Abstract
Biological oxidation of cyclic ketones normally results in formation of the corresponding dicarboxylic acids, which are further metabolized in the cell. Rhodococcus ruber strain SC1 was isolated from an industrial wastewater bioreactor that was able to utilize cyclododecanone as the sole carbon source. A reverse genetic approach was used to isolate a 10-kb gene cluster containing all genes required for oxidative conversion of cyclododecanone to 1,12-dodecanedioic acid (DDDA). The genes required for cyclododecanone oxidation were only marginally similar to the analogous genes for cyclohexanone oxidation. The biochemical function of the enzymes encoded on the 10-kb gene cluster, the flavin monooxygenase, the lactone hydrolase, the alcohol dehydrogenase, and the aldehyde dehydrogenase, was determined in Escherichia coli based on the ability to convert cyclododecanone. Recombinant E. coli strains grown in the presence of cyclododecanone accumulated lauryl lactone, 12-hydroxylauric acid, and/or DDDA depending on the genes cloned. The cyclododecanone monooxygenase is a type 1 Baeyer-Villiger flavin monooxygenase (FAD as cofactor) and exhibited substrate specificity towards long-chain cyclic ketones (C11 to C15), which is different from the specificity of cyclohexanone monooxygenase favoring short-chain cyclic compounds (C5 to C7).
The alicyclic hydrocarbons are a major component of petroleum, and microbial metabolism of these compounds has been reported in various microorganisms (5, 7, 9, 11, 13, 18, 26). Most of the microbes isolated to date that metabolize alicyclic hydrocarbons contain degradation pathways for small (C5, C6, and C7) cyclic compounds (7, 12, 13, 15). These microbes are phylogenetically diverse and appear to have similar biochemical pathways for degradation of the alicyclic hydrocarbons. One of the key enzymes in the biochemical pathway belongs to the class of flavoprotein monooxygenases, catalyzing the Baeyer-Villiger reaction of inserting an oxygen atom next to the alicyclic keto group (29). The Baeyer-Villiger monooxygenases have been exploited as valuable biocatalysts in chemoenzymatic synthesis and biotransformations (2, 4, 29), mainly due to their high enantioselectivity. However, the substrate specificity of these monooxygenase enzymes is generally limited to small ring sizes (5, 27) and thus cannot be used in applications where products with larger ring sizes are desired.
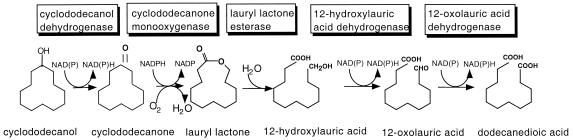
Isolation of strains able to degrade large cyclic hydrocarbons has recently been reported (24). J. D. Schumacher and R. M. Fakoussa isolated a strain, CD1-411, which used cyclododecane as the sole carbon source and typed the strain as Rhodococcus ruber. Based on metabolite analysis and enzyme inhibition studies, the proposed biochemical pathway (Fig. 1) for cyclododecane metabolism in R. ruber CD1-411 is analogous to the degradation of cyclohexane and cyclohexanol (9, 26). Cyclododecane is first hydroxylated to cyclododecanol, which is then oxidized to cyclododecanone. This alicylic ketone is then subject to a Baeyer-Villiger oxidation yielding the lactone oxacyclotridecan-2-one (lauryl lactone), which is hydrolyzed to 12-hydroxydodecanoic acid (12-hydroxylauric acid). The 12-hydroxylauric acid is further converted to 1,12-dodecanedioic acid (DDDA) by a two-step sequential oxidation via 12-oxolauric acid intermediate (23). Additionally, the cyclododecanone monooxygenase required for the oxidation of cycloketone to the corresponding lactone was purified (23). However, little is known about other enzymes or isolation of the genes that encode enzymes involved in the cyclododecane degradation pathway.
FIG. 1.
Proposed pathway of biochemical degradation of cyclododecanol or cyclododecanone by R. ruber SC1. The boxes above the reactions indicate the enzymes required for each step of the reaction.
We are interested in the comparison of the gene organization of alicyclic hydrocarbon-degradative pathways. Researchers reported earlier the characterization of genes involved in cyclohexanol degradation (7). In this paper we describe the characterization of a gene cluster involved in cyclododecanone degradation from R. ruber strain SC1. This strain was isolated from an industrial wastewater bioreactor by selecting for microbes that can utilize cyclododecanone as the sole carbon source. Characterization of a 10-kb gene cluster from R. ruber strain SC1 reveals genes for cyclododecanone monooxygenase, an esterase, an alcohol dehydrogenase, and an aldehyde dehydrogenase. These four genes were shown to be responsible for the biochemical conversions associated with cyclododecanone oxidation to DDDA. Gene function was confirmed by cloning combinations of these four genes into Escherichia coli and analyzing the accumulated intermediates in biotransformation experiments with cyclododecanone as the substrate.
MATERIALS AND METHODS
Strain isolation and 16S ribosomal DNA (rDNA) typing.
The bacterial strain used in this study that grows on cyclododecanone was isolated from an enrichment culture of activated sludge obtained from an industrial wastewater bioreactor. The enrichment culture was established by inoculating 1 ml of activated sludge into 20 ml of S12 medium (7) in a 125-ml screw-cap Erlenmeyer flask. The culture was supplemented with flakes of cyclododecanone (approximately 30 mg) added directly to the culture medium and was incubated at 35°C with reciprocal shaking. The enrichment culture was maintained by adding cyclododecanone flakes every 4 to 6 days. The culture was diluted every 5 to 11 days by replacing 18 ml of the culture with the same volume of S12 medium. After 24 days of incubation, 100 μl of the enrichment culture was spread onto S12 plates with cyclododecanone as the carbon source by sprinkling solids of the volatile compound on the interior of the petri dish lid. The petri dish was sealed with Parafilm and incubated at 37°C with the lid on the bottom. Bacteria that grew on the S12 minimal medium plates with cyclododecanone vapor were purified by streaking onto R2A agar media (Difco Laboratories, Bedford, Mass.). Single colonies were screened for the ability to grow in S12 liquid with cyclododecanone as the sole carbon and energy source. One of the isolates, SC1, was selected for further characterization.
Strain SC1 was typed using 16S rDNA analysis (16) as follows. SC1 was grown on R2A agar, and several colonies from the plate were suspended in 200 μl of Tris-EDTA plus RNase (100 μg/ml) and lysozyme (1 mg/ml) and incubated at 37°C for 4 h. To this mixture, 50 μg of proteinase K/ml and 0.5% sodium dodecyl sulfate (SDS) were added. The mixture was extracted with phenol-chloroform (25:24), and total DNA was precipitated with ethanol. The 889-bp 16S rDNA gene fragment was amplified from the total DNA by PCR using forward JCR15 primer GCCAGCAGCCGCGGTA (complementary to positions 517 to 532 on E. coli 16S rDNA) and reverse JCR14 primer ACGGGCGGTGTGTAC (complementary to positions 1406 to 1392 on E. coli 16S rDNA). PCR was performed in a Perkin-Elmer GeneAmp 9600 thermocycler (Foster City, Calif.). The samples were heated for 5 min at 94°C and then cycled 35 times at 94°C for 30 s, 55°C for 1 min, and 72°C for 1 min. The amplified 16S rRNA genes were purified using a QIAquick PCR Purification Kit (Qiagen, Valencia, Calif.) according to the manufacturer's instructions and sequenced on an automated ABI sequencer with the JCR14 and JCR15 primers. The 16S rRNA gene sequence of strain SC1 was used as the query sequence for a BLASTN search (1).
Purification of cyclododecanone monooxygenase.
Since SC1 grows slowly on cyclododecanone, cell mass was initially accumulated by growth of 1-liter batches in Luria broth (21) in a 2.8-liter Fernbach flask at 37°C with shaking for 24 h. Cells were collected by centrifugation in sterile bottles, washed twice in S12 medium, added to 1 liter of S12 and yeast extract (10 mg/liter) supplemented with about 8 to 10 mg of solid cyclododecanone, and allowed to grow at 37°C overnight. A second portion of cyclododecanone was added in the morning, and the culture was allowed to grow for an additional 6 to 8 h. Cells from the 3-liter culture were collected and suspended in 40 ml of 50 mM Na-HEPES buffer (pH 7.5). Lysozyme was added at 2 mg/ml, and the suspension was kept at 0°C for 0.5 to 2 h. Phenylmethylsulfonyl fluoride (0.1 M in ethanol; 0.2 ml) was then added, and the suspension was passed seven times through a French press at 16,000 psi. The phenylmethylsulfonyl fluoride treatment was repeated. The extract was centrifuged (9,000 × g; 20 min), and the supernatant was diluted in 250 ml of HB (50 mM Na-HEPES [pH 7.5] plus 10 mM 2-mercaptoethanol) and applied to a Q-Sepharose fast-flow column (column volume, 100 ml; Pharmacia, Piscataway, N.J.). The Q-Sepharose column was washed with 100 ml of HB and eluted with a linear NaCl gradient (0 to 0.7 M NaCl in HB, 500 ml each with a 2.4-ml/min flow rate). Active fractions (usually 40 ml) were pooled and diluted with HB to 200 ml and were applied to a Matrex red A agarose column (column volume, 5 ml; Amicon, Beverly, Mass.). The red A agarose column was washed with 15 ml of HB, and monooxygenase was eluted with 25 ml of NADPH (0.1 mM).
Activity of cyclododecanone monooxygenase in the fractions was monitored by substrate-dependent NADPH oxidation. Typically, the background rate of NADPH oxidation (340 nm; 6.22 mM) in a mixture of 0.2 ml of enzyme plus buffer (0.1 M glycine–NaOH, pH 8.8) was recorded spectrophotometrically. Cyclododecanone (10 mM in n-propanol; 1 μl) was added, and the A340 was examined for cyclododecanone-stimulated NADPH oxidation.
Cofactor of cyclododecanone monooxygenase.
Approximately 0.3 mg of Rhodococcus-derived enzyme was heated (10 min at 100°C) to precipitate the protein, which was pelleted by centrifugation. The supernatant was fractionated by thin-layer chromatography (silica; 4:3:3 n-butanol–acetic acid–water) with FAD (Rf, ∼0.4) and flavin mononucleotide (Rf, ∼0.84) as the standards. The cofactor content of the enzyme was estimated from the UV-visible spectrum of a sample of purified recombinant enzyme. A protein extinction coefficient at 278 nm of ∼110 mM−1 cm−1 was estimated from the deduced amino acid composition of the enzyme. An extinction coefficient for FAD at 278 nm of 25 mM−1 cm−1 was estimated from the absorption spectrum, as well as an extinction coefficient of 37 mM−1 cm−1 at 260 nm. Since FAD also has an extinction coefficient of 11.3 mM−1 cm−1 at 450 nm, the approximate A450/A278 ratio of a mixture of protein containing 1 FAD per mol and apoprotein would be 11.3χ/[135χ + 110(1−χ)], where χ is the mole fraction of holoenzyme.
Confirmation of cyclododecanone monooxygenase activity.
To confirm the activity of the purified cyclododecanone monooxygenase, the product of the cyclododecanone-dependent NADPH oxidation by the purified enzyme was also examined by gas chromatography/mass spectrometry (GC/MS) analysis as follows. Enzyme purified from the red agarose column was depleted of NADPH by repeated concentration and dilution cycles using HB buffer and a Centricon-30 concentrator (Amicon). Cyclododecanone (146 μg) in n-propanol was taken close to dryness in a 1-ml cuvette, diluted with 0.94 ml of HB buffer, and supplemented with NADPH (36 μl; 11 mM). The NADPH-depleted enzyme (20 μl; 5.8 mg/ml) was then added. After 20 min, the A340 had decreased to about 1/4 of its initial value. Additional NADPH was added at 20 min and at 1 h. At 90 min, the pH was adjusted to ∼3 and octadecane (146 μg) was added as an internal standard for GC/MS analysis. The sample was extracted with hexane three times (1 ml), dried with MgSO4, and analyzed by GC/MS, using a HP5890 Gas Chromatograph connected to a HP5971 mass-selective detector (Hewlett-Packard) and a DB-1 capillary column (J & W Scientific, Folsom, Calif.). The temperature program consisted of a 3-min hold at 75°C, an increase to 220°C at 10°C/min, an increase to 300°C at 20°C/min, and a 3.5-min hold at 300°C. Peaks were identified by comparison of their retention times and of their mass spectra with those of authentic standards.
Partial amino acid sequencing.
Protein purified from the red agarose column was separated on SDS-polyacrylamide gel electrophoresis (PAGE) and electroblotted to polyvinylidene difluoride membrane. The prominent 65-kDa protein band was excised from the membrane for N-terminal amino acid sequencing by Edman degradation. For internal sequencing, protein was further purified by reverse-phase high-pressure liquid chromatography using the Vydac C4 column (The Separations Group, Hesperia, Calif.). One milligram of protein recovered from the high-pressure liquid chromatography purification was dried, dissolved in 100 μl of 70% formic acid containing 3 mg of CNBr, and digested overnight. SDS gel loading buffer (50 μl) was added, plus enough 0.1 M Tris-Cl (pH 8) to bring the pH above 6. The volume was then reduced in a Centricon-10 concentrator (Amicon) for SDS-PAGE and electroblotting. One of the most prominent peptide fragments resulting from the CNBr digest was excised for amino acid sequencing.
Degenerate PCR.
Degenerate primers were designed based on the partial amino acid sequencing of the N-terminal and internal fragments of cyclododecanone monooxygenase. PCR was carried out as follows: 1 cycle of 94°C for 5 min; 35 cycles of 94°C for 1 min, 30°C for 1 min, and 72°C for 30 s; followed by 1 cycle of 72°C for 5 min. The PCR products obtained ranged from about 300 to 800 bp. The mixture of PCR products was cloned into the pCR2.1-TOPO cloning vector following the manufacturer's instructions (Invitrogen, Carlsbad, Calif.). Clones were sequenced using the standard M13 forward (−20) primer and M13 reverse (−48) primer from the vector.
Construction of cosmid library of strain SC1.
High-molecular-weight chromosomal DNA of strain SC1 was prepared as described previously (7). A cosmid library of SC1 was constructed using the SuperCos1 vector kit (Stratagene, La Jolla, Calif.). The DNA was packaged into lambda phage using Gigapack III XL packaging extract following the manufacturer's instructions (Stratagene). The packaged SC1 genomic DNA library contained a titer of 105 CFU per μg of DNA as determined by transfecting E. coli XL1-Blue MR included in the kit. Cosmid DNA isolated from 12 randomly chosen E. coli transformants was found to contain large inserts of DNA (30 to 40 kb).
Southern hybridization.
Southern hybridization was used to map the ends of the cosmids. Cosmid DNA was separately digested with restriction enzymes such as BglII, NcoI, or XhoI. The digests were separated on a 0.9% agarose gel and transferred to positively charged nylon membrane (Boehringer Mannheim, Indianapolis, Ind.) by alkaline downward capillary blotting (21). Probes for the cyclododecanone monooxygenase gene and the alcohol dehydrogenase gene were prepared with a PCR digoxigenin (DIG) labeling kit (Boehringer Mannheim) using DIG labeled deoxynucleoside triphosphate. Hybridization was carried out overnight at 37°C in Easy Hyb Solution (Boehringer Mannheim). The blot was washed at room temperature once with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) + 0.1% SDS and once with 0.1 × SSC + 0.1% SDS and developed with a DIG-luminescent detection kit (Boehringer Mannheim).
Construction of subclones of cosmids.
A small fragment of the cosmid insert was amplified and subcloned into pBluescript SK(+) vector (Stratagene). Primer KK1 (CCCCAAGCTTGAACCCAGCCCCTGCAAGAT) and primer KK2 (GGACTAGTTCAGTTCGAGCATCAGCCGCGG) were used to amplify a 4,243-bp fragment containing the cddA and cddB genes plus a 1,072-bp upstream region and a 101-bp downstream region. The PCR product was digested with HindIII and SpeI and cloned into the HindIII and SpeI sites of pBluescript SK(+) to construct pDCQ5. In pDCQ5, the cddAB genes were placed in the opposite orientation from the lacZ gene on the vector. A similar construct, pDCQ6, was made using primer KK3 (GGACTAGTGAACCCAGCCCCTGCAAGAT) and primer KK4 (CCCCAAGCTTGTAGGAGAGTGCACCCTGGA), which placed cddAB genes in the same orientation as the lacZ gene on the vector.
Subclones containing only the cddA gene were also constructed by deleting the downstream cddB gene from pDCQ5 or pDCQ6. A BglII site was located at the intergenic region between the cddA and cddB genes. The cddB gene was deleted from pDCQ5 or pDCQ6 by isolating and self-ligating the 5.9-kb filled Klenow BglII-XbaI fragment or BglII-HindIII fragment containing the vector and cddA gene. The resulting constructs, pDCQ7 and pDCQ8, were verified by restriction digestion.
Identification of diacids and other intermediates.
Recombinant E. coli XL1-Blue MR cells were grown in M9 medium (21) with 0.4% glucose as the sole carbon source. Substrates (250 mg/liter) for conversion were added at early log phase (optical density at 600 nm, 0.2), and cells were cultured at 30°C for 20 h. Cells were then frozen and thawed once and were centrifuged at 4,000 × g for 15 min. The supernatant was filtered through 0.2-μm-pore-size disc filters (Gelman Sciences, Ann Arbor, Mich.). The filtrate was first acidified to pH 2 using concentrated HCl. The acidified sample was extracted twice with methylene chloride or ethyl acetate. The extracts were combined and dried with anhydrous magnesium sulfate and were then filtered through Whatman 3MM paper and evaporated under a gentle stream of nitrogen. The dried residues were resuspended in 0.5 ml of methylene chloride as underivatized samples or were derivatized with 0.5 ml of BSTFA [bis (trimethylsilyl) trifluoroacetamide] silylation reagent (SUPELCO, Bellefonte, Pa.). The GC/MS analysis was performed using a Hewlett-Packard 5989B MS Engine GC/MS instrument. Samples were injected onto a 30-m MDN-5S capillary column (0.5-μm film thickness; SUPELCO) for separation. GC/MS analysis was conducted using electron impact ionization (70 eV). The intermediates were identified by comparison of their retention times and of their mass spectra with those of authentic standards. The standard compounds of DDDA, 12-hydroxylauric acid, and lauryl lactone were purchased from Aldrich (St. Louis, Mo.).
Nucleotide sequence accession number.
The nucleotide sequence reported in the paper has been deposited in GenBank under accession number AY052630.
RESULTS
Isolation and growth characteristics of R. ruber strain SC1.
An enrichment culture of bacteria that degrade cyclododecanone was established using sludge from an industrial aerobic wastewater bioreactor. The cyclododecanone degrader SC1 was isolated from the enrichment culture by selection on S12 medium with cyclododecanone as the sole carbon and energy source. The 16S rRNA gene sequence from strain SC1 was 99% identical to the 16S rRNA gene sequence of R. ruber. Phylogenetic analysis of the 16S rRNA gene sequences also showed that the SC1 strain was clustered together with other R. ruber strains (data not shown). Based on the 16S rRNA sequence analysis, strain SC1 was identified as R. ruber, which belongs to the GC-rich, gram-positive Actinomycetales group.
R. ruber SC1 was tested for growth on related C12 cyclic compounds. SC1 was also able to grow on cyclododecanol (C12 cyclic alcohol). However, it was unable to grow on cyclododecane (C12 cyclic alkane) used as the sole carbon and energy source.
R. ruber SC1 was also tested for growth on cyclic ketones of different chain lengths as the sole carbon and energy source. It grew well on cyclopentadecanone (C15 cyclic ketone), cyclotridecanone (C13 cyclic ketone), cyclododecanone (C12 cyclic ketone), and cycloundecanone (C11 cyclic ketone). It grew moderately well on cyclodecanone (C10 cyclic ketone). It did not grow on cyclooctanone (C8 cyclic ketone), cycloheptanone (C7 cyclic ketone), or cyclohexanone (C6 cyclic ketone).
Purification and characterization of cyclododecanone monooxygenase from R. ruber SC1.
After efforts to amplify the cyclododecanone monooxygenase gene with PCR primers based on other cycloketone monooxygenase gene sequences failed, purification of enzyme from strain SC1 was undertaken to enable cloning of the gene by a reverse genetic approach. The cyclododecanone monooxygenase was purified from R. ruber SC1 in two chromatographic steps. An oxygen uptake assay (5, 28) using cells grown with and without cyclododecanone indicated that cyclododecanone oxidation in SC1 is inducible (data not shown). For purification, the SC1 cells were grown on Luria broth and transferred to minimal medium, and expression of the cyclododecanone monooxygenase was induced by addition of cyclododecanone. The cells were broken by a French press and fractionated by anion exchange and a red A agarose affinity column. Similar NADPH elution of this resin was reported for purification of the Acinetobacter cyclohexanone monooxygenase (4). At this stage, the cyclododecanone monooxygenase was ∼80 to 90% pure, and the molecular mass was estimated to be 65 to 70 kDa, as judged by SDS gel electrophoresis (not shown). The reasons for the meager yield of about 20 μg/g of cell paste were not investigated; they may include suboptimal induction of the enzyme during the second growth period or inefficient breakage of Rhodococcus cells, in addition to losses during the purification. Large amounts of protein at higher purity can be obtained from the E. coli overexpression clone (TOP10/pDCQ9; Q. Cheng et al., unpublished results) by the two-column procedure reported above, which will greatly facilitate future biochemical studies.
The cyclododecanone monooxygenase enzyme was assayed by cyclododecanone-dependent NADPH oxidation as described in Materials and Methods. GC/MS analysis confirmed that lauryl lactone was the product of this reaction. The reaction mixture contained lauryl lactone, cyclododecanone, and the internal standard octadecane in an ∼1:0.08:1 area ratio. A parallel sample without enzyme contained only cyclododecanone and octadecane in an 0.75:1 area ratio. These data suggested that most of the cyclododecanone was converted to lauryl lactone in the presence of the purified cyclododecanone monooxygenase.
Cofactor of cyclododecanone monooxygenase.
The enzyme purified from Rhodococcus was yellow. Thin-layer chromatography of the supernatant from boiled enzyme showed that FAD, but not flavin mononucleotide, was present. Two separate dilutions of recombinant enzyme gave A450/A278 ratios of 0.081 and 0.090, close to the estimate of 0.084 for enzyme containing 1 mol of FAD/mol of enzyme.
Cloning of cyclododecanone monooxygenase gene from R. ruber SC1.
A reverse genetic approach was employed to clone the cyclododecanone monooxygenase gene from R. ruber SC1. Partial amino acid sequencing of the purified enzyme indicated the N-terminal sequence to be TTSIDREALRRKYAEERDKR and an internal amino acid sequence to be ERIRARVDEIG. These sequences were used to design degenerate primers for PCR amplification of a portion of the cyclododecanone monooxygenase gene. Primer CDDK4 (AARTAYGCNGARGARCGNGAYAA, where R = A or G, Y = C or T, and N = A or C or G or T) was derived from the N-terminal sequence, and primer CDDK10 (CCDATYTCRRCNACNCKNGC, where D = A or G or T, Y = C or T, R = A or G and N = A or C or G or T) was derived from the internal sequence. The PCR products obtained ranged from about 300 to 800 bp. One of the clones containing an insert of 556 bp had significant similarity to cyclohexanone monooxygenase from Acinetobacter sp. NCIB 9871 (6) and steroid monooxygenase from Rhodococcus rhodochrous accession no. BAA24454. The deduced amino acid sequence of both ends of the insert DNA matched well with the N-terminal and the internal amino acid sequences determined from sequencing of the purified protein. The amino-terminal methionine residue is apparently cleaved and thus not found in the mature protein.
Identification and characterization of gene cluster for cyclododecanone oxidation.
Specific primers of the cyclododecanone monooxygenase gene were designed based on the sequence obtained from the degenerate PCR product as described previously. Two primers, C12 MO TOP (ATGCAGAGGAGCGGGACAAG) and C12 MO BOTTOM (ACTTCGGTGTGGAACAGCGC), amplified the 430 bp of the N-terminal fragment of the cyclododecanone monooxygenase gene. These two primers were used in a PCR to screen the SC1 cosmid library for clones containing the cyclododecanone monooxygenase gene. Six positive clones (D12D, E9F, O8E, O11G, S8C, and T3C) were identified from two independent screens of a total of 2,000 clones. All six clones contain inserts of 35 to 40 kb spanning the cyclododecanone monooxygenase gene. Cosmids E9F and D12D were sequenced by a shotgun approach as described previously (7). Sequences of 400 clones were assembled, and a contig of 10,480 bp containing the cyclododecanone monooxygenase gene was formed.
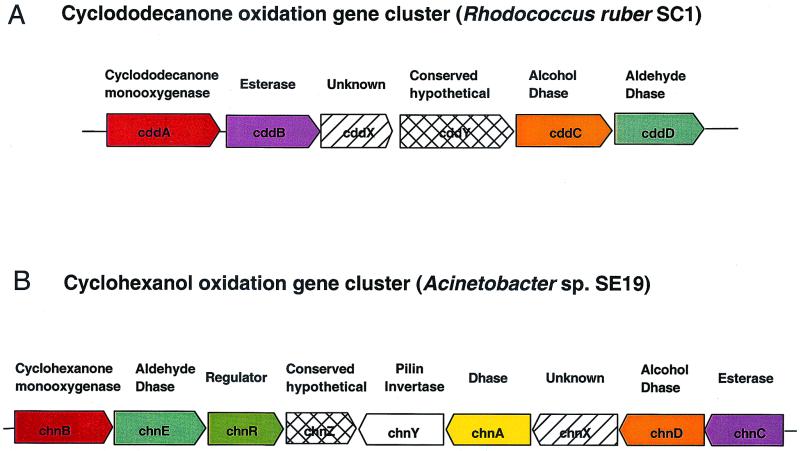
Six open reading frames (ORFs), designated cddA, cddB, cddX, cddY, cddC, and cddD (Table 1) for cyclododecanone degradation, were identified (10) from the 10,480-bp contig (Fig. 2A). BLAST analysis (1) indicated that cddA had the greatest similarity to a flavin monooxygenase gene from Acinetobacter sp. (6). The cyclohexanone monooxygenase from Acinetobacter catalyzes the Baeyer-Villiger oxidation of the C6 cyclic ketone to epsilon-caprolactone. The cddA gene of R. ruber SC1 encodes a cyclododecanone monooxygenase and was demonstrated to catalyze a similar reaction with the C12 cyclic ketone to form lauryl lactone. The deduced molecular mass of CddA is 67,494 Da, which agrees well with what we estimated from the SDS-PAGE (65 to 70 kDa). The cddB gene had the greatest similarity to the esterase A gene from Streptomyces chrysomallus (3). The cell-bound esterase A from Streptomyces specifically hydrolyzes short-chain p-nitrophenyl esters. The cddB gene presumably encodes an esterase that catalyzes the hydrolysis of lauryl lactone to 12-hydroxylauric acid. The cddB gene was only 83 bp downstream of cddA in R. ruber SC1. The greatest similarity to cddC was found in an alcohol dehydrogenase gene (adhB) from Mycobacterium tuberculosis (8). CddC belongs to a family of group I zinc-dependent long-chain alcohol dehydrogenases (20) and likely catalyzes the conversion of 12-hydroxylauric acid to 12-oxolauric acid. A putative aldehyde dehydrogenase gene from Streptomyces coelicolor (19) had the greatest similarity to cddD. The 23 strictly conserved residues identified in the multiple-sequence alignment of 16 aldehyde dehydrogenases (14) were also conserved in CddD. It is likely that cddD encodes an aldehyde dehydrogenase which catalyzes the conversion of 12-oxolauric acid to DDDA.
TABLE 1.
Homology of the ORFs with proteins in the nonredundant protein databases
| Gene name | Locationd (bp) | Homologous protein (source species) | % Identitya | % Similarityb | E valuec |
|---|---|---|---|---|---|
| cddA | 605–2416 | Cyclohexanone monooxygenase (Acinetobacter sp.) | 30 | 46 | 3 × 10−58 |
| cddB | 2499–3659 | Esterase A (S. chrysomallus) | 41 | 54 | 4 × 10−78 |
| cddX | 4346–4840 | No hits found | N/A | N/A | N/A |
| cddY | 5341–7236 | Hypothetical protein Rv3762c (M. tuberculosis) | 49 | 61 | 10−169 |
| cddC | 7295–8419 | Alcohol dehydrogenase adhB (M. tuberculosis) | 49 | 64 | 10−105 |
| cddD | 8459–9883 | Putative aldehyde dehydrogenase (S. coelicolor) | 50 | 63 | 10−127 |
Percent identity is defined as percentage of amino acids that are identical between the two proteins. N/A, not available.
Percent similarity is defined as percentage of amino acids that are identical or conserved between the two proteins.
Expect value. The Expect value estimates the statistical significance of the match, specifying the number of matches with a given score that is expected in a search of a database of this size absolutely by chance.
Location in reference to the 10,480-bp contig. The first ATG was chosen as the putative start codon of the proteins.
FIG. 2.
Comparison of the gene clusters for cyclododecanone oxidation in R. ruber SC1 and cyclohexanol oxidation in Acinetobacter sp. strain SE19. The blocks represent the genes, with the arrows indicating the direction of transcription. Genes encoding enzymes with similar functions in the two clusters were represented by the same color. The conserved hypothetical proteins and the unknown proteins in the two clusters are not related to each other.
Mapping the ends of the cosmids.
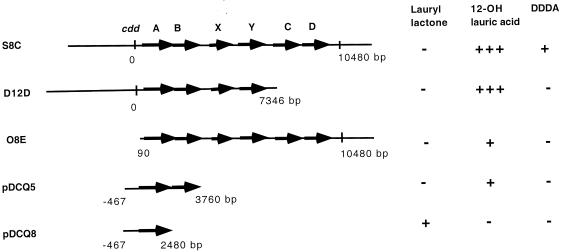
Southern hybridization and PCR analysis suggested that most cosmids such as S8C contain the full 10-kb gene cluster and substantial upstream and downstream regions. However, some cosmids contain only part of the 10-kb gene cluster (Fig. 3). Cosmid D12D contains approximately 30 kb of the upstream region and only a portion of the cdd gene cluster. The 3′ end of the insert was mapped to be within the cddC gene. Sequencing of the junction of cosmid D12D DNA using standard T7 primer on the cosmid vector showed that the 3′ end of the insert on D12D is at bp 7346 and contains only 51 bp of the N-terminal region of the cddC gene. Cosmid D12D thus contains only intact cddA, cddB, cddX, and cddY genes and does not contain cddC and cddD genes that encode the two dehydrogenases. On the other hand, cosmid O8E contains the six intact cdd genes and an approximately 25-kb-long downstream sequence but contains only a 515-bp-long upstream sequence of cddA as shown by sequencing.
FIG. 3.
Conversion of cyclododecanone by E. coli cosmid clones and subclones containing the indicated gene fragments. Region with arrows, the cdd genes identified on the 10,480-bp cluster. Solid horizontal line, flanking sequences of the cdd gene cluster represented on the cosmids or subclones. Vertical line, the start (0) or the end (bp 10480) of the cluster. Other numbers indicate the ends of the fragments relative to the start of the cluster on the cosmids or subclones (positive numbers for downstream, negative numbers for upstream). For product detection, −, absence of metabolite; +, presence of metabolite; +++, 6- to 10-fold more metabolite.
Assay of cyclododecanone oxidation by E. coli cosmid clones.
The six E. coli cosmid clones containing the cyclododecanone monooxygenase gene cluster were assayed for cyclododecanone oxidation by GC/MS as described in Materials and Methods. All six cosmid clones produced a major peak with a retention time around 24.8 min. MS analysis showed that the molecular mass of the compound in the peak was 360 Da, corresponding to trimethylsilylated 12-hydroxylauric acid. The vector control sample did not have a peak corresponding to this product. Furthermore, the cyclododecanone substrate peak with a retention time around 19.7 min (molecular mass of 182 Da) was detected in the vector control sample but was absent in the cosmid samples. Therefore, cyclododecanone was converted by the cosmid clones to 12-hydroxylauric acid. In addition, a small peak with a retention time around 25.6 min was also detected in some of the cosmid samples. MS analysis revealed the molecular mass of the peak compound to be 374 Da, corresponding to the trimethylsilylated DDDA.
A comparison of the biochemical activities of three cosmid clones was carried out by GC analysis of the culture supernatants (Table 2). The retention times of the peaks were compared to those of the authentic standards for 12-hydroxylauric acid or DDDA. The selected cosmids were S8C with an intact cdd gene cluster and significant upstream and downstream regions, D12D with a truncated cdd gene cluster and no downstream region, and O8E with an intact cdd gene cluster but a limited upstream region. The percent conversion of cyclododecanone to 12-hydroxylauric acid was as high as 56% with cosmid clone S8C, which suggested that the cyclododecanone monooxygenase encoded by cddA and the lauryl lactone hydrolase encoded by cddB were actively expressed in E. coli. A lower level of 12-hydroxylauric acid was detected in clones containing cosmid O8E (Table 2), suggesting that the limited upstream region of cddAB on O8E might have affected the expression of cddAB. The percent conversion for cyclododecanone to DDDA was 1.7% with cosmid clone S8C, which was 40-fold higher than that for either D12D or O8E. The inefficient metabolism of cyclododecanone to DDDA was likely due to poor expression of the two dehydrogenases CddC and CddD in E. coli. When cyclododecanol was used as the substrate, the conversion from cyclododecanol to 12-hydroxylauric acid was less than 1% with all cosmid clones, and no DDDA was detected in any of the clones (Table 2).
TABLE 2.
Production of 12-hydroxylauric acid and DDDA by E. coli cosmid clonesa
| Cosmid clone | % Conversionb
of:
|
|||
|---|---|---|---|---|
| 12-hydroxylauric acid
from:
|
DDDA from:
|
|||
| CDDAa | CDDK | CDDA | CDDK | |
| Vector control | 0 | 0 | 0 | 0 |
| S8C | 0.27 | 56.1 | 0 | 1.7 |
| D12D | 0.62 | 24.1 | 0 | 0.04 |
| O8E | 0.20 | 4.1 | 0 | 0.021 |
Assay was performed in M9 plus glucose medium. Cyclododecanol (CDDA) or cyclododecanone (CDDK) (7.5 mg of either) was added as the substrate for biotransformation.
Products were quantitated by GC analysis. Percent conversion was calculated as the percentage of 12-hydroxylauric acid or DDDA produced from either the CDDA or CDDK substrate.
Assay of cyclododecanone oxidation by E. coli subclones.
Since the six cosmids contain DNA inserts of 35 kb, it was of interest to know which of the genes on the cosmids are essential for conversion of cyclododecanone to 12-hydroxylauric acid and DDDA. Both cosmids D12D and O8E produced 12-hydroxylauric acid from cyclododecanone. The common regions of these cosmids contain the cddABXY genes (Fig. 3). To confirm if cddA and cddB were required for the conversion of cyclododecanone to 12-hydroxylauric acid, two sets of subclones were constructed. Subclones pDCQ5 and pDCQ6 contained the same insert, with the cddA and cddB genes and an approximately 1-kb-long upstream sequence, in opposite orientations. Subclones pDCQ7 and pDCQ8 contained the same insert with only the cddA gene and the 1-kb-long upstream sequence in opposite orientations. All four subclones were transformed into E. coli XL1-Blue MR cells, and two transformants of each construct were assayed for cyclododecanone oxidation by GC/MS. 12-Hydroxylauric acid was detected in both clones with pDCQ5 but not in any of the two clones with pDCQ6. Lauryl lactone was detected in the clones with pDCQ8 but not in the clones with pDCQ7. DDDA was not detected in any of the subclones. Therefore, introducing only cddAB genes on pDCQ5 conferred on E. coli the ability to convert cyclododecanone to 12-hydroxylauric acid. Conversion of cyclododecanone to lauryl lactone was achieved by the cyclododecanone monooxygenase encoded by cddA; subsequent hydrolysis of lauryl lactone to 12-hydroxylauric acid was achieved by the lauryl lactone esterase encoded by cddB.
Substrate specificity of CddAB.
We demonstrated that the E. coli cosmid strain containing S8C was able to efficiently convert cyclododecanone to 12-hydroxylauric acid. We explored whether the substrates could be expanded to cyclic ketones of different chain lengths and whether the corresponding hydroxy acids were produced. The S8C cosmid strain was grown as described above with different cyclic ketones. Cyclohexanone (C6), cycloheptanone (C7), cyclooctanone (C8), cyclodecanone (C10), cycloundecanone (C11), cyclododecanone (C12), cyclotridecanone (C13), and cyclopentadecanone (C15) at 250 mg/liter were tested as substrates for biotransformation. Identification of the corresponding hydroxy acids produced was performed by GC/MS analysis as described above. Significant levels of the corresponding hydroxy acids were detected from the S8C cosmid strain grown in the presence of C11, C12, C13 and C15 cyclic ketones. A small amount of hydroxy acids was detected from the strain grown in the presence of C6 and C10 cyclic ketones. Hydroxy acid was not detected when the strain was grown in the presence of C7 or C8 cyclic ketone. The substrate range of the monooxygenase and esterase on the S8C cosmid favors long-chain cyclic ketones (>C10). The substrate range of the bioconversions correlated well with the substrate utilization range (C10 to C15) of R. ruber SC1.
DISCUSSION
In this paper, we report the isolation of a gene cluster encoding all the enzymes required for the oxidative conversion of cyclododecanone to DDDA. This is the first report on molecular characterization of biological oxidation of large cyclic compounds. Six genes were present on a 10-kb cluster transcribed in the same direction. The first two genes, cddA and cddB, encode the enzymes catalyzing the first two steps of the cyclododecanone oxidation. The last two genes, cddC and cddD, encode the two dehydrogenases for oxidation of 12-hydroxy acid to generate DDDA. The cddAB and cddCD genes were separated by two open reading frames (ORFS) cddX and cddY, of unknown function. The organization of the cyclododecanone oxidation gene cluster (cdd cluster) from R. ruber SC1 (Fig. 2A) is very different from the organization of the previously characterized cyclohexanol oxidation cluster (chn cluster) from Acinetobacter sp. strain SE19 (Fig. 2B) (7). The order of the cdd genes matched well with the sequence of the biochemical reactions for cyclododecanone oxidation, whereas the chn genes appear to be in random order relative to the cyclohexanol oxidation pathway. The enzymes in the cyclododecanone oxidation pathway (cddA to-D) shared only moderate similarity (about 30% amino acid identity, 50 to 60% amino acid similarity) with the analogous proteins in the cyclohexanol oxidation pathway (chnB to E), explaining why initial attempts to clone cddA based on its similarity to the chnB gene failed. The cdd gene cluster also comprises one ORF (cddX) with no BLAST homologues in the database and one ORF (cddY) with similarity to a conserved hypothetical protein (8) (Fig. 2). However, these two ORFs were not homologous to the unknown protein (chnX) and the conserved hypothetical protein (chnZ) encoded by the chn gene cluster (7).
The homologues of two chn genes (chnA and chnR) essential for cyclohexanol oxidation were not present in the cdd gene cluster. The chnA homologue would encode a short-chain alcohol dehydrogenase (17) responsible for conversion of cyclododecanol to cyclododecanone. Although it was originally isolated from a cyclododecanone enrichment, R. ruber SC1 was also able to grow on the cyclic alcohol, cyclododecanol, as the sole carbon source. The cyclododecanol dehydrogenase should be encoded elsewhere in the chromosome of R. ruber SC1. Another gene that we did not identify on the cdd gene cluster was the positive transcriptional regulator. Although a small region of the ORF upstream of the cddA gene had weak similarity to a eukaryotic transcriptional regulator MLL protein (22), it did not appear to be required for expression of cdd genes in E. coli. It is not known whether the gene upstream of cddA functions as a transcriptional regulator in R. ruber SC1.
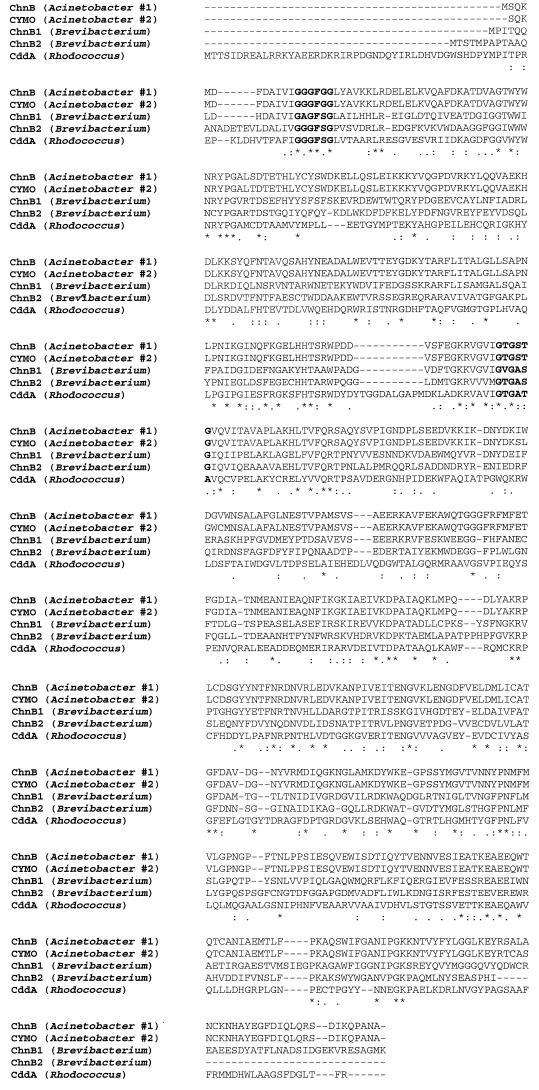
Cyclododecanone monooxygenase is a member of the type 1 Baeyer-Villiger monooxygenases (29). The type 1 Baeyer-Villiger monooxygenases are FAD-binding and NADPH-dependent enzymes. Figure 4 shows the multiple-sequence alignment of the cyclododecanone monooxygenase with several cyclohexanone monooxygenases. The N-terminal FAD-binding motif (GXGXXG) and the centered NADP-binding motif [GXGXX(G/A)] are identified in the cyclododecanone monooxygenase (25). Phylogenetic analysis indicated that the cyclododecanone monooxygenase was in a branch distant from other cyclohexanone monooxygenases. The substrate range of the cyclododecanone monooxygenase was examined by coupling the monooxygenase with the esterase. Both enzymes exhibited broad substrate specificity towards long-chain cyclic ketones (>C10). This is consistent with the in vitro substrate specificity data for the cyclododecanone monooxygenase from R. ruber CD4 (23). Cyclododecanone monooxygenase from strain CD4 had a very low level of activity towards short-chain cyclic ketones (C6 to C10). It is likely that the similar substrate specificity of cyclododecanone monooxygenase in SC1 contributed to the lack or low level of short-chain hydroxy acid production by the S8C cosmid strain. The specificity of the cyclododecanone monooxygenase was in contrast to that of monooxygenases isolated from strains able to grow on small cyclic ketones, which are active towards C4 to C8 ketones but not towards large rings (>C8) (5, 27). The cyclododecanone monooxygenase is approximately 10% larger than the cyclohexanone monooxygenases. Most of the extra amino acids in cyclododecanone monooxygenase appear to be located at the N-terminal portion (Fig. 4). It is conceivable that the extra amino acids might contribute to the larger pocket of the active site to accommodate larger substrates of the enzyme. Several models were proposed to study the active sites of the cyclohexanone monooxygenase (29). Crystal structures of the Baeyer-Villiger monooxygenases are also in progress and will shed more light on specificity as well as stereoselectivity of this class of enzymes.
FIG. 4.
Sequence alignment of the cyclododecanone monooxygenase (CddA) from R. ruber SC1 with the cyclohexanone monooxygenases in GenBank from Acinetobacter sp. strain SE19 (accession no. AF282240), Acinetobacter sp. strain NCIB 9871 (accession no. P12015), and Brevibacterium sp. strain HCU (accession no. AF257214 and AF257215). Alignment was performed with the ClustalW program. The N-terminal FAD-binding motif and the centered NADP-binding motif are highlighted in boldface.
The substrate specificity of other enzymes in the cyclic ketone metabolism, such as the alcohol dehydrogenase and the aldehyde dehydrogenase, has not been investigated. Since R. ruber SC1 could utilize large cyclic ketones, it is likely that the two dehydrogenases are also active on long-chain acids derived from the large cyclic ketones. A process could be designed to use a single, pure, cyclic ketone or a mixture of the large cyclic ketones as substrates in biocatalysis to produce one or a mixture of lactones, long-chain hydroxy acids, or diacids for industrial applications.
In conclusion, we have elucidated the gene function associated with the cyclododecanone oxidation pathway from R. ruber SC1. This cluster is likely to be responsible for oxidation of several large cyclic ketones (C11 to C15) that can be utilized by R. ruber SC1 for growth.
ACKNOWLEDGMENTS
We are grateful to Ray Jackson for DNA sequencing and Mario Chen for sequence assembly. We thank Ivan Turner, Sr. and Tom Miller for assistance with protein purification and peptide sequencing. We also thank James Valentine and Edward Davis for excellent analytical work. We thank Steve Picataggio and Steve Fahnestock for critical readings of the manuscript and Ethel Jackson for discussions and support of the project.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerjee A. Stereoselective microbial Baeyer-Villiger oxidations. In: Patel R N, editor. Stereoselective biocatalysis. New York, N.Y: Marcel Dekker, Inc; 2000. p. 867. [Google Scholar]
- 3.Berger R, Hoffmann M, Keller U. Molecular analysis of a gene encoding a cell-bound esterase from Streptomyces chrysomallus. J Bacteriol. 1998;180:6396–6399. doi: 10.1128/jb.180.23.6396-6399.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Branchaud B P, Walsh C T. Functional group diversity in enzymatic oxygenation reaction catalyzed by bacterial flavin-containing cyclohexanone oxygenase. J Am Chem Soc. 1985;107:2153–2161. [Google Scholar]
- 5.Brzostowicz P C, Gibson K L, Thomas S M, Blasko M S, Rouvière P E. Simultaneous identification of two cyclohexanone oxidation genes from an environmental Brevibacteriumisolate using mRNA differential display. J Bacteriol. 2000;182:4241–4248. doi: 10.1128/jb.182.15.4241-4248.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y-C J, Peoples O P, Walsh C T. Acinetobactercyclohexanone monooxygenase: gene cloning and sequence determination. J Bacteriol. 1988;170:781–789. doi: 10.1128/jb.170.2.781-789.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng Q, Thomas S M, Kostichka K, Valentine J, Nagarajan V. Genetic analysis of a gene cluster for cyclohexanol oxidation in Acinetobactersp. strain SE19 by in vitro transposition. J Bacteriol. 2000;182:4744–4751. doi: 10.1128/jb.182.17.4744-4751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole S T, et al. Deciphering the biology of Mycobacterium tuberculosisfrom the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 9.Donoghue N A, Trudgill P W. The metabolism of cyclohexanol by AcinetobacterNCIB 9871. Eur J Biochem. 1975;60:1–7. doi: 10.1111/j.1432-1033.1975.tb20968.x. [DOI] [PubMed] [Google Scholar]
- 10.Gish W, States D J. Identification of protein coding regions by database similarity search. Nat Genet. 1993;3:266–272. doi: 10.1038/ng0393-266. [DOI] [PubMed] [Google Scholar]
- 11.Griffin M, Trudgill P W. The metabolism of cyclopentanol by PseudomonasNCIB 9872. Eur J Biochem. 1972;129:595–603. doi: 10.1042/bj1290595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffin M, Trudgill P W. Purification and properties of cyclopentanone oxygenase of PseudomonasNCIB 9872. Eur J Biochem. 1976;63:199–209. doi: 10.1111/j.1432-1033.1976.tb10222.x. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa Y, Segawa T, Obata H, Tokuyama T. Metabolism of cycloheptanone by Nocardiasp. Nippon Nogeikagaku Kaishi. 1983;57:129–134. [Google Scholar]
- 14.Hempel J, Nicholas H, Lindahl R. Aldehyde dehydrogenases: widespread structural and functional diversity within a shared framework. Protein Sci. 1993;2:1890–1900. doi: 10.1002/pro.5560021111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwaki H, Hasegawa Y, Teraoka M, Tokuyama T, Bergeron H, Lau P C K. Identification of a transcriptional activator (ChnR) and a 6-oxohexanoate dehydrogenase (ChnE) in the cyclohexanol catabolic pathway in Acinetobactersp. strain NCIMB 9871 and localization of the genes that encode them. Appl Environ Microbiol. 1999;65:5158–5162. doi: 10.1128/aem.65.11.5158-5162.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kane M D, Poulsen L K, Stahl D A. Monitoring the enrichment and isolation of sulfate-reducing bacteria by using oligonucleotide hybridization probes designed from environmentally derived 16S rRNA sequences. Appl Environ Microbiol. 1993;59:682–686. doi: 10.1128/aem.59.3.682-686.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krozowski Z. The short-chain alcohol dehydrogenase superfamily: variations on a common theme. J Steroid Biochem Mol Biol. 1994;51:125–130. doi: 10.1016/0960-0760(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 18.Perry J J. Microbial metabolism of cyclic alkanes. In: Atlas R M, editor. Petroleum microbiology. New York, N.Y: Macmillan Publishing Co.; 1984. p. 63. [Google Scholar]
- 19.Redenbach M, Kieser H M, Denapaite D, Eichner A, Cullum J, Kinashi H, Hopwood D A. A set of ordered cosmids and a detailed genetic and physical map for the 8 Mb Streptomyces coelicolorA3(2) chromosome. Mol Microbiol. 1996;21:77–96. doi: 10.1046/j.1365-2958.1996.6191336.x. [DOI] [PubMed] [Google Scholar]
- 20.Reid M F, Fewson C A. Molecular characterization of microbial alcohol dehydrogenases. Crit Rev Microbiol. 1994;20:13–56. doi: 10.3109/10408419409113545. [DOI] [PubMed] [Google Scholar]
- 21.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 22.Schofield J, Isaac A, Golovleva I, Crawley A, Goodwin G, Tickle C, Brickell P. Expression of Drosophila trithorax-group homologues in chick embryos. Mech Dev. 1999;80:115–118. doi: 10.1016/s0925-4773(98)00207-x. [DOI] [PubMed] [Google Scholar]
- 23.Schumacher J D, Fakoussa R M. Degradation of alicyclic molecules by Rhodococcus ruberCD4. Appl Microbiol Biotechnol. 1999;52:85–90. doi: 10.1007/s002530051491. [DOI] [PubMed] [Google Scholar]
- 24.Schumacher J D, Fakoussa R M. Proceedings of the 9th International Conference on Coal Science. III. Hamburg, Germany: DGMK; 1997. Oxidation and cleavage of alicyclic structures using bacterial biocatalysts; pp. 1583–1586. [Google Scholar]
- 25.Stehr M, Diekmann H, Smau L, Seth O, Ghisla S, Singh M, Macheroux P. A hydrophobic sequence motif common to N-hydroxylating enzymes. Trends Biochem Sci. 1998;23:56–57. doi: 10.1016/s0968-0004(97)01166-3. [DOI] [PubMed] [Google Scholar]
- 26.Trower M K, Buckland M, Higgins R, Griffin M. Isolation and characterization of cyclohexane-metabolizing Xanthobactersp. Appl Environ Microbiol. 1985;49:1282–1289. doi: 10.1128/aem.49.5.1282-1289.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trower M K, Buckland R M, Griffin M. Characterization of an FMN-containing cyclohexanone monooxygenase from a cyclohexane-grown Xanthobactersp. Eur J Biochem. 1989;181:199–206. doi: 10.1111/j.1432-1033.1989.tb14711.x. [DOI] [PubMed] [Google Scholar]
- 28.Whittaker J W, Orville A M, Lipscomb J D. Protocatechuate 3,4-dioxygenase from Brevibacterium fuscum. Methods Enzymol. 1990;188:82–88. doi: 10.1016/0076-6879(90)88016-4. [DOI] [PubMed] [Google Scholar]
- 29.Willetts A. Structural studies and synthetic applications of Baeyer-Villiger monooxygenases. Trends Biotechnol. 1997;15:55–62. doi: 10.1016/S0167-7799(97)84204-7. [DOI] [PubMed] [Google Scholar]