Abstract
Intestinal atresia and hirschsprung disease are two common causes of bowel obstruction in neonates; simultaneous occurrence is rare. This report delineates a 36‐week newborn with ileal atresia and total colonic hirschsprung who was referred to our unit due to failure of meconium passage during the first 48 h after birth.
Keywords: aganglionosis, hirschsprung disease, ileum atresia
Colonic aganglionosis should be in mind in any operated infant with small intestinal atresia repair who continues to exhibit poor bowel function after corrective surgery.
1. INTRODUCTION
Intestinal atresia is a significant cause of primary bowel obstruction in neonates, accounting for 22.4% of all cases. 1 On the contrary, hirschsprung disease (HD) is the most frequent congenital intestinal motility disorder. 2 HD occurs when bowel segments are not supported by neuronal ganglion formation within the myenteric (Auerbach) and submucosal (Meissner) plexus, which promotes gastrointestinal movements.
And in 80% of cases, the absence of ganglion cells is limited to the rectosigmoid area. However, aganglionosis may extend through the colon with the distal ileum (10%) or more proximal segments. 3 Also, in these cases, a water‐soluble enema shows a “question mark” configuration in the foreshortened colon with no transitional zone. 4 The rectal biopsy can always confirm the diagnosis.
Concurrent small intestinal tract atresias associated with hirschsprung are <1%.
In this case report, we simultaneously present an infant born with ileal atresia and hirschsprung disease.
2. CASE PRESENTATION
A 36‐week male neonate was referred to the pediatric surgery service on the second day of life because of failure to pass meconium, abdominal distension, and bilious emesis.
The patient was born to a 23‐year‐old healthy primipara Iranian mother without a history of abortion via normal vaginal delivery. Her birth weight was 2500 g.
On physical examination, the primary vital signs were in the normal range, the bowel sounds were decreased, and the rectum was empty.
Initial abdominopelvic radiography revealed thumb‐sized intestinal loops with no gas in the rectum (Figure 1A). Small bowel contrast series confirmed dilated proximal blind end (Figure 1B).
FIGURE 1.
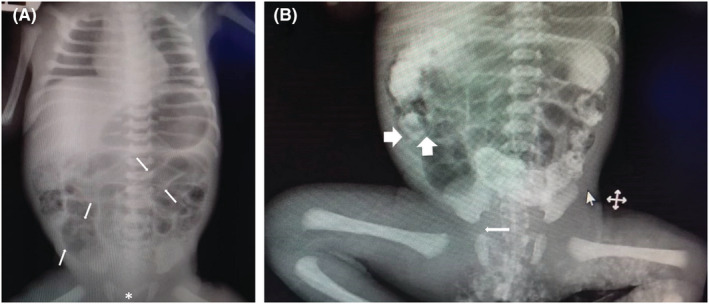
(A) several dilated gastrointestinal loops (Arrows) with no gas in the rectum (Asterisk). (B) Small bowel contrast study reveals dilated proximal blind end (Thick arrow).
Baseline biochemical and blood gas analyzes were in the normal range. On the third day, with a diagnosis of intestinal obstruction, the patient underwent an exploratory laparotomy after obtaining informed consent from the parent. We identified ileal atresia with a proximal dilated, blind‐ending 1 cm distal bud attached to the ileocecal region (Type IIIa). (Figure 2) The colon appeared as an unused microcolon throughout. After resecting the proximal dilated ileal pouch and ileocecal part, surgery was followed by an end‐to‐end ileo‐ascending colonic anastomosis.
FIGURE 2.
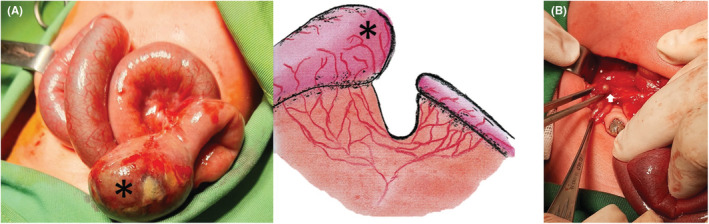
(A) Type (III‐a) atresia at the laparotomy. The proximal end of the ileum has been marked by the Asterisk. (B) The distal blind end of the ileum is seen as a bud in the ileocecal region (Arrow).
Permanent hematoxylin and eosin (H&E) pathological examination revealed the absence of ganglion cells in the cecum, the appendix, and the rectum and the abundance of ganglia in the ileum (Figure 3).
FIGURE 3.
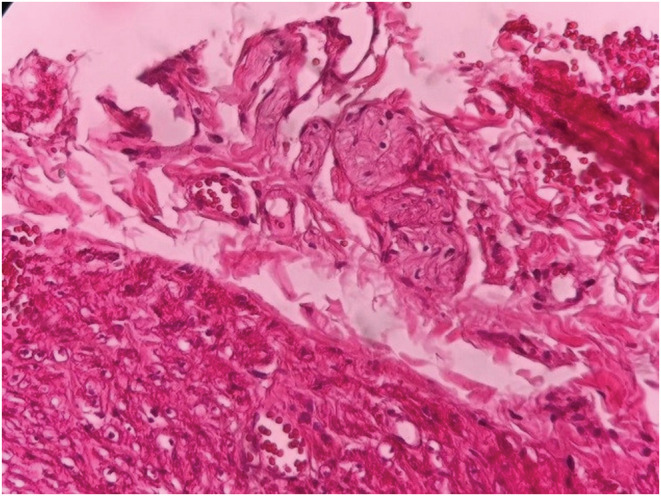
The hematoxylin and eosin study of the ileum in the left and colonic specimen in the right revealing the absence of neurologic ganglia compatible with HD (× 1000 magnification).
Due to the persistence of abdominal distention, no defecation on postoperative days, and a histopathologic report that was compatible with total segment HD, the patient was a candidate for re‐exploration. (Figure 4).
FIGURE 4.
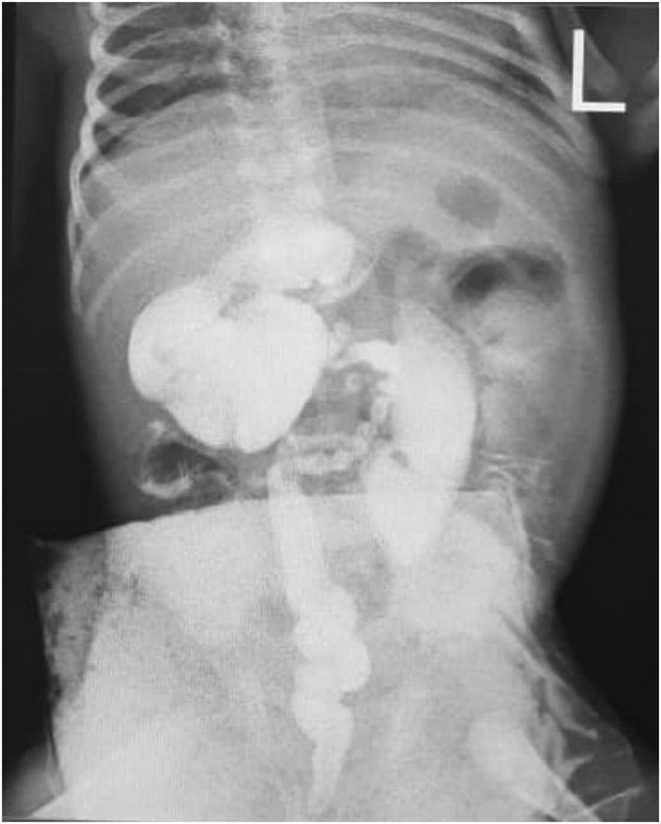
Contrast barium enema demonstrates an unused colon and a functional intestinal obstruction at the anastomosis site.
Our findings were a microcolon and a functional intestinal obstruction at the anastomosis site with no anastomotic leak.
After taking multiple levels of frozen section biopsies to reaffirm the total colonic aganglionosis, the patient underwent an ileostomy and colonic mucus fistula bypass.
The final permanent pathology studies by the calretinin test disclosed total aganglionic colon HD.
Swenson pull‐through surgery with removal of all colon and ileoanal anastomosis was performed on a three‐month‐old. The case tolerated oral feeding after 5 days and was discharged from the hospital in good condition.
3. DISCUSSION
Coexistence of ileal atresia and total colonic HD is a rare event. 5 , 6 Conforming to the currently accepted theories, jejunoileal atresia arises due to intrauterine ischemic vascular events in the third trimester, such as intussusception, perforation, volvulus, or thromboembolism; maternal smoking as a hypercoagulation state 7 , 8 and cocaine use as vasoconstrictive medications. 9
The migration of ganglion cells was completed through the gastrointestinal tract from proximal to distal by 13 weeks post conception. Therefore, early gestational atresia in the sixth to eighth weeks of gestation would result from an ischemic insult to interrupt the caudal migration of ganglion cells and lead to total colonic HD. 10 , 11 Finding a very small microcolon, no fibrotic of the left colon, and no meconium distal to atretic segments strengthens this theory.
The other hypothesis that can justify this concurrency is an increased colonic intraluminal pressure and subsequent perforation ileocecal portion due to a developed HD and the secondary small bowel atresia. However, in our case, there was no evidence of meconium spillage into the peritoneal space during our laparotomy, which weakens the second assumption.
As the common occurrence of microcolon in the cases of distal small intestinal atresia, it is tough to differentiate this colonic appearance during surgery from concomitant total colonic aganglionosis and small bowel atresia.
Therefore, it appears rational to do per‐operative colonic biopsies looking for ganglion cells in a frozen section to exclude or confirm the underlying HD in suspicious cases.
A definitive reconstructive operation should be planned once we have established the diagnosis and done a proximal ileostomy.
However, there are controversial questions about the correct timing and the most appropriate treatment options. 12 , 13
Several approaches have been described to treat this, such as primary pull‐through without ileostomy or total colectomy with standard techniques (Swenson, Duhamel, or Soave). Neither is superior to the others.
Albeit discriminating, the best operative approach should be constructed based on the surgeon's level of expertise 13 ; in our case, we did total colectomy with an ileoanal Swenson procedure when the patient status was allowed.
In conclusion, this rare concurrency should be considered in the cases of small bowel atresia with poor bowel function after the corrective operation.
AUTHOR CONTRIBUTIONS
Khashayar Atqiaee: Supervision; writing – review and editing. Mehran Hiradfar: Conceptualization; methodology. Mehdi Parvizi Mashhadi: Writing – review and editing. Ali Samady Khanghah: Writing – original draft.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interests relating to this original work.
ETHICAL APPROVAL
We confirm that all named authors have read and approved the manuscript. The protection of the intellectual property associated with this manuscript has been our consideration available on the DOI website when prepared.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
ACKNOWLEDGMENTS
The authors would like to acknowledge all the hospital staff who assisted us in treating the patient.
Atqiaee K, Hiradfar M, Mashhadi MP, Khanghah AS. Ileal atresia and total colonic hirschsprung disease in a 36‐week neonate: A case report. Clin Case Rep. 2023;11:e7079. doi: 10.1002/ccr3.7079
DATA AVAILABILITY STATEMENT
The authors declare that they have chosen “Data openly available in a public repository that issues datasets with DOIs” and the data that support the findings of this study will be openly available on the DOI website when prepared.
REFERENCES
- 1. Dhar PP, Mohanty U. A rare case report of infant ileal atresia with double appendix. Int J Surg Case Rep. 2020;74:226‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gfroerer S, Rolle U. Pediatric intestinal motility disorders. World J Gastroenterol: WJG. 2015;21(33):9683‐9687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gupta M, Beeram MR, Pohl JF, Custer MD. Ileal atresia associated with Hirschsprung disease (total colonic aganglionosis). J Pediatr Surg. 2005;40(9):e5‐e7. [DOI] [PubMed] [Google Scholar]
- 4. Das K, Mohanty S. Hirschsprung disease—current diagnosis and management. Indian J Pediatr. 2017;84(8):618‐623. [DOI] [PubMed] [Google Scholar]
- 5. Seo T, Ando H, Watanabe Y, et al. Colonic atresia and Hirschsprung's disease: the importance of histologic examination of the distal bowel. J Pediatr Surg. 2002;37(8):1‐3. [DOI] [PubMed] [Google Scholar]
- 6. Lauwers P, Moens E, Wustenberghs K, et al. Association of colonic atresia and Hirschsprung's disease in the newborn: report of a new case and review of the literature. Pediatr Surg Int. 2006;22(3):277‐281. [DOI] [PubMed] [Google Scholar]
- 7. Werler MM, Sheehan JE, Mitchell AA. Maternal medication use and risks of gastroschisis and small intestinal atresia. Am J Epidemiol. 2002;155(1):26‐31. [DOI] [PubMed] [Google Scholar]
- 8. Khen N, Jaubert F, Sauvat F, et al. Fetal intestinal obstruction induces alteration of enteric nervous system development in human intestinal atresia. Pediatr Res. 2004;56(6):975‐980. [DOI] [PubMed] [Google Scholar]
- 9. Werler MM, Sheehan JE, Mitchell AA. Association of vasoconstrictive exposures with risks of gastroschisis and small intestinal atresia. Epidemiology. 2003;14:349‐354. [PubMed] [Google Scholar]
- 10. Puri P, Montedonico S. Hirschsprung's disease: clinical features. Hirschsprung's Disease and Allied Disorders. Springer; 2008:107‐113. [Google Scholar]
- 11. Heanue TA, Pachnis V. Enteric nervous system development and Hirschsprung's disease: advances in genetic and stem cell studies. Nat Rev Neurosci. 2007;8(6):466‐479. [DOI] [PubMed] [Google Scholar]
- 12. Escobar MA, Grosfeld JL, West KW, et al. Long‐term outcomes in total colonic aganglionosis: a 32‐year experience. J Pediatr Surg. 2005;40(6):955‐961. [DOI] [PubMed] [Google Scholar]
- 13. Marquez TT, Acton RD, Hess DJ, Duval S, Saltzman DA. Comprehensive review of procedures for total colonic aganglionosis. J Pediatr Surg. 2009;44(1):257‐265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that they have chosen “Data openly available in a public repository that issues datasets with DOIs” and the data that support the findings of this study will be openly available on the DOI website when prepared.


