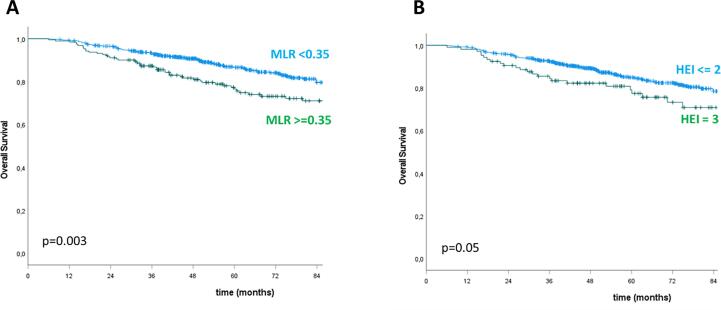
Table 3, Table 4, Table 5 show the results of the univariate and multivariate analysis for pCR, DFS and OS, respectively. The following inflammatory markers demonstrated a statistically significant association with the outcomes: NLR, PLR, SII and MLR with respect to pCR; MLR and HEI for DFS; NLR, MLR and HEI with regard to OS. At multivariate analysis, the only independent predictors of pCR were NLR for values >1.2 (p = 0.05) and SII for values >500 (p < 0.0001). Variables independently predictive of DFS were: age ≥ 65 years (p = 0.002), positive extramesorectal nodes (p = 0.02), RT dose ≥ 55 Gy (p = 0.008), MLR > 0.18 (p = 0.03), HEI 3 (p = 0.05). Finally, age ≥ 65 years (p < 0.0001), RT dose ≥ 55 Gy (p = 0.04), MLR > 0.35 (p = 0.028) and HEI 3 (p = 0.045) were independent predictors of OS. pCR rate was 48.3 % vs 24.1 % in patients with NLR < 1.2 vs ≥ 1.2 (p = 0.003) and 32.2 % vs 21.0 % in patients with SII < 500 vs ≥ 500 (p < 0.0001). Kaplan-Meier analysis confirmed that higher inflammatory values conferred a worse outcome: 5yDFS was 72.7 % vs 61.0 % for MLR < 0.18 vs ≥ 0.18 and 65.0 % vs 50.8 % for HEI ≤ 2 vs 3 (p = 0.006 and 0.009 respectively); 5yOS was 86.6 % vs 76.5 % for MLR < 0.35 vs ≥0.35 and 84.9 % vs 77.4 % for HEI ≤ 2 vs 3 (p = 0.003 and 0.05 respectively) (Fig. 1, Fig. 2).
Table 3.
Univariate and multivariate logistic regression of variables predicting complete response to nCRT (neoadjuvant chemoradiation).
| VARIABLE | pCR, n (%) | UNIVARIATE | MULTIVARIATE |
|---|---|---|---|
| GENDER Male Female |
113 (26.0) 65 (23.5) |
p = 0.45 1.15 (0.81–1.63) 1.00 |
|
| AGE, years <65 ≥65 |
81 (22.6) 97 (27.4) |
p = 0.14 1.00 1.29 (0.92–1.81) |
|
| cT 1–2 3 4 |
14 (31.1) 122 (24.9) 33 (21.4) |
p = 0.39 1.00 0.74 (0.38–1.43) 0.60 (0.29–1.26) |
|
| cN negative positive |
30 (23.3) 143 (24.9) |
p = 0.69 1.00 1.10 (0.70–1.72) |
|
| EXTRAMESORECTAL NODES no yes |
120 (24.7) 39 (25.2) |
p = 0.91 1.00 1.03 (0.68–1.56) |
|
| CONCOMITANT CT single agent double agent |
131 (25.5) 45 (23.4) |
p = 0.58 1.00 0.89 (0.61–1.32) |
|
| RT, Gy <55 ≥55 |
57 (21.7) 121 (26.9) |
p = 0.12 1.00 1.33 (0.93–1.91) |
|
| SURGICAL INTERVAL, weeks <12 ≥12 |
99 (23.2) 79 (27.7) |
p = 0.17 1.00 1.27 (0.90–1.79) |
|
| NLR <=1.2 >1.2 |
14 (48.3) 164 (24.0) |
p = 0.005 1.00 0.34 (0.16–0.72) |
p = 0.05 1.00 0.46 (0.21–1.00) |
| PLR <=200 >200 |
149 (27.6) 29 (16.9) |
p = 0.005 1.00 0.53 (0.34–0.83) |
--- |
| SII <=500 >500 |
83 (32.0) 95 (21.0) |
p = 0.001 1.00 0.56 (0.40–0.79) |
p = 0.009 1.00 0.62 (0.43–0.89) |
| MLR <=0.38 >0.38 |
158 (26.9) 20 (16.0) |
p = 0.012 1.00 0.52 (0.31–0.86) |
--- |
| HEI 0–1-2 3 |
158 (25.6) 20 (21.3) |
p = 0.37 1.00 0.79 (0.46–1.33) |
CT: chemotherapy; Gy: Gray; HEI: hemo-eosinophils inflammation index; MLR: monocyte-to-lymphocyte ratio; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; RT: radiotherapy; SII: systemic index of inflammation. In bold the statistically significant values.
Table 4.
Univariate and multivariate Cox regression analysis of variables predicting disease-free survival (DFS).
| VARIABLE | Events, n (%) | UNIVARIATE | MULTIVARIATE |
|---|---|---|---|
| GENDER Male Female |
207 (42.2) 111 (35.5) |
p = 0.16 1.18 (0.94–1.48) 1.00 |
|
| AGE, years <65 ≥65 |
131 (32.5) 187 (46.6) |
p < 0.0001 1.00 1.68 (1.34–2.10) |
p = 0.002 1.00 1.49 (1.16–1.94) |
| cT 1–2 3 4 |
27 (46.6) 214 (38.7) 72 (42.9) |
p = 0.16 1.00 0.74 (0.50–1.10) 0.90 (0.58–1.40) |
|
| cN negative positive |
53 (34.4) 264 (41.2) |
p = 0.034 1.00 1.38 (1.02–1.86) |
--- |
| EXTRAMESORECTAL NODES no yes |
198 (34.8) 80 (48.5) |
p < 0.0001 1.00 1.59 (1.23–2.07) |
p = 0.02 1.00 1.42 (1.07–1.88) |
| CONCOMITANT CT single agent double agent |
239 (40.4) 69 (34.3) |
p = 0.15 1.00 0.82 (0.63–1.07) |
|
| RT, Gy <55 ≥55 |
116 (36.6) 202 (41.5) |
p = 0.002 1.00 1.44 (1.14–1.82) |
p = 0.008 1.00 1.46 (1.10–1.94) |
| SURGICAL INTERVAL, weeks <12 ≥12 |
176 (39.6) 120 (37.6) |
p = 0.030 1.00 1.30 (1.03–1.66) |
--- |
| NLR <=2.5 >2.5 |
158 (38.6) 160 (40.5) |
p = 0.69 1.00 1.05 (0.84–1.30) |
|
| PLR <=100 >100 |
60 (35.9) 258 (40.5) |
p = 0.52 1.00 1.10 (0.83–1.45) |
|
| SII <=500 >500 |
118 (40.0) 200 (39.3) |
p = 0.89 1.00 1.02 (0.81–1.27) |
|
| MLR <=0.18 >0.18 |
52 (29.1) 266 (42.6) |
p = 0.01 1.00 1.46 (1.08–1.97) |
p = 0.03 1.00 1.43 (1.02–1.99) |
| HEI 0–1-2 3 |
267 (38.3) 51 (48.1) |
p = 0.009 1.00 1.49 (1.11–2.01) |
p = 0.05 1.00 1.35 (1.00–1.87) |
CT: chemotherapy; Gy: Gray; HEI: hemo-eosinophils inflammation index; MLR: monocyte-to-lymphocyte ratio; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; RT: radiotherapy; SII: systemic index of inflammation. In bold the statistically significant values.
Table 5.
Univariate and multivariate Cox regression analysis of variables predicting overall survival (OS).
| VARIABLE | Deaths, n (%) | UNIVARIATE | MULTIVARIATE |
|---|---|---|---|
| GENDER Male Female |
113 (23.0) 53 (16.9) |
p = 0.05 1.38 (0.99–1.91) 1.00 |
--- |
| AGE, years <65 ≥65 |
62 (15.4) 104 (25.9) |
p < 0.0001 1.00 2.09 (1.52–2.86) |
p < 0.0001 1.00 2.06 (1.50–2.83) |
| cT 1–2 3 4 |
12 (20.7) 112 (20.3) 38 (22.6) |
p = 0.54 1.00 0.83 (0.46–1.51) 1.01 (0.52–1.93) |
|
| cN negative positive |
30 (19.5) 135 (21.1) |
p = 0.24 1.00 1.27 (0.85–1.89) |
|
| EXTRAMESORECTAL NODES no yes |
97 (17.0) 29 (17.6) |
p = 0.20 1.00 1.32 (0.87–2.00) |
|
| CONCOMITANT CT single agent double agent |
120 (20.3) 40 (19.9) |
p = 0.62 1.00 0.91 (0.64–1.31) |
|
| RT, Gy <55 ≥55 |
91 (28.7) 75 (15.4) |
p = 0.04 1.00 0.72 (0.53–0.98) |
p = 0.04 1.00 0.73 (0.53–0.99) |
| SURGICAL INTERVAL, weeks <12 ≥12 |
109 (24.5) 44 (13.8) |
p = 0.66 1.00 0.92 (0.64–1.32) |
|
| NLR <=2.5 >2.5 |
70 (17.1) 96 (24.3) |
p = 0.05 1.00 1.36 (1.00–1.85) |
--- |
| PLR <=100 >100 |
24 (14.4) 142 (22.3) |
p = 0.10 1.00 1.44 (0.94–2.23) |
|
| SII <=500 >500 |
55 (18.6) 111 (21.8) |
p = 0.26 1.00 1.21 (0.87–1.67) |
|
| MLR <=0.35 >0.35 |
108 (17.6) 58 (30.4) |
p = 0.005 1.00 1.58 (1.15–2.18) |
p = 0.028 1.00 1.44 (1.04–1.98) |
| HEI 0–1-2 3 |
139 (19.9) 27 (25.5) |
p = 0.07 1.00 1.48 (0.98–2.23) |
p = 0.045 1.00 1.53 (1.01–2.32) |
CT: chemotherapy; Gy: Gray; HEI: hemo-eosinophils inflammation index; MLR: monocyte-to-lymphocyte ratio; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; RT: radiotherapy; SII: systemic index of inflammation. In bold the statistically significant values.
Fig. 1.
Disease-free survival stratified by MLR (A) and HEI (B).
Fig. 2.
Overall survival stratified by MLR (A) and HEI (B).
4. Discussion
Rectal cancer is a potentially curable disease after multimodality treatment; however, the disease has a non-negligible tendency to metastasize and globally 5-year-overall survival is around 65 % [19]. The identification of prognostic factors could lead to intensification of treatment for those patients predicted to be poor survivors [20]. Moreover, in LARC undergoing nCRT, the absence of residual tumor at the surgical specimen is associated with favorable survival outcomes; the prediction of pCR could promote the adoption of organ-preserving treatment strategies and save patients from surgery-related morbidity and mortality without compromising survival [21], [22].
We investigated the potential of pretreatment inflammatory markers (NLR, PLR, MLR, SII and HEI) to predict pathologic complete response rate and outcomes in a large retrospective multicentric cohort of patients with LARC who received nCRT followed by curative resection. To the best of our knowledge, based on the published retrospective data on LARC, there is only one study with a larger cohort and a multicentric design and it reports on the role of NLR and PLR [23].
To review this intriguing topic, we searched Pubmed for reports on LARC original series evaluating pre-nCRT inflammatory markers (NLR, PLR, MLR, SII). English language studies were reviewed, and results are summarized in Table 6. With regard to HEI, we borrowed this recently introduced composite marker, comprising SII, hemoglobin and eosinophils levels at baseline, from an experience in anal canal cancer patients undergoing CRT where it was found to predict DFS and OS and was externally validated [24], [25].
Table 6.
Summary of available studies reporting on composite inflammatory markers of interest in LARC patients undergoing nCRT. Cut-offs, p-values and statistics refer only to statistically significant associations at multivariate analysis between markers and outcomes, that are presented in bold.
| First author (year) | Design | Patients n° | Endpoints | Evaluated markers | Cut-off | p-value | Statistics, comments |
|---|---|---|---|---|---|---|---|
| Carruthers R (2012) [37] | Retrospective, monocentric | 115 | OS, DFS, TTLR |
NLR PLR |
5 | 0.001, 0.002, 0.014 | HR 7.0, 4.1, 3.8 |
| Kim IY (2014) [16] | Retrospective, monocentric | 102 | ypTNM | NLR | 3 | 0.04 | HR 5.2 |
| Shen L (2014) [30] | Retrospective, monocentric | 199 | OS, DFS, ypTNM | NLR | 2.8 | 0.018 | HR 2.123 |
| Nagasaki T (2015) [38] | Retrospective, monocentric | 201 | OS, RFS | NLR | 3 | 0.012 | HR 3.38 |
| Shen J (2017) [39] | Retrospective, monocentric | 202 | OS, DFS | NLR | n.s. | n.s. | – |
| Zhao J (2017) [40] | Retrospective, monocentric | 100 | OS |
LMR NLR, PLR |
3 | 0.002 | HR 0.43 |
| Vallard A (2018) [41] | Retrospective, monocentric | 257 | OS, PFS, LR, TRG | NLR | 2.8 | 0.02, 0.006, 0.03 | HR 2.23, 2.21,14.7 |
| Zhang X (2018) [42] | Retrospective, monocentric | 76 | OS | NLR | 2 | 0.025 | HR 7.707 |
| Braun LH (2019) [43] | Retrospective, monocentric | 220 | DFS |
NLR LMR, PLR |
4.06 | 0.017 | HR 0.3 |
| Dudani S (2019) [23] | Retrospective, multicentric | 1237 | pCR, OS, DFS | NLR, PLR | n.s. | n.s. | – |
| Kim TG (2019) [44] | Retrospective, monocentric | 176 |
TRG, OS, DFS TRG, OS, DFS |
NLR PLR |
2 133.4 |
0.008, 0.027, 0.014 <0.001 |
– |
| Lee J H (2020) [33] | Retrospective, two centres | 549 | OS, DFS | NLR, PLR | n.s. | n.s. | Significance only in MSI cases |
| Sun Y (2020) [17] | Retrospective, monocentric | 100 | TRG |
NLR PLR SII |
3.05 145.98 |
0.028 0.038 |
OR 4.025 OR 4.337 MACs |
| Timudom K (2020) [45] | Retrospective, monocentric | 111 | ypT, NAR | NLR, MLR, PLR | n.s. | n.s. | – |
| Zhang Y (2020) [15] | Retrospective, monocentric | 472 | OS, DFS |
NLR SII, MLR, PLR |
2.3 | 0.046, 0.044 | HR 1.797, 1.707 |
| Eraslan E (2021) [29] | Retrospective, monocentric | 188 | pCR |
SII NLR, LMR, PLR |
748 | 0.047 | OR 0.471 |
| Wang Y (2021) [46] | Retrospective, monocentric | 273 | TRG, OS, DFS |
PLR, NLR, LMR |
– | 0.013 | HR 0.992 |
DFS: disease-free survival; HR: hazard ratio; LMR: lymphocyte-to-monocyte ratio; LR: local recurrence; MACs: mucinous adenocarcinomas; MLR: monocyte-to-lymphocyte ratio; MSI: microsatellite instability; NAR: neoadjuvant rectal score; nCRT: neoadjuvant chemoradiotherapy; NLR: neutrophil-to-lymphocyte ratio; n.s.: not significant; OR: odds ratio; OS: overall survival; pCR: pathological complete response; PFS: progression-free survival; PLR: platelet-to-lymphocyte ratio; RFS: relapse-free survival; SII: systemic index of inflammation; TRG: tumor regression grade; TTLR: time to local recurrence.
In our experience, 22 % patients exhibited a pCR which is consistent with the published data [26], [27], [28]. Higher values of NLR, PLR, SII and MLR demonstrated an unfavourable association with pCR; however, only NLR and SII maintained a statistically significant association with pCR at the multivariate analysis. The literature does not clearly show a relationship between inflammatory indicators and nCRT response. In a series of 100 patients with mucinous rectal cancer, pretreatment lower NLR and PLR levels determined by receiver operating characteristic (ROC) analysis were independent predictors of favourable response to nCRT (TRG 0–1) while SII was not [17]. In contrast, a recent experience on 188 LARC patients reported that among baseline SII, NLR, and PLR, only SII was an independent predictive factor for pCR [29]. An et al. found that, among 168 LARC patients, neither PLR nor NLR were associated with pCR nor 5-year DFS and that only pre-treatment PLR could be used to predict OS; in this series, the cut-off PLR and NLR were set as the mean values [18]. In the aforementioned multicentric study by Dudani et al., baseline NLR and PLR values, with thresholds chosen on the basis of previous experience and confirmed by the authors’ statistical analysis, were neither prognostic for DFS and OS nor predictive of pCR [23].
In terms of survival, we found that HEI and MLR were related to DFS on both univariate and multivariate analysis; HEI and MLR were also independently predictive of OS, while NLR did not confirm its prognostic value for OS at the multivariate analysis. Previous studies have yielded mixed outcomes in this context as well. In contrast to the aforementioned experiences, Zhang et al. found that higher values of all the evaluated parameters (SII, NLR, PLR, MLR) were correlated with worse prognosis; NLR was an independent predictor of OS and DFS and the authors concluded that it was the most effective marker for systemic inflammation [15]. Also, Shen et al. found NLR to be an independent factor for worse OS in LARC [30]. With regard to HEI, we tested whether a new score developed on an anal cancer population could be applicable to rectal cancer patients [24], [25]; we maintained the score defining parameters described by the authors but found a different discriminating value (0–2 vs 3 rather than 0–1 vs 2–3). In our cohort, a higher HEI score was related to worse DFS and OS.
Our results only partially overlap with those in the literature, which are not univocal themselves. The reasons for these discrepancies could be many: population numerosity and inclusion criteria, methods for inflammation markers cut-offs choice, coexistence of confounding factors which have not been considered.
It is often observed that inflammatory indexes lose their predictive and prognostic potential when considered together with other variables as if inflammation is not independently related to the outcome but somehow linked to other disease or patient characteristics that interact in the complex host-tumor relationship. Additionally, we must consider the dynamicity of this relation and the possibility that immune response changes over the course of the disease; pre- and post-treatment variations in these same parameters could provide additional information [31].
To strengthen our findings and introduce them into clinical practice, the preferred option would be to prospectively evaluate the prognostic and predictive role of inflammatory markers; a model should be hopefully developed and validated able to identify patients at different risk levels and personalize therapies consequently.
A promising tool in the treatment of a subset of rectal cancer patients is represented by immune-checkpoint blockade, particularly after the recent publication of the surprising results obtained using dostarlimab [32]. In fact it is known that one key factor in shifting the peritumoral microenvironment in a pro-inflammatory direction is microsatellite instability (MSI), which can be found in 5–10 % of rectal adenocarcinomas and is predictive of a better response to immunotherapy. Lee et al. [33] found that NLR and PLR were predictive OS and DFS only in patient with MSI. It would be interesting to deepen the relation between MSI and inflammatory markers and to investigate the role of these drugs in the “hot” population.
We reported on a large cohort with fair follow-up time taken from well-experienced radiation oncology centers across Italy. We adopted a robust method for data processing and cut-offs identification, except for HEI which was borrowed from the literature given its originality. On the other hand, potential pitfalls of the study need to be recognized: the retrospective design, that prevented us from tracing some patient-related characteristics (e.g. symptom burden at diagnosis, comorbidities and anti-inflammatory medicines), which could have influenced the course of the disease and the inflammation indexes [34]; the long observation time; the heterogeneity in therapeutic procedures. Moreover, information on treatment (3D-CRT vs IMRT, adjuvant CT) and disease-related features (perineural invasion, extramural venous invasion, mucinous aspects, disease location, etc.) were lacking in most cases in our cohort but should be considered in future studies [35], [36].
5. Conclusions
Pre-treatment inflammatory composite markers may provide useful predictive and prognostic information on LARC patients, they are inexpensive and easy to obtain; NLR and SII may be independent predictors of pCR, while MLR and HEI seem able to prognosticate long term outcomes. However, findings from individual series should not be lightly generalized. Future large-scale prospective studies may provide more robust evidence and support the decision-making process in this population of patients.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The Authors thank the Scientific Committee and Board of the AIRO for the critical revision and final approval of the manuscript (Nr. 20/2022).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2023.100579.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Sauer R., Becker H., Hohenberger W., Rödel C., Wittekind C., Fietkau R., et al. German Rectal Cancer Study Group. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 2.Sebag-Montefiore D., Stephens R.J., Steele R., Monson J., Grieve R., Khanna S., et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373(9666):811–820. doi: 10.1016/S0140-6736(09)60484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glynne-Jones R., Wyrwicz L., Tiret E., Brown G., Rödel C., Cervantes A., et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv22–iv40. doi: 10.1093/annonc/mdx224. [DOI] [PubMed] [Google Scholar]
- 4.Stockton J.D., Tee L., Whalley C., James J., Dilworth M., Wheat R., et al. Complete response to neoadjuvant chemoradiotherapy in rectal cancer is associated with RAS/AKT mutations and high tumour mutational burden. Radiat Oncol. 2021;16(1) doi: 10.1186/s13014-021-01853-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinapoli N., Barbaro B., Gatta R., Chiloiro G., Casà C., Masciocchi C., et al. Magnetic resonance, vendor-independent, intensity histogram analysis predicting pathologic complete response after radiochemotherapy of rectal cancer. Int J Radiat Oncol Biol Phys. 2018;102(4):765–774. doi: 10.1016/j.ijrobp.2018.04.065. [DOI] [PubMed] [Google Scholar]
- 6.Greten F.R., Grivennikov S.I. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27–41. doi: 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balkwill F., Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 8.Yang R., Chang Q., Meng X., Gao N., Wang W. Prognostic value of Systemic immune-inflammation index in cancer: a meta-analysis. J Cancer. 2018;9(18):3295–3302. doi: 10.7150/jca.25691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.An S., Shim H., Kim K., Kim B., Bang H.-J., Do H., et al. Pretreatment inflammatory markers predicting treatment outcomes in colorectal cancer. Ann Coloproctol. 2022;38(2):97–108. doi: 10.3393/ac.2021.01004.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franco P., Montagnani F., Arcadipane F., Casadei C., Andrikou K., Martini S., et al. The prognostic role of hemoglobin levels in patients undergoing concurrent chemo-radiation for anal cancer. Radiat Oncol. 2018;13(1) doi: 10.1186/s13014-018-1035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rimini M, Franco P, Bertolini F, Berardino B, Giulia ZM, Stefano V, et al. The prognostic role of baseline eosinophils in HPV-related cancers: a multi-institutional analysis of anal SCC and OPC patients treated with radical CT-RT. J Gastrointest Cancer. 2022:1–10. doi: 10.1007/s12029-022-00850-y. Epub ahead of print. PMID: 35915202; PMCID: PMC9342937. [DOI] [PMC free article] [PubMed]
- 12.Ocana A., Nieto-Jiménez C., Pandiella A., Templeton A.J. Neutrophils in cancer: prognostic role and therapeutic strategies. Mol Cancer. 2017;16(1) doi: 10.1186/s12943-017-0707-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haemmerle M., Stone R.L., Menter D.G., Afshar-Kharghan V., Sood A.K. The platelet lifeline to cancer: challenges and opportunities. Cancer Cell. 2018;33(6):965–983. doi: 10.1016/j.ccell.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ménétrier-Caux C., Ray-Coquard I., Blay J.Y., Caux C. Lymphopenia in cancer patients and its effects on response to immunotherapy: an opportunity for combination with cytokines? J Immunother Cancer. 2019;7(1):85. doi: 10.1186/s40425-019-0549-5. Published 2019 Mar 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y., Liu X., Xu M., Chen K., Li S., Guan G. Prognostic value of pretreatment systemic inflammatory markers in patients with locally advanced rectal cancer following neoadjuvant chemoradiotherapy. Sci Rep. 2020;10(1):8017. doi: 10.1038/s41598-020-64684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim I.Y., You S.H., Kim Y.W. Neutrophil-lymphocyte ratio predicts pathologic tumor response and survival after preoperative chemoradiation for rectal cancer. BMC Surg. 2014;18(14):94. doi: 10.1186/1471-2482-14-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun Y., Huang Z., Chi P. An inflammation index-based prediction of treatment response to neoadjuvant chemoradiotherapy for rectal mucinous adenocarcinoma. Int J Clin Oncol. 2020;25(7):1299–1307. doi: 10.1007/s10147-020-01670-5. [DOI] [PubMed] [Google Scholar]
- 18.An S.H., Kim I.Y. Can pretreatment platelet-to-lymphocyte and neutrophil-to-lymphocyte ratios predict long-term oncologic outcomes after preoperative chemoradiation followed by surgery for locally advanced rectal cancer? Ann Coloproctol. 2022;38(3):253–261. doi: 10.3393/ac.2021.00633.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Cancer Society . American Cancer Society; Atlanta: 2022. Cancer Facts & Figures 2022. [Google Scholar]
- 20.Bahadoer R.R., Dijkstra E.A., van Etten B., Marijnen C.A.M., Putter H., Kranenbarg E.-K., et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):29–42. doi: 10.1016/S1470-2045(20)30555-6. [DOI] [PubMed] [Google Scholar]
- 21.Maas M., Nelemans P.J., Valentini V., Das P., Rödel C., Kuo L.-J., et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. LancetOncol. 2010;11(9):835–844. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 22.Habr-Gama A., Perez R.O., Nadalin W., Sabbaga J., Ribeiro U., Silva e Sousa A.H., et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240(4):711–718. doi: 10.1097/01.sla.0000141194.27992.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dudani S., Marginean H., Tang P.A., Monzon J.G., Raissouni S., Asmis T.R., et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictive and prognostic markers in patients with locally advanced rectal cancer treated with neoadjuvant chemoradiation. BMC Cancer. 2019;19(1) doi: 10.1186/s12885-019-5892-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rimini M., Franco P., De Bari B., Zampino M.G., Vagge S., Frassinetti G.L., et al. The prognostic value of the new combined hemo-eosinophil inflammation index (HEI Index): a multicenter analysis of anal cancer patients treated with concurrent chemo-radiation. Cancers (Basel) 2021;13(4):671. doi: 10.3390/cancers13040671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franco P., Porreca A., Mantello G., Valvo F., Gasparini L., Slim N., et al. External validation of a composite bio-humoral index in anal cancer patients undergoing concurrent chemoradiation. Radiother Oncol. 2022;177:9–15. doi: 10.1016/j.radonc.2022.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Gambacorta M.A., Masciocchi C., Chiloiro G., Meldolesi E., Macchia G., van Soest J., et al. Timing to achieve the highest rate of pCR after preoperative radiochemotherapy in rectal cancer: a pooled analysis of 3085 patients from 7 randomized trials. Radiother Oncol. 2021;154:154–160. doi: 10.1016/j.radonc.2020.09.026. [DOI] [PubMed] [Google Scholar]
- 27.Burbach J.P., den Harder A.M., Intven M., van Vulpen M., Verkooijen H.M., Reerink O. Impact of radiotherapy boost on pathological complete response in patients with locally advanced rectal cancer: a systematic review and meta-analysis. Radiother Oncol. 2014;113(1):1–9. doi: 10.1016/j.radonc.2014.08.035. [DOI] [PubMed] [Google Scholar]
- 28.Macchia G., Gambacorta M.A., Masciocchi C., Chiloiro G., Mantello G., di Benedetto M., et al. Time to surgery and pathologic complete response after neoadjuvant chemoradiation in rectal cancer: A population study on 2094 patients. Clin Transl Radiat Oncol. 2017;4:8–14. doi: 10.1016/j.ctro.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eraslan E., Adas Y.G., Yildiz F., Gulesen A.I., Karacin C., Arslan U.Y. Systemic immune-inflammation index (SII) predicts pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. J Coll Physicians Surg Pak. 2021;30(4):399–404. doi: 10.29271/jcpsp.2021.04.399. [DOI] [PubMed] [Google Scholar]
- 30.Shen L., Zhang H., Liang L., Li G., Fan M., Wu Y., et al. Baseline neutrophil-lymphocyte ratio (≥2.8) as a prognostic factor for patients with locally advanced rectal cancer undergoing neoadjuvant chemoradiation. Radiat Oncol. 2014;9(1) doi: 10.1186/s13014-014-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee I.H., Hwang S., Lee S.J., Kang B.W., Baek D., Kim H.J., et al. Systemic inflammatory response after preoperative chemoradiotherapy can affect oncologic outcomes in locally advanced rectal cancer. Anticancer Res. 2017;37(3):1459–1465. doi: 10.21873/anticanres.11470. [DOI] [PubMed] [Google Scholar]
- 32.Cercek A., Lumish M., Sinopoli J., Weiss J., Shia J., Lamendola-Essel M., et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N Engl J Med. 2022;386(25):2363–2376. doi: 10.1056/NEJMoa2201445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee J.H., Kang B.-H., Song C., Kang S.-B., Lee H.S., Lee K.-W., et al. Microsatellite instability correlated inflammatory markers and their prognostic value in the rectal cancer following neoadjuvant chemoradiotherapy: a hypothesis-generating study. In Vivo. 2020;34(4):2119–2126. doi: 10.21873/invivo.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao X., Wang X., Wang H., Xu G., Yu H. Systemic inflammation status relates to anti-inflammatory drug benefit and survival in rectal cancer. J Surg Res. 2022;269:249–259. doi: 10.1016/j.jss.2021.08.028. [DOI] [PubMed] [Google Scholar]
- 35.Kim C.H., Yeom S.-S., Lee S.Y., Kim H.R., Kim Y.J., Lee K.H., et al. Prognostic impact of perineural invasion in rectal cancer after neoadjuvant chemoradiotherapy. World J Surg. 2019;43(1):260–272. doi: 10.1007/s00268-018-4774-8. [DOI] [PubMed] [Google Scholar]
- 36.Schaap D.P., Voogt E.L.K., Burger J.W.A., Cnossen J.S., Creemers G.-J., van Lijnschoten I., et al. Prognostic implications of MRI-detected EMVI and tumor deposits and their response to neoadjuvant therapy in cT3 and cT4 rectal cancer. Int J Radiat Oncol Biol Phys. 2021;111(3):816–825. doi: 10.1016/j.ijrobp.2021.06.013. [DOI] [PubMed] [Google Scholar]
- 37.Carruthers R, Tho LM, Brown J, Kakumanu S, McCartney E, McDonald AC. Systemic inflammatory response is a predictor of outcome in patients undergoing preoperative chemoradiation for locally advanced rectal cancer. Colorectal Dis. 2012;14(10):e701-e707. doi:10.1111/j.1463-1318.2012.03147.x. [DOI] [PubMed]
- 38.Nagasaki T., Akiyoshi T., Fujimoto Y., Konishi T., Nagayama S., Fukunaga Y., et al. Prognostic impact of neutrophil-to-lymphocyte ratio in patients with advanced low rectal cancer treated with preoperative chemoradiotherapy. Dig Surg. 2015;32(6):496–503. doi: 10.1159/000441396. [DOI] [PubMed] [Google Scholar]
- 39.Shen J., Zhu Y., Wu W., Zhang L., Ju H., Fan Y., et al. Prognostic role of neutrophil-to-lymphocyte ratio in locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. Med Sci Monit. 2017;23:315–324. doi: 10.12659/MSM.902752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao J, Xu J, Zhang R. Clinical and prognostic significance of pathological and inflammatory markers in mucinous rectal cancer patients receiving neoadjuvant chemoradiotherapy and curative surgery. Med Sci Monit. 2017;23:4826-4833. doi:10.12659/msm.904116. [DOI] [PMC free article] [PubMed]
- 41.Vallard A., Garcia M.-A., Diao P., Espenel S., de Laroche G., Guy J.-B., et al. Outcomes prediction in pre-operative radiotherapy locally advanced rectal cancer: leucocyte assessment as immune biomarker. Oncotarget. 2018;9(32):22368–22382. doi: 10.18632/oncotarget.25023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang X, Li J, Peng Q, et al. Association of markers of systemic and local inflammation with prognosis of patients with rectal cancer who received neoadjuvant radiotherapy. Cancer Manag Res. 2018;11:191-199. doi:10.2147/CMAR.S187559. [DOI] [PMC free article] [PubMed]
- 43.Braun LH, Baumann D, Zwirner K, et al. Neutrophil-to-lymphocyte ratio in rectal cancer-novel biomarker of tumor immunogenicity during radiotherapy or confounding variable?. Int J Mol Sci. 2019;20(10):2448. doi:10.3390/ijms20102448. [DOI] [PMC free article] [PubMed]
- 44.Kim T.G., Park W., Kim H., Choi D.H., Park H.C., Kim S.-H., et al. Baseline neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in rectal cancer patients following neoadjuvant chemoradiotherapy. Tumori. 2019;105(5):434–440. doi: 10.1177/0300891618792476. [DOI] [PubMed] [Google Scholar]
- 45.Timudom K., Akaraviputh T., Chinswangwatanakul V., Pongpaibul A., Korpraphong P., Petsuksiri J., et al. Predictive significance of cancer related-inflammatory markers in locally advanced rectal cancer. World J Gastrointest Surg. 2020;12(9):390–396. doi: 10.4240/wjgs.v12.i9.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y, Chen L, Zhang B, Song W, Zhou G, Xie L, Yu D. Pretreatment inflammatory-nutritional biomarkers predict responses to neoadjuvant chemoradiotherapy and survival in locally advanced rectal cancer. Front Oncol. 2021;11:639909. doi: 10.3389/fonc.2021.639909. PMID: 33816284; PMCID: PMC8010250. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.