Abstract
Introduction
Limited and inconclusive evidence for the association of dietary potassium intake with serum potassium in chronic kidney disease (CKD) patients have been shown, though restricting dietary potassium has been recommended for CKD patients to prevent hyperkalemia. Multiple 24-hour urine collections are necessary to adequately assess potassium intake. We investigated associations of 24-hour urinary potassium excretion (UKV) with serum potassium in CKD outpatients based on multiple 24-hour urine collections.
Methods
This retrospective cohort study was based on outpatients with CKD stages G3 to G5, median age of 72.0 years; and median follow-up of 3.9 months and 8.9 months, respectively, for analyses using 3-time measurement (N = 290 and 870 observations) and 7-time measurements (N = 220 and 1540 observations). The outcome was serum potassium.
Results
Multivariable-adjusted mean difference in serum potassium (mEq/l) and odds ratio of hyperkalemia per 10 mEq/d increase in UKV were, respectively, 0.12 (95% confidence interval [CI]: 0.09–0.15) and 2.15 (1.70–2.73) in generalized estimating equations (GEEs) with 3-time measurements. The mean difference became more pronounced as CKD stages progressed: 0.08 (0.05–0.12), 0.12 (0.08–0.16), and 0.16 (0.12–0.20) for CKD G3, G4, and G5. Similar results were obtained from analyses using 7-time measurements and hierarchical Bayesian measurement error models treating measurement error of UKV adequately.
Conclusion
We suggest significant but weak associations (R2: 0.08, 0.14, and 0.18 for CKD G3, G4, and G5) between serum potassium and dietary potassium intake estimated by multiple 24-hour urine collections in CKD patients. Further studies are needed to validate nutritional and clinical aspects of the associations.
Keywords: chronic kidney disease, dietary potassium intake, hyperkalemia, nutrition, serum potassium, urinary potassium excretion
Graphical abstract
See Commentary on Page 403
Limited and inconclusive evidence for the association between dietary potassium intake and serum potassium in patients with CKD have been indicated by guidelines and systematic reviews summarizing observational studies on the basis of dietary assessment and interventional studies allocating restricted potassium intake,1, 2, 3, 4 which should urgently be investigated and improved because of the following reasons. First, in a randomized controlled trial and a meta-analysis of 2 other randomized controlled trials where serum potassium was not a primary outcome, patients with restricted potassium intake had significantly lower serum potassium compared with those with nonrestricted potassium intake; however, authors of the meta-analysis concluded that this was of poor quality because of a strongly suspected publication bias.3,5 On the other hand, previous observational studies reported weak correlations6 or no significant associations between dietary potassium intake and serum potassium.4,7 Second, despite the lack of conclusive evidence, restricting dietary potassium has been historically recommended for CKD patients to prevent hyperkalemia, which causes severe arrhythmia leading to death. However, restricting dietary potassium can deprive potential benefits of high dietary potassium intake on kidney function decline, end-stage of kidney disease, cardiovascular disease events, and premature deaths in non-CKD participants8, 9, 10 and patients with CKD.11,12
The limited and inconclusive evidence for the association of potassium intake with serum potassium in CKD patients3,4 may be due to reverse causation and measurement error. Reverse causation bias can occur in clinical customs where patients with high serum potassium are instructed to decrease dietary potassium intake, leading to spurious results. In addition, potassium intake has been assessed by methods vulnerable to measurement error (e.g., spot urine collections and single 24-hour urine collections) in previous observational studies that showed no significant associations of potassium intake with serum potassium in CKD.4
Multiple 24-hour urine collections are necessary to reduce measurement error and improve reproducibility of potassium intake13, 14, 15; however, to the best of our knowledge, no studies used 3 times and over 24-hour urinary potassium excretion (UKV) to investigate the associations. Long-term balance studies in which dietary potassium intake was fixed in highly controlled environments have shown that misclassification of potassium (i.e., differences between intake and excretion of >25 mmol/d) were 34%, 19%, and 13%, respectively, by 1-time, 3-time average, and 7-time average values of 24-hour urine collections at an individual level.13 Another previous study showed that 2-time to 3-time 24-hour urine collections were needed to achieve sufficient reproducibility of most urinary biomarker measurements, including UKV in epidemiologic studies.15
Possible biases can be reduced by multiple 24-hour urine collections that allow us to utilize statistical analyses that adequately handle measurement error of UKV and reverse causation biases. This study aimed to investigate associations of UKV with serum potassium in CKD outpatients, with serum potassium and UKV measurements on the basis of 24-hour urine collections of 4 or more times.
Methods
Study Design and Participants
This study was based on Multiple 24-hour Urine Collection Study,12 a retrospective cohort study registering outpatients with CKD in Daiko-Sunadabashi Clinic in Nagoya, Japan between September 2005 and April 2019. We summarized the present analyses design in Figure 1. As a treatment policy in this clinic, all CKD outpatients were asked to undergo 24-hour urine collections repeatedly. In addition, dietary guidance on salt intake of <6 g per day and protein intake of <0.8 g per body weight (kg) per day was provided regardless of CKD stage. Because potassium intake consequently becomes limited with restricted protein intake,16,17 no uniform dietary guidance was provided regarding potassium intake. Abnormalities found in serum potassium levels were ameliorated with a prescription of antihyperkalemic agents.
Figure 1.
Study design concept. CKD, chronic kidney disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; IQR, interquartile range; SBP, systolic blood pressure.
Eligible criteria of the present analyses were as follows (Supplementary Figure S1). First, we excluded low quality 24-hour urine samples, in which sample quality was assessed by comparing measured urinary creatinine excretion rate (CER) with expected CER.12,18,19 In general, 24-hour urine samples may be over- or under-collected by outpatients,19 and to confirm a correctly collected 24-hour urine sample, historically, measured urinary CER has been compared with an individual’s expected CER.18,19 Thus, we included data in which measured urinary CER was between 70% and 130% of estimated CER by Horio’s equation (n = 758) that can calculate the expected CER from age, sex, body weight, and body mass index.20,21 This equation was developed among Japanese patients,20 in which estimated creatinine clearance rate was highly correlated with measured creatinine clearance rate in the population (r = 0.882)20 and another Japanese population (r = 0.814).21 Second, we included data in which estimated glomerular filtration rate was (eGFR) <60 ml/min per 1.73 m2 (n = 567). Third, we included patients aged ≥20 years old with data of 4-time 24-hour urine samples collected over a period of 18 months, during which each interval was within 6 months (n = 290) for analyses of 3-time repeated data (the first collection data were used for covariates in statistical models). Fourth, we derived data of patients with 8-time 24-hour urine samples collected over a period of 42 months, during which each interval was within 6 months (n = 220) for analyses of 7-time repeated data (the first collection data were used for covariates in statistical models). This study was approved by the Ethics Committee of Fujita Health University. The need for patient consent was waived because of the retrospective nature of the present study and the absence of personally identifiable data collected.
The baseline of each patient was defined as the date of the second 24-hour urine collection because the first collection was only used for adjustment variables. Each patient was followed up by 3-time and 7-time measurements for analyses. Median values (interquartile range) for the completion of collections per patient was 3.9 (3.0–5.7) months for 3-time measurements and 8.9 (7.2–12.2) months for 7-time measurements.
Serum Potassium Level
The primary outcome of the present study was serum potassium assessed at each outpatient visit by our clinic laboratory and from blood samples drawn in fasting conditions after completing 24-hour urine collections corresponding to each outpatient visit. Serum potassium values were derived from medical records. This study defined hyperkalemia as serum potassium >5.5 mEq/l as a secondary outcome.
Multiple 24-Hour Urine Collections for Measurements of UKV and Other Urine Variables
As a treatment policy, CKD patients were provided detailed instructions to accurately collect 24-hour urine samples for their treatment. For these 24-hour collections, accumulated urine of a patient was stored in a vinyl chloride bag with a coolant in a dedicated heat-insulating container made of Styrofoam to prevent spoilage. This procedure was performed on all samples analyzed in this study. As results of the collections, we calculated UKV (mEq/24-hour) as well as 24-hour urinary sodium excretion (UNaV, mEq/24-hour), urine creatinine, and urine protein by multiplying their urine concentrations by urine volume.18 Stability of multiple measurements of UKV is summarized in Supplementary Methods.
Clinical Data
We derived the following information from medical records: age, sex, height, body weight, systolic blood pressure and diastolic blood pressure, underlying diseases of CKD (diabetic nephropathy, chronic glomerulonephritis, nephrosclerosis, and others), serum creatinine, eGFR, serum sodium, dietary protein intake per day corresponding to body weight (g/kg per day), medications used for hypertension and diabetes, medications used that could increase serum potassium level (including angiotensin-converting-enzyme inhibitor, angiotensin II receptor blocker, mineralocorticoid receptor antagonists, renin inhibitors, and potassium supplemental medication), and medication used that could decrease serum potassium level (including diuretic except mineralocorticoid receptor antagonists, sodium-glucose cotransporter 2 inhibitor, potassium binder, liquorice [Chinese herbal medicine “Kanzo”], and bicarbonate). Dietary protein intake was obtained by the 24-hour urine samples and the Maroni formula.22 Body mass index was obtained by weight (kg) divided by squared height (m2). To calculate eGFR, we used the following equation developed and validated in Japan: eGFR (ml/min per 1.73 m2) = 194 × serum creatinine−1.094 × Age−0.287 × 0.739 (if female).23 The equation is in regular use in clinical practice in Japan, and is recommended to assess eGFR by Japan’s Evidence-based Clinical Practice Guideline for CKD.24 We defined CKD stage G5 by eGFR < 15, G4 by 15 eGFR < 30, and G3 by 30 eGFR < 60 ml/min per 1.73 m2.24
Statistical Analyses
Baseline characteristics were summarized as medians (interquartile range) for continuous variables and N (%) for categorical variables. To investigate the associations of UKV with serum potassium, we used GEEs with unstructured working correlation accounting for repeated measures of each patient, and hierarchical Bayesian measurement error models with random intercept for each patient. In the statistical models, normal and binomial distributions were assumed, respectively, for continuous serum potassium and hyperkalemia. We performed the statistical analyses by using not only 3-time but also 7-time measurement data. We used the “geepack” package and “brms” in R statistical software version 4.2.1 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria).25 Significance tests were 2-sided with alpha error <0.05.
Our statistical model structures were as follows. Primary and secondary outcomes were continuous serum potassium and hyperkalemia, respectively, modeled as dependent variables. We used UKV as an independent variable of interest, modeled as a continuous and a categorical variable based on its tertile. For the primary outcome, their β-coefficients represented mean differences in continuous serum potassium related to a 1-unit increase in the continuous UKV (i.e., 10 mEq/d) and related to the UKV categories compared with the lowest tertile category as a reference. For the secondary outcome, their exponentiated β-coefficients showed odds ratios of hyperkalemia related to 10 mEq/d increase in continuous UKV and related to UKV categories compared with the lowest tertile category. We also obtained P-values to test the linear trend across the tertile category by using a variable with each median value of the tertile category. Furthermore, we included an interaction term between the continuous UKV and CKD stages to examine whether the association strengthened or weakened across CKD stages for the primary outcome. Its β-coefficients represented how much the mean differences in continuous serum potassium per 10 mEq/d increase in continuous UKV differed in CKD stages G4 and G5 compared with that in CKD stage G3.
These associations were adjusted as follows. Model 1 was an unadjusted model. Model 2 was adjusted for age, sex, eGFR, and urinary creatinine excretion as a surrogate marker of muscle mass.26,27 Model 3 was adjusted for Model 2 covariates, urine protein, diabetes (defined by any medication use of diabetes and diabetic nephropathy as an underlying disease for CKD), hypertension (defined by any use of antihypertensive, or systolic blood pressure ≥ 140 mm Hg or DBP ≥ 90 mm Hg, or nephrosclerosis as an underlying disease for CKD), medication use that could increase serum potassium level, and medication use that could decrease serum potassium level as established factors related to serum potassium.28,29 Model 3 was also adjusted for previous values of serum potassium and UKV, modeled by the sequential conditional mean model approach to deal with a possible reverse causation bias because of clinical customs where patients with high serum potassium are instructed by their physicians to decrease dietary potassium intake.30 The details of the sequential conditional mean model approach are described in Supplementary Methods. Furthermore, we used hierarchical Bayesian measurement error models to deal with possible measurement error of UKV adequately, for the association of continuous UKV with outcomes in the main 3 models. The hierarchical Bayesian measurement error models can estimate associations between exposures with measurement error and outcomes by incorporating uncertainty sources and measurement error.31,32 Their details are described in Supplementary Methods.
As sensitivity analyses, supplementary models were performed by GEEs, adjusted for the folowing: (i) urinary creatinine excretion only, (ii) eGFR only, (iii) Model 2 covariates, urine protein, diabetes, hypertension, and the medication use that could increase or decrease serum potassium level, and (iv) Model 3 covariates, UNaV, and calendar year at each outpatient visit. The 3 main models and the 4 supplementary models were also performed by GEEs, excluding data when patients received potassium binders.
Results
Characteristics of the Present Patients
We summarized the baseline characteristics of participants for analyses using 3-time measurements by tertiles of UKV in Table 1. The median (interquartile range) values were 72 (63–79) years old for baseline age, 23 (13.4–41.1) ml/min per 1.73 m2 for baseline eGFR, and 33.7 (22.8–45.0) mEq/d for baseline UKV corresponding to 1318 (892–1761) mg/d. Characteristics of the participants for analyses using 7-time measurements are summarized in Supplementary Table S1.
Table 1.
Baseline characteristics of present patients with CKD for analyses using 3-time measurements by tertiles of baseline UKV on the basis of 24-hour urine collections (N = 290)
| Variables |
Baseline UKV (mEq/d) on the basis of 24-hour urine collections |
||
|---|---|---|---|
| Tertiles |
Low tertile ( 26.0) |
Middle tertile (26.0–39.9) |
High tertile (>39.9) |
| N | 95 | 94 | 101 |
| Median (IQR) | |||
| Age, years old | 75.0 (66.5, 79.5) | 70.5 (63.2, 77.8) | 70.0 (60.0, 79.0) |
| BMI, kg/m2 | 22.1 (20.2, 24.3) | 22.6 (20.5, 24.6) | 22.4 (20.5, 25.0) |
| Height, cm | 160.0 (152.0, 165.3) | 162.0 (156.1, 167.0) | 163.0 (158.0, 170.0) |
| Body weight, kg | 54.5 (49.1, 64.2) | 60.3 (51.0, 67.0) | 60.0 (53.4, 68.0) |
| SBP, mm Hg | 130.0 (124.0, 136.0) | 130.0 (124.0, 134.0) | 128.0 (124.0, 134.0) |
| DBP, mm Hg | 74.0 (70.0, 78.0) | 74.0 (70.0, 78.0) | 74.0 (70.0, 78.0) |
| eGFR, ml/min/1.73 m2 | 16.1 (9.7, 22.5) | 28.0 (15.4, 42.0) | 35.1 (18.8, 49.3) |
| Serum creatinine, mg/dl | 3.1 (2.0, 4.8) | 1.7 (1.3, 3.0) | 1.5 (1.1, 2.7) |
| Serum potassium, mEq/l | 4.6 (4.2, 5.1) | 4.3 (4.0, 4.9) | 4.6 (4.1, 5.1) |
| Previous serum potassium, mEq/l | 4.7 (4.2, 5.2) | 4.4 (4.0, 4.9) | 4.6 (4.2, 5.0) |
| Serum sodium, mEq/l | 140.0 (138.0, 142.0) | 140.0 (138.0, 142.0) | 140.0 (139.0, 141.0) |
| Urinary measurements | |||
| UKV, mEq/d | 19.2 (16.1, 22.8) | 33.5 (29.3, 36.9) | 49.5 (44.7, 57.2) |
| UKV, mg/d | 753 (630, 891) | 1310 (1145, 1442) | 1935 (1746, 2236) |
| Previous UKV, mEq/d | 23.1 (16.8, 28.7) | 35.6 (29.0, 43.2) | 46.5 (36.4, 57.0) |
| SD of 3-time UKV within a patient, mEq/da | 0.4 (0.2, 0.5) | 0.6 (0.4, 0.8) | 0.7 (0.5, 1.0) |
| Dietary protein intake, g/kg/d | 0.7 (0.6, 0.8) | 0.9 (0.8, 1.0) | 1.0 (0.9, 1.1) |
| UNaV, mEq/d | 94.1 (74.7, 118.3) | 117.7 (94.5, 145.6) | 143.4 (114.6, 177.6) |
| UNaV, mg/d | 2164 (1717, 2720) | 2706 (2173, 3348) | 3296 (2635, 4084) |
| Urine protein, g/d | 0.5 (0.1, 1.5) | 0.5 (0.1, 1.5) | 0.4 (0.1, 2.1) |
| Urine creatinine, mg/dl | 53.0 (42.5, 71.0) | 53.5 (42.0, 67.8) | 55.0 (45.0, 74.0) |
| Urine volume, ml | 1580 (1130, 1938) | 1685 (1323, 2100) | 1900 (1500, 2150) |
| No. (%) | |||
| Male | 59 (62.1) | 64 (68.1) | 72 (71.3) |
| CKD stage | |||
| Stage 3 | 16 (16.8) | 45 (47.9) | 54 (53.5) |
| Stage 4 | 33 (34.7) | 26 (27.7) | 32 (31.7) |
| Stage 5 | 46 (48.4) | 23 (24.5) | 15 (14.9) |
| Underlying disease of CKD | |||
| CGN | 19 (20.0) | 22 (23.4) | 29 (28.7) |
| Diabetic nephropathy | 21 (22.1) | 30 (31.9) | 37 (36.6) |
| Nephrosclerosis | 39 (41.1) | 27 (28.7) | 18 (17.8) |
| Others | 16 (16.8) | 15 (16.0) | 17 (16.8) |
| Hypertensionb | 58 (61.1) | 68 (72.3) | 68 (67.3) |
| Any drug use for hypertension | 48 (50.5) | 62 (66.0) | 59 (58.4) |
| Diabetesc | 21 (22.1) | 34 (36.2) | 40 (39.6) |
| Any drug use for diabetes | 9 (9.5) | 23 (24.5) | 19 (18.8) |
| Drug use decreasing serum potassium | 34 (35.8) | 39 (41.5) | 44 (43.6) |
| Diuretics | 27 (28.4) | 37 (39.4) | 41 (40.6) |
| SGLT2i | 0 (0) | 0 (0) | 0 (0) |
| Potassium binder | 13 (13.7) | 4 (4.3) | 3 (3.0) |
| Liquorice extractd | 0 (0) | 0 (0) | 0 (0) |
| Bicarbonate | 1 (1.1) | 0 (0.0) | 0 (0.0) |
| Drug use increasing serum potassium | 35 (36.8) | 50 (53.2) | 45 (44.6) |
| ACEi/ARB | 33 (34.7) | 43 (45.7) | 41 (40.6) |
| MRA | 3 (3.2) | 11 (11.7) | 7 (6.9) |
| Renin inhibitor | 0 (0.0) | 1 (1.1) | 0 (0.0) |
| Potassium supplement | 0 (0.0) | 0 (0.0) | 1 (1.0) |
ACEi, angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CKD, chronic kidney disease; CGN, Chronic glomerulonephritis; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; IQR, interquartile range; MRA, mineralocorticoid receptor antagonists; SBP, systolic blood pressure; SGLT2i, sodium-glucose cotransporter 2 inhibitors; UKV, 24-hour urinary potassium excretion; UNaV, 24-hour urinary sodium excretion.
SD of UKV were calculated per patient on the basis of multiple 24-hour urine collections.
Hypertension was defined by any use of antihypertensive, or SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg, or nephrosclerosis as an underlying disease for CKD.
Diabetes was defined by any medication use of diabetes and diabetic nephropathy as an underlying disease for CKD.
Chinese herbal medicine “Kanzo.”
Associations of UKV With Serum Potassium in all Patients With CKD
Associations of UKV with serum potassium were evaluated by GEEs in patients (N = 290) with 3-time measurements (870 observations) (Table 2). Multivariable-adjusted means in serum potassium (mEq/l) were higher in higher tertiles of UKV as follows: their mean differences (95% CI) were 0.09 (0.004–0.17) and 0.14 (0.04–0.24) in the middle and highest tertiles of UKV compared with the lowest tertile (P-for-trend = 0.009) in Model 1. Similarly, after adjusting for age, sex, eGFR, and urine creatinine in Model 2, their mean differences (95% CI) in serum potassium were 0.16 (0.08–0.24) and 0.28 (0.18–0.38) in the middle and highest tertiles of UKV compared with the lowest tertile (P-for-trend < 0.001). Furthermore, similar results were obtained in Model 3 adjusted for full covariates, including medication uses related to serum potassium and previous values of UKV and serum potassium as follows: their mean differences (95% CI) in serum potassium (mEq/l) were 0.15 (0.06–0.23) and 0.30 (0.20–0.41) in the middle and highest tertiles of UKV compared with the lowest tertile (P-for-trend < 0.001). Based on Model 3, multivariable-adjusted means (i.e., estimated marginal means) of serum potassium were 4.40 (95% CI: 4.33–4.47), 4.54 (4.49–4.60), and 4.70 (4.63–4.77) mEq/l in the lowest, middle, and highest tertiles of UKV, respectively. Multivariable-adjusted mean differences in serum potassium (mEq/l) associated with per 10 mEq/d increase in continuous UKV were 0.12 (95% CI: 0.09–0.15) in Model 3. In addition, we evaluated associations of UKV with serum potassium in patients with 7-time measurements (N = 220 and 1540 observations), and their results were similar to the analyses of 3-time measurements (Table 2).
Table 2.
Associations of UKV with continuous serum potassium (mEq/l) on the basis of 3-time and 7-time measurements in all CKD patients by GEE regression analyses
| Models | Tertiles of UKV on the basis of 24-hour urine collections |
P-for-trend | Per 10 mEq/d increase in continuous UKVe | ||
|---|---|---|---|---|---|
| Low tertile | Middle tertile | High tertile | |||
| Analyzed patients (N = 290) with 3-time measurements (870 observations) | |||||
| UKV on the basis of 24-hour urine collections, mEq/d | 26.0 | 26.0–39.9 | >39.9 | ||
| N of observations | 287 | 288 | 295 | 870 | |
| Adjusted means (95% CI) of serum potassiuma | 4.40 (4.33–4.47) | 4.54 (4.49–4.60) | 4.70 (4.63 to 4.77) | ||
| Mean differences (95% CI) in serum potassium | |||||
| Model 1b | 0 [Ref] | 0.09 (0.004 to 0.17) | 0.14 (0.04 to 0.24) | 0.009 | 0.08 (0.05–0.11) |
| Model 2c | 0 [Ref] | 0.16 (0.08 to 0.24) | 0.28 (0.18 to 0.38) | P < 0.001 | 0.12 (0.09–0.15) |
| Model 3d | 0 [Ref] | 0.15 (0.06 to 0.23) | 0.30 (0.20 to 0.41) | P < 0.001 | 0.12 (0.09–0.15) |
| Analyzed patients (N = 220) with 7-time measurements (1540 observations) | |||||
| UKV on the basis of 24-hour urine collections, mEq/d | 27.3 | 27.3–39.9 | > 39.9 | ||
| N of observations | 508 | 510 | 522 | 1540 | |
| Adjusted means (95% CI) of serum potassium a | 4.42 (4.36–4.47) | 4.53 (4.48–4.58) | 4.65 (4.59–4.7) | ||
| Mean differences (95% CI) in serum potassium | |||||
| Model 1b | 0 [Ref] | 0.15 (0.07–0.23) | 0.23 (0.13–0.32) | P < 0.001 | 0.09 (0.06–0.12) |
| Model 2c | 0 [Ref] | 0.19 (0.11–0.26) | 0.28 (0.18–0.38) | P < 0.001 | 0.11 (0.08–0.14) |
| Model 3d | 0 [Ref] | 0.11 (0.05–0.18) | 0.23 (0.15–0.32) | P < 0.001 | 0.10 (0.07–0.13) |
ACE, angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blocker; CI, confidence interval; CKD, chronic kidney disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; GEE, generalized estimating equation; MRA, mineralocorticoid receptor antagonists; SBP, systolic blood pressure; SGLT2i, sodium-glucose cotransporter 2 inhibitors; UKV, 24-hour urinary potassium excretion.
Multivariable-adjusted means (95% CI) were based on estimated marginal means obtained from Model 3.
Model 1 was unadjusted.
Model 2 was adjusted for age, sex, eGFR, and urine creatinine.
Model 3 was adjusted for Model 2 covariates, urine protein, diabetes (defined by any medication use of diabetes and diabetic nephropathy as an underlying disease for CKD), hypertension (defined by any use of antihypertensive, or SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg, or nephrosclerosis as an underlying disease for CKD), medication use that could increase serum potassium level (including ACEi/ARB, MRA, renin inhibitors, potassium supplement medication), medication use that could decrease serum potassium level (including diuretics except MRA, SGLT2i, potassium binder, liquorice extract, and bicarbonate), and previous values of serum potassium and UKV.
This corresponds to a β coefficient of a continuous UKV on serum potassium.
Furthermore, associations of continuous UKV with serum potassium were evaluated by hierarchical Bayesian measurement error models incorporating SDs of 3-time or 7-time UKVs per patient based on multiple 24-hour urine collections (Figure 2), leading to consideration of possible measurement errors of UKV. Multivariable-adjusted mean differences in serum potassium associated with per 10 mEq/d increase in UKV were 0.14 (95% credible interval: 0.10–0.18) and 0.15 (0.11–0.18), respectively, for analyses of 3-time and 7-time measurements in Model 3.
Figure 2.
Associations of UKV with continuous serum potassium in all CKD patients by hierarchical Bayesian regression analyses c modeling possible measurement error of UKV on the basis of 3-time and 7-time measurements. CKD, chronic kidney disease; CI, credible interval; eGFR, estimated glomerular filtration rate; UKV, 24-hour urinary potassium excretion. aMultivariable-adjusted means were based on estimated marginal means obtained from Model 3 adjusted for age, sex, eGFR, urine creatinine, urine protein, diabetes, hypertension, medication use that could increase serum potassium level, medication use that could decrease serum potassium level, and previous values of serum potassium and UKV. These are shown in red lines with dashed lines for multivariable-adjusted means. bβ corresponds to multivariable-adjusted mean differences (95% CI) in serum potassium (mEq/l) corresponding to per unit increase in urinary potassium excretion (10 mEq/d) in Model 3. cIn the hierarchical Bayesian measurement error models, SDs of 3-time or 7-time UKVs per patient based on multiple 24-hour urine collections were incorporated to consider possible measurement errors of UKV. dR2 values were obtained from fixed effects of unadjusted models developed by the hierarchical Bayesian measurement error regression analyses.
Interactions Between CKD Stages and UKV in Relation to Serum Potassium
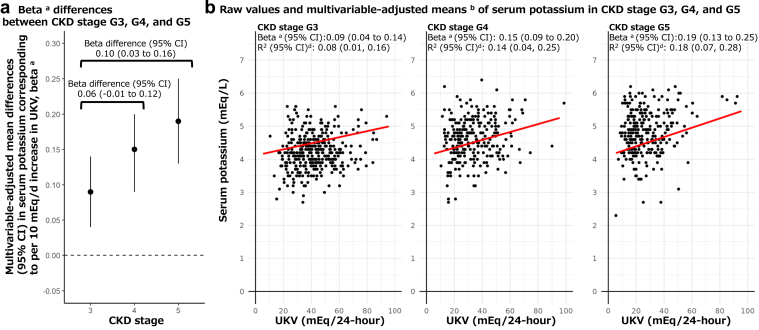
We investigated whether the associations of UKV with serum potassium strengthened or weakened as CKD stage progressed by evaluating interactions between CKD stages (G3, G4, and G5) and UKV in relation to serum potassium, using GEEs in patients (N = 290) with 3-time measurements (870 observations, Table 3). No significant interaction between G3 and G4 was found (P-for-interaction = 0.13 in Model 3). However, significant interaction between G3 and G5 was found (P-for-interaction = 0.002 in Model 3), showing associations of UKV with serum potassium differed. Multivariable-adjusted mean differences in serum potassium (mEq/l) associated with per 10 mEq/d increase in continuous UKV were 0.08 (95% CI: 0.05–0.12), 0.12 (0.08–0.16), and 0.16 (0.12–0.20), respectively, for CKD stage G3, G4, and G5 in Model 3. In addition, we evaluated associations of UKV with serum potassium in patients (N = 220) with 7-time measurements (1540 observations), and their results were similar to the analyses of 3-time measurements (Table 3).
Table 3.
Associations of UKV with continuous serum potassium (mEq/l) on the basis of 3-time and 7-time measurements in patients with CKD G3, G4, and G5 by GEE regression analyses
| Models |
CKD stage |
P-for-interaction |
|||
|---|---|---|---|---|---|
| CKD stage | G3 | G4 | G5 | G3 vs. G4 | G3 vs. G5 |
| Analyzed patients (N = 290) with 3-time measurements (870 observations) | |||||
| UKV on the basis of 24-hour urine collections, mEq/d, mean (SD) | 41.85 (14.66) | 33.5 (14.64) | 27.45 (13.97) | ||
| N of observations | 347 | 261 | 262 | ||
| Mean differences (95% CI) in serum potassium per 10 mEq/d increase in continuous UKVa | |||||
| Model 1b | 0.06 (0.02–0.10) | 0.10 (0.04–0.16) | 0.17 (0.12–0.22) | 0.27 | 0.001 |
| Model 2c | 0.07 (0.03–0.11) | 0.11 (0.05–0.16) | 0.17 (0.12–0.23) | 0.29 | 0.002 |
| Model 3d | 0.08 (0.05–0.12) | 0.12 (0.08–0.16) | 0.16 (0.12–0.20) | 0.13 | 0.002 |
| Analyzed patients (N = 220) with 7-time measurements (1540 observations) | |||||
| UKV on the basis of 24-hour urine collections, mEq/d, mean (SD) | 42.05 (14.67) | 34.07 (14.53) | 28.02 (14.22) | ||
| N of observations | 644 | 474 | 422 | ||
| Mean differences (95% CI) in serum potassium per 10 mEq/d increase in continuous UKV a | |||||
| Model 1b | 0.04 (0.01–0.07) | 0.12 (0.07–0.17) | 0.18 (0.12–0.24) | 0.003 | P < 0.001 |
| Model 2c | 0.04 (0.01–0.07) | 0.12 (0.07–0.18) | 0.18 (0.12–0.25) | 0.003 | P < 0.001 |
| Model 3d | 0.07 (0.04–0.10) | 0.11 (0.07–0.14) | 0.13 (0.09–0.17) | 0.06 | 0.004 |
CKD, chronic kidney disease; GEE, generalized estimating equation; UKV, 24-hour urinary potassium excretion; CI, confidence interval; SBP, systolic blood pressure; DBP, diastolic blood pressure; ACE, angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blocker; MRA, mineralocorticoid receptor antagonists; SGLT2i, sodium-glucose cotransporter 2 inhibitors.
This corresponds to a β-coefficient of a continuous UKV on serum potassium. Interaction term between CKD stage and continuous UKV, and their main effect terms in relation to serum potassium were included in statistical models.
Model 1 was unadjusted.
Model 2 was adjusted for age, sex, and urine creatinine.
Model 3 was adjusted for Model 2 covariates, urine protein, diabetes (defined by any medication use of diabetes and diabetic nephropathy as an underlying disease for CKD), hypertension (defined by any use of antihypertensive, or SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg, or nephrosclerosis as an underlying disease for CKD), medication use that could increase serum potassium level (including ACEi/ARB, MRA, renin inhibitors, potassium supplement medication), medication use that could decrease serum potassium level (including diuretics except MRA, SGLT2i, potassium binder, liquorice extract, and bicarbonate), and previous values of serum potassium and UKV.
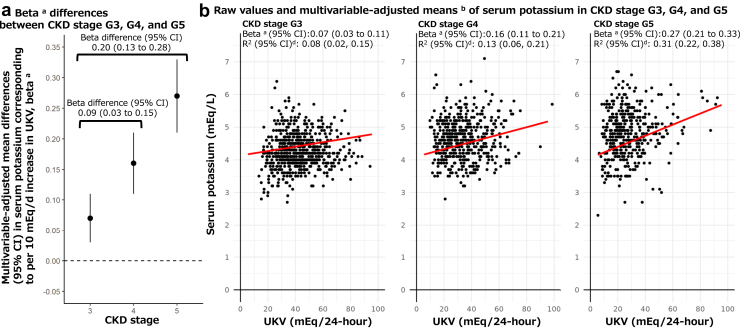
Furthermore, interactions were evaluated by hierarchical Bayesian measurement error models to consider possible measurement errors of UKV (Figure 3). Multivariable-adjusted mean differences in serum potassium associated with per 10 mEq/d increase in continuous UKV (i.e., β) were 0.09 (95% credible interval: 0.04–0.14), 0.15 (0.09–0.20), and 0.19 (0.13–0.25), respectively, for CKD stage G3, G4, and G5 in patients with 3-time measurement in Model 3. β-differences (95% credible interval) were 0.06 (−0.01 to 0.12) in G4 and 0.10 (0.03–0.16) in G5 compared with G3. In analyses of the 7-time measurement, those were 0.07 (0.03–0.11), 0.16 (0.11–0.21), and 0.27 (0.21–0.33), respectively, for CKD stage G3, G4, and G5 in Model 3. β-differences (95% credible interval) were 0.09 (0.03–0.15) in G4 and 0.20 (0.13–0.28) in G5 compared with G3 (Figure 4).
Figure 3.
Associations of UKV with continuous serum potassium in patients of CKD stage G3, G4, and G5 (N = 290) based on 3-time measurements (870 observations) by hierarchical Bayesian regression analyses c modeling possible measurement error of UKV. CKD, chronic kidney disease; CI, credible intervals; eGFR, estimated glomerular filtration rate; UKV, 24-hour urinary potassium excretion.
aβ corresponds to multivariable-adjusted mean differences (95% CI) in serum potassium (mEq/l) corresponding to per unit increase in UKV (10 mEq/d) in Model 3 adjusted for age, sex, eGFR, urine creatinine, urine protein, diabetes, hypertension, medication use that could increase serum potassium level, medication use that could decrease serum potassium level, and previous values of serum potassium and UKV. Interaction terms between CKD stage and continuous UKV, and their main effect terms in relation to serum potassium were included in the statistical model.
bMultivariable-adjusted means were based on estimated marginal means obtained from Model 3, shown in red lines with dashed lines for multivariable-adjusted means. cIn the hierarchical Bayesian measurement error models, SDs of 3-time UKVs per patient based on multiple 24-hour urine collections were incorporated to consider possible measurement errors of UKV. dR2 values were obtained from fixed effects of unadjusted models developed by the hierarchical Bayesian measurement error regression analyses in each CKD stage.
Figure 4.
Associations of UKV with continuous serum potassium in patients of CKD stage G3, G4, and G5 (N = 220) on the basis of 7-time measurements (1540 observations) by hierarchical Bayesian regression analyses cmodeling possible measurement error of UKV. CKD, chronic kidney disease; CI, credible intervals; eGFR, estimated glomerular filtration rate; UKV, 24-hour urinary potassium excretion. aβ corresponds to multivariable-adjusted mean differences (95% CI) in serum potassium (mEq/l) corresponding to per unit increase in UKV (10 mEq/d) in Model 3 adjusted for age, sex, eGFR, urine creatinine, urine protein, diabetes, hypertension, medication use that could increase serum potassium level, medication use that could decrease serum potassium level, and previous values of serum potassium and UKV. Interaction terms between CKD stage and continuous UKV, and their main effect terms in relation to serum potassium were included in the statistical model. bMultivariable-adjusted means were based on estimated marginal means obtained from Model 3, shown in red lines with dashed lines for multivariable-adjusted means. cIn the hierarchical Bayesian measurement error models, SDs of 7-time UKVs per patient based on multiple 24-hour urine collections were incorporated to consider possible measurement errors of UKV. dR2 values were obtained from fixed effects of unadjusted models developed by the hierarchical Bayesian measurement error regression analyses in each CKD stage.
Associations of UKV With Hyperkalemia in all Patients With CKD
We investigated associations of UKV with hyperkalemia (Table 4). UKV was significantly associated with hyperkalemia after adjustment for eGFR in Model 2 and 3 and Supplementary Model 2, 3, and 4. Compared with the lowest tertile of UKV, likelihood of hyperkalemia was higher in the middle and highest tertiles; its odds ratios (95% CI) were, respectively, 0.88 (0.38–2.06) and 2.49 (1.05–5.88) in Model 3 (P-for-trend = 0.03). In addition, odds ratio (95% CI) of hyperkalemia per 10 mEq/d increase in UKV was 2.15 (1.70–2.73) in Model 3.
Table 4.
Associations of UKV with hyperkalemia (>serum potassium 5.5 mEq/l) based on 3-time and 7-time measurements in all CKD patients by GEE regression analyses
| Models | Tertiles of UKV on the basis of 24-hour urine collections |
P-for-trend | Per 10 mEq/d increase in continuous UKVd | ||
|---|---|---|---|---|---|
| Low tertile | Middle tertile | High tertile | |||
| Analyzed patients (N = 290) with 3-time measurements (870 observations) | |||||
| UKV on the basis of 24-hour urine collections, mEq/d | 26.0 | 26.0–39.9 | > 39.9 | ||
| Hyperkalemia / N of observations (%) | 23/287 (8.0%) | 12/288 (4.2%) | 19/295 (6.4%) | 54/870 (6.2%) | |
| ORs (95% CI) of hyperkalemia by UKV | |||||
| Model 1a | 1 [Ref] | 0.60 (0.31–1.14) | 0.90 (0.46–1.76) | 0.85 | 1.15 (0.91–1.47) |
| Model 2b | 1 [Ref] | 1.05 (0.54–2.03) | 2.93 (1.40–6.13) | 0.007 | 1.61 (1.31–1.99) |
| Model 3c | 1 [Ref] | 0.88 (0.38–2.06) | 2.49 (1.05–5.88) | 0.03 | 2.15 (1.70–2.73) |
| Analyzed patients (N = 220) with 7-time measurements (1540 observations) | |||||
| UKV on the basis of 24-hour urine collections, mEq/d | 27.3 | 27.3–39.9 | > 39.9 | ||
| Hyperkalemia / N of observations (%) | 51/508 (10.0%) | 30/510 (5.9%) | 30/522 (5.7%) | 111/1540 (7.2%) | |
| ORs (95% CI) of hyperkalemia by UKV | |||||
| Model 1a | 1 [Ref] | 1.03 (0.60–1.77) | 1.35 (0.76–2.41) | 0.26 | 1.19 (1.01–1.41) |
| Model 2b | 1 [Ref] | 1.26 (0.72–2.20) | 2.09 (1.13–3.84) | 0.02 | 1.38 (1.19–1.59) |
| Model 3c | 1 [Ref] | 1.17 (0.69–1.97) | 2.45 (1.33–4.50) | 0.005 | 1.75 (1.43–2.15) |
ACE, angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blocker; CI, confidence interval; CKD, chronic kidney disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; GEE, generalized estimating equation; MRA, mineralocorticoid receptor antagonists; OR, odds ratio; SBP, systolic blood pressure; SGLT2i, sodium-glucose cotransporter 2 inhibitors; UKV, 24-hour urinary potassium excretion.
Model 1 was unadjusted.
Model 2 was adjusted for age, sex, eGFR, and urine creatinine.
Model 3 was adjusted for Model 2 covariates, urine protein, diabetes (defined by any medication use of diabetes and diabetic nephropathy as an underlying disease for CKD), hypertension (defined by any use of antihypertensive, or SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg, or nephrosclerosis as an underlying disease for CKD), medication use that could increase serum potassium level (including ACEi/ARB, MRA, renin inhibitors, potassium supplement medication), medication use that could decrease serum potassium level (including diuretics except MRA, SGLT2i, potassium binder, liquorice extract, and bicarbonate), previous hyperkalemia, and previous UKV value.
This corresponds to ORs of hyperkalemia by continuous UKV.
Sensitivity Analyses
Sensitivity results by GEEs using other covariate sets and patients with 3-time or 7-time measurements excluding data when patients received potassium binders, were similar to the main results (Supplementary Tables S2, S3, and S4). Detailed results for associations of continuous UKV with outcomes by the hierarchical Bayesian measurement error methods are shown in Supplementary Tables S5 and S6.
Discussion
Based on the present analyses using the 3-time and 7-time measurements of UKV and serum potassium, higher UKV was significantly associated with higher serum potassium in CKD patients, which became more pronounced as CKD stage progressed, especially in G5. In addition, the significant associations were relatively weak; R2 based on unadjusted models developed by the hierarchical Bayesian measurement error analyses in each CKD stage were 0.08, 0.14, and 0.18 for CKD G3, G4, and G5, respectively. Limited and inconclusive evidence for the association between potassium intake and serum potassium have been shown by previous guidelines, systematic reviews, randomized controlled trials, and observational studies.1, 2, 3, 4, 5, 6, 7 Note that significant associations were consistently observed after adjusting for important confounders including age, eGFR, and the medication uses related to serum potassium, and considering possible bias from measurement error of UKV.
We showed significant associations of higher UKV as a surrogate marker of dietary potassium intake with higher serum potassium, supported by previous meta-analysis of 2 randomized controlled trials.3 In the meta-analysis, patients with restricted potassium intake (1295 mg/d) had significantly lower serum potassium than those with nonrestricted potassium intake (1570 mg/d), of which mean difference (95% CI) was –0.22 mEq/l (–0.33 to –0.10, P = 0.0002).3 In addition, plasma potassium values were increased from their baseline values after patients with CKD stage G3b and G4 took potassium chloride supplementation (40 mmol/d for 2 weeks) in an uncontrolled, single-arm, open-label intervention study.33 CKD patients UKV can be interpreted as the quantity of dietary potassium intake because of the following. Potassium balance at the biologic level is largely handled by kidneys.34 It has been reported that of total dietary potassium intake, 77% are averagely excreted through kidneys into urine.10,35 On the other hand, previous observational studies reported weak correlation (r = 0.14, P < 0.05)6 between dietary potassium intake and serum potassium, and no significant association of dietary potassium intake or UKV with serum potassium and mortality because of hyperkalemia in CKD patients.3,4,7 This may be because of possible measurement error of potassium intake. Morris et al.3 and Ramos et al.7 pointed that dietary records and food frequency questionnaires can be problematic by known inaccuracies of self-reported dietary data,3,35 and lack of information in food labels about potassium content and loss of potassium in cooking processes. Furthermore, it can be insufficient to assess UKV by single 24-hour urine collections and especially by spot urine collections because their UKV measurements were reported to be vulnerable to misclassifications at an individual level and with limited reproducibility for epidemiologic studies.13, 14, 15 Note that our present significant associations of UKV with serum potassium were based on statistical analyses modeling possible UKV measurement error by treating SDs of the 3-time and 7-time UKV measurements per patient as uncertain information.
This study showed significant associations of higher UKV with higher serum to become more pronounced as CKD stage progresses, even after considering UKV measurement error, which may be the first observational, empirical evidence to the best our knowledge. Potassium excretion performance is considered to diminish as kidney function declines from theoretical insight and clinical experience.34,36 Thus, the present results can be interpreted that the risk of higher potassium intake on hyperkalemia can increase as kidney function declines, especially in CKD stage G5. Note that there were some CKD patients whose serum potassium levels were more than 6.0 mEq/l despite relatively low UKV (e.g., UKV < 20 mEq/d), especially in CKD stage G5. This may be because of other factors influencing serum potassium such as fecal excretion of potassium, acid-base equilibrium, glucose metabolism, and renin angiotensin aldosterone system.35, 36, 37
We believe it is essential to monitor serum potassium in CKD patients regardless of UKV values, especially those with CKD stage G5. This study showed significant associations of higher UKV with a higher likelihood of hyperkalemia and stronger associations of UKV with serum potassium in CKD stage G5 than in CKD stage G3, with a certain number of patients with hyperkalemia despite low UKV. Note that our findings are insufficient to support or oppose any dietary approaches to hyperkalemia because of the following. Although UKV is an objective biomarker of dietary potassium intake, this does not reflect all dietary potassium intake6 and is also determined by transcellular shift, medication uses, and so on.28 Data of dietary potassium intake by dietary assessment were unavailable in the present study. Associations of UKV with serum potassium were modest in the present CKD patients. Potassium intake consequently becomes limited because of restricted protein intake commonly recommended for CKD stage G3, G4, and G5,16,17 possibly resulting in lower serum potassium (Supplementary Information). Furthermore, strict restriction of dietary potassium intake may lead to disease-related outcomes including kidney function decline, end-stage of kidney disease, cardiovascular diseases, and premature death.8, 9, 10, 11, 12
This study has several limitations. First, we could not verify causal effects of UKV on serum potassium because the present study was observational. Second, our results were based on CKD patients whose four- or eight-time 24-hour urine samples (first 24-hour urine samples used as covariates in the statistical models) were collected, which might increase selection bias. We could not measure the degree of variability of the present results under different conditions because we did not have another cohort based on multiple 24-hour urine collections. However, there were no significant differences in important variables for the present aim, including age, serum potassium, or UKV between CKD patients with four-time 24-hour urine samples and those without such sufficient values (Supplementary Table S7). Although differences in several variables were suggested, significant associations of UKV with serum potassium were observed regardless of any adjustments for these variables. Third, we used data collected between 2006 and 2019. However, we could obtain similar results to main results even after adjusting for the baseline calendar year (Supplementary Tables S2–S4). Fourth, it is possible that the urine samples were not precise 24-hour samples (e.g., 23.5-hour or 24.5-hour urine samples). However, patients were given detailed instructions to accurately collect 24-hour urine samples for medical treatment.
This study had several strengths. First, we used 24-hour urine collections repeatedly measured and the hierarchical Bayesian measurement error models, which allowed us to model possible UKV measurement error when assessing association of UKV with serum potassium. Second, we used 24-hour urine collections of which measured urinary CER was between 70% and 130% of estimated CER by Horio’s equation, allowing us to use quality-assured 24-hour urine samples. This was important to avoid use of over- or under-collected 24-hour urine samples by outpatients.19
In conclusion, the present study suggests a significant but weak association between dietary potassium intake estimated by multiple 24-hour urine collections and serum potassium in CKD patients. This association became more pronounced as CKD stage progressed (especially in G5). Further studies are needed to validate the present results and other nutritionally and clinical aspects of this association.
Disclosure
SO and SN obtained funds for collaborative researches from HU Group Holdings, Inc. to conduct this study. KN obtained a fund for collaborative research from HU Group Holdings, Inc. to conduct another study. The other authors declared no competing interests in relation to this study.
Acknowledgments
We thank Issei Onji of Daiko-Sunadabashi Clinic for organizing the database of the present study and Kei Matsumaru for English proofreading. The present study was partially supported by funds for collaborative research from H.U. Group Holdings, Inc. (OP01-0038 and 336).
Author Contributions
SO, YA, and SN primarily had roles in study design, data acquisition, data analysis and interpretation, and drafting of the present paper. SY, YM, KN, KM (i.e., researchers from academic institutes) had roles in data interpretation, and reviewing and revising manuscript critically. SK, YO, and AS (i.e., researchers from HU Group Holdings Inc.) had no role in study design and data collection, but had roles in checking data analysis results twice and supporting in data interpretation and in writing of the statistical part from the viewpoint of data science experts.
Footnotes
Supplementary Methods. Stability of multiple measurements of UKVs; Sequential conditional mean model approach; Bayesian measurement error models.
Supplementary Information. Mediation analysis.
Figure S1. Detailed patient flow diagram.
Table S1. Baseline characteristics of present patients with CKD for analyses using 7-time measurements by tertiles of baseline UKV based on 24-hour urine collections (N = 220).
Table S2. Associations of UKV with serum potassium (mEq/l) based on 3-time and 7-time measurements in CKD patients by GEE regression analyses for sensitivity analyses.
Table S3. Associations of UKV with serum potassium (mEq/l) based on 3-time and 7-time measurements in patients with CKD G3, G4, and G5 by GEE regression analyses for sensitivity analyses.
Table S4. Associations of UKV with hyperkalemia (> serum potassium 5.5 mEq/l) on the basis of 3-time and 7-time measurements in all CKD patients by GEE regression analyses for sensitivity analyses.
Table S5. Mean differences in serum potassium (mEq/l) per 10 mEq/d increase in continuous UKV (i.e., β with 95% CI) based on 3-time and 7-time measurements in all patients and patients with CKD G3, G4, and G5 by hierarchical Bayesian measurement error models for sensitivity analyses.
Table S6. Associations of UKV with hyperkalemia (> serum potassium 5.5 mEq/l) on the basis of 3-time and 7-time measurements in all CKD patients by hierarchical Bayesian measurement error models for sensitivity analyses.
Table S7. Baseline characteristics of CKD patients with four-time 24-hour urine collections and CKD patients without such sufficient 24-hour urine collections not analyzed.
STROBE Statement.
Supplementary Material
Supplementary Methods. Stability of multiple measurements of UKVs; Sequential conditional mean model approach; Bayesian measurement error models.
Supplementary Information. Mediation analysis.
Figure S1. Detailed patient flow diagram.
Table S1. Baseline characteristics of present patients with CKD for analyses using 7-time measurements by tertiles of baseline UKV based on 24-hour urine collections (N = 220).
Table S2. Associations of UKV with serum potassium (mEq/l) based on 3-time and 7-time measurements in CKD patients by GEE regression analyses for sensitivity analyses.
Table S3. Associations of UKV with serum potassium (mEq/l) based on 3-time and 7-time measurements in patients with CKD G3, G4, and G5 by GEE regression analyses for sensitivity analyses.
Table S4. Associations of UKV with hyperkalemia (> serum potassium 5.5 mEq/l) on the basis of 3-time and 7-time measurements in all CKD patients by GEE regression analyses for sensitivity analyses.
Table S5. Mean differences in serum potassium (mEq/l) per 10 mEq/d increase in continuous UKV (i.e., β with 95% CI) based on 3-time and 7-time measurements in all patients and patients with CKD G3, G4, and G5 by hierarchical Bayesian measurement error models for sensitivity analyses.
Table S6. Associations of UKV with hyperkalemia (> serum potassium 5.5 mEq/l) on the basis of 3-time and 7-time measurements in all CKD patients by hierarchical Bayesian measurement error models for sensitivity analyses.
Table S7. Baseline characteristics of CKD patients with four-time 24-hour urine collections and CKD patients without such sufficient 24-hour urine collections not analyzed.
STROBE Statement.
References
- 1.Clase C.M., Carrero J.J., Ellison D.H., et al. Potassium homeostasis and management of dyskalemia in kidney diseases: conclusions from a Kidney Disease: improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2020;97:42–61. doi: 10.1016/J.KINT.2019.09.018. [DOI] [PubMed] [Google Scholar]
- 2.Ikizler T.A., Burrowes J.D., Byham-Gray L.D., et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis. 2020;76(3 suppl 1):S1–S107. doi: 10.1053/J.AJKD.2020.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Morris A., Krishnan N., Kimani P.K., Lycett D. Effect of dietary potassium restriction on serum potassium, disease progression, and mortality in chronic kidney disease: a systematic review and meta-analysis. J Ren Nutr. 2020;30:276–285. doi: 10.1053/J.JRN.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Picard K., Barreto Silva M.I., Mager D., Richard C. Dietary potassium intake and risk of chronic kidney disease progression in predialysis patients with chronic kidney disease: a systematic review. Adv Nutr. 2020;11:1002–1015. doi: 10.1093/ADVANCES/NMAA027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turban S., Juraschek S.P., Miller E.R., et al. Randomized trial on the effects of dietary potassium on blood pressure and serum potassium levels in adults with chronic kidney disease. Nutrients. 2021;13 doi: 10.3390/NU13082678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noori N., Kalantar-Zadeh K., Kovesdy C.P., et al. Dietary potassium intake and mortality in long-term hemodialysis patients. Am J Kidney Dis. 2010;56:338–347. doi: 10.1053/J.AJKD.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramos C.I., González-Ortiz A., Espinosa-Cuevas A., et al. Does dietary potassium intake associate with hyperkalemia in patients with chronic kidney disease? Nephrol Dial Transplant. 2021;36:2049–2057. doi: 10.1093/NDT/GFAA232. [DOI] [PubMed] [Google Scholar]
- 8.Kieneker L.M., Bakker S.J.L., de Boer R.A., et al. Low potassium excretion but not high sodium excretion is associated with increased risk of developing chronic kidney disease. Kidney Int. 2016;90:888–896. doi: 10.1016/j.kint.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Smyth A., Dunkler D., Gao P., et al. The relationship between estimated sodium and potassium excretion and subsequent renal outcomes. Kidney Int. 2014;86:1205–1212. doi: 10.1038/ki.2014.214. [DOI] [PubMed] [Google Scholar]
- 10.Ma Y., He F.J., Sun Q., et al. 24-hour urinary sodium and potassium excretion and cardiovascular risk. N Engl J Med. 2022;386:252–263. doi: 10.1056/NEJMOA2109794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenga M.F., Kieneker L.M., Soedamah-Muthu S.S., et al. Urinary potassium excretion, renal ammoniagenesis, and risk of graft failure and mortality in renal transplant recipients1-3. Am J Clin Nutr. 2016;104:1703–1711. doi: 10.3945/ajcn.116.134056. [DOI] [PubMed] [Google Scholar]
- 12.Ogata S., Akashi Y., Sakusabe T., et al. A multiple 24-hour urine collection study indicates that kidney function decline is related to urinary sodium and potassium excretion in patients with chronic kidney disease. Kidney Int. 2022;101:164–173. doi: 10.1016/J.KINT.2021.10.030. [DOI] [PubMed] [Google Scholar]
- 13.Birukov A., Rakova N., Lerchl K., et al. Ultra-long-term human salt balance studies reveal interrelations between sodium, potassium, and chloride intake and excretion. Am J Clin Nutr. 2016;104:49–57. doi: 10.3945/ajcn.116.132951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginos B.N.R., Olde Engberink R.H.G. Estimation of sodium and potassium intake: current limitations and future perspectives. Nutrients. 2020;12:1–14. doi: 10.3390/nu12113275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Q., Bertrand K.A., Franke A.A., et al. Reproducibility of urinary biomarkers in multiple 24-h urine samples. Am J Clin Nutr. 2017;105:159–168. doi: 10.3945/AJCN.116.139758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Japan nephrology society [Special issue: Clinical practice guidebook for diagnosis and treatment of chronic kidney disease 2012] Nihon Jinzo Gakkai Shi. 2012;54:1034–1191. [PubMed] [Google Scholar]
- 17.Japan nephrology society Dietary recommendations for chronic kidney disease, 2014. Nihon Jinzo Gakkai Shi. 2014;56:553–599. [PubMed] [Google Scholar]
- 18.Dougher C.E., Rifkin D.E., Anderson C.A., et al. Spot urine sodium measurements do not accurately estimate dietary sodium intake in chronic kidney disease. Am J Clin Nutr. 2016;104:298–305. doi: 10.3945/ajcn.115.127423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ix J.H., Wassel C.L., Stevens L.A., et al. Equations to estimate creatinine excretion rate: the CKD epidemiology collaboration. Clin J Am Soc Nephrol. 2011;6:184–191. doi: 10.2215/CJN.05030610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horio M., Orita Y., Manabe S., et al. Formula and nomogram for predicting creatinine clearance from serum creatinine concentration. Clin Exp Nephrol. 1997;12:110–114. doi: 10.1007/BF02479909. [DOI] [Google Scholar]
- 21.Kaburaki S., Yoshimura E., Kojima N., et al. Improvement of renal function estimation equations for elderly Japanese people. Heal Sci Rep. 2018;1:e85. doi: 10.1002/HSR2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maroni B.J., Steinman T.I., Mitch W.E. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int. 1985;27:58–65. doi: 10.1038/ki.1985.10. [DOI] [PubMed] [Google Scholar]
- 23.Matsuo S., Imai E., Horio M., et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 24.Okada H. Essential points from Evidence-based Clinical Practice Guidelines for Chronic Kidney Disease 2018. Clin Exp Nephrol. 2019;23:1–15. doi: 10.1007/s10157-018-1648-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.R Core Team. The R project for statistical computing. Published Online 2022. https://www.r-project.org/
- 26.Heymsfield S.B., Arteaga C., McManus C.M., et al. Measurement of muscle mass in humans: validity of the 24-hour urinary creatinine method. Am J Clin Nutr. 1983;37:478–494. doi: 10.1093/AJCN/37.3.478. [DOI] [PubMed] [Google Scholar]
- 27.Polinder-Bos H.A., Nacak H., Dekker F.W., et al. Low urinary creatinine excretion is associated with self-reported frailty in patients with advanced chronic kidney disease. Kidney Int Rep. 2017;2:676–685. doi: 10.1016/J.EKIR.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valdivielso J.M., Balafa O., Ekart R., et al. Hyperkalemia in chronic kidney disease in the New Era of kidney protection therapies. Drugs. 2021;81:1467–1489. doi: 10.1007/S40265-021-01555-5. [DOI] [PubMed] [Google Scholar]
- 29.Yamada S., Inaba M. Potassium metabolism and management in patients with CKD. Nutrients. 2021:13. doi: 10.3390/NU13061751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keogh R.H., Daniel R.M., VanderWeele T.J., Vansteelandt S. Analysis of longitudinal studies with repeated outcome measures: adjusting for time-dependent confounding using conventional methods. Am J Epidemiol. 2018;187:1085–1092. doi: 10.1093/AJE/KWX311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaw P.A., Gustafson P., Carroll R.J., et al. Stratos guidance document on measurement error and misclassification of variables in observational epidemiology: part 2-more complex methods of adjustment and advanced topics. Stat Med. 2020;39:2232–2263. doi: 10.1002/SIM.8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartlett J.W., Keogh R.H. Bayesian correction for covariate measurement error: a frequentist evaluation and comparison with regression calibration. Stat Methods Med Res. 2018;27:1695–1708. doi: 10.1177/0962280216667764. [DOI] [PubMed] [Google Scholar]
- 33.Gritter M., Wouda R.D., Yeung S.M.H., et al. Effects of short-term potassium chloride supplementation in patients with CKD. J Am Soc Nephrol. 2022;33:1779–1789. doi: 10.1681/ASN.2022020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cupisti A., Kovesdy C.P., D’Alessandro C., Kalantar-Zadeh K. Dietary approach to recurrent or chronic hyperkalaemia in patients with decreased kidney function. Nutrients. 2018;10 doi: 10.3390/NU10030261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holbrook J.T., Patterson K.Y., Bodner J.E., et al. Sodium and potassium intake and balance in adults consuming self-selected diets. Am J Clin Nutr. 1984;40:786–793. doi: 10.1093/AJCN/40.4.786. [DOI] [PubMed] [Google Scholar]
- 36.Palmer B.F. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med. 2004;351:585–592. doi: 10.1056/NEJMRA035279. [DOI] [PubMed] [Google Scholar]
- 37.St-Jules D.E., Goldfarb D.S., Sevick M.A. Nutrient non-equivalence: does restricting high-potassium plant foods help to prevent hyperkalemia in hemodialysis patients? J Ren Nutr. 2016;26:282–287. doi: 10.1053/J.JRN.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.