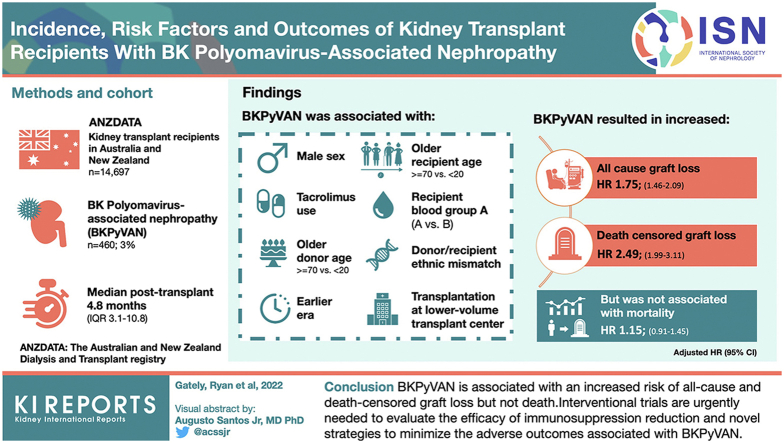
Abstract
Introduction
BK polyomavirus-associated nephropathy (BKPyVAN) is associated with graft dysfunction and loss; however, knowledge of immunosuppression reduction strategies and long-term graft, and patient outcomes across the disease spectrum is lacking.
Methods
This cohort study included 14,697 kidney transplant recipients in Australia and New Zealand (2005−2019), followed for 91,306 person years.
Results
BKPyVAN occurred in 460 recipients (3%) at a median posttransplant time of 4.8 months (interquartile range, 3.1−10.8). Graft loss (35% vs. 21%, P < 0.001), rejection (42% vs. 25%, P < 0.001), and death (18% vs. 13%, P = 0.002) were more common in the BKPyVAN group. The most frequent changes in immunosuppression after BKPyVAN were reduction (≤50%) in tacrolimus (172, 51%) and mycophenolate doses (134, 40%), followed by the conversion of mycophenolate to leflunomide (62, 19%) and tacrolimus to ciclosporin (20, 6%). Factors associated with the development of BKPyVAN included (adjusted hazard ratio [HR]; 95% confidence interval) male sex (1.66; 1.34−2.05), recipient age (≥70 vs. <20 [2.46; 1.30−4.65]), recipient blood group (A vs. B [2.00; 1.19−3.34]), donor age (≥70 vs. <20 [2.99; 1.71−5.22]), earlier era (1.74; 1.35−2.25), donor/recipient ethnic mismatch (1.52; 1.23−1.87), tacrolimus use (1.46; 1.11−1.91), and transplantation at a lower-volume transplant center (1.61; 1.24−2.09). The development of BKPyVAN was associated with an increased risk of all-cause (1.75; 1.46−2.09) and death-censored graft loss (2.49; 1.99−3.11), but not mortality (1.15; 0.91−1.45).
Conclusions
BKPyVAN is associated with an increased risk of all-cause and death-censored graft loss, but not death. Interventional trials are urgently needed to evaluate the efficacy of immunosuppression reduction and novel strategies to minimize the adverse outcomes associated with BKPyVAN.
Keywords: BKPyVAN, BKPyV, graft loss, kidney transplant, polyomavirus, registry
Graphical abstract
See Commentary on Page 401
BK polyomavirus (BKPyV) is a DNA virus that most frequently causes allograft dysfunction in kidney transplant recipients. Most of the population has had asymptomatic exposure to the virus during childhood, and adult seroprevalence is thought to approach 90%.1,2 The virus remains quiescent except when antiviral immunity is reduced because of chronic exposure to immunosuppression, wherein viral reactivation and clinical manifestations can occur. With the advent of potent immunosuppressive regimens, BKPyVAN has become a major cause of graft dysfunction and loss in kidney transplant recipients, affecting 1% to 10% of recipients.3, 4, 5 Early reports of allograft survival following BKPyVAN were dismal with up to 50% of patients losing their graft within 5 years of diagnosis,6 however, survival rates seem to have improved with the widespread adoption of BKPyV screening during the early posttransplant period.7
There are currently no pharmacologic therapies available that have proven efficacy in the treatment of BKPyV infection. Therefore, the mainstay of treatment involves a reduction in the intensity of immunosuppression to allow for the restoration of antiviral immunity to inhibit viral replication, however, such manipulations can trigger allograft rejection. Clinical practice guidelines recommend a stepwise approach to treatment as follows: an initial reduction in the dose of antimetabolite followed by a reduction in the dose of the calcineurin inhibitor with subsequent cessation of one of these agents should viral load not fall; however, the certainty of evidence supporting these recommendations is low.8,9 Although some studies have assessed longer-term graft and patient outcomes of patients with BKPyV infection,4,10,11 few have examined the epidemiology of BKPyV across the entire disease trajectory.
The aims of this study were to determine the incidence and risk factors of BKPyVAN, define the immunosuppression changes in response to BKPyVAN, and assess the longer-term overall graft and patient survival in those who developed BKPyVAN.
Methods
Study Design
This cohort study used data from The Australia and New Zealand Dialysis and Transplant (ANZDATA) registry. ANZDATA is a binational registry that collects annual contemporaneous data on patients with kidney failure throughout Australia and New Zealand.12 Ethical approval was attained from Metro South Human Research Ethics Committee (LNR/2020/QMS/67754). This study was designed and analyzed in accordance with the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines for cohort studies.13
Study Population
All patients who received a kidney transplant (or multiorgan including kidney) in Australia and New Zealand between January 2005 and December 2019 were included in this analysis. When patients had multiple transplants during the study period, each episode of transplantation was included and counted as a discrete event. Patients were censored at the end of the study period (December 2019), or if lost to follow-up, if no outcome of interest had occurred. Patients who died were considered to have had an outcome of interest in the all-cause graft loss analysis or were censored at the time of death in the death-censored graft loss analysis. Allograft loss referred exclusively to kidney allograft loss because data regarding nonkidney allograft loss were unavailable.
Data Collection
Data extracted from the ANZDATA registry included donor and recipient demographic characteristics at time of transplant (age, sex, ethnicity, blood-group, and body mass index), medical history (cause of kidney failure, number of prior kidney allografts, diabetes, coronary artery disease, lung disease, peripheral vascular disease, cerebrovascular disease, and smoking status), and transplant characteristics (transplant center volume [mean number of incident transplants per year categorized into tertiles], donor source, single or multiorgan transplant, human leucocyte antigen mismatches, induction [none, interleukin-2 receptor antibody, or T-cell depleting antibody] and maintenance immunosuppression [at months 0, 3, 12, 24, 36, 60, 84, and 120]), ischemic time, and delayed graft function). ANZDATA records rejection episodes and other biopsy-proven diseases (BKPyVAN, primary disease recurrence, or de novo glomerulonephritis) at months 3, 12, 24, 36, 60, 84, and 120.
Clinical Outcomes
The primary outcome was all-cause graft loss (defined as permanent return to dialysis, retransplantation, or death with a functioning graft); death-censored graft loss and patient death were secondary outcomes. The primary exposure of interest was BKPyVAN. BKPyVAN was diagnosed by each center’s pathologist in accordance with the Banff Polyomavirus Nephropathy classification system based on the presence and severity of characteristic histologic changes, including viral inclusions, interstitial infiltrates, tubular injury, and tubulitis.14 The indication for each biopsy was not recorded and the accuracy of histologic reporting by each center is not verified independently by the ANZDATA registry.
Statistical Analyses
Descriptive statistics were presented for the baseline parameters for patients and their transplants. Continuous variables were described by mean and standard deviation and count variables as a number and relative percentage of the total. Differences between groups were tested using the χ2 test, Wilcoxon rank sum test, or Fisher exact test, where appropriate.
When variables had less than 15% missing data, imputation was performed using the MissForest algorithm, a random forest imputation method optimized for mixed data types without constraint on the parametric nature of the variables it is imputing.15
Therapeutic changes in immunosuppression were determined by comparing doses of each immunosuppressive agent recorded before and after the diagnosis of BKPyVAN. Calcineurin inhibitor and antimetabolite dose reductions were categorized into 3 groups according to the degree of reduction as follows: less than or equal to 50% reduction, greater than 50% reduction, or elimination of the agent. Prednisolone dose reductions were not analyzed because most patients routinely underwent significant reductions in dosing within the first months of the posttransplant period. The substitution of therapies with ciclosporin, mechanistic target of rapamycin inhibitors, azathioprine, and leflunomide in response to BKPyVAN was also determined.
The cumulative incidence of BKPyVAN was calculated using the Kaplan-Meier (K-M) method and differences between groups were calculated using the log-rank test. Follow-up time was defined as the total time between transplant and outcome of interest. To assess the change in incidence of BKPyVAN over time, the study period was divided into three 5-year periods (2005−2009, 2010−2014, and 2015−2019) and the K-M cumulative incidence of BKPyVAN was compared between eras. Multivariable Cox-proportional hazard modeling was used to determine risk factors for the development of BKPyVAN. Stepwise regression was then used to determine the optimal set of variables to include in the final model based on the lowest Akaike’s Information Criterion value.
Three models were created to assess the association between BKPyVAN and patient and graft survival. These models included all-cause graft loss (Model 1), death-censored graft loss (Model 2) and patient survival (Model 3). BKPyVAN was considered a time-varying exposure to minimize the impact of immortal time bias. HRs were calculated for unadjusted models (BKPyVAN as the only independent variable) and full models that used all available independent variables. Stepwise regression was used to determine the optimal set of independent variables to include in the final model.
Sensitivity Analyses
Analyses in which death was treated as a competing risk for the development of BKPyVAN were performed using the Fine-Gray subdistribution hazards model.16 A shared frailty model was also performed to account for clustering in patients with repeated transplants during the study period.
A P-value of less than 0.05 was considered significant and all reported P-values were 2-tailed. Statistical analyses were carried out using R version 4.0.4.
Results
Baseline Characteristics of the Cohort
Demographic and transplant details are presented in Table 1. Overall, 15,176 kidney transplants (10,248 deceased and 4928 living donor; 14,455 kidney alone and 721 multiorgan) were performed in 14,697 patients between 2005 and 2019 at 33 different centers throughout Australia and New Zealand. These patients were followed up for a total of 91,306 patient-years during which time 460 patients (3%) developed BKPyVAN. The mean age of recipients was higher in the BKPyVAN group (50.1 years [±15.0] vs. 47.5 years [±15.3], P < 0.001) and a higher proportion of this group were males (73.9% vs. 62.6%, P < 0.001).
Table 1.
Baseline characteristics of the cohort (N = 15,176)
| Characteristic | BK negative (n = 14,716)a | BK positive (n = 460)a | P-value b |
|---|---|---|---|
| Male | 9217 (62.6%) | 340 (73.9%) | <0.001 |
| Age at transplant, yr | 47.5 ±15.3 | 50.1 ±15.0 | <0.001 |
| Age category | <0.001 | ||
| <20 | 787 (5.3%) | 17 (3.7%) | |
| 20–29 | 1170 (8.0%) | 34 (7.4%) | |
| 30–39 | 2148 (14.6%) | 58 (12.6%) | |
| 40–49 | 3174 (21.6%) | 75 (16.3%) | |
| 50–59 | 3883 (26.4%) | 131 (28.5%) | |
| 60–69 | 3058 (20.8%) | 119 (25.9%) | |
| ≥70 | 496 (3.4%) | 26 (5.7%) | |
| BMI category | 0.6 | ||
| Underweight | 1022 (7.2%) | 28 (6.2%) | |
| Healthy | 5295 (37.3%) | 181 (40.2%) | |
| Overweight | 4576 (32.3%) | 137 (30.4%) | |
| Obese | 3290 (23.2%) | 104 (23.1%) | |
| Ethnicity | 0.088 | ||
| Caucasian | 10,359 (75.3%) | 317 (72.0%) | |
| Asian | 1708 (12.4%) | 69 (15.7%) | |
| Pacific Islander | 479 (3.5%) | 18 (4.1%) | |
| Aboriginal or Torres Strait Islander | 465 (3.4%) | 12 (2.7%) | |
| Māori | 380 (2.8%) | 7 (1.6%) | |
| Other | 368 (2.7%) | 17 (3.9%) | |
| Primary disease | 0.5 | ||
| Glomerulonephritis | 5812 (39.5%) | 195 (42.4%) | |
| Diabetes | 2247 (15.3%) | 74 (16.1%) | |
| Cystic | 2103 (14.3%) | 66 (14.3%) | |
| Hypertension or vascular disease | 955 (6.5%) | 26 (5.7%) | |
| Other | 3599 (24.5%) | 99 (21.5%) | |
| Graft number | 0.5 | ||
| 1 | 12,978 (88.2%) | 414 (90.0%) | |
| 2 | 1481 (10.1%) | 37 (8.0%) | |
| 3 | 220 (1.5%) | 8 (1.7%) | |
| 4 | 33 (0.2%) | 1 (0.2%) | |
| 5 | 4 (0.0%) | 0 (0.0%) | |
| Multiorgan transplantation | 706 (4.8%) | 15 (3.3%) | 0.13 |
| Diabetic | 0.094 | ||
| No | 11,701 (79.5%) | 366 (79.6%) | |
| Type 1 | 890 (6.0%) | 18 (3.9%) | |
| Type 2 | 2125 (14.4%) | 76 (16.5%) | |
| Coronary artery disease | 0.026 | ||
| No | 13,130 (89.6%) | 397 (86.3%) | |
| Suspected | 294 (2.0%) | 8 (1.7%) | |
| Yes | 1233 (8.4%) | 55 (12.0%) | |
| Chronic lung disease | 0.4 | ||
| No | 13,878 (94.7%) | 441 (95.9%) | |
| Suspected | 204 (1.4%) | 3 (0.7%) | |
| Yes | 578 (3.9%) | 16 (3.5%) | |
| Peripheral vascular disease | 0.079 | ||
| No | 13,784 (94.0%) | 421 (91.5%) | |
| Suspected | 290 (2.0%) | 14 (3.0%) | |
| Yes | 587 (4.0%) | 25 (5.4%) | |
| Cerebrovascular disease | 0.074 | ||
| No | 14,170 (96.6%) | 436 (94.8%) | |
| Suspected | 82 (0.6%) | 4 (0.9%) | |
| Yes | 411 (2.8%) | 20 (4.3%) | |
| Male donor | 7,582 (51.5%) | 229 (49.8%) | 0.5 |
| Donor age, y | 46.3 ±15.6 | 49.8 ±15.5 | <0.001 |
| Donor age category | <0.001 | ||
| <20 | 965 (6.6%) | 20 (4.3%) | |
| 20–29 | 1502 (10.2%) | 42 (9.1%) | |
| 30–39 | 2008 (13.6%) | 41 (8.9%) | |
| 40–49 | 3291 (22.4%) | 92 (20.0%) | |
| 50–59 | 3711 (25.2%) | 131 (28.5%) | |
| 60–69 | 2682 (18.2%) | 94 (20.4%) | |
| ≥70 | 557 (3.8%) | 40 (8.7%) | |
| Donor Ethnicity | 0.3 | ||
| Caucasian | 12,867 (88.6%) | 395 (86.2%) | |
| Asian | 798 (5.5%) | 29 (6.3%) | |
| Pacific Islander | 135 (0.9%) | 4 (0.9%) | |
| Aboriginal or Torres Strait Islander | 254 (1.7%) | 10 (2.2%) | |
| Māori | 249 (1.7%) | 7 (1.5%) | |
| Other | 225 (1.5%) | 13 (2.8%) | |
| Donor source | 0.3 | ||
| Deceased | 9838 (66.9%) | 315 (68.5%) | |
| Living | 4788 (32.5%) | 140 (30.4%) | |
| Pathologic | 90 (0.6%) | 5 (1.1%) | |
| HLA mismatches | 0.039 | ||
| 0–2 | 4697 (32.4%) | 121 (26.9%) | |
| 3–4 | 5008 (34.6%) | 163 (36.2%) | |
| 5–6 | 4778 (33.0%) | 166 (36.9%) | |
| T-cell depletion at induction | 755 (5.1%) | 28 (6.1%) | 0.4 |
| mTOR Use Initially | 192 (1.3%) | 6 (1.3%) | >0.9 |
| Initial calcineurin inhibitor | 0.4 | ||
| Tacrolimus | 11,728 (80.3%) | 376 (81.7%) | |
| Ciclosporin | 2578 (17.7%) | 72 (15.7%) | |
| Neither | 292 (2.0%) | 12 (2.6%) | |
| Initial antimetabolite | 0.8 | ||
| Mycophenolate | 14,268 (97.7%) | 450 (97.8%) | |
| Azathioprine | 96 (0.7%) | 2 (0.4%) | |
| Neither | 234 (1.6%) | 8 (1.7%) | |
| Transplant center volume (terciles) | 0.003 | ||
| Low | 5131 (34.9%) | 184 (40.0%) | |
| Medium | 5727 (38.9%) | 187 (40.7%) | |
| High | 3858 (26.2%) | 89 (19.3%) |
n (%); Mean ±SD.
Chi-square test; Wilcoxon rank sum test; Fisher exact test.
Compared with those who never developed BKPyVAN, recipients who developed BKPyVAN were more likely to be of Asian ethnicity (15% vs. 11.6%, P = 0.039), have coronary artery disease (12% vs. 8.4%, P = 0.04), receive kidneys from older donors (P < 0.001), and be transplanted at a low volume transplant center (P = 0.003). Other characteristics were similar between groups.
Incidence of BKPyVAN in Transplant Recipients
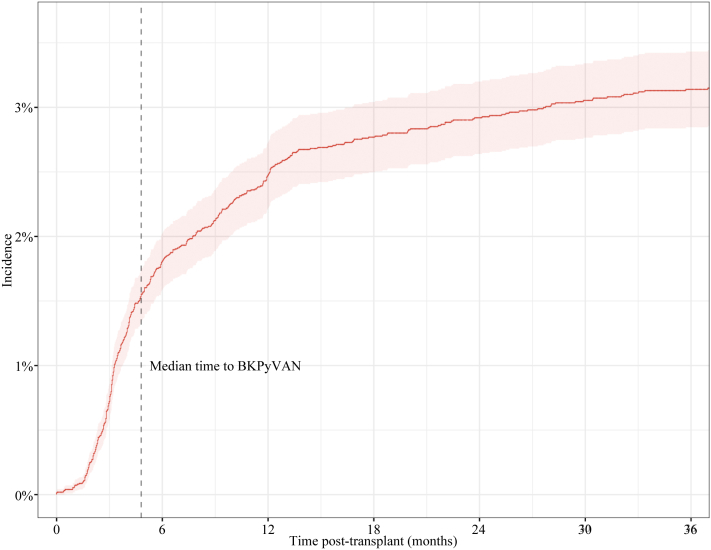
Patients had a median follow-up time of 64.8 months (±49.2 months). The K-M cumulative incidences of BKPyVAN at 1, 2, and 5 years were 2.47%, 2.92%, and 3.28%, respectively (Figure 1). The median time to BKPyVAN after transplant was 4.8 months (interquartile range, 3.1–10.8 months). The majority of BKPyVAN (350, 76%) occurred within the first 12 months of transplant, 91.5% of cases occurred within 24 months. Cumulative incidence varied by era (Supplementary Figure S1). The K-M cumulative incidences at 5 years for those transplanted in 2005 to 2009, 2010 to 2014, and 2015 to 2019 were 3.52%, 3.77%, and 2.53%, respectively (log-rank P = 0.004).
Figure 1.
Cumulative incidence of BKPyVAN. BKPyVAN, BK polyomavirus-associated nephropathy.
Incidence of Overall Allograft Loss and Death in Transplant Recipients With and Without BKPyVAN
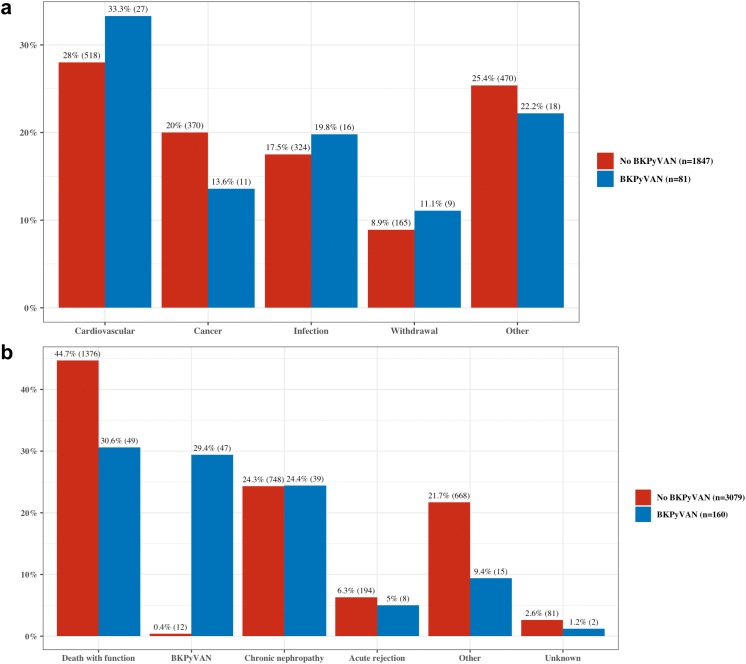
The rates of allograft loss, death, and rejection are presented in Table 2. A higher rate of graft loss was observed in the BKPyVAN group (160/460 [35%]) compared to those in the non-BKPyVAN group (3079/14,716 [21%]) (P < 0.001). There were also higher rates of death in the BKPyVAN group (81/460 [18%]) when compared to those who did not develop BKPyVAN (1866/14,716 [13%]) (P = 0.002) but no significant differences between groups were observed in the recorded causes of death (P = 0.6) (Figure 2a). Approximately 29% of graft loss in patients with BKPyVAN was attributed to BKPyVAN (Figure 2b). Death with a functioning graft was the most common cause of graft loss in this group (31%), followed by chronic allograft nephropathy (24%) and acute rejection (5%). In the non-BKPyVAN group, graft loss was predominantly because of death with a functioning graft (45%) and chronic allograft nephropathy (24%). Acute rejection was more common in the BKPyVAN group (P < 0.001) with 195 patients (42%) experiencing at least 1 episode of acute rejection posttransplant compared to 3717 patients (25%) in those without BKPyVAN. Of all patients, 179 had data available regarding the timing of their rejection and BKPyVAN episodes. In this group, acute rejection occurred before BKPyVAN in 78 patients (43.6%), after BKPyVAN in 73 patients (40.8%), and 28 patients (15.6%) had rejection both before and after their diagnosis of BKPyVAN.
Table 2.
Clinical outcomes (N = 15,176)
| Characteristic | BK Negative (n = 14,716)a | BK Positive (n = 460)a | P-valueb |
|---|---|---|---|
| Duration of follow-up | 1966 ±1510 | 2386 ±1,400 | <0.001 |
| Allograft loss | 3079 (21%) | 160 (35%) | <0.001 |
| Cause of allograft loss | <0.001 | ||
| Chronic allograft nephropathy | 748 (46%) | 39 (36%) | |
| Acute rejection | 194 (12%) | 8 (7.3%) | |
| BKPyVAN | 12 (0.7%) | 47 (43%) | |
| Other | 668 (41%) | 15 (14%) | |
| Death | 1866 (13%) | 81 (18%) | 0.002 |
| Cause of death | 0.5 | ||
| Cardiovascular | 518 (28%) | 27 (33%) | |
| Cancer | 370 (20%) | 11 (14%) | |
| Infection | 324 (18%) | 16 (20%) | |
| Withdrawal | 165 (8.9%) | 9 (11%) | |
| Other | 470 (25%) | 18 (22%) | |
| Acute rejection | 3717 (25%) | 195 (42%) | <0.001 |
Median ±SD; n (%).
Wilcoxon rank sum test; χ2 test; Fisher exact test
Figure 2.
(a) Cause of death. (b) Cause of graft loss. BKPyVAN, BK polyomavirus-associated nephropathy.
Risk Factors for Developing BKPyVAN
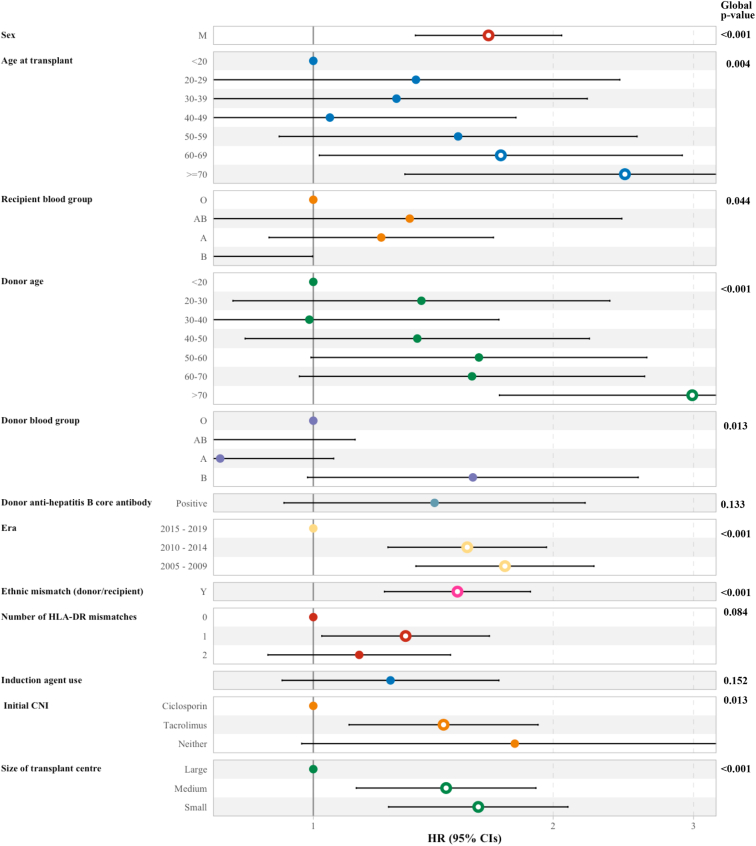
The risk factors for BKPyVAN are shown in Figure 3. These included (adjusted hazard ratio; 95% confidence interval) male sex (1.66; 1.34−2.05), older recipient age (categorized in decades, reference level patients <20; ≥70 [2.46; 1.30−4.65]; 60−69 [1.72; 1.02−2.90]), recipient blood group (reference level B; O [1.64; 1.00−2.68]; A [2.00; 1.19−3.34]; AB [2.17−4.21]), increasing donor age (categorized in decades, reference level patients <20; ≥70 [2.99; 1.71−5.22]), donor blood group (reference level AB; B [3.41; 1.37−8.48], era (reference level 2015−2019; 2005−2009 [1.74; 1.35−2.25]; 2010−2014 [1.56; 1.24−1.96]), ethnic mismatch between donor and recipient (1.52; 1.23−1.87), initial tacrolimus use (compared to ciclosporin use; 1.46; 1.11−1.91), and transplant center volume (compared to high volume; small [1.61; 1.24−2.09]). Neither T-cell induction therapy nor multiorgan transplant status added sufficient information to be included in the final model.
Figure 3.
Risk factors for the development of BKPyVAN. BKPyVAN, BK polyomavirus-associated nephropathy; CNI, x; HLA-DR, human leucocyte antigen.
Immunosuppression Changes After BKPyVAN
Out of the 460 patients that developed BKPyVAN, 444 had data regarding immunosuppression available (Supplementary Table S1). Of these, 334 recipients (75%) received prednisolone, tacrolimus, and mycophenolate before BKPyVAN. The changes made to immunosuppression in response to BKPyVAN in this group are shown in Table 3. The most frequent intervention was a reduction in tacrolimus dosing by ≤50% occurring in 172 patients (51%). Tacrolimus was reduced by >50% in 134 patients (40%) and eliminated in 28 patients (8.4%). Mycophenolate was reduced by ≤50%, reduced by >50%, or eliminated in 134 (40%), 94 (28%), and 106 (32%) patients, respectively. Other interventions included the initiation of leflunomide (62 patients, 19%), the initiation of ciclosporin (21, 6.3%), a conversion of tacrolimus to ciclosporin (20 patients, 6%), the initiation of an mechanistic target of rapamycin inhibitors (23 patients, 3.6%), and the initiation of azathioprine (10 patients, 3%). Between-group comparisons using χ2 test showed improved graft survival in those for whom mycophenolate dosing was reduced by >50% (P-value = 0.012) and worse graft survival in patients with a mycophenolate dose reduction of ≤50% (P-value = 0.014).
Table 3.
Immunosuppression changes after BKPyVAN (in patients on prednisolone, tacrolimus, mycophenolate)
| Characteristic | Overall, N = 334a,c | Graft survival, n = 225a,c | Graft loss, n = 109a,c | P-valueb |
|---|---|---|---|---|
| Tacrolimus reduced ≤50% | 172 (51%) | 113 (50%) | 59 (54%) | 0.5 |
| Tacrolimus reduced >50% | 134 (40%) | 94 (42%) | 40 (37%) | 0.4 |
| Tacrolimus stopped | 28 (8.4%) | 18 (8.0%) | 10 (9.2%) | 0.7 |
| Mycophenolate reduced ≤50% | 134 (40%) | 80 (36%) | 54 (50%) | 0.014 |
| Mycophenolate reduced >50% | 94 (28%) | 73 (32%) | 21 (19%) | 0.012 |
| Mycophenolate stopped | 106 (32%) | 72 (32%) | 34 (31%) | 0.9 |
| Ciclosporin started | 21 (6.3%) | 14 (6.2%) | 7 (6.4%) | >0.9 |
| mTOR started | 12 (3.6%) | 9 (4.0%) | 3 (2.8%) | 0.8 |
| Azathioprine started | 10 (3.0%) | 8 (3.6%) | 2 (1.8%) | 0.5 |
| Leflunomide started | 62 (19%) | 39 (17%) | 23 (21%) | 0.4 |
n (%).
Chi-square test; Fisher exact test.
Totals do not sum to 100% as multiple interventions are possible in each patient.
K-M Estimates of All-Cause, Death-Censored Graft and Patient Survival
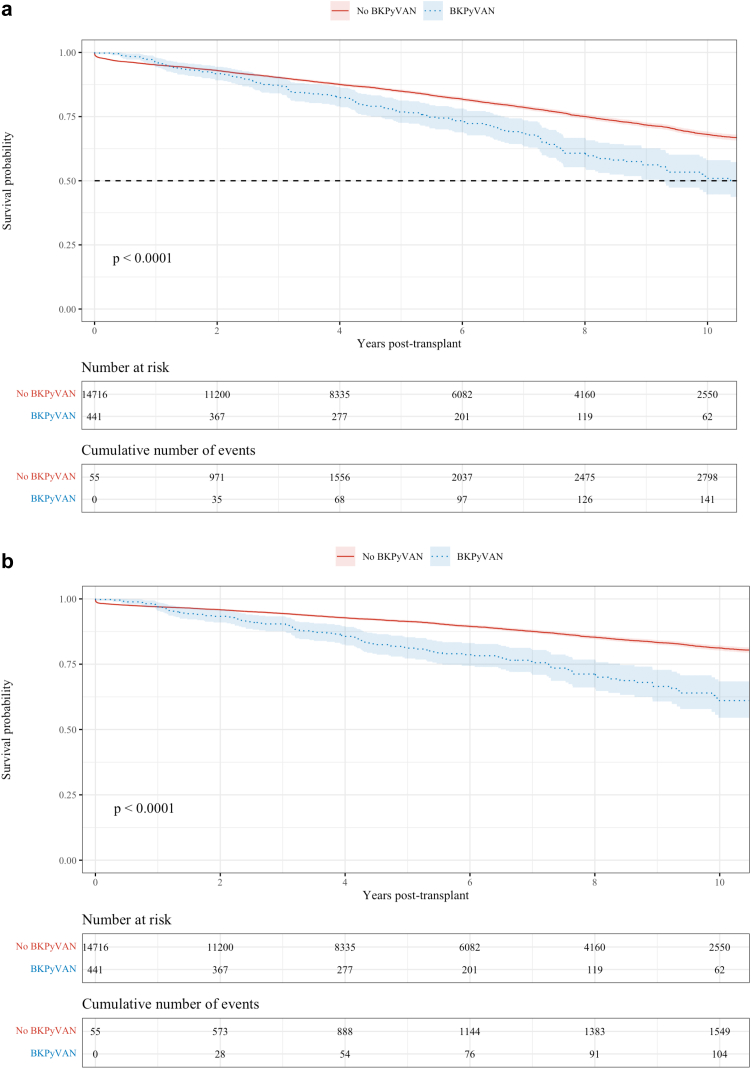
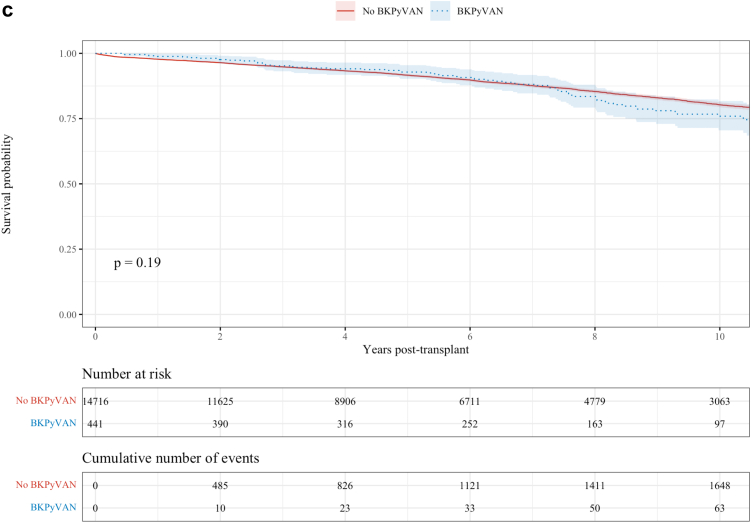
The K-M estimates of all-cause graft survival, death-censored graft survival and death are shown in Figure 4a–c.
Figure 4.
(a) All cause graft survival. (b) Death-censored graft survival. (Continued)
The all-cause graft survival rates at 1, 5, and 10 years for those with and without BKPyVAN were 95.8% versus 95.1%, 76.2% versus 84.8%, and 50.5% versus 68.0%, respectively (P < 0.0001). The death-censored graft survival rates in those with and without BKPyVAN at 1, 5, and 10 years were 96.6% versus 96.9%, 81.2% versus 91.3%, and 61.3% versus 81.2%, respectively (P < 0.0001). There was no difference in the observed overall patient survival between those with BKPyVAN and those without (P = 0.19). Survival rates at 1, 5, and 10 years in each group were 98.9 % versus 97.7%, 91.9% versus 91.5%, and 75.0% versus 80.3%, respectively. The all-cause graft survival after a diagnosis of BKPyVAN with the median survival time being 10.1 years is shown in Supplementary Figure S2.
Association Between BKPyVAN, Overall and Death-Censored Graft Loss and Death
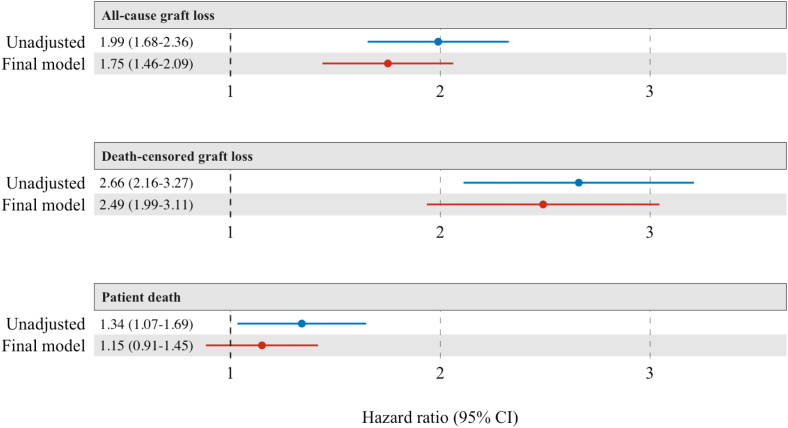
Compared to recipients without BKPyVAN, the adjusted HR (95% confidence interval) for all-cause graft loss, death-censored graft loss, and death in patients with BKPyVAN were 1.75 (1.46−2.09), 2.49 (1.99−3.11) and 1.15 (0.91−1.45), respectively (Figure 5). Adjusted survival curves for all-cause graft loss, death-censored graft loss, and patient survival are shown in Supplementary Figure S3.
Figure 5.
BKPyVAN hazard ratios in each model. BKPyVAN, BK polyomavirus-associated nephropathy.
Sensitivity Analyses
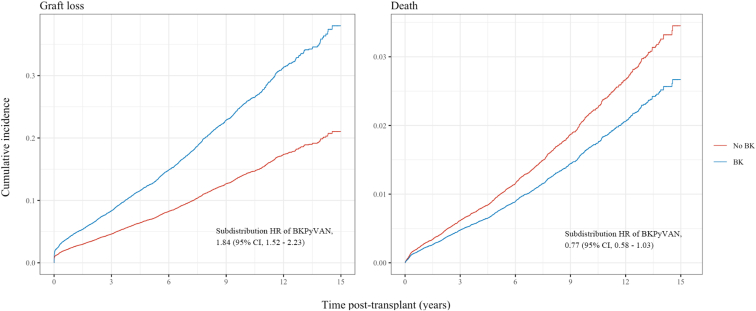
Fine-Gray hazard models were developed to assess the impact of BKPyVAN with death and graft loss treated as competing events. Compared to those without BKPyVAN, the subdistribution HR (95% confidence interval) for graft loss in patients with BKPyVAN was 1.84 (1.52−2.23), indicating an 84% increase in the relative incidence of graft loss in those who developed BKPyVAN. The subdistribution HR for death in those with BKPyVAN was 0.77 (0.58−1.03), indicating a nonsignificant reduction in the relative incidence of death compared to those without BKPyVAN. The Fine-Gray cumulative incidence functions for graft loss and death are shown in Figure 6.
Figure 6.
Cumulative incidence functions using Fine-Gray competing risk analysis comparing those with and without BKPyVAN. Graft loss and death are treated as competing events. BKPyVAN, BK polyomavirus-associated nephropathy; CI, confidence interval; HR, hazard ratio.
To account for the potential clustering effect of repeated transplant episodes within individual patients, a frailty model that included a unique patient identifier as a random effect was performed. This model did not converge when BKPyVAN was treated as a time-varying variable.
Discussion
Using contemporaneous data from a binational transplant registry, we found that the total posttransplant cumulative incidence of BKPyVAN was approximately 3.3%. The incidence of biopsy-proven disease appeared to decline over time and the lowest 5-year cumulative incidence (2.53%) was seen in patients transplanted between 2015 and 2019. The majority (406/445, 91.2%) of cases occurred within 2 years of transplantation. Compared to transplant recipients without BKPyVAN, those who developed the infection experienced a 1.75-fold increase in the risk of all-cause graft loss and a 2.5-fold increase in the risk of death-censored graft loss. Factors associated with the development of BKPyVAN included male sex, increasing donor and recipient age, donor and recipient blood group, ethnic mismatch between donor and recipient, tacrolimus use, earlier era, and undergoing transplant at a low volume center.
Although detectable BKPyV viruria and viremia are relatively common postkidney transplant (10%−35%7,17, 18, 19), the development of BKPyVAN remains rare. We observed an incidence of 3.3%, similar to a recent registry analysis from Europe (4.5%)20 and a 2006−2008 analysis from the United States (6.6%)4 with variations in the definition of BKPyV cases likely accounting for some of the variability. Despite its infrequent occurrence, the influence of BKPyVAN on patient relevant outcomes, such as graft loss and rejection,21 is substantial with the risk of all-cause graft loss and death-censored graft loss being 1.75 and 2.5 times higher, respectively, in those who develop BKPyVAN. The risk of acute rejection is also markedly higher, emphasizing the complex interplay between BKPyV and allograft rejection, which can be viewed as both a risk factor, and a complication of BKPyVAN. Indeed, 23.8% of patients with BKPyVAN experienced acute rejection after diagnosis. This compares to post-BKPyVAN rejection rates of 10% to 25% reported by other studies with the lowest rates seen in studies that included milder cases of BKPyV infection.10,22, 23, 24 Median graft survival rates of over 10 years in those with BKPyVAN compare favorably to historical cohorts25, 26, 27 in which graft loss was seen in up to two-thirds of patients within 2 years of diagnosis.
The key challenge for transplant clinicians in managing BKPyVAN is determining the optimal degree of immunosuppression reduction that prevents viral replication while balancing the risk of inducing allograft rejection. Guidelines offer broad recommendations regarding immunosuppression reduction,9 but substantial variation in clinical practice still exists.28 Treatment decisions are highly complex and need to be personalized on the basis of the severity of infection coupled with the patient’s immunologic risk for rejection. In this study, we compared immunosuppressive medications and doses before and after the diagnosis of BKPyVAN to determine the immunosuppressive changes that occurred in each patient. Before diagnosis, most patients (334, 75%) were taking standard, triple immunosuppression (prednisolone, tacrolimus, and mycophenolate). We found that reducing tacrolimus dosing by ≤50% was the most common intervention (172 patients, 51%) followed by tacrolimus reductions of >50% (134, 40%) and mycophenolate reductions of ≤50% (134, 40%). Leflunomide was the most frequently initiated therapy (62 patients, 19%). Few studies have retrospectively recorded the therapeutic interventions that occur in response to BKPyV infection. Comparison is made more difficult by the stepwise and sequential nature of the immunosuppression changes in each patient. Similar treatment strategies were seen in a European pediatric registry study,20 with tacrolimus reductions (70%) and mycophenolate reductions (36%) comprising the most common interventions. Leflunomide therapy was much less common, only occurring in 1 patient compared to almost 20% in our cohort. Other therapeutic agents, such as cidofovir, intravenous immunoglobulin, and fluroquinolones, are not recorded by ANZDATA.
Given the paucity of therapeutic options with BKPyV infection, risk factor avoidance assumes greater importance. We identified donor-recipient ethnic mismatches as well as donor and recipient blood group as novel factors associated with the development of BKPyVAN. We also demonstrated that increasing donor and recipient age, male sex, tacrolimus use, earlier era, and being transplanted at a smaller volume transplant center were risk factors for developing BKPyVAN. Where a difference existed between the ethnicities of the donor and recipient, the recipient had a 52% increased risk of developing BKPyVAN. After accounting for ethnic mismatch in the multivariable model, neither donor nor recipient ethnicity alone were shown to be risk factors for BKPyVAN. Previous studies have shown that African American29 and Asian30 populations were at an increased risk of BKPyVAN but without specifying the relationship between donor and recipient ethnicity. Both studies were undertaken in the United States where deceased donations predominantly occur from a White population31 in keeping with the overall ethnic mix of the country. A possible explanation for this relates to the different BKPyV subtypes that occur with varying frequency according to geographic region.32 Immunity to one subtype does not appear to offer similar protection across all subtypes and mismatches between donor and recipient neutralizing antibodies to specific BKPyV subtypes are known to be associated with BKPyVAN.33
Recipient blood group B was associated with a lower risk of BKPyVAN, whereas donor blood group B was associated with an increased risk. Multiple infections are known to occur with differing frequencies and varying virulence in people with different blood groups.34 Although specific human leucocyte antigen data were not available in the present study, it is likely that the different frequencies with which human leucocyte antigens were found in different blood groups likely contributed to this association. Various human leucocyte antigens have previously been shown to be associated with increased35 or decreased36 risk of BKPyV infection.
A nonlinear relationship between risk of BKPyV and age has been suggested, with higher incidence seen at the extremes of age.4 Although we did observe a markedly increased incidence in recipients aged over 70 years, there was no increase in recipients under 20 years, perhaps reflecting the lower incidence of biopsies undertaken in the pediatric population.37 Studies that have shown higher incidence of BKPyV in younger patients have included nonbiopsy-proven cases.4,20 The increased rate of BKPyVAN in the elderly is likely because of the reduction in antibody formation to BKPyV seen with increasing age. Low or absent antibody levels, which correlate with the BKPyV-specific cellular response, are significantly more common in those more than 50 years of age.38, 39, 40 Older patients who receive kidneys from older donors should be considered to be at very high risk for the development of BKPyV infection with consideration given to tailoring immunosuppression regimens accordingly.
Contrary to previous studies,4 we observed a falling incidence of BKPyVAN over time with those transplanted between 2015 and 2019 having a 43% lower risk of developing BKPyVAN compared to those transplanted between 2005 and 2009. However, the lower rates of BKPyVAN seen recently likely represent the increasing proportion of BKPyV infection being diagnosed and treated based solely on the detection of viremia instead of a true reduction in the burden of disease attributable to BKPyV.
Strengths of this study included the large, binational cohort of patients whose contemporaneous data were collected over a 15-year period allowing prolonged follow-up and detailed analysis of patient and graft survival and immunosuppression changes. Treating BKPyVAN as a time-varying exposure minimizes the risk of immortal time bias, and multiple imputation using random forest method optimized the available data. Limitations included those attributable to all registry studies such as its observational nature, the lack of an independent audit process to validate data accuracy and large amounts of missing data for some variables. Because of the similar histologic appearances of BKPyVAN and allograft rejection it is possible that some incidences of BKPyVAN or rejection have been misclassified. Between center differences in biopsy and screening practices would have influenced BKPyVAN detection rates. Despite the specification of BKPyVAN as a time-varying exposure, we cannot not account for the potential deleterious effect of BKPyV on the allograft before biopsy-proven disease. In addition, we have likely underestimated the burden of disease attributable to BKPyV infection because high viral loads of BKPyV, even without biopsy-proven disease, will prompt a reduction in immunosuppression and increase the likelihood of rejection and graft loss. Because immunosuppression was only recorded at prespecified times, there is the potential for multiple immunosuppressive changes between time points for reasons other than BKPyVAN, for example, medication intolerance or rejection.
In conclusion, BKPyVAN is associated with increased all-cause and death-censored graft loss but not patient survival. BKPyVAN affects approximately 3% of transplant recipients, although the incidence of biopsy-proven disease appears to be decreasing over time. Prospective, randomized trials are urgently required to assess the relative efficacies of immunosuppression reduction strategies and novel immune-based therapies.
Disclosure
All the authors declared no competing interests.
Footnotes
Figure S1. Cumulative incidence of BKPyVAN by era.
Figure S2. All cause graft survival after BKPyVAN.
Figure S3. Adjusted survival curves.
Table S1. Immunosuppressive regimen before BKPyVAN.
Supplementary Material
Figure S1. Cumulative incidence of BKPyVAN by era.
Figure S2. All cause graft survival after BKPyVAN.
Figure S3. Adjusted survival curves.
Table S1. Immunosuppressive regimen before BKPyVAN.
References
- 1.Knowles W.A., Pipkin P., Andrews N., et al. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol. 2003;71:115–123. doi: 10.1002/jmv.10450. [DOI] [PubMed] [Google Scholar]
- 2.Antonsson A., Green A.C., Mallitt K.A., et al. Prevalence and stability of antibodies to the BK and JC polyomaviruses: a long-term longitudinal study of Australians. J Gen Virol. 2010;91:1849–1853. doi: 10.1099/vir.0.020115-0. [DOI] [PubMed] [Google Scholar]
- 3.Nickeleit V., Hirsch H.H., Zeiler M., et al. BK-virus nephropathy in renal transplants-tubular necrosis, MHC-class II expression and rejection in a puzzling game. Nephrol Dial Transplant. 2000;15:324–332. doi: 10.1093/ndt/15.3.324. [DOI] [PubMed] [Google Scholar]
- 4.Dharnidharka V.R., Cherikh W.S., Abbott K.C. An OPTN analysis of national registry data on treatment of BK virus allograft nephropathy in the United States. Transplantation. 2009;87:1019–1026. doi: 10.1097/TP.0b013e31819cc383. [DOI] [PubMed] [Google Scholar]
- 5.Schold J.D., Rehman S., Kayle L.K., et al. Treatment for BK virus: incidence, risk factors and outcomes for kidney transplant recipients in the United States. Transpl Int. 2009;22:626–634. doi: 10.1111/j.1432-2277.2009.00842.x. [DOI] [PubMed] [Google Scholar]
- 6.Vasudev B., Hariharan S., Hussain S.A., et al. BK virus nephritis: risk factors, timing, and outcome in renal transplant recipients. Kidney Int. 2005;68:1834–1839. doi: 10.1111/j.1523-1755.2005.00602.x. [DOI] [PubMed] [Google Scholar]
- 7.Manzano Sanchez D., Jimeno Garcia L., Manzano Sanchez D., et al. Renal function impairment in kidney transplantation: importance of early BK virus detection. Transplant Proc. 2019;51:350–352. doi: 10.1016/j.transproceed.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Hirsch H.H., Randhawa P.S., AST Infectious Diseases Community of Practice BK polyomavirus in solid organ transplantation-guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33 doi: 10.1111/ctr.13528. [DOI] [PubMed] [Google Scholar]
- 9.Kidney disease: improving global outcomes (KDIGO) transplant work group KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1–S155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 10.Hardinger K.L., Koch M.J., Bohl D.J., et al. BK-virus and the impact of pre-emptive immunosuppression reduction: 5-year results. Am J Transplant. 2010;10:407–415. doi: 10.1111/j.1600-6143.2009.02952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malik O., Saleh S., Suleiman B., et al. Prevalence, risk factors, treatment, and overall impact of BK viremia on kidney transplantation. Transplant Proc. 2019;51:1801–1809. doi: 10.1016/j.transproceed.2019.03.035. [DOI] [PubMed] [Google Scholar]
- 12.McDonald S.P. Australia and New Zealand dialysis and transplant registry. Kidney Int Suppl (2011) 2015;5:39–44. doi: 10.1038/kisup.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Elm E., Altman D.G., Egger M., et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 14.Nickeleit V., Singh H.K., Randhawa P., et al. The Banff working group classification of definitive polyomavirus nephropathy: morphologic definitions and clinical correlations. J Am Soc Nephrol. 2018;29:680–693. doi: 10.1681/ASN.2017050477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stekhoven D.J., Bühlmann P. MissForest--non-parametric missing value imputation for mixed-type data. Bioinformatics. 2012;28:112–118. doi: 10.1093/bioinformatics/btr597. [DOI] [PubMed] [Google Scholar]
- 16.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 17.Brennan D.C., Agha I., Bohl D.L., et al. Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am J Transplant. 2005;5:582–594. doi: 10.1111/j.1600-6143.2005.00742.x. [DOI] [PubMed] [Google Scholar]
- 18.Brochot E., Descamps V., Handala L., et al. BK polyomavirus in the urine for follow-up of kidney transplant recipients. Clin Microbiol Infect. 2019;25:112.e111–112.e115. doi: 10.1016/j.cmi.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 19.Madden K., Janitell C., Sower D., Yang S. Prediction of BK viremia by urine viral load in renal transplant patients: an analysis of BK viral load results in paired urine and plasma samples. Transpl Infect Dis. 2018;20 doi: 10.1111/tid.12952. [DOI] [PubMed] [Google Scholar]
- 20.Hocker B., Schneble L., Murer L., et al. Epidemiology of and risk factors for BK polyomavirus replication and nephropathy in pediatric renal transplant recipients: an international CERTAIN registry study. Transplantation. 2019;103:1224–1233. doi: 10.1097/TP.0000000000002414. [DOI] [PubMed] [Google Scholar]
- 21.Tong A., Sautenet B., Poggio E.D., et al. Establishing a core outcome measure for graft health: a standardized outcomes in nephrology-kidney transplantation (SONG-TX) consensus workshop report. Transplantation. 2018;102:1358–1366. doi: 10.1097/TP.0000000000002125. [DOI] [PubMed] [Google Scholar]
- 22.Baek C.H., Kim H., Yu H., et al. Risk factors of acute rejection in patients with BK nephropathy after reduction of immunosuppression. Ann Transplant. 2018;23:704–712. doi: 10.12659/AOT.910483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schaub S., Hirsch H.H., Dickenmann M., et al. Reducing immunosuppression preserves allograft function in presumptive and definitive polyomavirus-associated nephropathy. Am J Transplant. 2010;10:2615–2623. doi: 10.1111/j.1600-6143.2010.03310.x. [DOI] [PubMed] [Google Scholar]
- 24.Bischof N., Hirsch H.H., Wehmeier C., et al. Reducing calcineurin inhibitor first for treating BK polyomavirus replication after kidney transplantation: long-term outcomes. Nephrol Dial Transplant. 2019;34:1240–1250. doi: 10.1093/ndt/gfy346. [DOI] [PubMed] [Google Scholar]
- 25.Randhawa P.S., Finkelstein S., Scantlebury V., et al. Human polyoma virus-associated interstitial nephritis in the allograft kidney. Transplantation. 1999;67:103–109. doi: 10.1097/00007890-199901150-00018. [DOI] [PubMed] [Google Scholar]
- 26.Binet I., Nickeleit V., Hirsch H.H., et al. Polyomavirus disease under new immunosuppressive drugs: a cause of renal graft dysfunction and graft loss. Transplantation. 1999;67:918–922. doi: 10.1097/00007890-199903270-00022. [DOI] [PubMed] [Google Scholar]
- 27.Randhawa P.S., Demetris A.J. Nephropathy due to polyomavirus type BK. N Engl J Med. 2000;342:1361–1363. doi: 10.1056/NEJM200005043421809. [DOI] [PubMed] [Google Scholar]
- 28.Wong G., Marsh J., Howell M., et al. Screening and management practices for polyoma (BK) viremia and nephropathy in kidney transplant recipients from the lands down under: addressing the unknowns and rationale for a multicenter clinical trial. Kidney Int Rep. 2020;5:1777–1780. doi: 10.1016/j.ekir.2020.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Theodoropoulos N., Wang E., Penugonda S., et al. BK virus replication and nephropathy after alemtuzumab-induced kidney transplantation. Am J Transplant. 2013;13:197–206. doi: 10.1111/j.1600-6143.2012.04314.x. [DOI] [PubMed] [Google Scholar]
- 30.Knight R.J., Gaber L.W., Patel S.J., et al. Screening for BK viremia reduces but does not eliminate the risk of BK nephropathy. Transplantation. 2013;96:e51. doi: 10.1097/TP.0b013e3182a68935. [DOI] [PubMed] [Google Scholar]
- 31.Adler J.T., Hyder J.A., Elias N., et al. Socioeconomic status and ethnicity of deceased donor kidney recipients compared to their donors. Am J Transplant. 2015;15:1061–1067. doi: 10.1111/ajt.13097. [DOI] [PubMed] [Google Scholar]
- 32.Zhong S., Randhawa P.S., Ikegaya H., et al. Distribution patterns of BK polyomavirus (BKV) subtypes and subgroups in American, European and Asian populations suggest co-migration of BKV and the human race. J Gen Virol. 2009;90:144–152. doi: 10.1099/vir.0.83611-0. [DOI] [PubMed] [Google Scholar]
- 33.Solis M., Velay A., Porcher R., et al. Neutralizing antibody-mediated response and risk of BK virus-associated nephropathy. J Am Soc Nephrol. 2018;29:326–334. doi: 10.1681/ASN.2017050532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cooling L. Blood groups in infection and host susceptibility. Clin Microbiol Rev. 2015;28:801–870. doi: 10.1128/CMR.00109-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bohl D.L., Storch G.A., Ryschkewitsch C., et al. Donor origin of BK virus in renal transplantation and role of HLA C7 in susceptibility to sustained BK viremia. Am J Transplant. 2005;5:2213–2221. doi: 10.1111/j.1600-6143.2005.01000.x. [DOI] [PubMed] [Google Scholar]
- 36.Wunderink H.F., Haasnoot G.W., de Brouwer C.S., et al. Reduced risk of BK polyomavirus infection in HLA-B51-positive kidney transplant recipients. Transplantation. 2019;103:604–612. doi: 10.1097/TP.0000000000002376. [DOI] [PubMed] [Google Scholar]
- 37.Tondel C., Vikse B.E., Bostad L., Svarstad E. Safety and complications of percutaneous kidney biopsies in 715 children and 8573 adults in Norway 1988–2010. Clin J Am Soc Nephrol. 2012;7:1591–1597. doi: 10.2215/CJN.02150212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gossai A., Waterboer T., Nelson H.H., et al. Seroepidemiology of human polyomaviruses in a US population. Am J Epidemiol. 2016;183:61–69. doi: 10.1093/aje/kwv155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kean J.M., Rao S., Wang M., Garcea R.L. Seroepidemiology of human polyomaviruses. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Egli A., Infanti L., Dumoulin A., et al. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis. 2009;199:837–846. doi: 10.1086/597126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.