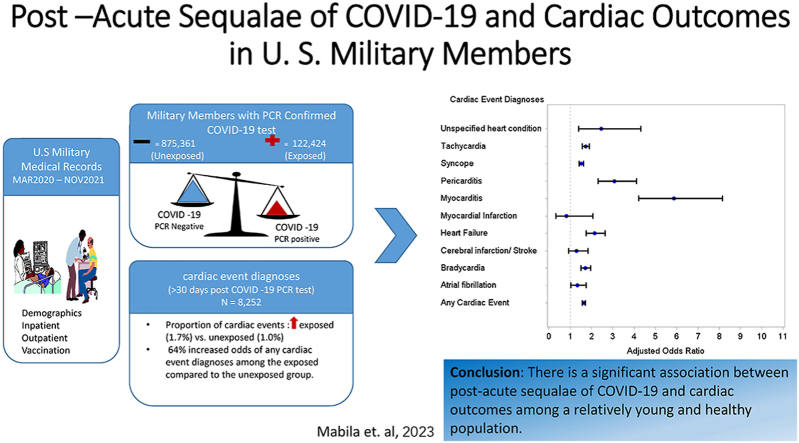
Abstract
Post –acute sequalae of COVID-19 (PASC) among U.S. military members remains unexplored. A cohort study of U. S. military members who had a COVID-19 test result, with the specimen collected between March 1, 2020 and November 30, 2021 was conducted. Demographic, inpatient and outpatient data including cardiac event diagnoses were extracted from electronic medical records and compared COVID-19 test-positive and COVID-19 test-negative service members. We used univariate and multivariable logistic regression methods to determine the effect PASC on select cardiac events. Among 997,785 service members, 15,779 (1.6%) were diagnosed with a cardiac event. In fully adjusted models, PASC was significantly associated with increased odds of any cardiac event [OR =1.64 (95% CI: 1.57, 1.71]. PASC was associated with increased odds of myocarditis [OR = 5.86 (95% CI: 4.22, 8.15)], pericarditis [OR =3.08 (95% CI: 2.31, 4.11)], syncope [OR =1.52 (95% CI: 1.41, 1.63)], tachycardia [OR =1.72 (95% CI: 1.56, 1.89)], heart failure [OR =2.15 (95% CI: 1.76, 2.63)], bradycardia [OR =1.71 (95% CI: 1.50, 1.96)], and atrial fibrillation [OR =1.33(95% CI: 1.02, 1.74)] in fully adjusted models. In a sensitivity analysis of military members with no history of cardiac events, PASC was still significantly associated with increased odds of any cardiac event [OR =1.75 (95% CI: 1.67, 1.84)]. In conclusion, we observed a significant association between PASC and cardiac outcomes including; myocarditis, pericarditis, and heart failure. These associations were observed in a relatively young and healthy population and among those without pre-existing cardiac diagnoses.
Keywords: Myocarditis, COVID-19, PASC, Pericarditis
Graphical abstract
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has impacted the health and readiness of the United States (U. S.) military, with over 650,000 positive cases identified in military members and military health system (MHS) beneficiaries. Patients with COVID-19 exhibit a wide range of symptoms and clinical events, having both short- and long-term health consequences [[1], [2], [3], [4]]. Although most people who have common COVID-19 symptomology recover completely within a few weeks, some people, referred to as “long haulers” or “long COVID-19″ experience symptoms long after their initial recovery (generally longer than 4 weeks after diagnosis) [5,6]. COVID-19 has been recognized as a multi-organ disease with effects on a broad spectrum of clinical manifestations [[7], [8], [9]]. Persistent symptoms have also been observed in survivors of previous coronavirus diseases, such as the severe acute respiratory syndrome (SARS) epidemic of 2003 and the Middle East respiratory syndrome (MERS) outbreak of 2012 [[10], [11], [12], [13], [14]]. Recent literature has specifically highlighted sequelae of COVID-19 on cardiovascular health [7,[15], [16], [17], [18], [19], [20], [21]]. Despite this preliminary evidence, the full extent of long-term outcomes of COVID-19 on cardiovascular health remains largely unknown. Cardiovascular disease has historically been considerably lower in military populations compared to the general public, likely due to military members being younger, more physically active, and in otherwise good health at time of entry into military service [22]. The aim of this study is to evaluate the effect of post-acute sequelae of COVID-19, and the odds of cardiac events among active component military service members.
2. Methods
2.1. Data source
The Armed Forces Health Surveillance Division (AFHSD) line list of COVID-19 cases was used in this study. AFHSD has been tasked with daily surveillance of COVID-19 cases. As part of this surveillance, AFHSD compiles a line list of COVID-19 tests among military members and beneficiaries. This list is updated daily and includes laboratory confirmed records of positive reverse transcription-polymerase chain reaction (RT-PCR) and antigen tests from Health Level 7 (HL-7) formatted Composite Health Care System (CHCS) and Military Health System (MHS) GENESIS data extracts provided by the Defense Centers for Public Health-Portsmouth EpiData Center (EDC). In addition, it contains confirmed and probable cases of COVID-19 reported in the Disease Reporting System Internet (DRSi) according to Department of Defense (DoD) and CDC reportable medical event case reporting guidelines [23].
The Defense Medical Surveillance System (DMSS); an electronic database that contains personnel, inpatient, outpatient, and in-theater medical encounters, and other data for the DoD was used to capture cardiac medical encounters using specific International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10) diagnostic codes. DMSS was also used to identify demographic information for the study population.
2.2. Study population
The study population included U. S. active component military members with a SARS-CoV-2 laboratory test conducted between March 1, 2020 and November 30, 2021. Military members from the Reserves or National Guard were excluded. The final study population consisted of 997,785 military members of which 122,424 (12.3%) were COVID-19 positive and 875,361 (87.7%) were COVID-19 negative. This active component U.S military retrospective study was reviewed by the Component Office for Human Research Protections (COHRP), Defense Health Agency (DHA) Office of Research Protections (ORP), which determined that the proposed activity does not constitute research as defined at 32 CFR 219.102(l) and as implemented through Department of Defense Instruction DoDI 3216.02.
2.3. COVID-19 status
The incident date of COVID-19 was defined as the earliest sample collection date of a positive RT-PCR test. The first incident date of COVID-19 was used for individuals who tested positive multiple times during the study period. Participants were COVID-19 exposed if they had a positive PCR test during the surveillance period and they were considered COVID-19 unexposed if they had a negative PCR test and negative antigen or “unknown” test in instances where they had a PCR test and another test (antigen or ‘unknown’). Immunization records were queried to determine COVID-19 vaccination status (Yes/No). An individual was considered COVID-19 vaccinated on the date they completed a vaccine series based on the vaccine used.
2.4. Cardiac medical encounters
Inpatient, outpatient, and in-theater medical encounter data were queried for the following cardiac outcomes based on the specified ICD-10 diagnostic code in the first and second diagnosis position. An asterisk indicates that any subsequent digit or character was included.
-
•
Myocardial infarction (I21*)
-
•
Cerebral infarction/Stroke (I63*)
-
•
Pericarditis (I30.0, I30.1, I30.8, I30.9, I32, M32.12)
-
•
Atrial fibrillation (I48.91, I48.0)
-
•
Syncope (R55)
-
•
Tachycardia (R00.0)
-
•
Heart Failure (I50.9, I51.7)
-
•
Bradycardia (R00.1)
-
•
Myocarditis (I51.4, I40.0, I40.1, I40.8, I40.9, I01.2, I09.0)
-
•
Unspecified heart disease (I51.9)
-
•
And a total group of any of the above cardiac events; “any cardiac events”
An individual was counted once for each cardiac outcome that occurred. If a military member had multiple cardiac events of the same type, the cardiac event closest to the COVID-19 test date was used.
2.5. Statistical analyses
SAS V9.4 software was used for all statistical analysis (SAS Institute, Cary, NC)). The outcome of interest was cardiac event and the independent variable was COVID-19 test status. Covariates of interest in this study were: age, race/ethnicity, sex, service, rank, vaccination status, and education. Time-varying characteristics were measured at the time of the COVID-19 test. Age group was stratified into seven groups by 5-year increments from <20 to 45+, sex was dichotomized to male and female, and vaccination status was dichotomized into yes or no. Race/ethnic group was stratified as non-Hispanic White, non-Hispanic Black, Hispanic, and unknown/other. Military rank [Enlisted (E), officers (O), and warrant officers (WO)] was grouped into 5 groups: E1-E4, E5-E9, O1–O3, O4–O10, and WO. Education was grouped into 4 categories: High school or less, some education, bachelors or advanced degree, and other/unknown.
Distribution of COVID-19 test status by demographic characteristics was determined. We determined how many military members were lost to follow up at the end of the study surveillance period (November 30, 2021). The incidence of each cardiac event in our population during the 20 month-long surveillance period was calculated and we also assessed whether cardiac events occurred before or after an individual was fully vaccinated.
To determine whether cardiac events occurred as a result of complications of acute COVID-19, or they were due to post-acute sequalae of COVID-19 (PASC), a 30 day lag was used as a proxy. Cardiac events occurring within 30 days after a COVID-19 test were considered complications of acute COVID-19 and cardiac events occurring after 30 days from COVID-19 test date were considered PASC.
Covariates included in the fully adjusted models were determined a priori. To ensure that regression analysis assessed cardiac events that are attributed to PASC, only cardiac events that occurred >30 days after COVID-19 testing were included in the analysis. Crude logistic regression models were built to evaluate PASC and diagnosis with select cardiac events. Multivariable logistic regression models were then used to assess the association between PASC and select cardiac events adjusting for age, race/ethnicity, sex, service, rank, vaccination status, and education. Due to some military members having a history of cardiac events (Supplementary Table 1), a sensitivity analysis of military members without any prior history of cardiac events was performed.
3. Results
In this retrospective cohort of 997,785 military service members who had a PCR confirmed COVID-19 test between March 1, 2020 and November 30, 2021, there was a total of 122,424 military members who had COVID-19 (exposed group) accounting for 12.3% of the study population. The study population was predominantly male (80.2%), non-Hispanic white (54.2%), in the Army (43.6%), and fully vaccinated during the surveillance period (88.5%) (Table 1). The mean age at COVID-19 testing was 27.8 years (STD = 8.2). There was a statistically significant difference between the COVID-19 test -positive and the COVID-19 test -negative group for all included demographic variables (P < 0.01). All military members in the study were confirmed to be active duty at the beginning of the study and 6.1% (N = 68,882) were lost to follow-up at the end of the study. Of those lost to follow-up, 69.8% (N = 48,065) separated from the military. Descriptive statistics by COVID-19 status are detailed in Table 1.
Table 1.
Demographic Characteristics for COVID -19 tested U. S. military members, March 1, 2020–November 30, 2021.
| Variable | COVID-19 test -positive |
COVID-19 test -negative |
Total |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Overall | 122,424 (100) | 875,361 (100) | 997,785 (100) |
| Sex | |||
| Male | 98,990 (80.86) | 701,090 (80.09) | 800,080 (80.19) |
| Female | 23,434 (19.14) | 174,271 (19.91) | 197,705 (19.81) |
| Age Group | |||
| <20 | 14,813 (12.1) | 106,654 (12.18) | 121,467 (12.17) |
| 20–24 | 42,375 (34.61) | 277,882 (31.74) | 320,257 (32.1) |
| 25–29 | 26,425 (21.58) | 183,052 (20.91) | 209,477 (20.99) |
| 30–34 | 16,601 (13.56) | 124,427 (14.21) | 141,028 (14.13) |
| 35–39 | 11,949 (9.76) | 92,554 (10.57) | 104,503 (10.47) |
| 40–44 | 6114 (4.99) | 50,147 (5.73) | 56,261 (5.64) |
| 45+ | 4147 (3.39) | 40,645 (4.64) | 44,792 (4.49) |
| Race/Ethnic group | |||
| Non-Hispanic White | 62,789 (51.29) | 478,419 (54.65) | 541,208 (54.24) |
| Non-Hispanic Black | 23,751 (19.4) | 147,292 (16.83) | 171,043 (17.14) |
| Hispanic | 24,141 (19.72) | 151,519 (17.31) | 175,660 (17.6) |
| Other/Unknown | 11,743 (9.59) | 98,131 (11.21) | 109,874 (11.01) |
| Military Branch of Service | |||
| Army | 56,341 (46.02) | 378,460 (43.23) | 434,801 (43.58) |
| Navy | 26,919 (21.99) | 197,052 (22.51) | 223,971 (22.45) |
| Air Force | 25,397 (20.75) | 202,438 (23.13) | 227,835 (22.83) |
| Marines | 13,767 (11.25) | 97,411 (11.13) | 111,178 (11.14) |
| Rank | |||
| E1-E4 | 63,386 (51.78) | 427,774 (48.87) | 491,160 (49.23) |
| E5-E9 | 42,939 (35.07) | 304,376 (34.77) | 347,315 (34.81) |
| O1–O3 | 9375 (7.66) | 78,153 (8.93) | 87,528 (8.77) |
| O4–O10 | 5126 (4.19) | 52,845 (6.04) | 57,971 (5.81) |
| WO | 1598 (1.31) | 12,213 (1.4) | 13,811 (1.38) |
| Education | |||
| High School or less | 83,483 (68.19) | 556,672 (63.59) | 640,155 (64.16) |
| Some College | 13,971 (11.41) | 104,835 (11.98) | 118,806 (11.91) |
| Bachelors or advanced degree | 22,255 (18.18) | 189,863 (21.69) | 212,118 (21.26) |
| Other/unknown | 2715 (2.22) | 23,991 (2.74) | 26,706 (2.68) |
Prepared by Armed Forces Health Surveillance Division.
Public Health Directorate, Defense Health Agency.
Source: Defense Medical Surveillance System (DMSS), Composite Health Care System (CHCS) Health Level 7 (HL-7), Military health System (MHS) Genesis data, and Disease Reporting System Internet (DRSi) October 2022.
A total of 15,779 (1.6%) military members were diagnosed with a cardiac event; 2896 were COVID-19 positive and 12,883 were COVID-19 negative. The proportion of cardiac events that occurred on the day of and after COVID-19 vaccination were slightly higher among the exposed compared to the unexposed; 0.77% versus 0.68%. Prior to being vaccinated, the proportion of cardiac events diagnoses was significantly higher in the exposed compared to the unexposed; 1.22% and 0.55%, respectively. The average number of days between COVID-19 testing and any cardiac event was 144.2 days (STD = 124.4). The highest number of cardiac events was among military members in the age category of 20–24 years with 30.6% (n = 4828) of all cardiac diagnoses (data not shown). The proportion of hospitalization due to cardiac events was relatively low overall (0.01%) but it was higher for the exposed compared to the unexposed group; 0.03% and 0.01%, respectively.
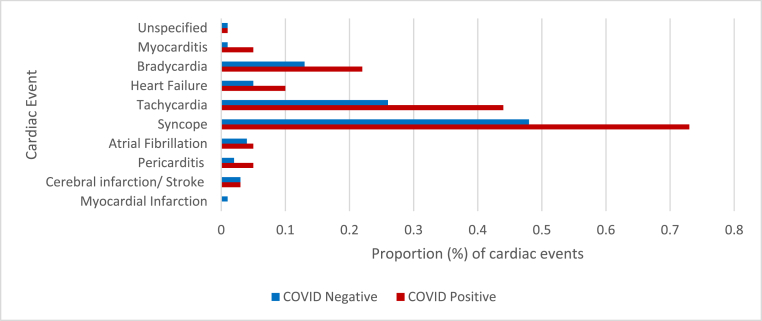
Overall, 29.3% (n = 4631) of all cardiac events occurred within 30 days after testing positive for COVID-19. Among those who tested positive for COVID-19 and had a cardiac event (n = 2986), 28.2% (n = 816) of cardiac events were a result of complications of acute COVID-19 (occurred within 30 days after testing positive for COVID-19) and 71.8% (n = 2080) were attributed to PASC (occurred >30 days after COVID -19 positive test). Fig. 1 shows the proportion of cardiac events attributed to PASC for the exposed and unexposed. The top three cardiac events were the same for the exposed and unexposed group: syncope, tachycardia, and bradycardia (Fig. 1). However, the proportion of cardiac events was slightly higher among the exposed compared to the unexposed (Fig. 1); overall, 1.7% of the exposed group had a cardiac event compared to 1.0% among the unexposed (results not shown).
Fig. 1.
Proportion (%) of all COVID-19 exposed and unexposed U.S. military service members that had a cardiac diagnosis, March 1, 2020–November 30, 2021.
For logistic regression, we only counted cardiac events that were diagnosed >30 days after COVID-19 testing to delineate the impact of long COVID-19 in the study population. In crude models, compared to the unexposed, PASC was significantly associated with 61% increased odds of any cardiac event [OR = 1.61 (95% CI: 1.54, 1.68)] (Table 2). PASC was also significantly associated with increased odds of all cardiac outcomes except for myocardial infarction, cerebral infarction/stroke and atrial fibrillation (Table 2). After adjusting for age, race/ethnic, sex, service, rank, vaccination status, and education, PASC was still significantly associated with increased odds of any cardiac event [OR = 1.64 (95% CI: 1.57, 1.71)]. Compared to the unexposed, COVID-19 was associated with >5-fold increased odds of myocarditis [OR = 5.86 (95% CI: 4.22, 8.15)] (Table 2). Moreover, COVID-19 was significantly associated with increased odds of pericarditis [OR = 3.08 (95% CI: 2.31, 4.11)], syncope [OR = 1.52 (95% CI: 1.41, 1.63)], tachycardia [OR = 1.72 (95% CI: 1.56, 1.89)], heart failure [OR = 2.15 (95% CI: 1.76, 2.63)], bradycardia [OR = 1.71 (95% CI: 1.50, 1.96)], and atrial fibrillation [OR = 1.33(95% CI: 1.02, 1.74)] (Table 2).
Table 2.
Association of COVID -19 and cardiac event diagnoses among U. S. military members, March 1, 2020–November 30, 2021.
| Cardiac Events | Crude OR (95%CI) | Adjusted ORa (95%CI) |
|---|---|---|
| Myocardial Infarction | 0.75 (0.3,1.87) | 0.82 (0.33,2.07) |
| Cerebral infarction/Stroke | 1.22 (0.87,1.71) | 1.3 (0.93,1.83) |
| Pericarditis | 3.15 (2.37,4.2) | 3.08 (2.31,4.11) |
| Atrial fibrillation | 1.18 (0.9,1.53) | 1.33 (1.02,1.74) |
| Syncope | 1.51 (1.4,1.62) | 1.52 (1.41,1.63) |
| Tachycardia | 1.68 (1.53,1.85) | 1.72 (1.56,1.89) |
| Heart Failure | 2.08 (1.71,2.54) | 2.15 (1.76,2.63) |
| Bradycardia | 1.65 (1.44,1.88) | 1.71 (1.5,1.96) |
| Myocarditis | 5.89 (4.24,8.17) | 5.86 (4.22,8.15) |
| Unspecified | 2.24 (1.28,3.94) | 2.46 (1.4,4.32) |
| Any Cardiac Event | 1.61 (1.54,1.68) | 1.64 (1.57,1.71) |
Prepared by Armed Forces Health Surveillance Division.
Public Health Directorate, Defense Health Agency.
Source: Defense Medical Surveillance System (DMSS), Composite Health Care System (CHCS) Health Level 7 (HL-7), Military health System (MHS) Genesis data, and Disease Reporting System Internet (DRSi), October 2022.
Adjusted for age, race/ethnic, sex, service, rank, vaccination status, and education.
In a sensitivity analysis of military members without any history of any cardiac event diagnosis prior to COVID-19 testing, we observed similar findings (Table 3). COVID-19 was still significantly associated with increased odds of any cardiac event [OR = 1.75 (95% CI: 1.67, 1.84)] compared to COVID-19 negative group in a fully adjusted model (Table 3). There was also an almost >7 times increased odds of myocarditis [OR = 6.97 (95% CI: 4.78, 10.15)] and over 3 times increased odds of pericarditis [OR = 3.16 (95% CI: 2.27, 4.41)] in the exposed group compared to the unexposed group (Table 3). In addition, there were significant odds of syncope, tachycardia, heart failure, bradycardia, and unspecified heart disease (Table 3). Adjusted odds of myocardial infarction are 35% higher in the exposed group even though this increase is not statistically significant.
Table 3.
Association of COVID -19 and cardiac event diagnoses among U. S. military members without any prior cardiac events, March 1, 2020–November 30, 2021.
| Cardiac Events | Crude OR (95%CI) | Adjusted ORa (95%CI) |
|---|---|---|
| Myocardial Infarction | 1.23 (0.48,3.18) | 1.35 (0.52, 3.51) |
| Cerebral infarction/Stroke | 1.12 (0.71, 1.76) | 1.18 (0.75, 1.85) |
| Pericarditis | 3.28 (2.35, 4.57) | 3.16 (2.27, 4.41) |
| Atrial fibrillation | 1.35 (0.90, 2.02) | 1.47 (0.98, 2.21) |
| Syncope | 1.53 (1.41,1.66) | 1.53 (1.41, 1.66) |
| Tachycardia | 1.75 (1.58, 1.95) | 1.78 (1.60, 1.98) |
| Heart Failure | 2.45 (1.95, 3.10) | 2.50 (1.98, 3.16) |
| Bradycardia | 1.69 (1.46, 1.96) | 1.74 (1.50, 2.02) |
| Myocarditis | 7.00 (4.81, 10.20) | 6.97 (4.78, 10.15) |
| Unspecified | 2.85 (1.53, 5.30) | 3.07 (1.65, 5.72) |
| Any Cardiac Event | 1.73 (1.65, 1.82) | 1.75 (1.67, 1.84) |
Prepared by Armed Forces Health Surveillance Division.
Public Health Directorate, Defense Health Agency.
Source: Defense Medical Surveillance System (DMSS), Composite Health Care System (CHCS) Health Level 7 (HL-7), Military health System (MHS) Genesis data, and Disease Reporting System Internet (DRSi), October 2022.
Adjusted for age, race/ethnic, sex, service, rank, vaccination status, and education.
4. Discussion
This retrospective cohort investigates the relationship between PASC and various cardiac events in a U. S. military cohort using laboratory diagnoses and medical encounter records. We observed a strong association (75% increased odds) between PASC and diagnosis with any cardiac event among military members who had no history of any cardiac events. There were increased odds of pericarditis, syncope, heart failure, bradycardia and myocarditis among the exposed group compared to the unexposed group. This was observed in a relatively young population where the average age at cardiac diagnoses was <30 years old. Our findings are similar to other studies that have shown the association between PASC and cardiac events. In a study of Veteran Health Administration (VHA) who had acute COVID-19 (n = 73,435) compared to all other VHA users (n = 4,990,835), there was a significant increased risk of cardiovascular outcomes including acute coronary disease, bradycardia, heart failure, and tachycardia post-acute COVID-19 [24]. With the exception of acute coronary disease, these associations were evident in individuals who were not hospitalized with COVID-19 [24]. Similar findings were also observed in another study. In a cross-sectional study of 442 adults enrolled in the COVID-19 Hopkins Opportunity Participant Engagement (HOPE) registry, more than one third of people who had acute COVID-19 reported cardiac-related PASC symptoms [25].
Hospitalization due to COVID-19 was also very low in this study (0.4%). This could be attributed to the healthy worker effect in the U. S. Military population. However, PASC is still significantly associated with increased odds of cardiac event diagnoses. This evidence further suggests that PASC is still a health concern for non-hospitalized individuals who might have experienced mild-to-moderate COVID-19 symptoms.
Of all the cardiac events investigated in this study, COVID-19 had the strongest association with increased odds of myocarditis [OR = 5.86 (95% CI: 4.22, 8.15)]. Myocarditis has been shown to be a complication of COVID-19 [[26], [27], [28], [29]]. The increased odds of myocarditis post COVID-19 are therefore not surprising due to the general high incidence of cardiac injury that has been reported among COVID-19 patients. Residual post myocarditis abnormalities or new onset of cardiac injury during the convalescent phase after COVID-19 are to be expected. Even though there are still no demonstrations of COVID-19 genome in cardiac tissue of patients with clinical myocarditis, there is evidence of mechanisms that leads to increased myocardial injury [30]. In a recent systematic review of cardiac sequelae after COVID-19 recovery, there was evidence to suggest that COVID-19 is associated with persistent cardiac injury after recovery, especially myocardial injury [31]. The review showed that COVID-19 survivors had a higher risk of heart failure, arrhythmias, and myocardial infarction. In this study we also see an onset or persistence of cardiac events >30 days after COVID-19 diagnoses; of the 2896 cardiac diagnoses among the exposed, 71.8% (n = 2080) occurred >30 days after a positive COVID-19 test. We also observed a significant association between PASC and heart failure [OR = 2.50 (95% CI: 1.98, 3.16)]. Even after excluding military members with any history of cardiac events, 1639 (78.8%) cardiac diagnoses occurred >30 days after a positive COVID-19 test and there was 75% increased odds of any cardiac event diagnoses.
There has been evidence to show that vaccinated individuals have less severe symptoms if they get COVID-19 [32,33]. As a result, the number of cardiac events among military members might decrease as they become fully vaccinated. The military mandated that all active duty service members must be fully vaccinated by the end of 2021 and this change potentially changed the incidence of COVID-19 and PASC in 2021. The proportion of fully vaccinated military members was relatively the same among the exposed (87.6%) and unexposed (88.6%) at the end of the surveillance period in this study. However, the number of any cardiac events among the exposed was lower post-vaccination compared to pre-vaccination; 1495 compared to 928 cardiac events, respectively. There was also a small number of cardiac events that occurred on the day the military member completed a vaccination series (n = 17).
4.1. Limitations
One major limitation of the study was the lack of data on recovery time. To account for this, we used a 30-day proxy for recovery and only counted cardiac outcomes that occurred after >30 days post COVID-19 testing in regression analysis. This is in agreement with the CDC's definition (4-week time frame) of post-COVID-19 conditions to emphasize the importance of clinical evaluation and care early. Another limitation is that we used the first COVID-19 test date and this does not consider individuals who might have gotten COVID-19 multiple times during the study period. As a result, our results may be overstating the effect of PASC as it relates to cardiac outcome; an individual who had COVID-19 multiple times and their cardiac outcomes were due to acute COVID-19 symptoms are not excluded in this study. Future studies should consider either excluding these individuals and or restarting their time to recovery as multiple COVID-19 infections may inflict multiple cardiac insults. Another limitation of this study is that it did not exclude vaccination-related cardiac events that have been seen in COVID-19 vaccines, especially mRNA vaccines. There has been evidence to suggest that mRNA COVID-19 vaccines can result in myocarditis post-vaccination with the highest incidence being among young males [34,35]. Since this study consists of predominantly young males, further investigating the effect of vaccination-related cardiac events would be beneficial in future studies. However, in our study there was a noticeable change in the percent of cardiac events among those who had COVID-19 when comparing pre and post vaccination cardiac events (1.22% vs 0.76%, respectively) indicating a positive effect of COVID-19 vaccination.
Due to lack of comprehensive smoking information in our data, this study did not adjust for smoking, a major cause of cardiac disease. This might result in the overestimation of PASC as it relates to cardiac outcomes. There is evidence to suggest that smoking results in increased risk of major adverse cardiovascular events among patients hospitalized with SARS-CoV-2 infection [35]. Moreover, this study did not adjust for non-cardiac conditions and or medications that military members were using prior to COVID-19 infection or medications that they might have been prescribed to manage their COVID-19 symptoms. Our population consists of relatively young and healthy military members who tend not to be heavily medicated hence reducing the likelihood of pre-COVID-19 infection medication impact on PASC related cardiac events. Furthermore, only 0.4% of the study population was hospitalized meaning that our population mostly had mild to moderate COVID-19 symptoms that most likely did not require medication to manage acute COVID-19. However, future studies should consider the impact of medications that might have unfavorable or protective effects on cardiac conditions.
Also, other concomitant conditions that might lead to cardiac injury onset or exacerbate cardiac conditions were not adjusted for in this study. These conditions might have an impact on the effect of PASC and cardiac events. This study did not consider COVID-19 variants and potential differences in cardiac outcomes. Finally, this study is not generalizable to the entire U. S. population since it uses a U. S. military population, which is relatively younger and healthier than the general U. S. population.
5. Conclusion
In this study of U. S. military members who were tested for SARS-CoV-2, we examined the relationship between COVID-19 and select cardiac events. We observed a significant association between PASC and myocarditis, pericarditis, syncope, bradycardia, and heart failure. This was observed in a population with very low COVID-19 related hospitalizations. These associations were observed in a relatively young and healthy population and among those without pre-existing cardiac diagnoses.
Handling Editor: D Levy
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcrp.2023.200183.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Salepci E., Turk B., Ozcan S.N., Bektas M.E., Aybal A., Dokmetas I., Turgut S. vol. 278. European Archives of Oto-Rhino-Laryngology; 2021. pp. 525–535. (Symptomatology of COVID-19 from the Otorhinolaryngology Perspective: a Survey of 223 SARS-CoV-2 RNA-Positive Patients). 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dixon B.E., Wools-Kaloustian K.K., Fadel W.F., Duszynski T.J., Yiannoutsos C., Halverson P.K., Menachemi N. Symptoms and symptom clusters associated with SARS-CoV-2 infection in community-based populations: results from a statewide epidemiological study. PLoS One. 2021;16(3) doi: 10.1371/journal.pone.0241875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boscolo-Rizzo P., Borsetto D., Fabbris C., Spinato G., Frezza D., Menegaldo A., Mularoni F., Gaudioso P., Cazzador D., Marciani S., Frasconi S., Ferraro M., Berro C., Varago C., Nicolai P., Tirelli G., Da Mosto M.C., Obholzer R., Rigoli R., Polesel J., Hopkins C. Evolution of altered sense of smell or taste in patients with mildly symptomatic COVID-19. JAMA Otolaryngol Head Neck Surg. 2020;146(8):729–732. doi: 10.1001/jamaoto.2020.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wanga V., Chevinsky J.R., Dimitrov L.V., Gerdes M.E., Whitfield G.P., Bonacci R.A., Nji M.A.M., Hernandez-Romieu A.C., Rogers-Brown J.S., McLeod T., Rushmore J., Lutfy C., Bushman D., Koumans E., Saydah S., Goodman A.B., Coleman King S.M., Jackson B.R., Cope J.R. Long-term symptoms among adults tested for SARS-CoV-2—United States, January 2020–April 2021. MMWR (Morb. Mortal. Wkly. Rep.) 2021;70:1235. doi: 10.15585/mmwr.mm7036a1. 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubin R. As their numbers grow, COVID-19 “long haulers” stump experts. JAMA. 2020;14:1381–1383. doi: 10.1001/jama.2020.17709. 324. [DOI] [PubMed] [Google Scholar]
- 6.Marshall M. The lasting misery of coronavirus long-haulers. Nature. 2020;585(7825):339–342. doi: 10.1038/d41586-020-02598-6. [DOI] [PubMed] [Google Scholar]
- 7.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., Cook J.R., Nordvig A.S., Shalev D., Sehrawat T.S., Ahluwalia N., Bikdeli B., Dietz D., Der-Nigoghossian C., Liyanage-Don N., Rosner G.F., Bernstein E.J., Mohan S., Beckley A.A., Seres D.S., Choueiri T.K., Uriel N., Ausiello J.C., Accili D., Freedberg D.E., Baldwin M., Schwartz A., Brodie D., Garcia C.K., Elkind M.S.V., Connors J.M., Bilezikian J.P., Landry D.W., Wan E.Y. Post-acute COVID-19 syndrome. Nat. Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Behzad S., Aghaghazvini L., Radmard A.R., Gholamrezanezhad A. Extrapulmonary manifestations of COVID-19: radiologic and clinical overview. Clin. Imag. 2020;66:35–41. doi: 10.1016/j.clinimag.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korompoki E., Gavriatopoulou M., Hicklen R.S., et al. Epidemiology and organ specific sequelae of post-acute COVID19: a narrative review. J. Infect. 2021;83(1):1–16. doi: 10.1016/j.jinf.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed H., Patel K., Greenwood D.C., Halpin S., Lewthwaite P., Salawu A., Eyre L., Breen A., O'Connor R., Jones A., Sivan M. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. J. Rehabil. Med. 2020;52(5) doi: 10.2340/16501977-2694. [DOI] [PubMed] [Google Scholar]
- 11.Hui D.S., Joynt G.M., Wong K.T., Gomersall C.D., Li T.S., Antonio G., Ko F.W., Chan M.C., Chan D.P., Tong M.W., Rainer T.H., Ahuja A.T., Cockram C.S., Sung J.J. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60(5):401–409. doi: 10.1136/thx.2004.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S.H., Shin H.S., Park H.Y., Kim J.L., Lee J.J., Lee H., Won S.D., Han W. Depression as a mediator of chronic fatigue and post-traumatic stress symptoms in Middle East respiratory syndrome survivors. Psychiatry Investig. 2019;16(1):59–64. doi: 10.30773/pi.2018.10.22.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ong K.C., Ng A.W., Lee L.S., Kaw G., Kwek S.K., Leow M.K., Earnest A. Pulmonary function and exercise capacity in survivors of severe acute respiratory syndrome. Eur. Respir. J. 2004;24(3):436–442. doi: 10.1183/09031936.04.00007104. [DOI] [PubMed] [Google Scholar]
- 14.Moldofsky H., Patcai J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol. 2011;11:37. doi: 10.1186/1471-2377-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johansson M., Ståhlberg M., Runold M., Nygren-Bonnier M., Nilsson J., Olshansky B., Bruchfeld J., Fedorowski A. Long-haul post-COVID-19 symptoms presenting as a variant of postural orthostatic tachycardia syndrome: the Swedish experience. JACC Case Rep. 2021;3(4):573–580. doi: 10.1016/j.jaccas.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan W., Aboulhosn J. The cardiovascular burden of coronavirus disease 2019 (COVID-19) with a focus on congenital heart disease. Int. J. Cardiol. 2020;309:70–77. doi: 10.1016/j.ijcard.2020.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saed Aldien A., Ganesan G.S., Wahbeh F., Al-Nassr N., Altarawneh H., Al Theyab L., Saed Aldien S., Tomerak S., Naveed H., Elshazly M.B., Zakaria D. Systemic inflammation may induce cardiac injury in COVID-19 patients including children and adolescents without underlying cardiovascular diseases: a systematic review. Cardiovasc. Revascularization Med. 2022;35:169–178. doi: 10.1016/j.carrev.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magadum A., Kishore R. Cardiovascular manifestations of COVID-19 infection. Cells. 2020;9(11):2508. doi: 10.3390/cells9112508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tajbakhsh A., Gheibi Hayat S.M., Taghizadeh H., Akbari A., Inabadi M., Savardashtaki A., Johnston T.P., Sahebkar A. COVID-19 and cardiac injury: clinical manifestations, biomarkers, mechanisms, diagnosis, treatment, and follow up. Expert Rev. Anti Infect. Ther. 2021;19(3):345–357. doi: 10.1080/14787210.2020.1822737. [DOI] [PubMed] [Google Scholar]
- 20.Bandyopadhyay D., Akhtar T., Hajra A., Gupta M., Das A., Chakraborty S., Pal I., Patel N., Amgai B., Ghosh R.K., Fonarow G.C., Lavie C.J., Naidu S.S. COVID-19 pandemic: cardiovascular complications and future implications. Am. J. Cardiovasc. Drugs. 2020;20(4):311–324. doi: 10.1007/s40256-020-00420-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puntmann V.O., Carerj M.L., Wieters I., Fahim M., Arendt C., Hoffmann J., Shchendrygina A., Escher F., Vasa-Nicotera M., Zeiher A.M., Vehreschild M., Nagel E. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Donnell F.L., Stahlman S., Oetting A.A. Incidence rates of diagnoses of cardiovascular diseases and associated risk factors, active component, U. S. Armed Forces. MS. 2018;25(3):12–18. 2007-2016. [PubMed] [Google Scholar]
- 23.Center for Disease Control and Prevention (Cdc) Reporting COVID-19 cases to CDC. 2021. https://www.cdc.gov/coronavirus/2019-ncov/downloads/php/COVID19-CSV-Case-Reporting-Instructions.pdf Published November. Accessed.
- 24.Al-Aly Z., Xie Y., Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594(7862):259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- 25.Ogungbe O., Gilotra N.A., Davidson P.M., Farley J.E., Himmelfarb C.R.D., Post W.S., Commodore-Mensah Y. Cardiac postacute sequelae symptoms of SARS-CoV-2 in community-dwelling adults: cross-sectional study. Open Heart. 2022;9(2) [Google Scholar]
- 26.Farshidfar F., Koleini N., Ardehali H. Cardiovascular complications of COVID-19. JCI Insight. 2021;6(13) doi: 10.1172/jci.insight.148980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhatraju P.K., Ghassemieh B.J., Nichols M., et al. Covid-19 in critically ill patients in the seattle region - case series. N. Engl. J. Med. 2020 May 21;382(21):2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., Gong W., Liu X., Liang J., Zhao Q., Huang H., Yang B., Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in wuhan, China. JAMA Cardiol. 2020 Jul 1;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramadan M.S., Bertolino L., Zampino R., Durante-Mangoni E. Monaldi Hospital Cardiovascular Infection Study Group. Cardiac sequelae after coronavirus disease 2019 recovery: a systematic review. Clin. Microbiol. Infect. 2021 Sep;27(9):1250–1261. doi: 10.1016/j.cmi.2021.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chidambaram V., Kumar A., Calcaterra G., Mehta J.L. Persistent cardiac injury - an important component of long COVID-19 syndrome. EBioMedicine. 2022 Mar;77 doi: 10.1016/j.ebiom.2022.103892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stahlman S., Hiban K., Mahaney H., Ford S. Incident COVID-19 infections, active and reserve components. MS. 2021 Dec;28(12):14–21. 1 January 2020-31 August 2021. [PubMed] [Google Scholar]
- 33.Sadoff J., Gray G., Vandebosch A., Cárdenas V., Shukarev G., Grinsztejn B., Goepfert P.A., Truyers C., Fennema H., Spiessens B., Offergeld K., Scheper G., Taylor K.L., Robb M.L., Treanor J., Barouch D.H., Stoddard J., Ryser M.F., Marovich M.A., Neuzil K.M., Corey L., Cauwenberghs N., Tanner T., Hardt K., Ruiz-Guiñazú J., Le Gars M., Schuitemaker H., Van Hoof J., Struyf F., Douoguih M., ENSEMBLE Study Group Safety and efficacy of single-dose Ad26.COV2.Svaccine against COVID-19. N. Engl. J. Med. 2021;384(23):2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Witberg G., Barda N., Hoss S., Richter I., Wiessman M., Aviv Y., Grinberg T., Auster O., Dagan N., Balicer R.D., Kornowski R. Myocarditis after covid-19 vaccination in a large health care organization. N. Engl. J. Med. 2021 Dec 2;385(23):2132–2139. doi: 10.1056/NEJMoa2110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montgomery J., Ryan M., Engler R., Hoffman D., McClenathan B., Collins L., Loran D., Hrncir D., Herring K., Platzer M., Adams N., Sanou A., Cooper L.T., Jr. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021 Oct 1;6(10):1202–1206. doi: 10.1001/jamacardio.2021.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.