Abstract
With restricted usage of growth-promoting antibiotics, identifying alternative feed additives that both improve intestinal barrier function and reduce inflammation is the center to improve chickens’ health. This study examined the effects of a microencapsulated feed additive containing citric acid, sorbic acids, thymol, and vanillin on intestinal barrier function and inflammation status. A total of 240 birds were assigned to either a commercial control diet or control diet supplemented with 500 g/MT of the microencapsulated additive product. Birds were raised by feeding a 2-phase diet (starter, d 1 to d 21; and grower, d 15 to d 42). Growth performance was recorded weekly. At d 21 and d 42, total gastrointestinal tract permeability was evaluated by FITC-dextran (FD4) oral gavage. Jejunum-specific barrier functions were evaluated by Ussing chamber. Intestinal gene expression of selected epithelial cell markers, tight junction (TJ) proteins, inflammatory cytokines, and endocannabinoid system (ECS) markers were determined by RT-PCR. Statistical analysis was performed using Student t test. Results showed significant improvement of feed efficiency in the birds supplemented with the blend of organic acids and botanicals. At d 21, both oral and jejunal FD4 permeability were lower in the supplemented group. Jejunal transepithelial resistance was higher in the supplemented birds. At d 21, expression of TJs mRNA (CLDN1 and ZO2) was both upregulated in the jejunum and ileum of supplemented birds, while CLDN2 was downregulated in cecum. Proliferating cell marker SOX9 was higher expressed in jejunum and ceca. Goblet cell marker (MUC2) was upregulated, while Paneth cell marker (LYZ) was downregulated in the ileum. Proinflammatory cytokine expressions of IL1B, TNFA, and IFNG were downregulated in jejunum, while anti-inflammatory IL10 expression was higher in jejunum, ileum, cecum, and cecal tonsil. The ECS markers expressions were upregulated in most intestinal regions. Together, these results demonstrated that the blend of organic acids and botanical supplementation reduced inflammation, improved the TJs expression and intestinal barrier function, and thus improved chicken feed efficiency. The activated ECS may play a role in reducing intestinal tissue inflammation.
Key words: botanical, organic acid, gut barrier, tight junction, endocannabinoid
INTRODUCTION
The intestinal barrier consists of a single layer of epithelial cells that selectively transport dietary nutrients while blocking the diffusion of toxins and pathogens in the lumen. Although the definition of optimal gut health may varies among conditions (Kogut and Arsenault, 2016), the improved gut barrier function is most likely the central goal in the current agricultural animal industry (Broom, 2018). The intestinal barrier includes an extrinsic secreted barrier, such as mucus and antimicrobial peptides, as well as intrinsic epithelial cell lining and tight junction (TJ) complexes. The proper development of intestinal epithelium, including absorptive enterocytes and secretory goblet cells, enteroendocrine cells and Paneth cells, dynamically regulates intestinal barrier function. During early posthatch development, the interplay between nutrition and microbiota might regulate intestinal epithelial cell composition and homeostasis (Bayer et al., 2021; Gieryńska et al., 2022). Intestinal inflammation from chronic stress or pathogenic challenges always negatively affects barrier function. Given the breeding selection toward traits like high feed intake and rapid growth in modern broilers, a low-grade chronic inflammation due to noninfectious stimuli from feed components has been suggested (Kogut et al., 2018). This type of sterile inflammation may increase intestinal permeability at a basal level, facilitating pathogens' translocation.
The removal of growth-promoting antibiotics from poultry production has pushed the demand for identifying alternative feed additives that improve gut health and animal production. Multiple natural dietary additives have been actively investigated for their beneficial effects on animals’ health. Among alternatives, organic acids and botanicals improve intestinal health via antimicrobial activity (Bonetti et al., 2020), antioxidation and enhanced digestive enzymes (Hashemipour et al., 2013), and anti-inflammatory and immunomodulatory (Swaggerty et al., 2020b) in pigs (Tugnoli et al., 2020) and chickens (Rossi et al., 2020; Gholami-Ahangaran et al., 2022). Considering multiple modes of actions of these functional additives, the mechanism facilitating the anti-inflammatory effects is of great interests to animal productions. One recently proposed anti-inflammatory property of botanicals is their ability to activate endocannabinoid system (ECS) via modulating either the cannabinoid receptors, or their ligand biogenesis enzymes N-acylphosphatidylethanolamine-specific phospholipase D (NAPE-PLD) and fatty acid amide hydrolase (FAAH) (Bisogno et al., 2005; Johnson et al., 2020). In fact, the activation of ECS system by endogenous, plant-derived, or synthetic cannabinoids and botanicals may exert beneficial effects on intestine via inhibiting intestinal inflammation (Di Marzo and Izzo, 2006; Hryhorowicz et al., 2021). Moreover, a recent study of dietary thymol supplementation in pigs suggested that the modulation of the ECS could partially explain the ability of some botanicals to control intestinal inflammation (Toschi et al., 2020b).
A commercial blend of microencapsulated organic acids and botanicals (OA+B) has shown beneficial effects on modulating gut kinase activities and boosting the immune function of chickens (Swaggerty et al., 2020a), as well as reducing ileal cytokine gene expression in vitro (Toschi et al., 2020a) and in vivo in weaned pigs (Grilli et al., 2015). Whether this blend regulates intestinal epithelium proliferation and maturation in chickens has not been fully evaluated. Moreover, it is of interest to evaluate whether the blend of organic acid and botanicals influence intestinal inflammation as well as the involvement of the ECS system in the fast-growing modern broilers. For this reason, the aim of the present study was to evaluate the effect of a microencapsulated blend of sorbic acid, citric acid, thymol, and vanillin on growth performance, intestinal barrier function, intestinal epithelium cell markers, inflammatory cytokines, and ECS markers on broiler chickens.
MATERIALS AND METHODS
Animals, Housing, and Experimental Design
All animal procedures followed guidelines established by the University of Delaware Institutional Animal Care and Use Committees and were approved by this committee (AUP#101R-2019). The experiments were conducted in accordance with the recommended code of practice for the care and handling of poultry and followed the ethical principles according to the Guide for the Care and Use of Laboratory Animals (NRC, 2011).
Ross 308 eggs were obtained from a local commercial hatchery (Moyer's Chicks, Inc., Quakertown, PA) and incubated at 37.5°C and 60% humidity for 21 d. At the day of hatch, a total of 240 straight-run birds (BW, 0.0426 ± 0.003 kg) were removed from the incubator, and randomly assigned to either a control (Con) or organic acids and botanicals supplemented diet (OA+B), with 15 birds per pen, 8 replicate floor pens per treatment (n = 120). Chickens were given ad libitum access to water and an unmedicated corn and soybean meal-based diet (Supplementary Table 1) that met or exceeded the established nutrient requirements (NRC, 1994). Two phases of broiler diet (the starter, d 1–21; and the grower, d 22–42) were manufactured from local feed mill (Gehman Feed Mill, Inc., Denver, PA) in mash form. Those assigned to the OA+B supplement-diet were given free access to the same basal diet supplemented with 500 g/MT of a microencapsulated blend of citric (25%) and sorbic (16.7%) acids, thymol (1.7%), and vanillin (1.0%) (AviPlusP; Vetagro S.p.A., Reggio Emilia, Italy). The birds did not receive any vaccine nor medications during the study. All the birds were raised under the same condition, recommended by commercial broiler industry. Each house was maintained around 32°C for the first week and then decreased by 1.5°C weekly until a final temperature of 21°C was reached. A heat lamp was set up in each pen and removed on d 7. The birds were provided with 24 h of light for the entire length of the trial. The BW and feed intake (FI) were recorded weekly. The average daily feed intake (ADFI), average daily gain (ADG), and feed conversion ratio (FCR) were calculated. At 21 and 42 d of age, 4 birds from each replicate pen (n = 32 for each group) were euthanized for sample collections.
FITC-Dextran 4000 (FD4) Oral Gavage
On the d 21 and d 42 of age, 3 birds in each pen were randomly selected for fluorescein isothiocyanate-dextran 4000 (Sigma Aldrich, St. Louis, MO) oral gavage. The oral dose of FD4 was adjusted by bird BW to reach 8.8 mg/kg BW. Blood (1–2 mL) was collected via the brachial vein at 2.5 h postgavage. Serum samples were collected freshly for FD4 measurement. FD4 levels were quantified by using SpectraMax M2e microplate reader (Molecular Devices, Downingtown, PA), using pooled nongavaged serum as blanks.
Sample Collection
Birds (n = 32) were euthanized by cervical dislocation on d 21 and 42 of age. The jejunum, ileum, ceca, and cecal tonsil were collected and separated by using landmarks of duodenal loop, the Meckel's diverticulum, and ileo-cecal junction. For jejunum and ileum samples, a 2-cm length segment from the distal end was fixed in 10% neutral buffered formalin (NBF) for histological analysis; the next 4-cm segment was saved for Ussing chamber analysis; the next 5 to 7-cm segment was frozen in liquid nitrogen and stored at −80°C until further analysis.
Histology
Small intestinal segments were fixed into 10% NBF, then transferred to the University of Delaware Comparative Pathology Laboratory (Newark, DE) for paraffin embedding, sectioning into 5-μm thickness slides, and H&E staining. Stained slides were scanned onto an imaging analysis software (Leica). The villus height was measured from the tip to the villus-crypt junction. Villus width was measured at one-third and two-thirds of the length of the villus. The villus width is reported as the average between these 2 values. The crypt depth was defined as the base of the villus to the mucosa. A total of 10 to 15 measurements per animal (n = 6) were taken for each trait from intact well-oriented crypt-villus units. Villus height and width were measured at 4-times objective magnification and the crypt depth at 10-times objective magnification. The longitudinal and circular muscle thicknesses were measured at 4 separate locations at 20-times objective on the same sample piece to obtain an average for each section.
Ussing Chamber Experiment
Electrophysiological parameters and FD4 permeability of jejunum (n = 32) at 21 and 42 d of age were evaluated. Immediately postmortem, a 5-cm piece of jejunum was cut open along the mesentery and rinsed to remove digesta particles, then tissue was immediately put into cold Ringer's solution, which was pregassed with carbogen gas (95% O2–5% CO2). The Ringer's solution consisted of the following composition (mmol/L): NaCl, 115; KCl, 5; CaCl2, 1.5; MgCl2, 1.2; NaHCO3, 25; Na2HPO4, 2.4; NaH2PO4 1.2, glutamate, 1;mannitol, 20. The opened intestinal sheet was pinned down in Ringer's solution with 10 μM of indomethacin, for blunt dissection of the outer longitudinal/circular muscle layers under a dissecting microscope. A piece of 0.5 cm2 mucosa was mounted on the Ussing chambers. The tissue was bathed in 5 mL of 40°C Ringer's solution, continuously gassed with carbogen to allow for oxygenation and circulation of the buffer by gas lift. A 10 mM glucose was added to the serosal side as tissue energy source, and 10 mM mannitol was added to the mucosal side for balancing osmotic pressure. The temperature was maintained at 40°C by a circulating thermostatic water jacket. After mounting, tissues were allowed for a 20 min equilibration period, then the tissue was short-circuited by clamping the voltage to zero. The potential difference (mV), short-circuit current (ISC, μA/cm2), and transepithelial resistance (TER, Ω × cm2) were continuously measured by the chamber software (Acquire and Analyze 2.3, Physiologic Instruments Inc., Reno, NV). After the TER readings stabilized, FD4 (1 mg/mL) was added to the luminal side to examine the tissue permeability.
RNA Isolation and cDNA Synthesis
On d 21 of age, the jejunum, ileum, ceca, and cecal tonsil samples (n = 8) were obtained and the total RNA were isolated via a commercially available kit (RNeasy Mini kit, Qiagen, Germantown, MD) according to the manufacturer's instructions. A NanoDrop (ThermoFisher Scientific, Waltham, MA) was used to determine RNA quantity. The RNA quality was checked by formaldehyde denaturing gel electrophoresis. The synthesis of single-stranded complementary DNA (cDNA) from 3 µg of total RNA was performed using a Maxima First Strand cDNA Synthesis Kit (ThermoFisher Scientific). The DNase treatment was performed to avoid contamination. The resulting cDNA products were stored at −20°C until further analysis.
RT-PCR
Relative gene expressions were performed using a QuantStudio 3 Real-Time PCR system (ThermoFisher Scientific) and PowerUp SYBR Green Master mix (Applied Biosystems, Waltham, MA). Each reaction was performed in duplicate. Primers are listed in Table 1. The following amplification program was used: a hold stage for one cycle of 95°C for 2 min, a PCR stage for 40 cycles of 95°C for 1 s and 60°C for 30 s. The melt curve was analyzed at the end of the run to determine specific product amplification. The comparative Ct method (2–ΔΔCT method) was used to quantify gene expression. The Ct values of each gene were compared to the geometric mean of 3 housekeeping genes (HMBS, RPL1, and TBP) (Schmittgen and Livak, 2008).
Table 1.
Primers for qRT-PCR assay.
| Genes | Accession number | Primer sequence (forward; reverse) | Amplicon (bp) | Concentration (nM) |
|---|---|---|---|---|
| HMBS | XM_417846 | GGTTGAGATGCTCCGTGAGTTT; GGCTCTTCTCCCCAATCTTAGAA |
153 | 300 |
| RPL1 | NM_205322.1 | TCTCCACGACGACGAAGTCA; CCGCCGCCTTGATGAG |
63 | 600 |
| TBP | NM_205103 | CTTCGTGCCCGAAATGCT; GCGCAGTAGTACGTGGTTCTCTT |
82 | 300 |
| CLDN1 | NM_001013611.2 | AGACCCAGGAGTATGGCGGC; AGACCCAGGAGTATGGCGGC |
170 | 600 |
| CLDN2 | NM_001277622.1 | GACGTGAACCATTCGCAGTCC; GTTTTGTGAGGGCACAGGCA |
151 | 300 |
| TJP1 | XM_015278981.2 | TGAAGGACCCCAGTGACACG; CACTCATGGCTGGGAGCGTA |
75 | 600 |
| ZO2 | NM_204918.1 | GCCCCAGCCAAAAGCAGTTA; CCAGCAAGTCGCAGACCAAC |
126 | 600 |
| OCLN | NM_205128.1 | CGCTACGGCAAAGCCAACAT; CCATCCGCCACGTTCTTCAC |
92 | 600 |
| LGR5 | XM_046909876.1 | ACGTCTTGCAGGAAATGGCT; TTGGCATCCAGGCGTAGAG |
159 | 300 |
| SOX9 | NM_204281.1 | CCCCACCATGTCGGATGACT; CCTTCTTCAGGTCCGGGTCG |
119 | 600 |
| CHGA | XM_015287755.2 | AAGCCAACACTGATGAAGAAGG; CTGAGGTGAGTACTGGGAGC |
70 | 300 |
| MUC2 | XM_421035.2 | ATTGAAGCCAGCAATGGTGT; TTGTTGGCCTTGTCATCAAA |
125 | 300 |
| LYZ | NM_205281 | GACGATGTGAGCTGGCAG; GGATGTTGCACAGGTTCC |
225 | 600 |
| IL1B | NM_204524.1 | TGCCTGCAGAAGAAGCCTCG; AGGTCGCTGTCAGCAAAGTCC |
170 | 600 |
| TNFA |
NM_204267.1 |
CCCTACCCTGTCCCACAACC; TGGGCGGTCATAGAACAGCA |
150 | 300 |
| IFNG | NM_205149.1 | ATGTAGCTGACGGTGGACCT; GCGGCTTTGACTTGTCAGTGT |
135 | 300 |
| IL10 | NM_001004414.2 | CACCGCTTCTTCACCTGCGA; CCCGTTCTCATCCATCTTCTCG |
81 | 800 |
| CNR1 | XM_015284635.2 | TTCCAAAGACGTCTGCACCCC; GTTGGAGCCCACGTAAAGGA |
185 | 300 |
| FAAH | XM_422450.5 | TCCCTCGGTGGACATGGTCT; TGGGTCTCGCTTTCCTGC |
174 | 300 |
| NAPEPLD | NM_001030730.3 | GAAGAGGACGGGACTGCTCG; GTCACTGCCCCTGGATCCTC |
153 | 300 |
Statistical Analysis
Significance was determined using a Student t test. Data are presented as means ± SEM. A P value less than 0.05 (*, P ≤ 0.05) is considered statistically significant. A P value less than 0.10 (#, P ≤ 0.10) represents a trend in the data.
RESULTS
Growth Performance
No significant changes in the ADG were observed in the chickens fed OA+B diet from hatch to d 42 of age. A significant reduction of ADFI was shown at the first 3 wk of age in OA+B group (Table 2). During the first 3 wk and the overall 6 wk of growth period, the FCR was significantly improved in the OA+B-treated birds compared with the control (Table 2).
Table 2.
Effects of dietary supplementation on ADG (average daily gain), ADFI (average daily feed intake), and FCR (feed conversion ratio) in broilers.
| Performance | Con | OA+B |
|---|---|---|
| BW, g | ||
| d 1 | 40.21 ± 0.30 | 40.09 ± 0.18 |
| d 42 | 2750.67 ± 94.41 | 2688.64 ± 113.15 |
| ADG, g | ||
| d 1–21 | 44.09 ± 0.86 | 42.20 ± 1.32 |
| d 1–42 | 64.53 ± 2.25 | 63.06 ± 2.69 |
| ADFI, g | ||
| d 1–21 | 59.46 ± 2.73 | 47.56 ± 1.83* |
| d 1–42 | 134.01 ± 6.32 | 122.89 ± 5.20 |
| FCR | ||
| d 1–21 | 1.35 ± 0.06 | 1.13 ± 0.05* |
| d 1–42 | 2.07 ± 0.03 | 1.95 ± 0.03* |
indicates significant difference (P ≤ 0.05) between 2 groups at each growth period. Dietary treatments were as follows: Con, basal diet; OA+B, basal diet + 500 ppm microencapsulated organic acids and botanicals.
Intestinal Histology and Intestinal Barrier Functions
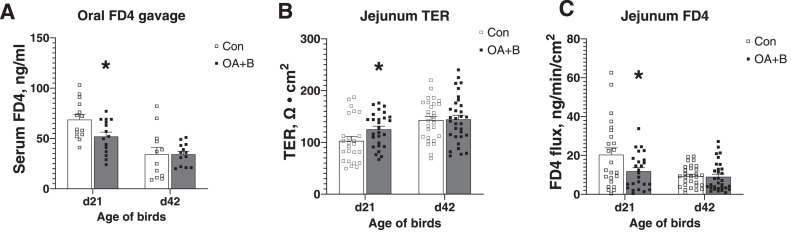
No significant difference was found in the histology of jejunum or ileum after OA+B supplementation (data not shown). For intestinal barrier functions, the whole gastrointestinal (GI) tract permeability was first examined by measuring FD4 levels in the serum 2.5-h postoral FD4 gavage. At the age of d 21, OA+B-treated chickens exhibited significant reduction (P ≤ 0.05) of oral-FD4 leakage to the blood. No differences were observed at d 42 of age (Figure 1A). To examine the intestinal-specific region, jejunum mucosa was isolated and mounted on the Ussing chamber. At d 21 of age, in comparison with the control birds, the jejunum from OA+B-treated birds showed significantly higher TER and significantly lower FD4 flux rate (Figure 1B and C), both indicated an enhanced gut barrier function. There were no significant differences in jejunum of the d 42 birds.
Figure 1.
Intestinal barrier integrity of birds fed with control or supplemented diet. Serum FD4 concentration measured after FD4 oral gavage in birds at d 21 and d 42 of age (A). Transepithelial resistance (TER) of the jejunum mucosa of birds at d 21 and d 42 of age on Ussing chambers (B). Tissue FD4 flux rate of the jejunum mucosa of birds at d 21 and d 42 of age on Ussing chambers (C). Difference between 2 groups at each growth period was labeled. * indicates significant difference (P ≤ 0.05). Dietary treatments were as follows: Con, basal diet; OA+B, basal diet + 500 ppm encapsulated organic acids and botanicals.
Intestinal Tight Junction Proteins mRNA Expression
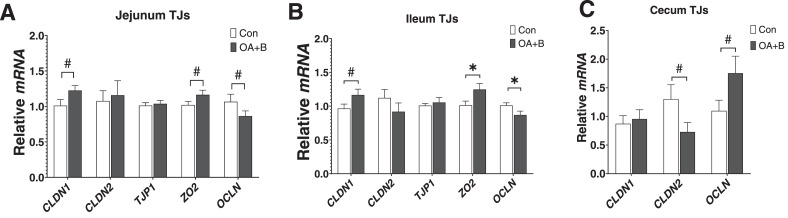
For gene expression analysis of TJ proteins in the intestine, the attention was focused on the d 21 time point. The expression levels of CLDN1 and ZO2 tended (P = 0.07 and 0.09, respectively) to be increased by OA+B supplement in the jejunum, while the expression of OCLN tended (P = 0.06) to be decreased at d 21 of age (Figure 2A). In the ileum, similar pattern of gene expression was observed. OA+B treatment tended to increase CLDN1 (P = 0.07) ileal expression and significantly increased ZO2 mRNA expression, but significantly reduced OCLN expression in ileum (Figure 2B). A different pattern of expression showed in the cecum, where CLND2 expression tended (P = 0.07) to be reduced but OCLN expression was increased (P = 0.08) in the OA+B-treated birds (Figure 2C). The data indicated that OA+B treatment upregulated certain TJ proteins mRNA expression throughout the intestine.
Figure 2.
Intestinal tight junction proteins mRNA expression of birds fed with control or supplemented diet. At d 21 of age, gene expressions in jejunum (A), ileum (B), and cecum (C). Difference between 2 groups at each growth period was labeled. * indicates significant difference (P ≤ 0.05). # indicates trend difference (0.05 < P ≤ 0.1). Dietary treatments were as follows: Con, basal diet; OA+B, basal diet + 500 ppm encapsulated organic acids and botanicals. Abbreviations: CLDN1, claudin 1; CLDN2, claudin 2; OCLN, occludin; TJP1, tight junction protein 1; ZO2, Zonula occludins 2.
Intestinal Epithelium Cell Marker mRNA Expression
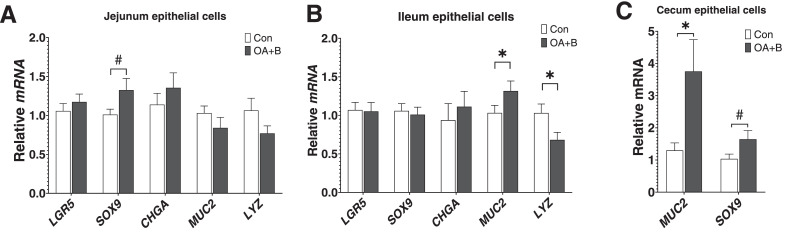
At d 21 of age, the expressions of intestinal epithelial-specific marker genes in the isolated intestinal segments were further examined. In jejunum, the proliferating cell marker SOX9 showed a tendency (P = 0.10) of increased expression in the OA+B-treated birds (Figure 3A). In the ileum, marker gene expression for goblet cells (MUC2) was upregulated, but for Paneth cells marker (LYZ) was downregulated the in the OA+B group (Figure 3B). In the cecum, the SOX9 mRNA tended (P = 0.06) to be increased, while the MUC2 mRNA was significantly upregulated in the OA+B-treated birds (Figure 3C). These data indicated OA+B treatment upregulated secretory epithelial cell components and increased proliferation of epithelium throughout the intestine.
Figure 3.
Intestinal epithelial cell markers mRNA expression of birds fed with control or supplemented diet. At d 21 of age, gene expressions in jejunum (A), ileum (B), and cecum (C). Difference between 2 groups at each growth period was labeled. * indicates significant difference (P ≤ 0.05). # indicates trend difference (0.05 < P ≤ 0.1). Dietary treatments were as follows: Con, basal diet; OA+B, basal diet + 500 ppm encapsulated organic acids and botanicals. Abbreviations: CHGA, chromogranin A; LGR5, leucine-rich repeat-containing G-protein coupled receptor 5; LYZ, lysozyme; MUC2, mucin 2; SOX9, SRY-box transcription factor 9.
Intestinal Cytokines mRNA Expression
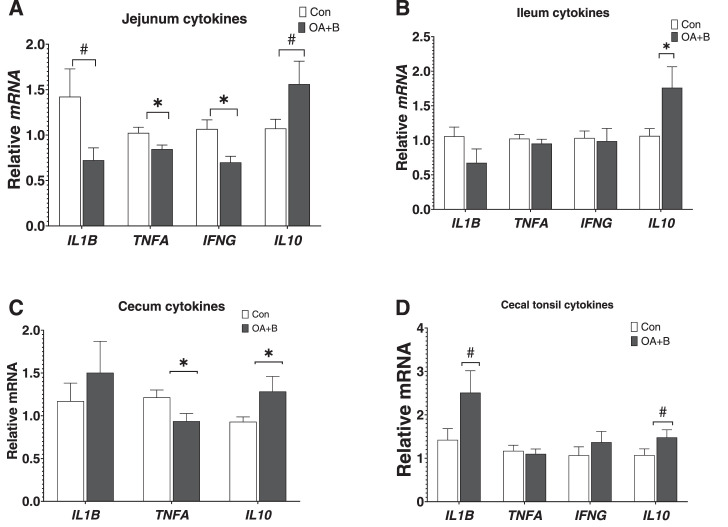
The mRNA expression of selected proinflammatory and anti-inflammatory cytokines in the intestine was examined. At d 21 of age, OA+B treatment significantly reduced the relative mRNA of proinflammatory cytokines including IL1B (P = 0.06), TNFA, and IFNG, while tended to increase mRNA of anti-inflammatory cytokine IL10 (P = 0.09) (Figure 4A). In the ileum, the expression of IL1B was numerically decreased and expression of IL10 was significantly increased in the OA+B-treated birds (Figure 4B). A similar expression pattern was shown in the cecum, where TNFA mRNA expression was decreased and IL10 mRNA expression was increased by OA+B supplementation (Figure 4C). In the cecal tonsil, the expression level of IL1B and IL10 both tended to be upregulated (P = 0.06 and 0.08, respectively) in the OA+B-treated birds. Other selected cytokines expression did not show any difference between groups (Figure 4D). The data suggested a robust anti-inflammation tone throughout the intestine in the OA+B-treated birds.
Figure 4.
Intestinal cytokines mRNA expression of birds fed with control or supplemented diet. At d 21 of age, gene expressions in jejunum (A), ileum (B), cecum (C), and cecal tonsil (D). Difference between 2 groups at each growth period was labeled. * indicates significant difference (P ≤ 0.05). # indicates trend difference (0.05 < P ≤ 0.1). Dietary treatments were as follows: Con, basal diet; OA+B, basal diet + 500 ppm encapsulated organic acids and botanicals. Abbreviations: IFNG, interferon gamma; IL, interleukin; TNFA, tumor necrosis factor alpha.
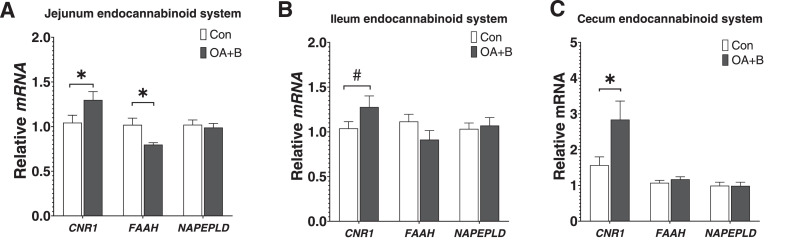
Intestinal Endocannabinoids System Marker mRNA Expression
To investigate the potential mechanism of the anti-inflammatory effect of OA+B supplementation in the intestine, the expression of marker genes that governing 3 fundamental constituents: receptors, signaling molecules, and enzymes involved in ligand biosynthesis and degradation in the ECS system were examined. The mRNA expression of cannabinoid receptor 1 (CNR1) was upregulated in the jejunum, ileum (P = 0.10), and cecum of birds treated with OA+B at d 21 of age (Figure 5A–C). The expression of ligand degrading enzyme (fatty acid amide hydrolase, FAAH) was significantly decreased in the jejunum while numerically reduced in the ileum of OA+B birds. These data suggested an activated ECS system throughout the intestine of OA+B birds.
Figure 5.
Intestinal endocannabinoids system markers mRNA expression of birds fed with control or supplemented diet. At d 21 of age, gene expressions in jejunum (A), ileum (B), and cecum (C). Difference between 2 groups at each growth period was labeled. * indicates significant difference (P ≤ 0.05). # indicates trend difference (0.05 < P ≤ 0.1). Dietary treatments were as follows: Con, basal diet; OA+B, basal diet + 500 ppm encapsulated organic acids and botanicals. Abbreviations: CNR1, cannabinoid receptor 1; FAAH, fatty acid amide hydrolase; NAPEPLD, N-acylphosphatidylethanolamine-specific phospholipase D.
DISCUSSION
With restrictions in the usage of growth-promoting antibiotics in the poultry industry, identifying effective feed additive alternatives that improves gut health, especially the barrier function, is of significant importance of current poultry health research. In the present study, chickens were fed with a microencapsulated blend of citric acid, sorbic acid, thymol, and vanillin (OA+B) for 42 d; then growth performance and intestinal barrier function parameters were evaluated. Although the growth rate was not changed in the birds with OA+B supplementation, the feed efficiency was significantly improved throughout the experimental period. Similar improvement in regard to feed efficiency has been shown from other groups as well (Polycarpo et al., 2017). Under nonchallenged condition, the addition of thymol and carvacrol reduced feed intake and improved feed efficiency in broilers (Hashemipour et al., 2013). These phytogenic additives might have multiple mode of action for feed efficiency, such as flavor effect, digestive enzyme stimulation, and endocrine stimulation, etc. (Yang et al., 2018; Su et al., 2021), yet are not under the scope of the present study. Given the fact that the modern broiler breeding selection for a rapid growth often leads to a low-grade of chronic inflammation (Kogut et al., 2018), one would assume such improvement of feed efficiency in the present study could be the result of strengthened gut. This may be achieved by improving the barrier function and anti-inflammatory status, which overall reduced the cost of immune activation and inflammation for the bird.
An effective intestinal barrier could prevent paracellular diffusion of luminal pathogens and toxins into the bloodstream. In the present study, the addition of microencapsulated organic acids and botanicals significantly reduced the leakage of oral FD4 administration at d 21 of age, indicating an improved whole GI tract barrier function. However, there are potential limitations in the FD4 in vivo evaluation of GI permeability (Quigley, 2016; Ilchmann-Diounou et al., 2021), which could be confounded by the amount of feed digesta in the GI tract, mucus thickness, integrity of epithelial cells TJ, and intestinal motility. Moreover, the data from whole GI tract permeability could also mask the various effects among different intestinal segments from duodenum, jejunum, ileum, to ceca. To further reveal the effects of the supplementation on the epithelial TJ barrier function, the Ussing chamber technique was applied. The TER measurement indicated the resistance of free ion movement across TJs, which reflects integrity of epithelium (Ghiselli et al., 2021). The FD4 flux measurement indicated the paracellular permeability of exogenous molecules. With increased TER and reduced FD4 flux rate shown in the present study, the results of Ussing chamber measurement clearly indicated that OA+B treated birds have improved TJ barrier function in the jejunum up to d 21 of age.
To reveal the potential mechanism by which the blend supplementation improved intestinal barrier function, the gene expression of important TJ proteins throughout the gut was further performed. The expression of major TJs, CLDN1 and ZO2, was upregulated throughout different intestinal regions, while the pore-forming TJ protein, CLND2, was downregulated. These mRNA expressions of TJs were in line with the Ussing chamber measurements of intestinal barrier functions. Interestingly, the expression of another major TJ protein, OCLN, was downregulated in jejunum and ileum, but upregulated in the cecum. With enhanced barrier function supported by Ussing chamber measurements, this disconnection between mRNA expression and protein function of occludin can be explained by different post-transcriptional or post-translational regulations of occludin protein (Rao, 2009). Unlike claudin 1 that serves as a major structural TJ protein of epithelial cells, the occludins are dynamically regulated by physiological conditions via endocytosis (Marchiando et al., 2010). The reduction in mRNA expression of occludin might reflect a reduction of the replenishment demand from occludin endocytosis.
The intestinal epithelium consists of 4 major cell types, including absorptive enterocytes, mucin producing goblet cells, antimicrobial peptide producing Paneth cells, and hormone producing enteroendocrine cells (Grün et al., 2015). The rapid turnover of the entire epithelium is maintained by epithelial stem cell and proliferating cells. It has been suggested that dietary component interplays with microbiota to regulate epithelial cell population and functions (Bayer et al., 2021; Gieryńska et al., 2022). To understand if the addition of organic acids and botanicals to the basal diet can influence the epithelium turnover, the expression of epithelial-specific cell markers gene (Grün et al., 2015) were measured. Although the stem cell marker (LGR5) expression remained unchanged, the proliferating cell marker (SOX9) was upregulated in jejunum and cecum, which indicated a rapid turnover of epithelial cells to replenish the dead cells. It may also explain the upregulated gene expression of TJ protein (CLDN1), as the structural component in newly proliferated cells. The goblet cell marker (MUC2) was upregulated in ileum and cecum, which indicated either maturation of goblet cells or increased number of differentiated goblet cells. As the mucus layer is an important extrinsic part of intestinal barrier, such increases suggested a strengthened gut barrier function, especially in the lower gut, where the highest number of microbes resides. The Paneth cell marker (LYZ) expression was downregulated in the ileum. A recent study in enteroids reported continuous secretion of lysozyme granules when exposed to luminal bacteria, as well as the de novo synthesis and replenishment of the granule after secretion (Yokoi et al., 2019). Therefore, a decreased transcription of lysozyme might indicate a reduction of luminal bacteria load in OA+B-treated birds, which could be supported by the well-established antimicrobial activities of organic acids and botanicals in the blend products. Given the rapid turnover rate in the intestinal epithelium, the changes in these epithelial-specific marker genes might also suggest the proportion of epithelial cell types were influenced by the continuous dietary stimulation. Future investigations via cell lineage-specific staining or single cell sequencing are needed to further elucidate these aspects.
Inflammatory cytokines are believed to be major mediators that regulate intestinal barrier function (Al-Sadi et al., 2009). Proinflammatory cytokines such as IL-1β, IFN-γ, and TNF-α increase permeability by inducing disruption of TJs, whereas anti-inflammatory cytokines such as IL-10, tend to protect the epithelial integrity. In the present study, the expression pattern of cytokines was similar across different regions in the intestine. The addition of microencapsulated organic acids and botanicals significantly reduced expression of proinflammatory cytokines (IL1B, TNFA, and IFNG), while upregulated expression of anti-inflammatory cytokine (IL10). Interestingly, although a higher level of inflammation was expected in the ileum region, the anti-inflammatory effects of the OA+B blend were more robust in the jejunum and cecum than in the ileum. The breeding selection of modern broilers pushed the traits toward high feed intake and high growth rate, both of which could increase the basal level of inflammation without pathogen challenge (Kogut et al., 2018). This low grade, chronic sterile inflammation has been proposed from high level of feed immune stimulation factors, such as mannose in the soybean meal (Kogut et al., 2018). Therefore, under a nonchallenged condition, a higher basal inflammation level in the jejunum could be expected. In the cecal tonsils, which are considered as the largest gut associated lymphoid tissue, the IL10 and IL1B mRNA expression was upregulated. A previous study reported that broilers supplemented with this combination of organic acids and botanicals exhibited the upregulated immune activities, indicated by increased IL10 and IL1B mRNA expression in the isolated heterophils and monocytes (Swaggerty et al., 2020b). In the present study, with no pathogen challenges and improved intestinal barrier in the OA+B-treated birds, less pathogenic or commensal bacteria stimulation was expected in the gut lymphoid aggregated tissue. Therefore, the upregulated IL1B may also indicate enhanced immune activity that would rapidly respond to pathogen challenges.
Another partial explanation of the positive effects of the blend in modulating intestinal barrier and immune function could be related to the modulation of ECS. In fact, the ability of the ECS to mitigate the inflammatory status has been widely investigated in human medicine, arousing growing interest in veterinary medicine as well (DiPatrizio, 2016). The ECS has long been known for its effects on comforting abdominal pain and GI stress. Accumulating evidence indicated that ECS plays key modulatory role in GI pathophysiology (Ambrose and Simmons, 2019; Lian et al., 2022). Briefly, the function of ECS relies on the activation of main cannabinoid receptors (CB1 and CB2), and the endogenous enzymatic reactions for the ligand synthesis (NAPE-PLD or DGL) and degradation (FAAH or MGL) (Lee et al., 2016). A recent study in pigs indicated that thymol modulated ECS system and the inflammatory status in small intestine (Toschi et al., 2020b), suggesting that botanical components of the blend could drive the activation of the ECS. In the present study, interesting results were obtained from the analysis of gene expression of ECS markers, as mRNA of CNR1 (CB1 analogue in chickens), NAPE-PLD and FAAH were detected. This is the first study assessing the presence of cannabinoid markers in the GI tract of chickens, confirming the presence of this system in the mucosa of poultry. Moreover, consistently upregulated expression of CNR1 was observed across the gut in jejunum, ileum, and cecum of broilers. This increased activation of CNR1 receptor could be related with the reduction of FAAH enzyme expression in ileum. In fact, a recent study found that increased activation of CB1 receptors possibly reflect the augmented concentration of endocannabinoid ligands after FAAH inhibition in mice (Moreira et al., 2008). Moreover, the activation of cannabinoid receptors partially regulates feeding behaviors, GI contractility and enzyme secretion (DiPatrizio, 2016).
CONCLUSIONS
In conclusion, our data showed that dietary supplement of a blend of organic acids and botanicals activated the ECS system and reduced inflammatory gene expressions throughout the intestine of broilers. Moreover, the supplementation improved intestinal epithelial barrier functions including upregulated secretory mucus and antimicrobial peptide barrier, as well as upregulated the TJ expressions and epithelial barrier, eventually improved growth performance and feed efficiency in broiler chickens.
ACKNOWLEDGMENTS
This study was supported by Vetagro S.p.A. (ANSC432286). We acknowledge the support of the College of Agriculture and Natural Resources in providing the research facilities and partial funding to conduct this work. The authors thank the Comparative Pathology Laboratory of University of Delaware for their advice and technical expertise with regards to histology samples processing.
DISCLOSURES
Ester Grilli serves as an advisor of Vetagro S.p.A. Andrea Toschi is an employee of Vetagro S.p.A. All other authors declare no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.psj.2022.102460.
Appendix. Supplementary materials
REFERENCES
- Al-Sadi R., Boivin M., Ma T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front. Biosci. (Landmark Ed.) 2009;14:2765–2778. doi: 10.2741/3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose T., Simmons A. Cannabis, cannabinoids, and the endocannabinoid system – is there therapeutic potential for inflammatory bowel disease? J. Crohns Colitis. 2019;13:525–535. doi: 10.1093/ecco-jcc/jjy185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer F., Dremova O., Khuu M.P., Mammadova K., Pontarollo G., Kiouptsi K., Soshnikova N., May-Simera H.L., Endres K., Reinhardt C. The interplay between nutrition, innate immunity, and the commensal microbiota in adaptive intestinal morphogenesis. Nutrients. 2021;13 doi: 10.3390/nu13072198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T., Ligresti A., Di Marzo V. The endocannabinoid signalling system: biochemical aspects. Pharmacol. Biochem. Behav. 2005;81:224–238. doi: 10.1016/j.pbb.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Bonetti A., Tugnoli B., Rossi B., Giovagnoni G., Piva A., Grilli E. Nature-identical compounds and organic acids reduce E. coli K88 growth and virulence gene expression in vitro. Toxins (Basel) 2020;12 doi: 10.3390/toxins1208046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broom L.J. Gut barrier function: effects of (antibiotic) growth promoters on key barrier components and associations with growth performance. Poult. Sci. 2018;97:1572–1578. doi: 10.3382/ps/pey021. [DOI] [PubMed] [Google Scholar]
- Di Marzo V., Izzo A.A. Endocannabinoid overactivity and intestinal inflammation. Gut. 2006;55:1373–1376. doi: 10.1136/gut.2005.090472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPatrizio N.V. Endocannabinoids in the gut. Cannabis Cannabinoid Res. 2016;1:67–77. doi: 10.1089/can.2016.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiselli F., Rossi B., Piva A., Grilli E. Assessing intestinal health. Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.723387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholami-Ahangaran M., Ahmadi-Dastgerdi A., Azizi S., Basiratpour A., Zokaei M., Derakhshan M. Thymol and carvacrol supplementation in poultry health and performance. Vet. Med. Sci. 2022;8:267–288. doi: 10.1002/vms3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieryńska M., Szulc-Dąbrowska L., Struzik J., Mielcarska M.B., Gregorczyk-Zboroch K.P. Integrity of the intestinal barrier: the involvement of epithelial cells and microbiota – a mutual relationship. Animals (Basel) 2022, 145;12 doi: 10.3390/ani12020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli E., Tugnoli B., Passey J.L., Stahl C.H., Piva A., Moeser A.J. Impact of dietary organic acids and botanicals on intestinal integrity and inflammation in weaned pigs. BMC Vet. Res. 2015;11:96. doi: 10.1186/s12917-015-0410-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grün D., Lyubimova A., Kester L., Wiebrands K., Basak O., Sasaki N., Clevers H., van Oudenaarden A. Single-cell messenger Rna sequencing reveals rare intestinal cell types. Nature. 2015;525:251–255. doi: 10.1038/nature14966. [DOI] [PubMed] [Google Scholar]
- Hashemipour H., Kermanshahi H., Golian A., Veldkamp T. Effect of thymol and carvacrol feed supplementation on performance, antioxidant enzyme activities, fatty acid composition, digestive enzyme activities, and immune response in broiler chickens. Poult. Sci. 2013;92:2059–2069. doi: 10.3382/ps.2012-02685. [DOI] [PubMed] [Google Scholar]
- Hryhorowicz S., Kaczmarek-Ryś M., Zielińska A., Scott R.J., Słomski R., Pławski A. Endocannabinoid system as a promising therapeutic target in inflammatory bowel disease - a systematic review. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.790803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilchmann-Diounou H., Buleon M., Bacquie V., Theodorou V., Denis C., Menard S. Revisiting definition and assessment of intestinal trans-epithelial passage. Cell. Mol. Life Sci. 2021;78:8157–8164. doi: 10.1007/s00018-021-04000-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S.A., Rodriguez D., Allred K. A systematic review of essential oils and the endocannabinoid system: a connection worthy of further exploration. Evid. Based Complement. Alternat. Med. 2020;2020 doi: 10.1155/2020/8035301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogut M.H., Arsenault R.J. Editorial: gut health: the new paradigm in food animal production. Front. Vet. Sci. 2016;3:71. doi: 10.3389/fvets.2016.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogut M.H., Genovese K.J., Swaggerty C.L., He H., Broom L. Inflammatory phenotypes in the intestine of poultry: not all inflammation is created equal. Poult. Sci. 2018;97:2339–2346. doi: 10.3382/ps/pey087. [DOI] [PubMed] [Google Scholar]
- Lee Y., Jo J., Chung H.Y., Pothoulakis C., Im E. Endocannabinoids in the gastrointestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol. 2016;311:G655–G666. doi: 10.1152/ajpgi.00294.2015. [DOI] [PubMed] [Google Scholar]
- Lian J., Casari I., Falasca M. Modulatory role of the endocannabinoidome in the pathophysiology of the gastrointestinal tract. Pharmacol. Res. 2022;175 doi: 10.1016/j.phrs.2021.106025. [DOI] [PubMed] [Google Scholar]
- Marchiando A.M., Shen L., Graham W.V., Weber C.R., Schwarz B.T., Austin J.R., Raleigh D.R., Guan Y., Watson A.J., Montrose M.H., Turner J.R. Caveolin-1-dependent occludin endocytosis is required for Tnf-induced tight junction regulation in vivo. J. Cell. Biol. 2010;189:111–126. doi: 10.1083/jcb.200902153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira F.A., Kaiser N., Monory K., Lutz B. Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (Faah) is mediated by Cb1 receptors. Neuropharmacology. 2008;54:141–150. doi: 10.1016/j.neuropharm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- NRC . 9th ed. National Academy Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- NRC . National Academy Press; Washington, DC: 2011. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- Polycarpo G.V., Andretta I., Kipper M., Cruz-Polycarpo V.C., Dadalt J.C., Rodrigues P.H.M., Albuquerque R. Meta-analytic study of organic acids as an alternative performance-enhancing feed additive to antibiotics for broiler chickens. Poult. Sci. 2017;96:3645–3653. doi: 10.3382/ps/pex178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley E.M. Leaky gut - concept or clinical entity? Curr. Opin. Gastroenterol. 2016;32:74–79. doi: 10.1097/MOG.0000000000000243. [DOI] [PubMed] [Google Scholar]
- Rao R. Occludin phosphorylation in regulation of epithelial tight junctions. Ann. N. Y. Acad. Sci. 2009;1165:62–68. doi: 10.1111/j.1749-6632.2009.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi B., Toschi A., Piva A., Grilli E. Single components of botanicals and nature-identical compounds as a non-antibiotic strategy to ameliorate health status and improve performance in poultry and pigs. Nutr. Res. Rev. 2020;33:218–234. doi: 10.1017/S0954422420000013. [DOI] [PubMed] [Google Scholar]
- Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Su G., Wang L., Zhou X., Wu X., Chen D., Yu B., Huang Z., Luo Y., Mao X., Zheng P., Yu J., Luo J., He J. Effects of essential oil on growth performance, digestibility, immunity, and intestinal health in broilers. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaggerty C.L., Arsenault R.J., Johnson C., Piva A., Grilli E. Dietary supplementation with a microencapsulated blend of organic acids and botanicals alters the kinome in the ileum and jejunum of Gallus gallus. PLoS One. 2020;15 doi: 10.1371/journal.pone.0236950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaggerty C.L., He H., Genovese K.J., Callaway T.R., Kogut M.H., Piva A., Grilli E. A microencapsulated feed additive containing organic acids, thymol, and vanillin increases in vitro functional activity of peripheral blood leukocytes from broiler chicks. Poult. Sci. 2020;99:3428–3436. doi: 10.1016/j.psj.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toschi A., Rossi B., Tugnoli B., Piva A., Grilli E. Nature-identical compounds and organic acids ameliorate and prevent the damages induced by an inflammatory challenge in Caco-2 cell culture. Molecules. 2020;25:4296. doi: 10.3390/molecules25184296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toschi A., Tugnoli B., Rossi B., Piva A., Grilli E. Thymol modulates the endocannabinoid system and gut chemosensing of weaning pigs. BMC Vet. Res. 2020;16:289. doi: 10.1186/s12917-020-02516-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugnoli B., Giovagnoni G., Piva A., Grilli E. From acidifiers to intestinal health enhancers: how organic acids can improve growth efficiency of pigs. Animals (Basel) 2020;10 doi: 10.3390/ani10010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Xin H., Yang C. Impact of essential oils and organic acids on the growth performance, digestive functions and immunity of broiler chickens. Anim. Nutr. 2018;4:388–393. doi: 10.1016/j.aninu.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi Y., Nakamura K., Yoneda T., Kikuchi M., Sugimoto R., Shimizu Y., Ayabe T. Paneth cell granule dynamics on secretory responses to bacterial stimuli in enteroids. Sci. Rep. 2019;9:2710. doi: 10.1038/s41598-019-39610-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.