Abstract
Introduction
Although the investigation of chronic kidney disease of uncertain etiology (CKDu) has identified many possible influencing factors in recent years, the exact pathomechanism of this disease remains unclear.
Methods
In this study, we collected 13 renal biopsies from patients with symptomatic CKDu (Sym-CKDu) from Sri Lanka with well-documented clinical and socioeconomic factors. We performed light microscopy and electron microscopic evaluation for ultrastructural analysis, which was compared with 100 biopsies from German patients with 20 different kidney diseases.
Results
Of the 13 Sri Lankan patients, 12 were men (92.3%), frequently employed in agriculture (50%), and experienced symptoms such as feeling feverish (83.3%), dysuria (83.3%), and arthralgia (66.6%). Light microscopic evaluation using activity and chronicity score revealed that cases represented early stages of CKDu except for 2 biopsies, which showed additional signs of diabetes. Most glomeruli showed only mild changes, such as podocyte foot process effacement on electron microscopy. We found a spectrum of early tubulointerstitial changes including partial loss of brush border in proximal tubules, detachment of tubular cells, enlarged vacuoles, and mitochondrial swelling associated with loss of cristae and dysmorphic lysosomes with electron-dense aggregates. None of these changes occurred exclusively in Sym-CKDu; however, they were significantly more frequent in these cases than in the control cohort.
Conclusion
In conclusion, our findings confirm the predominant and early alterations of tubular structure in CKDu that can occur without significant glomerular changes. The ultrastructural changes do not provide concrete evidence of the cause of CKDu but were significantly more frequent in Sym-CKDu than in the controls.
Keywords: chronic kidney disease of uncertain etiology (CKDu), lysosomal alteration, mitochondrial swelling, renal biopsy, transmission electron microscopy, tubular injury
Graphical abstract
Environmental nephropathies have become a primary focus for scientific studies because of challenges in identifying the exact causative agents. There have been several epidemics of geographically confined nephropathies, namely the Itai-Itai disease in Japan (cadmium), the Balkan endemic nephropathy (aristolochic acid), lead nephropathy in Australia, and CKD.1,2 CKDu was first reported in the 1990s in Sri Lanka and Central America almost simultaneously.3 Subsequently, the disease was reported in India, Egypt, Taiwan, and Tunisia. Typically, all these environmental nephropathies were described as primary tubular interstitial diseases and supported toxin-mediated kidney injury.4,5 Initially, fluoride-aluminum complexes in drinking water were suspected to cause CKDu in Sri Lanka. Agrochemicals that have been excessively used in these farming areas were subsequently considered as the etiological agents. Natural substances, as well as contaminants, such as cadmium, arsenic, fluoride, calcium, and some other heavy metals, were hypothesized in the causation of this still mysterious disease.6 Infections by Leptospira and Hantavirus, for instance, were among other suspected etiologies.7
Since its inception, the diagnosis of CKDu has been challenging. Initially, the diagnosis was made by excluding known causes such as diabetes, hypertension, and glomerular diseases. Subsequently, an evolving diagnostic criterion was proposed to define CKDu in endemic regions of the country.8 Later, more objective, less complicated diagnostic models based on serum creatinine, urine albumin, and serum albumin–based diagnostic models were proposed with a good performance in a biopsy-based prospective study.9 A novel approach using a panel of urinary markers for screening and diagnosis of CKDu has been reported by Fernando et al.10 Nevertheless, as in most other kidney diseases, despite its invasiveness, renal biopsy remains the gold standard of diagnosis.10
The main histological features in Sri Lankan CKDu are tubular atrophy (TA), interstitial fibrosis (IF), and periglomerular fibrosis (PGF) in the absence of or with a mild degree of interstitial mononuclear inflammatory cell infiltration and tubulitis.11,12 The electron microscopic analysis of relatively early stages of CKDu cases from patients in Central America revealed mild wrinkling of the glomerular basement membrane and subendothelial edema with segmental effacement of podocyte foot processes.13 Partial tubular cell degeneration was also observed. Interestingly, no cadmium or arsenic toxicity features such as dysmorphic mitochondria or accumulation of metallothioneins were evident.13 Recently, large dysmorphic proximal tubular lysosomal lesions were described predominantly in CKDu but also identified in transplanted kidneys, drug-induced CKDs, and light chain disease.14 Thus, the specificity of these lysosomal lesions remained questionable.15
Moreover, on immunologic evaluation, IgG, IgM, IgA, and complement factors were reported as consistently negative in the absence of electron-dense deposits in blood vessels or glomeruli. Most histological features of CKDu in Sri Lanka were similar to those reported for meso-American nephropathy. However, differences in the frequency and severity of glomerular and tubulointerstitial changes existed.16 In contrast to the chronic lesions in asymptomatic CKDu, significant interstitial inflammation and widespread tubulitis have been reported in patients with Sym-CKDu in Sri Lanka.17 Similarly, severe injury to the tubulointerstitial compartments, more specifically to proximal tubules, besides unaffected glomeruli and blood vessels, have been identified on electron microscopic evaluation of patients with tubular interstitial nephritis in Central America.13
Although the initiating pathomechanism of the kidney disease is difficult to investigate in already chronically altered kidneys in CKDu, the investigation of patients with CKDu with clinical symptoms during the early phase may be more promising. Understanding the importance of ultrastructural assessment of acute interstitial nephritis of unknown origin provided the basis for identifying the underlying pathophysiological mechanisms and their relationship with CKDu. This led to this cross-sectional study of clinically suspected patients with Sym-CKDu.
Methods
This descriptive study was conducted on 13 randomly selected patients with Sym-CKDu from CKDu endemic regions in Sri Lanka identified through a surveillance program. Hundred routine biopsies with different pathologies obtained from patients across Germany were selected as controls. All procedures and interview-based data collection were conducted after obtaining informed written consent from patients. The ethical approval for this study was obtained from the institutional ethical review committees at National Hospital, Kandy, Sri Lanka (THK/ERC/75/2017) and Friedrich–Alexander University of Erlangen-Nürnberg, Germany (No. 4415).
Patient Recruitment and Biopsy Indication
In our study, patients (25–65 years) were enrolled through the surveillance program in endemic areas for CKDu. We included patients who presented with fever, malaise, arthralgia, nausea, dysuria, and dyspepsia with elevated plasma creatinine concentrations and normal-sized kidneys (>9 cm), who were suspected to have Sym-CKDu and referred for kidney biopsy. Children (<18 years), people with morphologic kidney abnormalities, patients with positive urine culture, patients who were exposed to identifiable nephrotoxins such as drugs, patients who had significant proteinuria (+ or more), patients with hematuria, or patients with an alternative explanation for renal dysfunction were excluded from the study. Glomerular filtration rate was calculated based on creatinine and the CKD-epidemiology collaboration formula.18 Every fourth patient who had undergone renal biopsy was included in ultrastructural investigations in this study. One hundred controls with a wide spectrum of kidney disease etiologies including IgA nephropathy (n = 35), diabetic nephropathy (n = 12), hypertensive nephropathy (n = 6), minimal change glomerulopathy (n = 6), primary focal segmental glomerulosclerosis (n = 4), interstitial nephritis (n = 5), or others (n = 22) and kidney transplant biopsies (n = 9) were selected from routine biopsies diagnosed at the Department of Nephropathology, Friedrich–Alexander University Erlangen-Nürnberg, Germany, for comparison. All control biopsies also showed tubulointerstitial changes, such as TA and fibrosis, graded from mild to severe. Furthermore, among controls, 60% of cases had weak, 15% had moderate, and 1 had severe concomitant inflammation. All diagnoses and the extent of tubulointerstitial changes of the control group are listed in Supplementary Table S1.
Questionnaire-Based Interview for Suspected Sym-CKDu
All patients who were supposed to undergo a biopsy were admitted to the Nephrology Unit, National Hospital Kandy, Kandy, Sri Lanka, the day before the biopsy. A questionnaire-based interview was carried out by investigators before the biopsy procedure to obtain patient demographic data, including living area, drinking water source, and unhealthy habits (smoking, drinking alcohol, and betel chewing). Medical history was retrieved; blood pressure, weight, and height were also recorded. Subsequently, ultrasound imaging of kidneys and essential laboratory evaluations were conducted according to the study protocol.
Patients Undergoing Renal Biopsy
All ultrasound-guided biopsies were performed by nephrologists following strict aseptic conditions and their respective protocols. For the patients with Sym-CKDu, 3 cores were taken as follows: a formalin-fixed core for light microscopy, a fresh core for immunofluorescence, and another core for electron microscopic evaluation. The core was collected into a screw-capped plastic container with buffered 4% formaldehyde solution at pH 7. Thereafter, the patient’s identification details were labeled on the container and stored at 4 °C until transportation. The same fixative was used to preserve electron microscopy specimens of the control collective from Germany.
Biopsy Review
Histologic analyses were performed at the Department of Pathology, Faculty of Medicine, University of Peradeniya, Sri Lanka, and at the Department of Nephropathology of Friedrich–Alexander University Erlangen-Nürnberg, Germany. Formalin-fixed paraffin-embedded sections were stained with hematoxylin and eosin stain and with the silver methenamine stain for basement membranes. The fresh tissue was used for frozen sections and stained for IgG, IgA, IgM, and C3 using the direct immunofluorescence method. To determine the origin of tubular casts, formalin-fixed paraffin-embedded sections were stained for uromodulin using a mouse antihuman uromodulin (a gift from Professor J. Scherberich, LMU München, Germany) and a standard protocol for immunohistochemistry as described previously.19
Histologic changes assessed explicitly for the quantification were glomerulosclerosis (GS), TA, IF, tubulitis, and interstitial mononuclear inflammation. Quantification of histologic changes was performed based on the percentage of total area affected by tubulointerstitial lesions and the percentage of sclerosed glomeruli. The histologic scores were given as follows: histologic score 0 indicating no lesions, score 1 indicating 25% lesions, score 2 indicating 26% to 50%, and scores 3 indicating >50% lesions. The activity index (AI) is the sum of tubulitis grade and interstitial mononuclear inflammatory infiltration grade. The AI was grouped as AI-1 (sum of tubulitis and interstitial inflammation between 1 and 2), AI-2 (sum of tubulitis and interstitial inflammation between 3 and 4), and AI-3 (sum of tubulitis and interstitial inflammation between 5 and 6). The sum of TA, IF, PGF, and GS scores was considered for the chronicity index. The index grouping is considered as chronicity index-1 (sum of TA, IF, PGF, and GS between 1 and 4), chronicity index-2 (sum of TA, IF, PGF, and GS between 5 and 8), and chronicity index-3 (sum of TA, IF, PGF, and GS between 9 and 12).11
Electron Microscopy
For electron microscopy, a separate renal biopsy from patients with Sym-CKDu was fixed in 4% formalin buffered in 0.1 M phosphate buffered saline pH 7.6 following the same protocol as control biopsies. Renal biopsies were washed in 0.1 M phosphate buffered saline pH 7.6, treated with 0.5% OsO4 for 60 minutes, and stained with 1% uranyl acetate in 70% ethanol. After the dehydration, tissue blocks were embedded in epoxy araldite resin (Serva Electrophoresis GmbH, Heidelberg, Germany). After that, 80 nm ultrathin sections were cut on a UC6 ultramicrotome (Leica, Wetzlar, Germany), rinsed in lead citrate buffer before analysis, and analyzed using a SIGMA scanning electron microscope equipped with a scanning transmission electron microscopy detector at 28 kV (Zeiss, Oberkochen, Germany). Sym-CKDu and control specimens were examined for detached tubular cells, mitochondrial changes, myeloid bodies (MBs), and increased vacuolization in the tubular compartment. These changes were graded as follows: 0 = absent, 1 = rare (occurring in only 1–2 single nephrons), and 2 = frequent (occurring in more than 2 nephrons). In addition, tubuli were investigated for the presence of lysosomes filled with electron-dense material. Here, we further distinguished between small round lysosomes and lysosomes larger than 1.2 μm and dysmorphic in shape. For this purpose, at least 4 randomly selected tubulointerstitial areas in the renal cortex were evaluated for each sample at a magnification of 5000× and an area of 400 μm2. In addition, images of the glomeruli were taken to assess podocyte effacement.
Results
Clinical and Demographic Data
Thirteen randomly selected patients with Sym-CKDu (male 12, female 1) were included in the study. All demographic data are presented in Table 1. Of these, 50% of the participants were engaged in farming, and 42% were engaged in at least one of the following occupations: sand mining, fishing, or as a mason or laborer. Their water consumption pattern was diverse; less than 50% used water less likely to be contaminated with agrochemical or natural substances. Of the participants, 33% depended on public water supplies, whereas 17% used spring water. Thirty-three percent and 8% of participants regularly consumed groundwater extracted from shallow dug wells and deep tube wells, respectively, whereas the rest used all sources (Table 1).
Table 1.
Baseline clinical characteristics of patients
| Characteristics | Male (%) n = 12 | Female (%) n = 1 | |
|---|---|---|---|
| Work | Working | 92 | - |
| Nonworking | - | 8 | |
| Only farming | 50 | - | |
| Occupation | Farming with other heavy physical work | 50 | - |
| Drinking water source | Public supply | 25 | 8 |
| Tube well | 8 | - | |
| Protected dug well | 33 | - | |
| Spring | 17 | - | |
| Public, tube well, bottle | 8 | - | |
| Tobacco and alcohol use | Alcohol | 75 | - |
| Betel | 75 | - | |
| Smoking | 33 | - | |
| Type of alcohol used | Alcohol | 78 | - |
| Illicit alcohol | 11 | - | |
| Alcohol and illicit alcohol | 11 | - | |
| Composition of betel | Tobacco | 89 | - |
| Areca nut | 89 | - | |
| Lime | 67 | - | |
| Type of smoking | Cigarette | 25 | - |
| Beedi | 25 | - | |
| Cigarette and beedi | 50 | - | |
| Symptoms present | |||
| Symptoms | Fever | 83 | 8 |
| Back/loin pain | 8 | 8 | |
| Arthralgia | 67 | - | |
| Dysuria | 83 | 8 | |
| Dyspepsia | 58 | - | |
Considering risk factors, a majority (75%) consumed alcohol and chewed betel, and only 33% smoked. At the time point of biopsy, all were symptomatic, and a majority (92%) had fever and dysuria as the main symptoms. Of the participants, 17% presented with back pain/loin pain. Another 67% and 17% had arthralgia and back or loin pain, respectively, as symptoms; 58% of the participants, all of them male, presented with dyspepsia (Table 1).
All patients had a significantly reduced glomerular filtration rate at presentation (mean estimated glomerular filtration rate 31.25 ml/min per 1.73 m2) without marked proteinuria (Table 2, Table 3), with subsequent improvement at the time of biopsy within a period less than 4 weeks (Table 3). Follow-up data were available in 7 of 13 cases and were used to calculate estimated glomerular filtration rate slopes over time (Figure 1).
Table 2.
Urine dipstick results at the time of biopsy
| Urine dipstick | Frequency | Percentage (%) n = 13 |
|---|---|---|
| Urine protein | ||
| Nil | 46 | |
| Trace | 46 | |
| + | 8 | |
| Urine pus cells | ||
| Occasionally | 8 | |
| 0–9 | 62 | |
| 10–19 | 23 | |
| 20–30 | 0 | |
| >30 | 8 | |
| Urine red cells | ||
| Nil | 54 | |
| 0–10 | 46 |
Table 3.
Mean values of clinical data (baseline and at the time of biopsy)
| Clinical parameter | Baseline | At the time of biopsy |
|---|---|---|
| eGFR | 31.25 ± 13.09 | 41.66 ± 11.12 |
| Hemoglobin (g/dl) | 13.56 ± 0.93 | 12.59 ± 1.09 |
| Serum sodium (mmol/l) | 140.81 ± 2.45 | 140.80 ± 4.63 |
| Serum potassium (mmol/l) | 3.97 ± 0.75 | 4.0 ± 1.21 |
| Serum calcium (mg/dl) | 8.98 ± 1.02 | 9.27 ± 0.87 |
| Serum uric acid (mg/dl) | 6.02 ± 0.56 | 6.27 ± 0.37 |
| Serum protein (g/dl) | 7.75 ± 0.65 | 7.02 ± 0.39 |
| Serum phosphate (mmol/l) | 1.60 ± 0.47 | 1.47 ± 0.92 |
| Serum cholesterol (mg/dl) | 184.64 ± 66.45 | 164.5 ± 12.02 |
| Blood pressure (mm Hg) | 118/79 ± 3/4 | 125/80 ± 2/4 |
eGFR, estimated glomerular filtration rate.
Figure 1.
Renal function in the follow-up of patients with Sym-CKDu. The eGFR slope of patients with acute interstitial nephritis with uncertain etiology over time is shown. GFR, glomerular filtration rate.
Histologic Analysis by Light Microscopy
Light microscopic evaluation of all participants revealed a tubulointerstitial disease; the only glomerular change observed was GS in a few cases. Most had mild chronic interstitial nephritis, with only 2 having a moderate disease. Activity without chronicity was observed in 1 case. Early hypertensive vasculopathy was noted in 2 cases. None had features suggestive of pyelonephritis. In terms of tubulointerstitial disease, signs of both activity and chronicity, were present in 10 cases, whereas 1 had activity only, and 2 had chronicity only (Table 4). Thus, this case series, with a similar clinical presentation, represents a spectrum of pathological changes that differ in the degree of active inflammatory reaction and chronic changes. These range from cases with very low inflammatory response and mild chronic changes (Figure 2a) to cases in which inflammatory activity dominates the renal histology (Figure 2b), to cases with atrophic tubules, IF, and very low inflammatory activity (Figure 2c). Two participants were subsequently diagnosed to have diabetes mellitus. However, neither had nodular diabetic glomerulopathy. The results of the clinical and histologic analyses of each Sym-CKDu case at the time of biopsy collection are summarized in Table 5.
Table 4.
Histologic activity and chronicity indices of patients who underwent biopsy (n = 13)
| Histologic activity or chronicity index | 0 | 1 | 2 | 3 |
| Activity in number of patients | 2 | 10 | 1 | - |
| Chronicity in number of patients | 1 | 8 | 4 | - |
Figure 2.
Light microscopy of study cases (hematoxylin and eosin stain). (a) Mild chronicity only characterized by mild IF and mild TA. The glomerulus is unremarkable. (b) Significant activity characterized by tubulitis and interstitial inflammation mediated by lymphocytes with a few PC casts. (c) Typical established CKDu appearance with TA with IF without/minimal activity. IF, interstitial fibrosis; PC, pus cell; TA, tubular atrophy; TI, tubulitis and interstitial inflammation.
Table 5.
Baseline clinical characteristics (at the time of symptoms onset) and histopathology
| ID | Age (yr)/sex | Serum creatinine (μmol/ml)/eGFR (ml/min) | Upn | Upc | Urc | Hb (g/dl) | Serum Na+/K+ (mmol/l) | Serum Ca2+/PO43− (mg/dl) | Serum UA (mg/dl)/Pn (total) (g/dl) | BP (mm Hg) | Kidney size R/L (cm) | Histologic finding | EM findings: FP, DTC, MS, MB, AL, V (0 = absent; 1 = rare/small lysosomes only; 2 = frequent/dysmorphic lysosomes; # = no glomeruli in section) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 38/M | 196/26.52 | Plus | 1–2 | Nil | 14.7 | 142.6/4.22 | 9.89/1.46 | 6.91/7.7 | 120/80 | 10.9 × 4.7/10.0 × 5.5 | Mild chronic interstitial nephritis AI-1 CI-1 |
FP-0, DTC-0, MS-2, MB-2, AL-2, V-1 |
| 2 | 44/M | 128/42.09 | Trace | 1–2 | Nil | 12.7 | 144.7/4.18 | 8.97/2.4 | 5.46/7.4 | 121/81 | 9.0 × 4.3/9.3 × 4.8 | Chronic interstitial nephritis AI-1 CI-2 |
FP-1, DTC-0, MS-2, MB-0, AL-0, V-2 |
| 3 | 58/M | 128/40 | Trace | 8–10 | Nil | 14.3 | 9.52/1.14 | 6.19/7.81 | 118/79 | 9.1 × 4.6/9.1 × 4.7 | Mild chronic interstitial nephritis AI-1 CI-1 |
FP-2, DTC-1, MS-0, MB-0, AL-2, V-1 | |
| 4 | 60/M | 126/40.24 | Nil | occ | 2–4 | 13.9 | 125/81 | Mild interstitial fibrosis AI-0 CI-1 |
FP-1, DTC-1, MS-2, MB-2, AL-2, V-1 | ||||
| 5 | 53/M | 173/28.63 | Plus | occ | 8–10 | 12.6 | 142/3.6 | 8.9/1.58 | 6.54/7.07 | 119/79 | 8.8 × 5.1/9.5 × 4.5 | Chronic interstitial fibrosis AI-0 CI-2 |
FP-0, DTC-2, MS-2, MB-2, AL-2, V-2 |
| 6 | 49/M | 193/26 | 14.4 | 139/3.29 | 110/70 | 9.9 × 4.2/10.4 × 4.7 | Mild chronic interstitial nephritis AI-1 CI-1 |
FP-0, DTC-2, MS-1, MB-0, AL-2, V-2 | |||||
| 7 | 54/M | 321/13.97 | Plus | 100–150 | 1–2 | 13.2 | 119/80 | 10.9 × 4.3/10.4 × 4.5 | Mild chronic interstitial nephritis AI-1 CI-1 |
FP-0, DTC-2, MS-2, MB-2, AL-1, V-2 | |||
| 8 | 55/M | 169/29.19 | Trace | 30–40 | 2–3 | 118/75 | 9.1 × 4.8/9.7 × 5.8 | Mild chronic interstitial nephritis AI-1 CI-1 |
FP-1, DTC-2, MS-1, MB-0, AL-2, V-2 | ||||
| 9 | 40/M | 90/64.43 | Nil | 4–5 | Nil | 15 | 139/3.6 | 5.7/NA | 120/85 | Mild chronic interstitial nephritis AI-1 CI-1 |
FP-#, DTC-1, MS-0, MB-0, AL-2, V-1 | ||
| 10 | 32/M | 220/24 | Plus | 60–70 | 2–3 | 137.7/3.44 | 7.05 | 5.95/NA | 119/72 | 11.3 × 4.5/11.9 × 4.9 | Acute on chronic interstitial nephritis AI-2 CI-2 |
FP-#, DTC-0, MS-2, MB-0, AL-1, V-2 | |
| 11 | 61/F | 248/18 | Trace | 5–8 | 12.6 | 9.58/1.43 | 5.41/8.81 | 118/81 | 10.1/10 | Acute tubular injury AI-1 CI-0 |
FP-#, DTC-2, MS-1, MB-1, AL-0, V-2 | ||
| 12 | 33/M | 170/32 | 13.2 | 120/85 | 10.1 × 5.9/9.6 × 5.7 | Chronic interstitial nephritis AI-1 CI-2 |
FP-0, DTC-1, MS-0, MB-2, AL-2, V-1 | ||||||
| 13 | 62/M | 218/21 | Trace | 30–40 | Nil | 12.5 | 140.7/5.47 | NA/7.7 | 119/79 | 10.6 × 4.4/11.4 × 5.4 | Mild chronic interstitial nephritis AI-1 CI-1 |
FP-#, DTC-0, MS-1, MB-2, AL-1, V-2 |
AI, activity index; AL, altered lysosomes; BP, blood pressure; CI, chronicity index; DTC, detached tubular cell; eGFR, estimated glomerular filtration rate; EM, electron microscopy; FP, foot process; Hb, hemoglobin; ID, identification; L, left kidney; M, male; MB, myeloid body; MS, mitochondrial swelling; NA, not available; Pn, protein; R, right kidney; UA, uric acid; Upc, urinary pus cell; Upn, urinary protein; Urc, urinary red cell; V, vacuolization.
The other histopathologic changes observed are as follows: most biopsies (n = 6) showed typical features of CKDu with low-grade activity and chronicity, with mild ischemic-like injury to low-grade glomerulosclerosis (Figure 2c and Figure 3a and b) on the light microscopic analysis. Ultrastructural examination of the glomeruli in this group also revealed intact podocyte foot processes (Figure 3c and d) and foot process effacement in rare cases (Figure 3e and f). Glomerular basement membranes were typically formed and showed neither thickening nor immune complex deposition (Figure 3d and f). PGF is consistently absent in all 6 cases, indicating low-grade or early disease. In the 6 cases with typical CKDu appearance, the tubules were inconspicuous on light microscopic examination. However, electron microscopic examination showed partial brush border loss as a sign of early tubular damage in these cases and single detached cells in the proximal tubule region (Figure 4a and b). In 2 other Sym-CKDu cases, light microscopy revealed uromodulin-positive tubular casts (Figure 4c), with no apparent tubule damage visible on light microscopy. In the ultrastructural analysis of these 2 cases, we could confirm the absence of cellular debris in the tubular lumen. Therefore, these histopathologic changes probably represent the earliest stages of tubular damage because of the development of chronicity.
Figure 3.
Ultrastructural glomerular changes in Sym-CKDu biopsies. (a) Semithin sections of biopsies from patients with Sym-CKDu showed normal glomeruli or (b) only mild ischemic-like injury. (c,d) Ultrastructural examination of the glomeruli in biopsies with typical CKDu appearance revealed intact podocyte foot processes. (e,f) Foot process effacement was only observed in rare cases. EC, endothelial cell; FP, foot process; GBM, glomerular basement membrane; N, nucleus; RBC, red blood cell.
Figure 4.
Tubular damage in biopsies with Sym-CKDu. (a) Sym-CKDu with typical CKDu appearance showed partial brush border loss (black arrowheads) and (b) detached cells in the lumen of proximal tubules. (c) Uromodulin-positive tubular casts were seen in 2 cases with Sym-CKDu, as assessed by immunohistochemistry. (d) Two other cases with evidence of tubular epithelial cell damage showed detached cells in the lumen of proximal tubular cells. (e) Also confirmed by electron microscopy. (f) Tubulorhexis was seen in one case without chronic changes. (g) Cell detritus was also seen in some collecting ducts. (h) Distal parts of the tubules were frequently atrophic and showed ultrastructural changes in vacuolization and mitochondrial structure. BB, brush border; BM, basement membrane; CD, cellular debris; DTC, distal tubule cell; EM, extracellular matrix; N, nucleus; TC, tubular cast; THP, Tamm-Horsefall protein;TL, tubular lumen; V, vacuole.
In contrast, in 2 cases, there was evidence of tubular epithelial cell damage associated with inflammatory cell infiltrates based on the light microscopic evaluation (Figure 4d). In one of the latter 2 cases, there was only tubulorrhexis on light and electron microscopic evaluation (Figure 4e) in the absence of chronic changes, which was not described in CKDu earlier. Cell detritus was not restricted to the lumen of proximal tubules but could also be seen in other tubular segments, including collecting ducts (Figure 4f). Cellular debris or detached cells in the lumen of tubuli were absent in 4 of 13, rare in another 4 of 13, and frequent in 5 of 13 cases with Sym-CKDu. Tubular damage occurred primarily within the proximal tubular compartment; however, distal tubules were frequently atrophic and also showed ultrastructural changes in vacuolization and mitochondrial structure (Figure 4h). All investigated cases of Sym-CKDu showed high numbers of intracellular vacuoles with increased size (Figure 5a). In the one case without chronic changes but acute tubular necrosis with strong vacuolization, we observed lymphocyte and eosinophilic inflammation (Figure 5b). Interestingly, this case lacked altered lysosomes with electron-dense material that were found in 11 of 13 cases (Figure 5c and d). The second case with definite tubule epithelial damage showed chronic changes such as arterial wall thickening (Figure 5e), marked IF, frequent tubules with altered lysosomes with electron-dense material, and mitochondrial swelling. In only 3 of the 13 Sym-CKDu cases examined, all mitochondria were of standard size and had regularly shaped cristae (Figure 5f). In all other investigated Sym-CKDu cases, we observed mitochondrial swelling and rudimental cristae in at least some of the analyzed tubuli in proximal as well as distal parts (Figure 5g and h).
Figure 5.
Ultrastructural changes of cells and organelles in the tubulointerstitium of biopsies from patients with Sym-CKDu. (a) Sym-CKDu cases showed high numbers of intracellular vacuoles with increased size. (b) Eosinic infiltration in the tubulointerstitium. (c,d) Small (back arrowheads) and dysmorphic lysosomes (white arrowheads) with electron-dense aggregates were frequently observed in Sym-CKDu. (e) Arterial wall thickening was observed in several cases with Sym-CKDu. (f) Some biopsies showed mitochondria with regular shape and intact cristae, (g,h) whereas mitochondrial swelling is frequently observed. BB, brush border; CF, collagen fibrils; EC, endothelial cell; EOS, eosinophil granulocyte; L, lysosome; M, mitochondria; N, nucleus; TL, tubular lumen; V, vacuole.
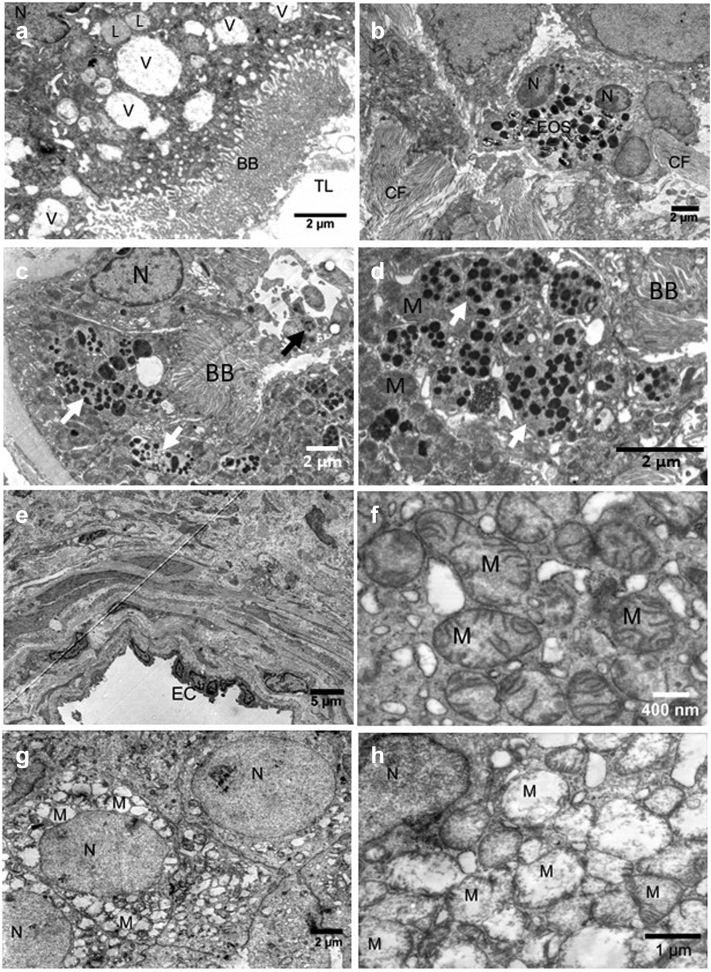
Of the 13 Sym-CKDu cases examined, 3 cases stand out. One case showed pyelonephritis-like pathology with significant acute interstitial nephritis and occasional pus cell casts in a chronic background. However, this subject’s urine cultures were consistently negative, and no infection could be detected. Electron microscopy revealed mild glomerular changes with intact podocyte foot processes (Figure 6a). In contrast, the tubules showed severe vacuolization (Figure 6b, V) and IF (Figure 6c). In addition, this biopsy showed detached cells in the tubular lumen (Figure 6c), mitochondrial swelling, and altered lysosomes as described for other cases.
Figure 6.
Ultrastructural changes in biopsies with Sym-CKDu and pyelonephritis-like or diabetic appearance. (a) Podocyte foot processes appeared intact. (b) Tubules showed severe vacuolization and interstitial fibrosis with (c) luminal pus cells. (d) Glomeruli from patients with diabetes showed podocyte foot process effacement and (e) progressed thickening of the glomerular basement membrane in one case. (f) Mitochondrial swelling, (g) huge altered lysosomes with electron-dense material, and (h) myeloid body formation were characteristic in these biopsies. BB, brush border; CD, cellular debris; CF, collagen fibrils; FP, foot process; GBM, glomerular basement membrane; L, lysosome; M, mitochondria; MB, myeloid body; N, nucleus; PC, pus cell; RBC, red blood cell; TA, tubular atrophy; TBM, tubular basement membrane; TC, tubular cast; V, vacuole.
Finally, the case series included 2 cases later diagnosed with diabetes mellitus. The chronic changes here are more advanced and dominated by diabetic disease but seemed to be also influenced by Sym-CKDu. This includes mild to advanced glomerular basement membrane thickening with podocyte foot process effacement (Figure 6d and e, glomerular basement membrane) and hyaline arteriosclerosis. In these cases, the swelling of mitochondria (Figure 6f) and the number and size of altered lysosomes with electron-dense material (Figure 6g and h) were particularly pronounced. Conspicuous were MBs that arose in autophagosomes from membrane material found in the proximal tubules’ lumen (Figure 6h, MB). These MBs were also observed in 3 of 10 of the other cases but with lower frequency. However, it remains unclear whether the patients developed CKDu in addition to diabetic renal changes.
Altered Dysmorphic Lysosomes Are a Conspicuous but Nonspecific Finding in Sym-CKDu
Because lysosomal changes have been described in previous studies, we wanted to investigate whether this change is a specific feature of Sym-CKDu or if it also occurs in other renal diseases. For this purpose, kidney biopsies from 100 patients with common kidney diseases diagnosed in the Department of Nephropathology at Friedrich–Alexander University in Erlangen, Germany, were examined ultrastructurally to serve as controls to the CKDu cases from Sri Lanka. Lysosomes with electron-dense material appeared in Sym-CKDu biopsies either round with 500 nm to 700 nm diameter (Figure 7b) or in an irregular, dysmorphic shape with a minimum diameter of 1.2 μm, but often reaching several micrometers in size (Figure 7a and c). These lysosomes were exclusively found in the proximal and not in the distal part of the tubuli. The small round lysosomes with electron-dense aggregates were indeed found in 85% of the cases with Sym-CKDu (including all categories) (Figure 7d) but also observed in biopsies from patients with different diseases within the control cohort (Figure 7d), as shown for IgA nephropathy (Figure 7g and h, black arrowheads). In cases with hypertensive nephropathy, these lysosomes were observed in almost equal frequency and in IgA nephropathy and after renal transplantation in more than 40% of cases (Figure 7d). The occurrence of large dysmorphic lysosomes with electron-dense aggregates was also found in 50% of cases with hypertensive nephropathy and in other renal diseases in approximately 20% of cases (Figure 7e).
Figure 7.
Lysosomal changes are more frequent but not restricted to renal biopsies from patients with Sym-CKDu. (a) Lysosomes with electron-dense aggregates were seen in proximal tubules and (b) occurred in a small round (black arrowheads) and/or (c) dysmorphic shape (white arrowhead). (d) Evaluation of the percentage of cases with lysosomes with electron-dense aggregates irrespective of shape and size or (e) restricted to dysmorphic lysosomes in biopsies from the Sym-CKDu and the control cohort with different renal diseases. (f) n of dysmorphic lysosomes with electron-dense aggregates per 400 μm2 in biopsies from Sym-CKDu and the control cohort with different renal diseases. (g,h) Representative electron microscopic pictures of tubuli seen in biopsies from patients with IgA nephropathy showing small round (black arrowheads) and dysmorphic (white arrowheads) lysosomes with electron-dense aggregates. BB, brush border; CKDu, chronic kidney disease of uncertain etiology; DN, diabetic nephropathy; FSGS, focal segmental glomerulosclerosis; IgAN, IgA nephropathy; KTx, kidney transplantation; M, mitochondria; MCGP, minimal change glomerulopathy; NM, nephropathy; RBC, red blood cell; TBM, tubular basement membrane. ∗∗∗P < 0.001, ∗∗ P < 0.01, ∗ P < 0.05 vs. Sym-CKDu group as assessed by Kruskal-Wallis test followed by Dunn’s multiple comparison test.
Even in Sym-CKDu, these dysmorphic lysosomes were detected in only 62% of the cases examined (Figure 7e), but the number was significantly higher in Sym-CKDu cases compared with transplant biopsies or biopsies from patients with diabetic nephropathy, FSGS, or minimal change glomerulopathy (Figure 7f).
The Ultrastructural Changes Occur Significantly More Frequently in Sym-CKDu Than in the Control Cohort
The comparison between the Sym-CKDu case series and the control group clearly showed that the ultrastructural characteristics observed in the Sym-CKDu group, such as lysosomal changes, mitochondrial swelling, MBs, and vacuolization, were more frequent than in the control group, both qualitatively and quantitatively (Figure 8). In detail, especially dysmorphic lysosomes with electron-dense aggregates were 3 times more frequent in the Sym-CKDu group than in the control cohort (Figure 8a).
Figure 8.
Distribution and frequency of ultrastructural changes in Sym-CKDu compared with controls. The percentage of cases with (a) dysmorphic or small and round lysosomes with electron-dense aggregates, (b) mitochondrial swelling, (c) myeloid bodies and (d) vacuoles with increased size and number in patients with Sym-CKDu and the control cohort. CKDu, chronic kidney disease of uncertain etiology.
Mitochondria swelling with rudimentary cristae was observed in the Sym-CKDu group in different tubule sections in 10 of all 13 cases, being 3 times more frequent than in the control group (Figure 8b). In the control group, frequent mitochondrial swelling was rare at 4%, whereas this was 10 times more frequent in the Sym-CKDu group (Figure 8b). MBs also occurred in the Sym-CKDu group in only 46% of all cases (6/13) but were observed in the control group in only 7% of all biopsies examined (Figure 8c). Indeed, we observed increased vacuolization in both proximal and distal tubules in all Sym-CKDu cases, which was even frequent in 62% of cases (Figure 8d). In contrast, only 3% of control biopsies showed increased vacuolization frequently, whereas more than 60% showed only average vacuolization (Figure 8d). These results indicate that the observed ultrastructural changes of tubular cells, though not specific for Sym-CKDu, are more frequent in this disease.
Discussion
Renal biopsy remains the gold standard of diagnosis in most renal diseases, including CKDu. Despite years of research to elucidate the etiology of CKDu in Sri Lanka,6,20 no clear pathomechanism or pathognomic features were identified. CKDu predominantly occurs in males in the dry zone of Sri Lanka, where agriculture is the main source of income. Numerous hypotheses on the causation of the disease have been put forward. However, a proven link of a single biological, agrochemical, or hydrogeochemical factor with disease development has not been established thus far.21,22
Although the majority of CKDu cases are asymptomatic, a group of patients exhibited symptoms such as fever, back pain, dysuria, and general ill health (Sym-CKDu).17 Most of the previous studies on CKDu in Sri Lanka were on asymptomatic patients with established CKDu on clinical, biochemical, and histologic evaluation. Most of these studies demonstrated predominantly tubule-interstitial involvement with GS, PGF, TA, IF, and mononuclear cell infiltration on histologic evaluation.5,23, 24, 25 In contrast, in Sym-CKDu, widespread tubulitis and plasma lymphocytic cellular infiltrate were reported in Sri Lanka and Central America.11,13 Because Sym-CKDu was proposed as the early phase of the disease,11 clinical, biochemical, and light and electron microscopic evaluations were performed in this study to identify typical abnormalities in early disease.
Notably, all investigated patients with Sym-CKDu were symptomatic, and there were minimal chronic disease manifestations on clinical, biochemical evaluation, or imaging. Consequently, all biopsies were classified as early stages of CKDu apart from the cases later diagnosed for diabetes mellitus and showed diabetic changes on histologic evaluation. Overall, our light microscopic evaluation revealed a spectrum of histopathologic changes ranging from 1 case with active inflammation without chronic alteration to cases showing both active and chronic lesions. In contrast, only patients with diabetes showed exclusive chronic lesions. Furthermore, the site and type of injury varied, as detected by detached cells or casts within the tubular lumen. The electron microscopic evaluation confirmed the findings of light microscopic observations. Previous ultrastructural studies focused on glomerular changes in CKDu, describing podocyte foot process effacement between 18% and 57%.13,26,27
In contrast, our biopsy study of patients with Sym-CKDu revealed a lack of mild podocyte foot process effacement in most glomeruli and only a few cases with frequent pathologic changes in podocyte structure. In contrast to established CKDu, biopsies from patients with Sym-CKDu showed more active inflammation. Therefore, we hypothesized that CKDu might develop from acute interstitial nephritis and that its progression may be due to repeated attacks of acute interstitial nephritis. However, what triggers the acute interstitial nephritis remains unclear. Tissue damage and inflammatory responses influence each other. Therefore, it is unclear whether the tubular damage triggers the inflammatory response or whether the inflammatory response damages the tubules. A systemic proinflammatory environment can also be caused by heat stress,28,29 one of the suspected causes of CKDu.29,30
Because in CKDu, it is generally agreed that the primary damage occurs in the tubulointerstitium,23,24 we paid special attention to this compartment. There was extensive vacuolization and frequent detachment of tubular cells in Sym-CKDu cases. Tubular damage in Sym-CKDu is not limited to the proximal tubule, but loss of the brush border, detachment of cells, and lysosomal changes have occurred most frequently in this part of the tubule. Given that heat stress–induced renal damage was predominantly localized in the distal tubular parts and the outer medulla,31 Sym-CKDu does not appear to be primarily induced by heat stress.
Mitochondrial swelling, cell death, cell detachment, and increased vacuolization were often reported after toxic injury.23,31,32 As an early sign of kidney damage, we observed mitochondrial swelling that was absent in only 3 of 13 Sym-CKDu biopsies, rare in 4 of 13, and frequently observed in 6 of 13 Sym-CKDu cases. On the other hand, mitochondrial swelling can be induced by acute kidney injury as reported for renal ischemia32,33 or sepsis.34 Fluoride, suspected of contributing to the pathology of CKDu,35 is also a known trigger of mitochondrial dysfunction, leading to reduced activity, excessive fission, and disruption of fusion.36 Heat stress also affects mitochondrial function and induces mitochondrial condensation.37 However, mitochondrial swelling has been reported neither after fluoride intoxication nor during heat stress. Given that the triggers for mitochondrial swelling can be manifold, no clear conclusions can be drawn about the cause.
Interestingly, none of the patients were aware of exposure to any of the above nephrotoxins that could contribute to renal injury preceding the illness. MBs represent lysosomes filled with phospholipids. Such lysosomal phospholipidosis is known for aminoglycoside nephrotoxicity.38 MBs are lacking in most Sym-CKDu cases, consistent with CKDu histology, but occur more often in cases with advanced injury. Similar to mitochondrial swelling, the presence of MBs indicates a disturbance in the lysosomal function that might be caused by a toxin but not necessarily through an aminoglycoside. However, in more than 84% of all investigated biopsies from patients with CKDu, we detected lysosomes with electron-dense aggregates, and in 65% of Sym-CKDu cases, these were large and dysmorphic. Similar lysosomal changes were recently described in a collection of patients, suggesting that these changes characterize CKDu.14
In contrast, we found dysmorphic lysosomes with electron-dense aggregates in a large proportion of kidney biopsies from Germany with a wide range of kidney diseases. Dysmorphic lysosomes were furthermore frequently described in light chain–mediated renal disease39 and showed high frequency in cases with hypertensive nephropathy in our control cohort. In Sym-CKDu, the formation of dysmorphic lysosomes does not seem to be caused by hypertension because all patients showed normal blood pressure. In common diseases such as IgA nephropathy, more than 20% of the biopsies showed dysmorphic lysosomes. Nevertheless, changes were particularly pronounced in Sym-CKDu. Of the 19% of controls with large dysmorphic lysosomes, 2 (10.5%) had interstitial nephritis. However, all other cases had an acute (58%) or chronic (31.5%) concomitant inflammation. Only 2 of all control cases with large dysmorphic lysosomes were transplant biopsies, and in the only biopsy with known acute toxic damage to the kidney in the control cohort, no such lysosomes were observed (Supplementary Table S1), suggesting that large dysmorphic lysosomes occur even in the absence of a toxic insult. Similar findings indicating that lysosomal changes do not exclusively occur in CKDu were reported by Wijkström et al.15
The small sample size and the small study area, which was limited to 2 CKDu endemic regions close to each other, were obvious limitations of this study. As a result, no significant associations between ultrastructural changes and sociodemographic or clinical parameters could be established in this study. Although our control group covers a broad spectrum of kidney diseases and shows chronic fibrotic aspects with concomitant inflammation (Supplementary Table S1), a larger collective would be necessary for future studies. Strengths of the study include the systematic and comprehensive assessment of tubulointerstitial changes, the large control group analyzed by the same investigators and the use of the same fixative in cases and controls, making fixation artifacts as a cause of the observed differences unlikely.
Overall, in this study, we detected various ultrastructural changes in Sym-CKDu that increased in severity and frequency as the disease progressed. However, none of the described alterations, including the lysosomal changes, seem to be specific for Sym-CKDu but also occurred albeit less frequently in our control cohort, which includes a wide variety of renal diseases. Our findings confirm the tubular interstitium’s predominant and early involvement even without significant glomerular changes. Tubular interstitial changes are not identical in all cases. However, they present a spectrum of the disease that a nephrotoxin may have likely induced. Nonetheless, such features can also be influenced by other environmental factors such as heat stress. In parallel with other studies, we found no clear evidence of immune complex–mediated disease in CKDu.
Disclosure
KUE reports supporting the GCKD study by Bayer, FMC, Evotec, AstraZeneca, and Vifor and consulting or lecture fees from AstraZeneca, Akebia, and FMC. PE reports funding from DFG, Jäckstädt Stiftung, and BIH as well as lecture fees from GSK, AstraZeneca, Bayer, Akakdemie der Nieren, and NAW Berlin. All the other authors declared no competing interests.
Acknowledgments
This research was cofunded by the German Federal Ministry of Education and Research in a project named Sri-Kid-H2O (Grant Number 01DP17042) to RC, NN, CZ, KA, PE, and JB and by the Deutsche Forschungsgemeinschaft (German Research Foundation), project number 387509280, SFB 1350 TP C2 to CD and KA.
Footnotes
Table S1. Ultrastructural changes, inflammation, and fibrosis in control cohort.
Supplementary Material
Table S1. Ultrastructural changes, inflammation, and fibrosis in control cohort.
References
- 1.Gifford F.J., Gifford R.M., Eddleston M., Dhaun N. Endemic nephropathy around the world. Kidney Int Rep. 2017;2:282–292. doi: 10.1016/j.ekir.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loghman-Adham M. Renal effects of environmental and occupational lead exposure. Environ Health Perspect. 1997;105:928–938. doi: 10.1289/ehp.97105928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Annual health bulletin 2012 Medical Statistics Unit. Ministry of Health. http://www.health.gov.lk/moh_final/english/public/elfinder/files/publications/AHB/Annual%20Health%20Bulletin%20-%202012.pdf Published 2012. Accessed December 15, 2022.
- 4.Herath C., Jayasumana C., De Silva P., De Silva P.H.C., Siribaddana S., De Broe M.E. Kidney diseases in agricultural communities: a case against heat-stress nephropathy. Kidney Int Rep. 2018;3:271–280. doi: 10.1016/j.ekir.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wijetunge S., Ratnatunga N.V., Abeysekera D.T., Wazil A.W., Selvarajah M., Ratnatunga Retrospective analysis of renal histology in asymptomatic patients with probable chronic kidney disease of unknown aetiology in Sri Lanka. Ceylon Med J. 2013;58:142–147. doi: 10.4038/cmj.v58i4.6304. [DOI] [PubMed] [Google Scholar]
- 6.Pearce N., Caplin B. Let’s take the heat out of the CKDu debate: more evidence is needed. Occup Environ Med. 2019;76:357–359. doi: 10.1136/oemed-2018-105427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gamage C.D., Sarathkumara Y.D. Chronic kidney disease of uncertain etiology in Sri Lanka: are leptospirosis and hantaviral infection likely causes? Med Hypotheses. 2016;91:16–19. doi: 10.1016/j.mehy.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Caplin B., Yang C.W., Anand S., et al. The International Society of Nephrology’s International Consortium of Collaborators on Chronic Kidney Disease of Unknown Etiology: report of the working group on approaches to population-level detection strategies and recommendations for a minimum dataset. Kidney Int. 2019;95:4–10. doi: 10.1016/j.kint.2018.08.019. [DOI] [PubMed] [Google Scholar]
- 9.Anand S., Montez-Rath M.E., Adasooriya D., et al. Prospective biopsy-based study of CKD of unknown etiology in Sri Lanka. Clin J Am Soc Nephrol. 2019;14:224–232. doi: 10.2215/CJN.07430618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernando B., Alli-Shaik A., Hemage R.K.D., et al. Pilot study of renal urinary biomarkers for diagnosis of CKD of uncertain etiology. Kidney Int Rep. 2019;4:1401–1411. doi: 10.1016/j.ekir.2019.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Badurdeen Z., Nanayakkara N., Ratnatunga N.V., et al. Chronic kidney disease of uncertain etiology in Sri Lanka is a possible sequel of interstitial nephritis! Clin Nephrol. 2016;2016:106–109. doi: 10.5414/CNP86S115. [DOI] [PubMed] [Google Scholar]
- 12.Nanayakkara S., Senevirathna S.T., Karunaratne U., et al. Evidence of tubular damage in the very early stage of chronic kidney disease of uncertain etiology in the North Central Province of Sri Lanka: a cross-sectional study. Environ Health Prev Med. 2012;17:109–117. doi: 10.1007/s12199-011-0224-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer R.S.B., Vangala C., Truong L., et al. Early detection of acute tubulointerstitial nephritis in the genesis of Mesoamerican nephropathy. Kidney Int. 2018;93:681–690. doi: 10.1016/j.kint.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Vervaet B.A., Nast C.C., Jayasumana C., et al. Chronic interstitial nephritis in agricultural communities is a toxin-induced proximal tubular nephropathy. Kidney Int. 2020;97:350–369. doi: 10.1016/j.kint.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Wijkström J., Elinder C.G., Hultenby K., Söderberg M., Wernerson A. “Dysmorphic” lysosomes in proximal tubular cells are not specific for CINAC/CKDu and do not provide evidence that CINAC/CKDu is a toxin-induced disease. Kidney Int. 2020;98:786–787. doi: 10.1016/j.kint.2020.04.057. [DOI] [PubMed] [Google Scholar]
- 16.Gunawardena S., Dayaratne M., Wijesinghe H., Wijewickrama E. A systematic review of renal pathology in chronic kidney disease of uncertain etiology. Kidney Int Rep. 2021;6:1711–1728. doi: 10.1016/j.ekir.2021.03.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kafle K., Balasubramanya S., Horbulyk T. Prevalence of chronic kidney disease in Sri Lanka: a profile of affected districts reliant on groundwater. Sci Total Environ. 2019;694 doi: 10.1016/j.scitotenv.2019.133767. [DOI] [PubMed] [Google Scholar]
- 18.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer L.M., Fichte L.A., Büttner-Herold M., et al. Complement in renal disease as a potential contributor to arterial hypertension. Kidney Blood Press Res. 2021;46:1–15. doi: 10.1159/000515823. [DOI] [PubMed] [Google Scholar]
- 20.Campese V.M. The unresolved epidemic of chronic kidney disease of uncertain origin (CKDu) around the world: a review and new insights. Clin Nephrol. 2021;95:65–80. doi: 10.5414/CN110186. [DOI] [PubMed] [Google Scholar]
- 21.Weaver V.M., Fadrowski J.J., Jaar B.G. Global dimensions of chronic kidney disease of unknown etiology (CKDu): a modern era environmental and/or occupational nephropathy? BMC Nephrol. 2015;16:145. doi: 10.1186/s12882-015-0105-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lunyera J., Mohottige D., Von Isenburg M., Jeuland M., Patel U.D., Stanifer J.W. CKD of uncertain etiology: a systematic review. Clin J Am Soc Nephrol. 2016;11:379–385. doi: 10.2215/CJN.07500715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Athuraliya N.T., Abeysekera T.D., Amerasinghe P.H., et al. Uncertain etiologies of proteinuric-chronic kidney disease in rural Sri Lanka. Kidney Int. 2011;80:1212–1221. doi: 10.1038/ki.2011.258. [DOI] [PubMed] [Google Scholar]
- 24.Nanayakkara S., Komiya T., Ratnatunga N., et al. Tubulointerstitial damage as the major pathological lesion in endemic chronic kidney disease among farmers in North Central Province of Sri Lanka. Environ Health Prev Med. 2012;17:213–221. doi: 10.1007/s12199-011-0243-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wijetunge S., Ratnatunga N.V., Abeysekera T.D., Wazil A.W., Selvarajah M. Endemic chronic kidney disease of unknown etiology in Sri Lanka: correlation of pathology with clinical stages. Indian J Nephrol. 2015;25:274–280. doi: 10.4103/0971-4065.145095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wijkström J., González-Quiroz M., Hernandez M., et al. Renal morphology, clinical findings, and progression rate in Mesoamerican nephropathy. Am J Kidney Dis. 2017;69:626–636. doi: 10.1053/j.ajkd.2016.10.036. [DOI] [PubMed] [Google Scholar]
- 27.Wijkström J., Jayasumana C., Dassanayake R., et al. Morphological and clinical findings in Sri Lankan patients with chronic kidney disease of unknown cause (CKDu): similarities and differences with Mesoamerican Nephropathy. PLoS One. 2018;13 doi: 10.1371/journal.pone.0193056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heled Y., Fleischmann C., Epstein Y. Cytokines and their role in hyperthermia and heat stroke. J Basic Clin Physiol Pharmacol. 2013;24:85–96. doi: 10.1515/jbcpp-2012-0040. [DOI] [PubMed] [Google Scholar]
- 29.Kulasooriya P.N., Jayasekara K.B., Nisansala T., et al. Utility of self-reported heat stress symptoms and NGAL biomarker to screen for chronic kidney disease of unknown origin (CKDu) in Sri Lanka. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph181910498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pett J., Mohamed F., Knight J., Linhart C., Osborne N.J., Taylor R. Two decades of chronic kidney disease of unknown aetiology (CKDu) research: existing evidence and persistent gaps from epidemiological studies in Sri Lanka. Nephrol (Carlton, Vic. 2022;27:238–247. doi: 10.1111/nep.13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato Y., Roncal-Jimenez C.A., Andres-Hernando A., et al. Increase of core temperature affected the progression of kidney injury by repeated heat stress exposure. Am J Physiol Ren Physiol. 2019;317:F1111–F1121. doi: 10.1152/ajprenal.00259.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kallingal G.J., Weinberg J.M., Reis I.M., Nehra A., Venkatachalam M.A., Parekh D.J. Long-term response to renal ischaemia in the human kidney after partial nephrectomy: results from a prospective clinical trial. BJU Int. 2016;117:766–774. doi: 10.1111/bju.13192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shanley P.F., Brezis M., Spokes K., Silva P., Epstein F.H., Rosen S. Hypoxic injury in the proximal tubule of the isolated perfused rat kidney. Kidney Int. 1986;29:1021–1032. doi: 10.1038/ki.1986.102. [DOI] [PubMed] [Google Scholar]
- 34.Gao Y., Zeng Z., Li T., et al. Polydatin inhibits mitochondrial dysfunction in the renal tubular epithelial cells of a rat model of sepsis-induced acute kidney injury. Anesth Analg. 2015;121:1251–1260. doi: 10.1213/ANE.0000000000000977. [DOI] [PubMed] [Google Scholar]
- 35.Liyanage D.N.D., Diyabalanage S., Dunuweera S.P., Rajapakse S., Rajapakse R.M.G., Chandrajith R. Significance of Mg-hardness and fluoride in drinking water on chronic kidney disease of unknown etiology in Monaragala, Sri Lanka. Environ Res. 2022;203 doi: 10.1016/j.envres.2021.111779. [DOI] [PubMed] [Google Scholar]
- 36.Wei M., Ye Y., Ali M.M., Chamba Y., Tang J., Shang P. Effect of fluoride on cytotoxicity involved in mitochondrial dysfunction: a review of mechanism. Front Vet Sci. 2022;9 doi: 10.3389/fvets.2022.850771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vlad M., Şerboiu C., Ispas A.T., Giuvărăşteanu I., Ungureanu E., Ionescu N. Electron microscopy of the morphological changes in rat viscera during experimental hyperthermic shock. J Med Life. 2013;6:55–60. [PMC free article] [PubMed] [Google Scholar]
- 38.Lopez-Novoa J.M., Quiros Y., Vicente L., Morales A.I., Lopez-Hernandez F.J. New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int. 2011;79:33–45. doi: 10.1038/ki.2010.337. [DOI] [PubMed] [Google Scholar]
- 39.Büttner-Herold M., Krieglstein N., Chuva T., et al. Light Chain Restriction in Proximal Tubules-implications for light chain proximal tubulopathy. Front Med (Lausanne) 2022;9 doi: 10.3389/fmed.2022.723758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.