Highlights
-
•
Increasing BED in SBRT is associated with improved overall survival in renal cancer patients.
-
•
Increasing dose should be encouraged when safe.
-
•
Prospective investigation needed to validate.
Keywords: Renal cancer, SBRT, Stereotactic Body Radiotherapy, Biologically equivalent dose
Abstract
Purpose /Objectives Materials/Methods
The National Cancer Database (NCDB) was queried (2004–2017) for patients with RCC who did not have surgical resection but received definitive SBRT. Kaplan-Meier analysis with log-rank test was used to evaluate overall survival (OS). Univariable (UVA) and multivariable (MVA) analysis were conducted using cox proportional hazard models to determine prognostic factors for OS.
Results
A total of 344 patients with median age 77 (IQR 70–85) were included in this study. Median BED3 was 180 Gy (IQR 126.03–233.97). Median OS was 90 months in the highest quartile compared to 36–52 months in the lower three quartiles (p < 0.01). On UVA, the highest BED3 quartile was a positive prognostic factor (HR 0.67, p < 0.01 CI 0.51–0.91) while age, tumor size, T-stage, metastasis, renal pelvis location, and transitional cell histology were negative factors. On MVA, the highest BED3 quartile was remained significant (HR 0.69, p = 0.02; CI 0.49–0.95) as a positive factor, while age, metastasis were negative factors.
Conclusion
Higher BED may be associated with improved OS. Prospective investigation is needed to clearly define optimal BED for SBRT used to treat RCC.
Introduction
Renal Cell Carcinoma (RCC) is the sixth most common cancer in men and the ninth most common cancer in women worldwide with an estimated 79,000 total new cases and 13,920 estimated deaths in 2022 [1]. Surgical resection is standard of care for the initial management of localized RCC [2]. However, many RCC patients are not good candidates for surgery, particularly those who are elderly or have multiple comorbidities where active surveillance or ablative therapies may be recommended.
Historically radiation therapy has been thought to have limited utility in treating RCC given its radioresistant nature [3]. However stereotactic body radiation therapy (SBRT), an emerging therapy that has seen increased utilization for unresectable RCC, due to its radiobiologic properties that potentially overcome the radioresistance of RCC [4]. This technique has been incorporated into the National Comprehensive Cancer Network’s guidelines and is a category 2A recommendation for T1a RCC [5]. SBRT offers many benefits, such as a low toxicity profile and the noninvasiveness of this procedure. SBRT is usually given in one to five fractions in the US which shortens the overall treatment logistics and burden on patients as when compared to conventionally fractionated radiation.
It is believed that from a radiobiologic standpoint SBRT is also advantageous compared to conventionally fractionated, lower dose radiation (1.8–2.0 Gy/fraction). In vitro studies suggest that RCC has an α/β ratio between 2.6 Gy and 6.92 Gy, which predicts higher tumor cell killing with higher fraction size [6]. While different SBRT fractionation schemes are utilized with a wide range of biologically equivalent doses (BED) for management of RCC, there is an absence of data evaluating the prognostic impact of BED for disease control.
Materials and Methods
The National Cancer Data Base (NCDB) is a collaboration between the Commission on Cancer of the American College of Surgeons and the American Cancer Society. It consists of de-identified information that characterizes tumors, patient demographics, therapies given, and patient survival. The data used in this study is from the NCDB database and as it is de-identified, is exempt from institutional review board evaluation.
The NCDB was queried from 2004 to 2017 and identified patients with RCC (C64.9). Inclusion criteria for this study included patients aged 18 or older and patients with newly diagnosed RCC were identified using the Internal Classification of Disease for Oncology (ICD-O) third edition codes with RCC subtypes including papillary (8050, 8260), clear cell adenocarcinoma (8310, 8312, 8255), unspecified (8000, 8010, 8290) as well as others (8070, 8317, 8318, 8120, 8130). Patients were excluded if they received surgical resection (codes 0 – 29).
A record of radiation doses and modality/technique/target volume were required to be included in this study, specifically requiring patients to have had stereotactic body radiotherapy doses, defined as > 400 cGy per fraction, delivered to a kidney volume. Patients treated to lymph nodes or metastasis were excluded. BED was calculated using the α/β ratio of 3 given the in vitro studies previously mentioned, and patients were required to have received a BED3 of > 60 Gy, above the palliative dose of 30 Gy in 10 fractions.
All statistical tests were two-sided, with threshold of p < 0.05 for statistical significance. IBM SPSS Statistics 25 was used for data analysis. Kaplan-Meier analysis with log-rank test was used to evaluate overall survival (OS). Univariable (UVA) and multivariable (MVA) analysis were conducted using cox proportional hazard models to determine which clinical and treatment factors were prognostic for overall survival. Overall survival was defined as the time interval in months between initial diagnosis and date of death. Patients were censored otherwise at their last known date of contact.
Results
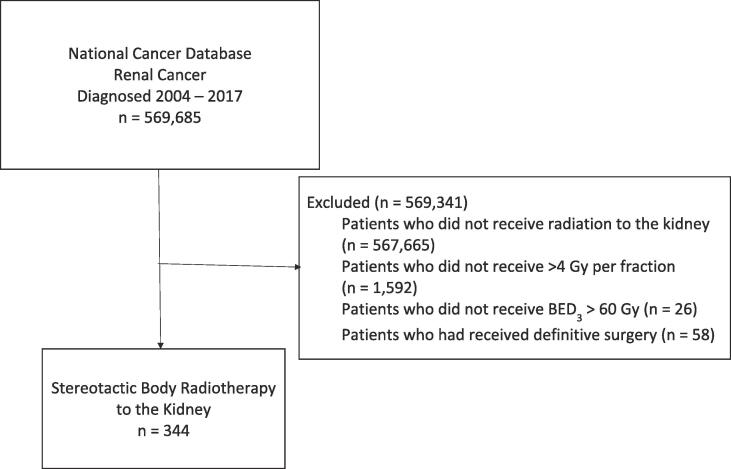
Fig. 1 displays the patient selection diagram for this study. A total of 344 patients with median age 77 (IQR 70–85) were included (Table 1.). Histology was clear cell in 72.7 %, papillary in 6.4 %, transitional in 14.2 %, and other/unknown in 6.7 %. T stage was cT1a in 46.8 %, cT1b in 25.9 %, cT2 in 4.9 %, cT3 in 6.1 %, and cT4 in 1.2 %. A total of 2.3 % of patients were cN1, 6.1 % of patients were cM1. Median BED3 was 180 Gy (IQR 126.03–233.97) and different radiation schemes are depicted in Table 2, with common fractionations being between 30 Gy and 60 Gy in 3 fractions and 30 Gy – 50 Gy in 5 fractions.
Fig. 1.
Patient Selection Diagram.
Table 1.
Patient Characteristics.
| Quartiles BED3 (Gy) | <145 | 145–180 | 180–225 | 225 | p-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (% | ||||||
| Total: | 76 | (22.1) | 112 | (32.6) | 46 | (13.4) | 110 | (32) | |||||
| Age (Median): | 79 | 77 | 73 | 77 | 0.62 | ||||||||
| Race: | 0.67 | ||||||||||||
| White | 66 | (86.8) | 96 | (85.7) | 41 | (89.1) | 97 | (88.2) | |||||
| Black | 8 | (10.5) | 15 | (13.4) | 1 | (2.2) | 9 | (8.2) | |||||
| Hispanic/Other | 2 | (2.6) | 1 | (0.9) | 1 | (2.2) | 3 | (2.7) | |||||
| Sex: | 0.06 | ||||||||||||
| Male | 40 | (52.6) | 79 | (70.5) | 32 | (69.6) | 75 | (68.2) | |||||
| Female | 36 | (47.4) | 33 | (29.5) | 14 | (30.4) | 35 | (31.8) | |||||
| Charlson-Deyo Score: | 0.34 | ||||||||||||
| 0 | 62 | (81.6) | 80 | (71.4) | 32 | (69.6) | 84 | (76.4) | |||||
| >0 | 14 | (18.4) | 32 | (28.6) | 14 | (30.4) | 26 | (23.6) | |||||
| Tumor Size (mm)(Median): | 42 | 37 | 35 | 35 | 0.08 | ||||||||
| Clinical T Stage: | <0.01 | ||||||||||||
| cT1a | 24 | (31.6) | 49 | (43.8) | 18 | (39.1) | 70 | (63.6) | |||||
| cT1b | 23 | (30.3) | 37 | (33) | 12 | (26.1) | 17 | (15.5) | |||||
| cT2 - cT4 | 14 | (18.4) | 9 | (8) | 9 | (19.6) | 10 | (9.1) | |||||
| Clinical N Stage: | <0.01 | ||||||||||||
| cN0 | 58 | (76.3) | 98 | (87.5) | 37 | (80.4) | 100 | (90.9) | |||||
| cN1 | 7 | (9.2) | 2 | (1.8) | 1 | (2.2) | 1 | (0.9) | |||||
| Clinical M Stage: | 0.33 | ||||||||||||
| cM0 | 63 | (82.9) | 93 | (83) | 41 | (89.1) | 94 | (85.5) | |||||
| cM1 | 8 | (10.5) | 8 | (7.1) | 1 | (2.2) | 6 | (5.5) | |||||
| Primary Site: | 0.64 | ||||||||||||
| Renal Parenchyma | 64 | (84.2) | 100 | (89.3) | 40 | (87) | 99 | (90) | |||||
| Renal Pelvis | 12 | (15.8) | 12 | (10.7) | 6 | (13) | 11 | (10) | |||||
| Laterality: | 0.55 | ||||||||||||
| Right | 43 | (56.6) | 55 | (49.1) | 27 | (58.7) | 63 | (57.3) | |||||
| Left | 33 | (43.4) | 57 | (50.9) | 19 | (41.3) | 47 | (42.7) | |||||
| Histology: | 0.06 | ||||||||||||
| Clear Cell Adenocarcinoma | 48 | (63.2) | 87 | (77.7) | 35 | (76.1) | 80 | (72.7) | |||||
| Papillary Renal Cell Carcinoma | 4 | (5.3) | 3 | (2.7) | 5 | (10.9) | 10 | (9.1) | |||||
| Transitional | 15 | (19.7) | 15 | (13.4) | 6 | (13) | 13 | (11.8) | |||||
| Other/Unspecified | 9 | (11.8) | 7 | (6.3) | 0 | (0) | 7 | (6.4) |
Table 2.
Radiation Fractionation Schemes.
| Quartiles BED3 (Gy) | <145n (%) | 145–180n (%) | 180–225n (%) | >225n(%) |
|---|---|---|---|---|
| Dose Fractionation | ||||
| 30 Gy in 3 fractions | 19 (40.4 %) | |||
| 30 Gy in 5 fractions | 16 (34.0 %) | |||
| 35 Gy in 5 fractions | 12 (25.5 %) | |||
| 36 Gy in 3 fractions | 19 (18.3 %) | |||
| 40 Gy in 4 fractions | 73 (70.2 %) | |||
| 45 Gy in 5 fractions | 12 (11.5 %) | |||
| 39 Gy in 3 fractions | 15 (37.5 %) | |||
| 50 Gy in 5 fractions | 25 (62.5 %) | |||
| 45 Gy in 3 fractions | 15 (17.9 %) | |||
| 48 Gy in 3 fractions | 47 (51.1 %) | |||
| 60 Gy in 3 fractions | 9 (11.0 %) | |||
| 48 Gy in 4 fractions | 13 (15.9 %) |
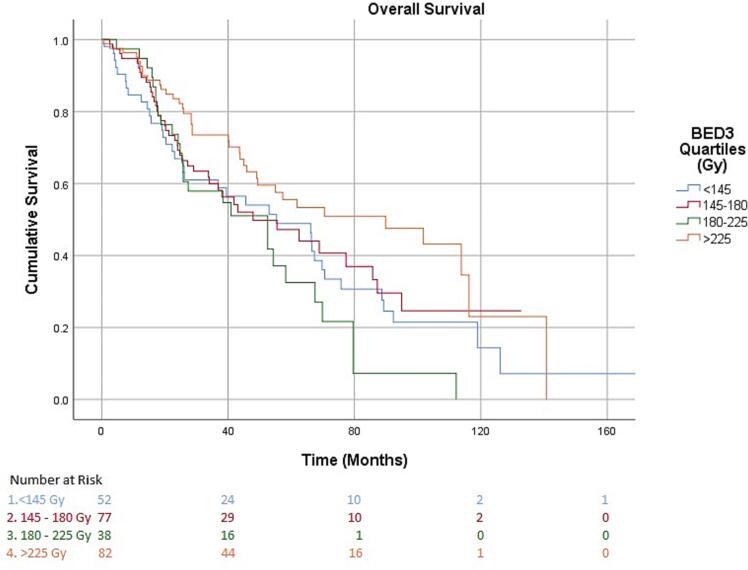
Fig. 2 shows the Kaplan Meier curve for OS. OS for the overall group at 2 years, 4 years, and 10 years was 72.2 %, 52.1 %, and 16.5 % respectively. Median OS was 90 months in the highest quartile, >225 Gy BED3, compared to 43 months, 43 months, and 52 months in the lower three quartiles, <145 Gy BED3, 145 – 180 Gy BED3, and 180 – 225 Gy BED3 (p < 0.01). Table 3 depicts the prognostic factors for survival on univariate analysis and highlights that higher BED3 was a positive prognostic factor both as a continuous variable (HR 0.99, p < 0.01; CI 0.99–0.99) and as a categorical variable when stratifying patients into quartiles of BED3 (HR 0.67, p < 0.01 CI 0.51–0.91). Table 4 depicts the prognostic factors for survival on multivariate analysis and shows that only the highest quartile BED3 was found to be a positive prognostic factor (HR 0.69, p = 0.02; CI 0.49–0.95).
Fig. 2.
Table 3.
Prognostic Factors for Survival on Univariate Analysis.
| HR [95 % CI] | p-value | ||
|---|---|---|---|
| Age (Continuous, year): | 1.03 [1.01–1.04] | <0.01 | |
| Race: | |||
| White | 1(ref) | ||
| Black | 0.69 [0.40–1.19] | 0.19 | |
| Hispanic | 1.07 [0.34–3.34] | 0.91 | |
| Sex: | |||
| Male | 1(ref) | ||
| Female | 0.87 [0.64–1.19] | 0.39 | |
| Charlson-Deyo Score: | |||
| 0 | 1(ref) | ||
| >0 | 1.02 [0.71–1.48] | 0.89 | |
| Tumor Size (Continuous, mm): | 1.02 [1.01–1.02] | <0.01 | |
| Clinical T Stage: | |||
| cT1a | 1(ref) | ||
| cT1b | 1.74 [1.22–2.49] | <0.01 | |
| cT2 - cT4 | 2.30 [1.45–3.67] | <0.01 | |
| Clinical N Stage: | |||
| cN0 | 1(ref) | ||
| cN1 | 5.81 [2.93–11.53] | <0.01 | |
| Clinical M Stage: | |||
| cM0 | 1(ref) | ||
| cM1 | 9.80 [5.77–16.65] | <0.01 | |
| Primary Site: | |||
| Renal Parenchyma | 1(ref) | ||
| Renal Pelvis | 1.64 [1.05–2.57] | 0.03 | |
| Laterality: | |||
| Right | 1(ref) | ||
| Left | 0.78 [0.58–1.05] | 0.09 | |
| Histology: | |||
| Clear Cell Adenocarcinoma | 1(ref) | ||
| Papillary Renal Cell Carcinoma | 1.07 [0.60–1.90] | 0.81 | |
| Transitional | 1.90 [1.26–2.87] | <0.01 | |
| Other/Unspecified | 0.95 [0.54–1.68] | 0.86 | |
| BED3 (Continuous, Gy): | 0.99 [0.99–0.99] | <0.01 | |
| BED3 (Quartiles): | |||
| <145 | 1(ref) | ||
| 145–180 | 0.84 [0.58–1.23] | 0.38 | |
| 180–225 | 0.97 [0.61–1.53] | 0.89 | |
| >225 | 0.53 [0.36–0.79] | <0.01 |
Table 4.
Prognostic Factors for Survival on Multivariate Analysis.
| HR [95 % CI] | p-value | ||
|---|---|---|---|
| Age (Continuous, year): | 1.03 [1.01–1.05] | <0.01 | |
| Tumor Size (Continuous, mm): | 1.01 [0.99–1.02] | 0.23 | |
| Clinical T Stage: | |||
| cT1a | 1(ref) | ||
| cT1b | 1.14 [0.86–1.52] | 0.36 | |
| cT2 - cT4 | 0.94 [0.62–1.42] | 0.77 | |
| Clinical N Stage: | |||
| cN0 | 1(ref) | ||
| cN1 | 1.34 [0.77–2.32] | 0.30 | |
| Clinical M Stage: | |||
| cM0 | 1(ref) | ||
| cM1 | 3.81 [2.33–6.23] | <0.01 | |
| Primary Site: | |||
| Renal Parenchyma | 1(ref) | ||
| Renal Pelvis | 0.54 [0.20–1.47] | 0.23 | |
| Histology: | |||
| Clear Cell Adenocarcinoma | 1(ref) | ||
| Papillary Renal Cell Carcinoma | 0.67 [0.34–1.29] | 0.23 | |
| Transitional | 3.03 [0.83–11.09] | 0.09 | |
| Other/Unspecified | 0.69 [0.35–1.39] | 0.31 | |
| BED3 (Quartiles): | |||
| <145 | 1(ref) | ||
| 145–180 | 0.95 [0.69–1.31] | 0.76 | |
| 180–225 | 1.68 [1.16–2.42] | <0.01 | |
| >225 | 0.69 [0.49–0.95] | <0.01 |
Negative prognostic factors on UVA included age (HR 1.03, p < 0.01; CI 1.01–1.04), tumor size (HR 1.02, p < 0.01; CI 1.01–1.02), cT1b (HR 1.74, p < 0.01; CI 1.22–2.49), cT2-cT4 (HR 2.30, p < 0.01; CI 1.45–3.67), cN1 (HR 5.81, p < 0.01; CI 2.93–11.53), cM1 (HR 9.80, p < 0.01; CI 5.77–16.65), renal pelvis location (HR 1.64, p = 0.03; CI 1.05–2.57), transitional cell histology (HR 1.89, p < 0.01; CI 1.26–2.87). It is also seen that the median size of tumors in the lowest BED3 quartile, <145 Gy, was 42 mm, compared to 35 – 37 mm in the other quartiles, though not statistically significant (p = 0.08). However, when looking at MVA, only age (HR 1.03, p = 0.001; CI 1.01 – 1.05), cM1 (HR 3.81, p < 0.01; CI 2.33–6.23), BED3 180 Gy – 225 Gy (HR 1.68, p < 0.01; CI 1.16–2.42) were significant negative prognostic factors while transitional cell histology trended towards significance (HR 3.03, p = 0.09; CI 0.83–11.09).
Table 5 shows the statistics for subgroups analysis. When excluding patients that were node positive or with metastasis, 268 patients were included in the analysis, of which the OS at 2 years, 4 years, and 10 years was 75.8 %, 53.0 %, and 16.2 % respectively. When looking at positive prognostic factors, the highest BED3 quartile, >225 Gy BED3, trended toward significance on UVA (HR 0.65, p = 0.07; CI 0.41 – 1.03), however this significance was lost on MVA (HR 0.73, p = 0.25; CI 0.42 – 1.26). Age was also seen to be a negative prognostic factor on MVA (HR 1.03, p < 0.01; CI 1.01 – 1.05).
Table 5.
Subgroup Analysis.
| HR [95 % CI] | p-value | ||
|---|---|---|---|
| Multivariate Analysis Excluding Node Positive or Metastasis | |||
| BED3 (Quartiles): | |||
| <145 | 1(ref) | ||
| 145–180 | 1.06 [0.62 – 1.82] | 0.83 | |
| 180–225 | 1.79 [0.99 – 3.26] | 0.06 | |
| >225 | 0.73 [0.42 – 1.26] | 0.25 | |
| Age | 1.03 [1.01 – 1.05] | <0.01 | |
| Multivariate Analysis Excluding Transitional Cell Histology | |||
| BED3 (Quartiles): | |||
| <145 | 1(ref) | ||
| 145–180 | 1.12 [0.67 – 1.87] | 0.66 | |
| 180–225 | 1.84 [1.03 – 3.29] | 0.04 | |
| >225 | 0.71 [0.41 – 1.21] | 0.21 | |
| Age | 1.03 [1.01 – 1.05] | <0.01 | |
| Clinical T Stage: | |||
| cT1a | 1(ref) | ||
| cT1b | 1.56 [1.04 – 2.34] | 0.03 | |
| cT2 – cT4 | 1.25 [0.67 – 2.25] | 0.52 | |
| Clinical M Stage | |||
| cM0 | 1(ref) | ||
| cM1 | 9.32 [3.86 – 22.51] | <0.01 | |
When excluding patients with transitional cell histology, 295 patients were included in the analysis. The OS at 2 years, 4 years, and 10 years was similar to the whole cohort at 74.2 %, 55.6 %, and 17.1 % respectively. On UVA, the highest BED3 quartile, >225 Gy BED3, was seen to be a positive prognostic factor (HR 0.57, p = 0.01; CI 0.37 – 0.89) however this significance was lost on MVA (HR 0.71, p = 0.21; CI 0.41 – 1.21). On MVA the second highest BED3 quartile was actually seen to be a negative prognostic factor (HR 1.84, p = 0.04; CI 1.03 – 3.29). Other negative prognostic factors on MVA included age (HR 1.03, p < 0.01; CI 1.01 – 1.05), cT1b (HR 1.56, p = 0.03; CI 1.04 – 2.34), and metastasis (HR 9.3, p < 0.02; CI 3.86 – 22.51).
Discussion
SBRT for treatment of RCC is a particularly attractive option for patients who cannot undergo surgical intervention. It is a noninvasive method for local treatment and has been shown to be used increasingly given its safety and efficacy profile. The International Radiosurgery Oncology Consortium for Kidney (IROCK) published their consensus statement for SBRT for RCC in 2016, suggesting some fractionation scheme using 1 – 12 fractions to a total dose of 25 Gy – 80 GyE with recommendations for target parameters as well as dose constraints for organs at risk [7]. As far as local tumor control, it has been shown through many retrospective studies and meta-analyses that SBRT offers very high rates, well above 90 % [3], [8], [9], [10], [11].
When looking at toxicities of SBRT for the treatment of primary RCC, grade 1 and grade 2 toxicities occur at various rates and include symptoms such as fatigue, pain, dermatitis. Grade 3 and grade 4 toxicities seem to be minimal; many studies do not report any incidence of grade 3 and grade 4 toxicities while other series report rates of up to 25 % [8], [10]. When specifically looking at renal function, studies have shown a dose response to renal function measured as glomerular filtration rate (GFR) [12], while others suggest no lasting dysfunction to the kidneys measured in GFR and creatinine levels [13], [14], [15]. Some series reported some patients needing long term-dialysis after SBRT to the kidneys, though they noted these patients had baseline chronic kidney disease stage 4–5 [16]. Some groups have looked at using nuclear medicine imaging techniques with Tc-DMSA SPECT/CT to assist in delineating treatment volumes and monitoring response, concluding that there is a dose effect relationship between SBRT with renal function [17], [18].
Given the high rates of control and safety profile of SBRT, it has demonstrated its utility in a certain subset of patient, now being incorporated in the NCCN guidelines under “ablative techniques” for the treatment of T1a kidney cancers. Despite these recommendations, NCCN does not give a specific dose fractionation scheme or a BED recommendation, which likely stems from the various doses and fractionations seen across different institutions and across the different studies.
Dose escalation has been explored in prospective trials, though mostly single-institution. One group explored dose escalation up to 48 –60 Gy (BED3 182.4 Gy – 276.0 Gy) in 3 fractions demonstrating that SBRT for renal cell cancers is safe; in this study, the only acute radiation related toxicities seen were grade 1 fatigue and nausea, with no acute or late toxicity of patients needing to undergo long term dialysis [13], [19]. Local control rates at 3 and 5 years were both 96.2 %, which is similar to rates reported in other trials. Other groups looking at dose escalation reached similar conclusions, showing high rates of local control as well as minimal toxicities that included fatigue, pain, nausea, and worsening renal function [20], [21].
When looking at large database studies, there are often many limitations. Though they include larger numbers of patients, they often lack detailed information such as toxicities and local control, oftentimes using OS as a surrogate marker for treatment response. One large database study looked at patients with renal cancers and compared surgery, tumor ablation, SBRT, and observation [22]. In their study, about 20 % of patients had died at 5-years with multivariable analysis showing treatment with either surgery, tumor ablation, or SBRT was associated with lower risk of death as compared to observation. They further looked at BED10 cutoff of 100 Gy, approximately equivalent to BED3 of 217 – 270 Gy, suggesting decreased risk of death with BED10 greater than or equal to 100 Gy.
This current study uses updated NCDB data, from 2004 − 2017, particularly looking at only patients with pathologically confirmed renal cancers treated with SBRT to the kidney and suggests benefit to OS with high BED3, particularly above BED3 of 225 Gy. We do note that BED3 180 – 225 Gy was found to be a negative prognostic factor on MVA which highlights some of the limitations of this type of study. We looked at different subgroups to try to explain why this quartile did poorly; when excluding patients that were node positive or with metastasis, BED3 180 – 225 Gy was not seen as a negative prognostic factor, however when excluding transitional cell histology it was. This suggests that metastatic patients are likely driving this quartile to do worse, or that there may be a lack of statistical power when we exclude parts of the whole cohort. Compared to other studies, this cohort also does seem to exhibit lower OS, 72.2 % and 52.1 % at 2 and 4 years respectively, compared to an OS of 82.1 % and 70.7 % at 2 and 4 years respectively seen by Siva et al.[9] however when excluding node positive or metastatic patients we did not see a drastic improvement in OS. We also want to note that though median tumor size between all quartiles was not significantly different, BED3 < 145 Gy did have larger tumors at 42 mm; this study would suggest delivering high BED3 to tumors irrespective of size, if deemed safe by treating clinicians.
This study does have limitations that are associated with retrospective, hospital based data sets. Though it uses a large database, this study has a relatively low patient volume and lacks granularity. There also are likely both selection and reporting bias that inherently exist in retrospective studies. In the case of NCDB studies specific metrics such as cancer specific mortality, treatment-related mortalities, toxicities, and local control are not reported. Information on performance status of the patients included is also lacking. Without this specific information it is difficult to say whether OS is driven by death due to RCC or death due to other causes. It is seen that in these patients, distant failures is more common than local failure and we theorize that there is some immunologic response related to SBRT of primary renal cancers that is driving this OS benefit [23].
This study suggests that there may be a benefit in increasing BED in treating RCC with SBRT, however, we await the results of a prospective, multi-institutional study, such as the TROG 15.03 FASTRACT II study which utilizes 42 Gy in 3 fractions (BED3 of 238 Gy) or 26 Gy in 1 fraction (BED3 of 251 Gy) to confirm it [24].
Conclusion
SBRT is an increasingly utilized modality for the treatment of RCC, particularly in patients who are poor surgical candidates. There have been many studies that show high rates of local control with minimal toxicities or treatment-related deaths. This study suggests the OS benefits associated with higher BED for treatment of RCC and future trials should consider using regimens of BED3 > 225 Gy.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Ljungberg B., Bensalah K., Canfield S., Dabestani S., Hofmann F., Hora M., et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67(5):913–924. doi: 10.1016/j.eururo.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Ishiyama H., Blanco A.I., Lo S.S., Butler E.B., Teh B.S. Stereotactic body radiotherapy (SBRT)/stereotactic ablative body radiotherapy(SABR) for “radioresistant” renal cell carcinoma (RCC) J Radiat Oncol. 2014;3(4):339–346. [Google Scholar]
- 4.Haque W., Verma V., Lewis G.D., Lo S.S., Butler E.B., Teh B.S. Utilization of radiotherapy and stereotactic body radiation therapy for renal cell cancer in the USA. Future Oncol. 2018;14(9):819–827. doi: 10.2217/fon-2017-0536. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. Kidney Cancer. Version 4.2022. www.nccn.org/professionals/physician_gls/pdf/kidney.pdf.
- 6.Ning S., Trisler K., Wessels B.W., Knox S.J. Radiobiologic studies of radioimmunotherapy and external beam radiotherapy in vitro and in vivo in human renal cell carcinoma xenografts. Cancer. 1997;80(12 Suppl):2519–2528. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2519::aid-cncr26>3.3.co;2-t. [DOI] [PubMed] [Google Scholar]
- 7.Siva S., Ellis R.J., Ponsky L., Teh B.S., Mahadevan A., Muacevic A., et al. Consensus statement from the International Radiosurgery Oncology Consortium for Kidney for primary renal cell carcinoma. Future Oncol. 2016;12(5):637–645. doi: 10.2217/fon.16.2. [DOI] [PubMed] [Google Scholar]
- 8.Siva S., Kothari G., Muacevic A., Louie A.V., Slotman B.J., Teh B.S., et al. Radiotherapy for renal cell carcinoma: renaissance of an overlooked approach. Nat Rev Urol. 2017;14(9):549–563. doi: 10.1038/nrurol.2017.87. [DOI] [PubMed] [Google Scholar]
- 9.Siva S., Louie A.V., Warner A., Muacevic A., Gandhidasan S., Ponsky L., et al. Pooled analysis of stereotactic ablative radiotherapy for primary renal cell carcinoma: A report from the International Radiosurgery Oncology Consortium for Kidney (IROCK) Cancer. 2018;124(5):934–942. doi: 10.1002/cncr.31156. [DOI] [PubMed] [Google Scholar]
- 10.Correa R.J.M., Louie A.V., Zaorsky N.G., Lehrer E.J., Ellis R., Ponsky L., et al. The Emerging Role of Stereotactic Ablative Radiotherapy for Primary Renal Cell Carcinoma: A Systematic Review and Meta-Analysis. Eur Urol Focus. 2019;5(6):958–969. doi: 10.1016/j.euf.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Francolini G., Detti B., Ingrosso G., Desideri I., Becherini C., Carta G., et al. Stereotactic body radiation therapy (SBRT) on renal cell carcinoma, an overview of technical aspects, biological rationale and current literature. Crit Rev Oncol Hematol. 2018;131:24–29. doi: 10.1016/j.critrevonc.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Siva S., Jackson P., Kron T., Bressel M., Lau E., Hofman M., et al. Impact of stereotactic radiotherapy on kidney function in primary renal cell carcinoma: Establishing a dose-response relationship. Radiother Oncol. 2016;118(3):540–546. doi: 10.1016/j.radonc.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 13.Grubb W.R., Ponsky L., Lo S.S., Kharouta M., Traughber B., Sandstrom K., et al. Final results of a dose escalation protocol of stereotactic body radiotherapy for poor surgical candidates with localized renal cell carcinoma. Radiother Oncol. 2021;155:138–143. doi: 10.1016/j.radonc.2020.10.031. [DOI] [PubMed] [Google Scholar]
- 14.Svedman C., Karlsson K., Rutkowska E., Sandström P., Blomgren H., Lax I., et al. Stereotactic body radiotherapy of primary and metastatic renal lesions for patients with only one functioning kidney. Acta Oncol. 2008;47(8):1578–1583. doi: 10.1080/02841860802123196. [DOI] [PubMed] [Google Scholar]
- 15.Svedman C., Sandström P., Pisa P., Blomgren H., Lax I., Kälkner K.-M., et al. A prospective Phase II trial of using extracranial stereotactic radiotherapy in primary and metastatic renal cell carcinoma. Acta Oncol. 2006;45(7):870–875. doi: 10.1080/02841860600954875. [DOI] [PubMed] [Google Scholar]
- 16.Chang J.H., Cheung P., Erler D., Sonier M., Korol R., Chu W. Stereotactic Ablative Body Radiotherapy for Primary Renal Cell Carcinoma in Non-surgical Candidates: Initial Clinical Experience. Clin Oncol (R Coll Radiol) 2016;28(9):e109–e114. doi: 10.1016/j.clon.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Chevli N., Chiang S.B., Farach A.M., Haque W., Satkunasivam R., Bernicker E.H., et al. DMSA-SPECT: A Novel Approach to Nephron Sparing SBRT for Renal Cell Carcinoma. Adv. Radiat Oncol. 2021;6(6):100719. doi: 10.1016/j.adro.2021.100719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson P., Foroudi F., Pham D., Hofman M.S., Hardcastle N., Callahan J., et al. Short communication: timeline of radiation-induced kidney function loss after stereotactic ablative body radiotherapy of renal cell carcinoma as evaluated by serial 99mTc-DMSA SPECT/CT. Radiat Oncol. 2014;9(1) doi: 10.1186/s13014-014-0253-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponsky L., Lo S.S., Zhang Y., Schluchter M., Liu Y., Patel R., et al. Phase I dose-escalation study of stereotactic body radiotherapy (SBRT) for poor surgical candidates with localized renal cell carcinoma. Radiother Oncol. 2015;117(1):183–187. doi: 10.1016/j.radonc.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 20.Correa R.J.M., Ahmad B., Warner A., Johnson C., MacKenzie M.J., Pautler S.E., et al. A prospective phase I dose-escalation trial of stereotactic ablative radiotherapy (SABR) as an alternative to cytoreductive nephrectomy for inoperable patients with metastatic renal cell carcinoma. Radiat Oncol. 2018;13(1) doi: 10.1186/s13014-018-0992-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McBride S.M., Wagner A.A., Kaplan I.D. A phase 1 dose-escalation study of robotic radiosurgery in inoperable primary renal cell carcinoma. Int J Radiat Oncol Biol Phys. 2013;87(2):S84. doi: 10.1016/j.ijrobp.2013.06.218. [DOI] [Google Scholar]
- 22.Grant S.R., Lei X., Hess K.R., Smith G.L., Matin S.F., Wood C.G., et al. Stereotactic Body Radiation Therapy for the Definitive Treatment of Early Stage Kidney Cancer: A Survival Comparison With Surgery, Tumor Ablation, and Observation. Adv. Radiat Oncol. 2020;5(3):495–502. doi: 10.1016/j.adro.2020.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rabinovitch R.A., Zelefsky M.J., Gaynor J.J., Fuks Z. Patterns of failure following surgical resection of renal cell carcinoma: implications for adjuvant local and systemic therapy. J Clin Oncol. 1994 Jan;12(1):206–212. doi: 10.1200/JCO.1994.12.1.206. [DOI] [PubMed] [Google Scholar]
- 24.Siva S., Chesson B., Bressel M., Pryor D., Higgs B., Reynolds H.M., et al. TROG 15.03 phase II clinical trial of Focal Ablative STereotactic Radiosurgery for Cancers of the Kidney - FASTRACK II. BMC Cancer. 2018;18(1) doi: 10.1186/s12885-018-4916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]