Introduction
Immune checkpoint inhibitors (CPIs), including pembrolizumab, are becoming common oncological treatments and have revolutionized the treatment of cancers. CPIs have been associated with a significant risk of developing immune-related adverse events affecting various organs including kidney. The reported incidence of these events with CPIs used as monotherapy is about 2% to 3%, and 5% when used in combination.1 Most kidney immune-related adverse events observed are acute kidney injury, typically caused by acute tubulo-interstitial nephritis.2,3 Glomerular diseases are rarely seen with immunotherapy.2,4 In addition, the association of malignancy with some forms of secondary glomerular disease makes it more challenging to distinguish from CPI-associated glomerular disease. Here, we present the case of a 71-year-old man with stage 4 lung adenocarcinoma who developed proteinuria and membranous nephropathy (MN) during treatment with pembrolizumab.
Case Presentation
A 71-year-old man with history of stage 4 lung adenocarcinoma, diabetes mellitus, and hypertension was referred to outpatient nephrology clinic for proteinuria. The patient was diagnosed with stage 4 lung adenocarcinoma 2 years prior and has completed 6 cycles of paclitaxel and pembrolizumab therapy. Initial laboratory results in the outpatient clinic showed serum creatinine at baseline of 0.8 mg/dl, serum albumin at 4.2 mg/dl and spot urine total protein-to-creatinine ratio significantly elevated at 4.2. Work up for secondary causes of nephrotic range proteinuria were done. The work up revealed negative results for hepatitis B surface antigen, hepatitis C core antibody, HIV, serum antinuclear antibodies levels, cytoplasmic antineutrophil cytoplasmic antibodies levels, perinuclear antineutrophil cytoplasmic antibodies levels, serum double stranded DNA antibody levels, serum anti-phospholipase A2 receptor (anti-PLA2R) antibodies and glomerular basement membrane (GBM) antibodies. Serum C3 complement levels were within range 131 and serum C4 complement levels were also in range at 31. Serum free light chain kappa/ lambda ratio was 0.9. To evaluate the etiology for nephrotic range proteinuria, the patient underwent a kidney biopsy.
The Kidney Biopsy Findings
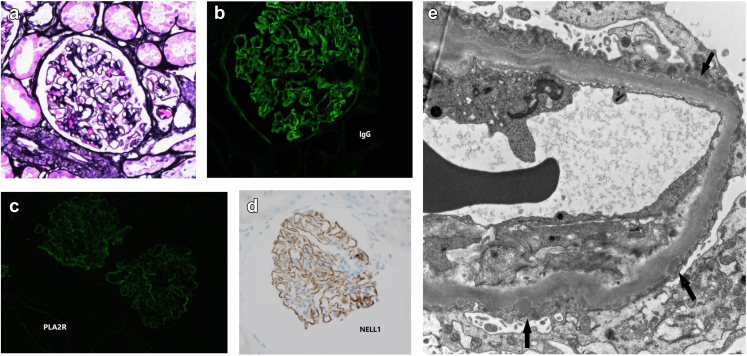
On light microscopy, the glomeruli show rigid capillary walls, without overt spike formation or vacuolization (Figure 1a). The capillary loops and urinary spaces were patent without significant inflammatory cell infiltrate. The mesangial areas were in normal thickness. There was minimal interstitial fibrosis and tubular atrophy. Immunofluorescence microscopy showed granular staining in capillary walls for IgG (2+, see Figure 1b), C3 (trace+) and kappa (1–2+), and lambda (1–2+) light chains, and PLA2R (trace to 1+, see Figure 1c). There was no significant glomerular staining for IgA, IgM, C1q, albumin, THSD7A, and fibrinogen. Additional immunohistochemical staining for nerve epidermal growth factor-like 1 (NELL-1) marker was done, which showed granular staining along glomerular basement membranes (see Figure 1d). Electron microscopy revealed immune type electron dense deposits in subepithelial locations (Figure 1e). Immune type electron dense deposits were not seen in mesangial or subendothelial areas. There was no significant basement membrane reaction to the deposits. Overlying epithelial cells showed marked effacement of foot processes.
Figure 1.
Panel of the kidney biopsy findings. (a) Jones stain (×200): rigid glomerular capillary walls. (b) Immunofluorescence stain of IgG (×400): granular staining along glomerular capillary walls. (c) Immunofluorescence stain of PLA2R (×400): trace staining along glomerular capillary walls. (d) Immunohistochemical stain of NELL1 (×200): positive along glomerular capillary walls. (e) Electron microscopy (×4000): scattered electron dense deposits in subepithelial spaces (arrow). NELL-1, nerve epidermal growth factor-like 1. PLA2R, phospholipase A2 receptor.
Diagnosis and Follow-Up
A diagnosis of NELL-1 MN was made. Given NELL-1's association with cancer, the concern was if this was lung cancer related or could this NELL-1 disease be triggered by immunotherapy. Repeat computerized tomography scan of the chest was done, which showed unchanged lung findings with no new lung cancer related lesion. After a multidisciplinary meeting with oncology and nephrology, the decision was made to hold pembrolizumab and monitor the proteinuria. Over the course of 2 months, spot urinary total protein-to-creatinine ratio improved to 1.2 g/g and subsequently to 0.8 g/g. Given the improvement of proteinuria after holding immunotherapy, the patient likely had nephrotic range proteinuria secondary to NELL-1 MN because of the use of pembrolizumab in lung cancer treatment.
Discussion
MN is a common cause of proteinuria and nephrotic syndrome. It can be subdivided into primary and secondary forms. The primary form is an autoimmune disease clinically characterized by nephrotic syndrome and slow progression. It accounts for approximately 70% of cases in MN. In the remaining cases, MN may be secondary to well-defined causes, including infections, drugs, solid organ cancer, or autoimmune diseases, such as systemic lupus erythematosus, rheumatoid arthritis, sarcoidosis, Sjogren syndrome, systemic sclerosis, or ankylosing spondylitis. The clinical presentation is similar in primary and secondary MN. However, the outcome may be different and often related to that of the original disease in secondary MN. In addition, the treatment may be different, being targeted to the etiologic cause in secondary MN. Therefore, the differential diagnosis between primary and secondary MN is critical and should be based not only on history and clinical features of the patient but also on immunofluorescence and electron microscopy analysis of kidney biopsy as well as on the research of circulating antibodies.5
MN and association with malignancy has been well described within the literature.6 However, MN secondary to drug exposure is also not infrequent. Rihova et al. in their study conducted in a single center in Czech Republic revealed that out of 129 patients with MN, an underlying cause was identified in 40 cases (31%). In 18 of the 40 (45%) MN was secondary to drugs.7
There have been many reports of glomerular diseases associated with CPIs therapies (Table 1).4 Both nephritic and nephrotic syndromes have been described. In a recent systematic review of all biopsy proven published cases or series of glomerular pathology associated with CPIs, the most frequent pathology findings were pauci-immune glomerulonephritis (27%), podocytopathies (24%), and C3 glomerulonephritis.4
Table 1.
Glomerular pathology associated with immune check point inhibitors (CPIs)
| Nephrotic syndrome | Nephritic syndrome |
|---|---|
| Podocytopathies (24%) | Pauci-immune vasculitis (26.7%) |
| AA amyloidosis (8.9%) | IgA nephropathy (8.9%) |
| Membranous nephropathy (2.2%) | Anti GBM disease (6.7%) |
| Thrombotic microangiopathy (4.4%) | |
| Immune complex glomerulonephritis (4.4%) | |
| Lupus like nephritis (2.2%) |
GBM, glomerular basement membrane.
The mechanisms underlying the development of drug-induced MN have not been fully elucidated. One of the implicated pathogeneses of drug-induced MN is probably because of an immune response to the drug or to a by-product that acts as planted antigen on the subepithelial position of the GBM. The most plausible mechanism is that cationic drug–derived antigens traverse the GBM, are planted in the subepithelial space, and are targeted in situ by circulating antibodies directed against these antigens.8 In our case, the patient had a history of solid organ malignancy (lung cancer) which is well established as a cause for secondary MN.9 Even though, initial serologic work up was negative for PLA2R antibodies, the kidney biopsy revealed weak anti-PLA2R antibody staining. Additional staining was negative for THSD7A antigen but came back positive for NELL-1 marker. There have been few case reports describing MN associated with CPIs inhibitors (Supplementary Table S1). Recent studies have shown that NELL1-associated MN is more often associated with malignancy than other known types of MN.S4,S5 Recently Kurien et al. in their study described the association of NELL-1 positivity in MN with traditional indigenous medicine use.S6 However, the prevalence of NELL-1 positivity in MN associated with other drug classes including CPIs has not been studied. No specific kidney biopsy findings can help differentiate drug induced MN from primary forms of MN. In the past, the most frequent drugs that caused MN were gold salts, penicillamine, and bucillamine that contained a sulfhydryl group, also called Thiol group.S7 However, the use of these drugs has progressively reduced after the introduction of biological agents in the treatment of rheumatoid arthritis.S8 Drug-induced MN is considered when: (i) there is no other apparent cause for MN, (ii) proteinuria develops within 6 months to 12 months of drug therapy, and (iii) discontinuation of the drug leads to resolution of the proteinuria. Our patient was receiving pembrolizumab as part of treatment for lung cancer. In our case, the MN was noted after CPI start of therapy and cancer status remained unchanged despite improvement of proteinuria. It is still possible that the NELL-1 induced MN in our case is related to the cancer and was a triggered immune response with the use of CPI. It is plausible that this particular individual is prone to getting NELL-1 MN and the CPI trigged this specific immune disorder. After discontinuation of pembrolizumab, we observed significant improvement in the spot urine total protein-to-creatinine ratio within 8 weeks, hence establishing a potential association of pembrolizumab use and MN in our patient. One of the limitations of this case is the lack of NELL-1 antibody testing and correlation of that with discontinuation of the CPI.
Conclusion
This is the first case, to best of our knowledge, of NELL-1 MN with potential association with pembrolizumab or any CPI form of therapy. It is possible that besides malignancy, NELL-1 associated MN may be associated with medications and drugs and might be the next most common antigen after PLA2R.S9 With increasing use of CPIs for management of malignancy, patients need to be monitored for such rare but significant complications. The management includes discontinuation of CPIs (Table 2).
Table 2.
Teaching points
|
|
|
MN, membranous nephropathy; NELL-1, nerve epidermal growth factor-like 1.
Disclosure
KDJ is a founder and co-president of the American Society of Onco-Nephrology; reports consultancy agreements with Secretome, George Clinicals, PMV pharmaceuticals, and Calliditas. KDJ reports honoraria from the American Society of Nephrology, the International Society of Nephrology, and UpToDate.com; reports serving on the editorial boards of American Journal of Kidney Diseases, CJASN, Clinical Kidney Journal, Journal of Onconephrology, Kidney International, and Nephrology Dialysis Transplantation; reports serving as Editor-in-Chief of ASN Kidney News and section editor for onconephrology for Nephrology Dialysis Transplantation. All the authors declared no competing interests.
Patient Consent
Patient consent was obtained for this report.
Footnotes
Supplementary References.
Table S1. Summary of published reports of membranous nephropathy associated with immune checkpoint inhibitor therapy.
Supplementary Material
Supplementary References.
Table S1. Summary of published reports of membranous nephropathy associated with immune checkpoint inhibitor therapy.
References
- 1.Cortazar F.B., Kibbelaar Z.A., Glezerman I.G., et al. Clinical features and outcomes of immune checkpoint inhibitor-associated AKI: a multicenter study. J Am Soc Nephrol. 2020;31:435–446. doi: 10.1681/ASN.2019070676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seethapathy H., Herrmann S.M., Sise M.E. Immune checkpoint inhibitors and kidney toxicity: advances in diagnosis and management. Kidney Med. 2021;3:1074–1081. doi: 10.1016/j.xkme.2021.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mamlouk O., Selamet U., Machado S., et al. Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: single-center experience. J Immunother Cancer. 2019;7:1–3. doi: 10.1186/s40425-018-0478-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitchlu A., Jhaveri K.D., Wadhwani S., et al. A systematic review of immune checkpoint inhibitor–associated glomerular disease. Kidney Int Rep. 2021;6:66–77. doi: 10.1016/j.ekir.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moroni G., Ponticelli C. Secondary membranous nephropathy. A narrative review. Front Med (Lausanne) 2020;7 doi: 10.3389/fmed.2020.611317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Row P.G., Cameron J.S., Turner D.R., et al. Membranous nephropathy: long-term follow-up and association with neoplasia. Q J M. 1975;44:207–239. [PubMed] [Google Scholar]
- 7.Rihova Z., Honsova E., Merta M., et al. Secondary membranous nephropathy—one center experience. Ren Fail. 2005;27:397–402. doi: 10.1081/JDI-200065304. [DOI] [PubMed] [Google Scholar]
- 8.Hogan J.J., Markowitz G.S., Radhakrishnan J. Drug-induced glomerular disease: immune-mediated injury. Clin J Am Soc Nephrol. 2015;10:1300–1310. doi: 10.2215/CJN.01910215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leeaphorn N., Kue-A-Pai P., Thamcharoen N., Ungprasert P., Stokes M.B., Knight E.L. Prevalence of cancer in membranous nephropathy: a systematic review and meta-analysis of observational studies. Am J Nephrol. 2014;40(1):29–35. doi: 10.1159/000364782. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.