Abstract
The US Food and Drug Administration (FDA) has publicly recognized the importance of improving drug development efficiency, deeming translational biomarkers a top priority. The use of imaging biomarkers has been associated with increased rates of drug approvals. An appropriate level of validation provides a pragmatic way to choose and implement these biomarkers. Standardizing imaging modality selection, data acquisition protocols, and image analysis (in ways that are agnostic to equipment and algorithms) have been key to imaging biomarker deployment. The best known examples come from studies done via precompetitive collaboration efforts, which enable input from multiple stakeholders and data sharing. Digital health technologies (DHTs) provide an opportunity to measure meaningful aspects of patient health, including patient function, for extended periods of time outside of the hospital walls, with objective, sensor‐based measures. We identified the areas where learnings from the imaging biomarker field can accelerate the adoption and widespread use of DHTs to develop novel treatments. As with imaging, technical validation parameters and performance acceptance thresholds need to be established. Approaches amenable to multiple hardware options and data processing algorithms can be enabled by sharing DHT data and by cross‐validating algorithms. Data standardization and creation of shared databases will be vital. Pre‐competitive consortia (public‐private partnerships and professional societies that bring together all stakeholders, including patient organizations, industry, academic experts, and regulators) will advance the regulatory maturity of DHTs in clinical trials.
INTRODUCTION
The path from drug discovery to regulatory approval can be long, 1 and there is an urgent need for increased expediency and efficiency. In 2004, the US Food and Drug Administration (FDA) publicly recognized the importance of improving efficiency in drug development with the 2004 Critical Path Initiative Challenges and Opportunities, in which the development of biomarkers was deemed a top priority. 2 The use of medical imaging to develop and deploy biomarkers in support of clinical trial end points has been associated with increased drug approvals. 3 Many imaging biomarkers have been deemed fit‐for‐purpose. They are used judiciously and selectively to answer critical development questions, alongside other biomarkers. Corresponding imaging biomarkers are derived from an array of imaging platforms, including magnetic resonance imaging (MRI), nuclear medicine techniques, such as single photon emission computed tomography, positron emission tomography (PET), computerized tomography, ultrasound, and other scanning techniques. Imaging technologies have become indispensable for addressing questions about dose selection, pharmacokinetic (PK)/pharmacodynamic (PD) relationships, drug mechanism of action, and establishing proof of concept (POC) in clinical trials (Figure 1). Imaging biomarkers allow a unique visualization of anatomic specificity, physiology, and drug activity in ways that are not possible with other biomarker approaches. 4 Imaging biomarkers play different roles across the stages of clinical development, and can be categorized as:
Fit‐for‐purpose biomarkers enable internal decision making by sponsors; for example, they can be used in early‐phase drug development, including POC studies. Here, imaging biomarkers are often aimed at obtaining information about tissue distribution, target engagement, or measures of downstream pharmacology. 5
Qualified biomarkers for a specific purpose – for example, hippocampal volumes as an enrichment tool in early‐stage Alzheimer's disease (AD) trials 6 – are done via established regulatory pathways.
Regulatory accepted endpoints as surrogates of clinical outcomes.
FIGURE 1.
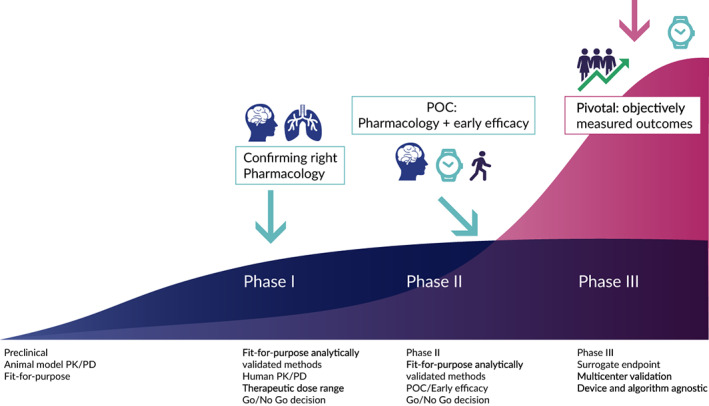
Imaging and digital biomarkers across the stages of clinical development. PK/PD, pharmacokinetic/pharmacodynamic; POC, proof of concept
Specific examples of imaging biomarker use and their impact in clinical investigations are presented in Table 1.
TABLE 1.
Examples of successful imaging biomarker applications in early‐ and late‐stage clinical trials
| Disease area | Biomarker/Imaging technology | Application | Impact | Regulatory endorsement |
|---|---|---|---|---|
| Multiple sclerosis | Gd‐enhanced T1‐ and T2‐lesions/MRI | Proof of concept | De‐risking pivotal studies | Fit for purpose, drug candidate Go/No Go decision making |
| Rheumatoid arthritis | Information and structural damage/MRI | Mechanism of action | Reducing sample size and shortening the follow‐up | Fit for purpose, drug candidate Go/No Go decision making |
| Polycystic kidney disease | Total kidney volume/MRI | Patient enrichment, efficacy | Reducing sample size and shortening study duration 85 |
Full Biomarker Qualification (FDA) as reasonably likely surrogate of efficacy 27 EMA qualification opinion as a prognostic biomarker 28 |
| Alzheimer's disease | Hippocampal volume /MRI | Patient enrichment | Clinical trial patient stratification for trials targeting predementia stages of AD. Identification of a subset of MCI participants at the predementia stage with low baseline hippocampal volume with a defined rate of disease progression for enrollment in clinical trials |
EMA full qualification opinion 6 FDA letter of support 86 |
| Parkinson's disease | Molecular neuroimaging of the dopamine transporter (DAT) as an enrichment biomarker for clinical trials for early Parkinson's disease by SPECT neuroimaging | Early disease Confirm hallmark pathology of Parkinson's disease as an enrichment biomarker use by SPECT neuroimaging to exclude SWEDD participants who do not progress | Reducing sample size 87 |
FDA letter of support 36 EMA qualification opinion 37 |
Abbreviations: AD, Alzheimer’s disease; EMA, European Medicines Agency; FDA, US Food and Drug Administration; MCI, mild cognitive impairment; MRI, magnetic resonance imaging; SPECT, single photon emission computed tomography; SWEDD, scans without evidence of dopaminergic deficit.
Whereas imaging biomarkers have been applied broadly in clinical trials, they do require some specific considerations. Acquiring imaging data relies upon a patient visiting an imaging site: this needs to be planned carefully into the trial design. Patient burden, trial complexity, and cost are important factors that mean imaging biomarkers need to be used selectively, where they will have the most impact on decision making, concerns around radiation exposure, and patient comfort associated with noise and claustrophobia. In general, imaging biomarkers describe pharmacology or pathophysiology, rather than patient function or perception of benefit.
In recent years, the rapid emergence and evolution of digital health technologies (DHTs; Table 2) has opened the possibility of measuring meaningful aspects of patient health, including patient function, for extended periods of time outside of the clinic with objective, sensor‐based measures in addition to patient self‐reporting. 7 DHTs can capture people's activities under everyday living conditions (Table 3). The concept of digital endpoints supported by DHTs is a nascent but quickly developing field that requires the expertise of multiple stakeholders, including drug developers, healthcare providers, the technology sector, and patient organizations, among others – all under the guidance of health regulators. The potential of DHTs as drug development tools in clinical trials has been clarified through emerging regulatory guidance documents that provide basic frameworks and definitions. 8 , 9 , 10 Nevertheless, both regulators and the scientific community will benefit greatly by following concrete examples of validation and deployment of these technologies in drug development.
TABLE 2.
Definitions of digital health technology, medical devices, biomarkers, and clinical outcome assessments
| Term | Definitionreference |
|---|---|
| Digital Health Technology (DHT) | A system that uses computing platforms, connectivity, software, and sensors for healthcare and related uses. These technologies span a wide range of uses, from applications in general wellness to applications as a medical device. They include technologies intended for use as a medical product, in a medical product, or as an adjunct to other medical products (devices, drugs, and biologics). They may also be used to develop or study medical products. 38 |
| Medical device | Devices cleared as medical devices; may require a prescription from a healthcare professional and trained personnel to configure and deploy 88 |
| Biomarker | A defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or a response to an exposure or intervention, including therapeutic interventions 38 |
| Digital biomarker |
A characteristic or set of characteristics, collected from digital health technologies, that is measured as an indicator of normal biological processes, pathogenic processes, or responses to an exposure or intervention, including therapeutic interventions 8 An objective, quantifiable measure of physiology and/or behavior used as an indicator of biological, pathological process, or response to an exposure or an intervention that is derived from a digital measure. The clinical meaning is established by a reliable relationship to an existing, validated endpoint. 10 |
| Clinical outcome assessment (COA) | A report by a clinician, a patient, a non‐clinician observer or through a performance‐based assessment. There are four types of COAs.
38
|
TABLE 3.
Comparison of imaging biomarkers and digital health technologies
| Comparison parameters | Imaging biomarkers | DHTs |
|---|---|---|
| Examples of use in drug development | Target occupancy and dose selection, establishing PK/PD relationships, drug mechanism of action, and a proof of concept, efficacy assessments as well as identifying and enriching clinical trial populations | Measuring meaningful aspects of patient health by means of objective, sensor‐based measures in addition to patient self‐report. Can capture physiological aspects of health (e.g., cardiac function or sleep). Moreover, can measure how a patient is feeling and functioning remotely or in the clinic |
| Technology examples | PET, MRI, CT | Accelerometers, pulse oximeters, blood pressure monitors |
| Technology origin | Medical diagnostic tools | Commercial technologies (e.g., fitness trackers), or medical devices with regulatory clearance |
| Biomarker performance characteristics with appropriate controls |
Technical: Measure and, bias, linearity, precision, reference value, repeatability, repeatability conditions, reproducibility, reproducibility conditions, truth, or true value 62 Clinical: Cross‐sectional signal difference between indication and healthy Cross‐sectional signal versus disease stage Short term test–retest as the easiest way to estimate longitudinal variability Longitudinal change and variability |
V3 concept: verification of a sensor versus bench standard, analytical validation of the data processing algorithm, clinical validation to evaluate if the technology acceptably measures a concept of interest 41 Design and operation of DHT, verification, validation, and usability 9 |
| Establishing method specifications/ device calibration | Equipment, manufacturers have established calibration and verification methods – for clinical use. These methods cover most clinical trial biomarker needs. If extra requirements are needed for a biomarker – keeping it simple is highly desirable |
Calibration of a sensors under appropriate use conditions (e.g., temperature and humidity) Filtering out invalid data based on predefined rules (data processing algorithm) |
| Human factor testing/usability | N/A ‐ other than defining the risk associated with imaging data procedure | Usability needs to be established as a part of validation 9 |
| Data collection environment | Hospital/doctor's office | Hospital or under free living conditions |
| Data structure | Limited number of timepoint acquisitions during a clinical trial | Frequent as specified (e.g., daily, multiple times a day, or semi‐continuously for extended periods of time) |
Abbreviations: CT, computed tomography; DHT, digital health technology; MRI, magnetic resonance imaging; N/A, not applicable; PET, positron emission tomography; PK/PD, pharmacokinetic/pharmacodynamic.
Imaging and DHT‐enabled measures, including digital biomarkers, 8 are both able to contribute critical information in the development of a new therapeutic. However, their impacts may be different when we consider whether we can measure noninvasive pharmacology, individual patient function, or patient diagnosis. The continuous spectrum of biomarker technological applications in drug development is outlined in Figure 1.
In this paper, we identify key aspects that contribute to successful acceptance of imaging technologies in drug development and posit that the lessons learned can enable the adoption and widespread use of DHTs to help develop novel treatments. Below, we provide detailed summaries of each of those aspects, noting the applicable stage of clinical development.
EARLY‐STAGE CLINICAL DEVELOPMENT
Early clinical development is focused on characterizing PK/PD relationships, achieving proof of mechanism (POM) and POC, and de‐risking future investment in late‐stage trials by evaluating the totality of data from phase I and II studies.
The use of imaging technologies in preclinical models in both rodents and primates is well‐established, and has informed translational strategies, including the characterization of PK/PD relationships across many different diseases. 11 Critical aspects of this early stage include building confidence in a compound and a target, which requires systems pharmacology approaches in conjunction with disease modification. 12 Clinical imaging studies to assess parameters, such as receptor occupancy or a target engagement pathway in POM studies, provide useful information to de‐risk moving novel drug candidates into the next phase of development by ensuring target coverage throughout the dosing interval 4 or by ascertaining that a specific pathway was modulated according to the mechanism of action (MOA). 13 Additionally, imaging technologies can provide early signs of drug efficacy in POC studies (Table 1 and Figure 2).
FIGURE 2.
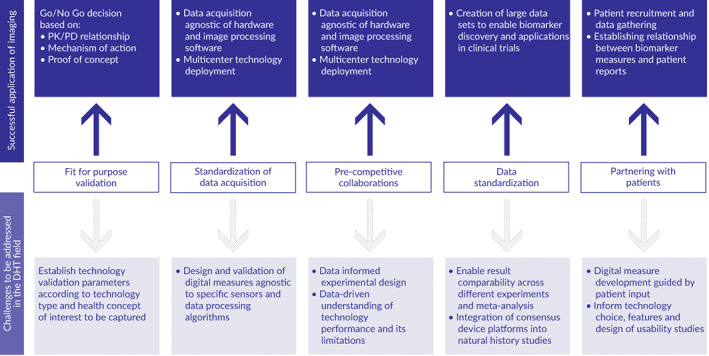
Leveraging insights from imaging technologies to enable the advancement of digital heath technologies. PK/PD, pharmacokinetic/pharmacodynamic
The choice of technology, and the rigor of its validation, including both analytical (sometimes also referred as technical) and clinical validation, is usually done according to the “fit‐for‐purpose” principle: a biomarker is useful only when it is valid in the context of its specific use. 14 In the case of imaging biomarkers, this involves an assessment of the biomarker's technology performance clinically, but it can be also supported by preclinical or tissue studies. Characteristics, such as the cross‐sectional signal difference between diseased and healthy subjects, the presence of a signal related to a disease stage, or short‐term test–retest as the easiest way to estimate longitudinal variability (Table 3), are usually required to gain confidence in imaging biomarker performance. Pragmatic considerations are equally important. For example, will the method be deployable in the required trial setting, for example, expandable to multiple centers? Will the scanning protocol be acceptable in terms of patient burden?
In deciding on the level of evidence required for validation as fit‐for‐purpose it is important to consider separately the technical validation of the methods and the overall clinical validation of the biomarker. The aim of developing an imaging biomarker is to have a noninvasive measure of pathophysiology. The steps toward the overall clinical validation of the imaging biomarker are mentioned in the previous paragraph. However, there may be multiple methods capable of measuring the clinical pathophysiological endpoint in a particular context of use. Technical validation of the various methods that can be used to ensure reproducibility and minimize the variability are equally critical to the overall performance. A nonexclusive list of technical parameters important for imaging end points has been published in Clinical Trial Imaging Endpoint Process Standards Guidance for Industry. 15 Development of precise, sensitive, and reproducible acquisition and analysis methods for early phase trials is a competitive research and development field.
Sponsors want to be sure that novel medicines are advanced where they can truly bring value for patients, and considerable time is spent to evaluate the science around these biomarkers to ensure that decisions are made with appropriate rigor. There are no explicit regulatory requirements to qualify biomarkers for use in clinical trials, although guidelines and qualification advice processes exist. It is important to distinguish the validation concept from the qualification process, which is a formal regulatory review to ascertain that a certain measure can be relied upon to have a specific interpretation and application in medical product development and regulatory review within the stated context of use. 16 Health authority qualification of biomarkers (e.g., through the European Medicines Agency [EMA] “Qualification of Novel Methodologies”) would require very strong evidence that the interpretation is straightforward and that it cannot be misinterpreted within the stated context of use. In order to achieve this, well‐documented use of the biomarker in multiple studies and a clear specification of the framework of implementation are needed. This is partially because the benefit/risk of the biomarker is being assessed in a relatively broad context, without reference to risk assessment for a specific investigational medicine. With this background it is understandable why the weight of evidence is high and the context of use are restricted, to the effect that it may take many years to generate the necessary evidence to achieve a full qualification. A number of biomarkers have been qualified by the FDA full list of qualification recommendations 17 and EMA biomarker qualification opinions full list. 18
Because of the multiple studies and weight of evidence required for a qualification balanced with the pace at which new biomarkers need to be implemented for novel indications and targets, 19 sponsors mainly use imaging biomarkers as fit‐for‐purpose. Ultimately, decisions about deploying a specific imaging biomarker are made by the sponsor in collaboration with the regulatory authorities. The results of clinical investigations that use the biomarker are usually discussed with regulatory authorities by individual sponsors advancing candidate therapeutics, and only in the specific context of the benefit/risk profile of that novel treatment to justify therapeutic doses. This information may be included in the clinical pharmacology or MOA section of the label to describe the relationship with preclinical findings, provide rationale for dose selection, and ascertain an MOA, but it typically does not support efficacy claims.
Consensus recommendations have been developed by experts, professional societies, and precompetitive consortia to help assure alignment of how to define success as it relates to imaging biomarker validation, standardization and fit‐for‐purpose decision making. 20 Some precompetitive work is repeated independently by multiple sponsors, and often not disclosed to the broader scientific community. Nevertheless, the best‐known examples come from precompetitive collaborations because they draw experts from multiple fields and institutions, and because this information is available in the public domain (Table 1).
LATE‐STAGE CLINICAL DEVELOPMENT
When planning to use biomarkers to support primary end points in pivotal trials, it is strongly advisable to consult with regulatory authorities to determine what evidence will be needed to validate a candidate biomarker before starting the trial. Depending on the intent of the use of the data, regulators may request clinical validation study results for review.
The successful application of imaging biomarkers in late‐stage clinical trials poses unique demands in terms of validation. Technical validation requires that reliable results can be derived by different institutions (comparability) and on widely available platforms. Reproducibility studies for imaging biomarkers are typically carried out in natural history biomarker studies, where inclusion of a repeat baseline study can be carried out. To demonstrate that known perturbations in biology alter the imaging biomarker signal in a way that supports the measurement characteristics assigned to the biomarker, multicenter technical validation using standardized protocols are likely carried out after initial biological validation. Summarizing the experience and lessons learned from many examples of imaging biomarker applications in drug development, the FDA has issued guidance to aid in the application of imaging biomarkers in clinical development. 15
Below, we provide a close examination of several case studies carried out beyond a single pharmaceutical product, which were impactful in understanding disease pathophysiology in the context of drug development and which, at the same time, advanced the field of imaging biomarkers.
CASE STUDIES
Rheumatoid arthritis magnetic resonance imaging scoring system
The rheumatoid arthritis scoring system was developed using MRI data, which included improved image acquisition, and variable definition. 21 This approach allowed researchers to demonstrate inhibition of inflammation and structural damage progression with fewer patients and shorter follow‐up compared to conventional radiography. 22 Importantly, the relationship between the imaging scoring system and patient‐reported outcomes was established, linking the imaging biomarkers to outcomes meaningful to patients. 21 It is recognized that there is an increasing need for a more sensitive end point for monitoring structural progression, because placebo arms are kept necessarily short and improved treatments result in less structural progression in active comparator arms.
This is an example of an imaging modality that can be incorporated in early‐stage clinical trials, enrolling patients with rheumatoid arthritis (RA), that would provide an early readout of an experimental medicine's efficacy. Despite 20 years of progress using MRI in RA, the method is not considered fully validated, 23 because it is largely used as fit for purpose and not for regulatory approvals.
Gadolinium enhanced‐T1 and T2 lesion count and volume by MRI in multiple sclerosis
Similarly, gadolinium enhanced‐T1 and T2 lesion count and volume are determined by MRI and used as a biomarker in relapsing remitting multiple sclerosis. This is used in short, small‐sample‐size trials to assess the impact of a novel treatment before the effects on clinical symptoms, such as frequency of disease flares, become apparent. After phase II, MRI T2 lesions are often measured in phase III trials, and the predictive value of the early‐phase results of this biomarker for later‐phase outcomes has been clearly shown. 24 As yet, T2 lesions have not been established as surrogate biomarkers in late‐stage trials. Despite the well‐established evidence of use of these biomarkers to assess effect and the success of treatments that showed effect, these biomarkers are not formally qualified.
Total kidney volume in polycystic kidney disease
One example of establishing surrogacy for imaging biomarkers is the collaborative initiative led by Critical Path Institute's Polycystic Kidney Disease Outcomes Consortium (PKDOC). The progression and outcome of polycystic kidney disease is associated with changes in the volume of the kidneys, and this can be measured by a structural MRI. In addition, this information can help identify patients who will benefit from treatments, and that knowledge can enrich and shorten clinical trials. PKDOC has brought together members of the drug‐development community, patient‐advocacy groups including the Polycystic Kidney Disease Foundation, academic researchers, industry, and representatives from regulatory agencies around the world, to gain consensus about aspects of autosomal dominant polycystic kidney disease (ADPKD): specifically, disease progression, biomarker application, alternative endpoints, and innovative trial design. There has been an urgent need for biomarkers, given that diagnosis is now based on measures that occur very late in the disease continuum. 25 Initially, PKDOC focused on developing data standards for ADPKD, amalgamating natural history data sets collected by stakeholders over many years. These data were applied to a disease‐progression model that was used to qualify the relationship between total kidney volume and known clinical outcomes of ADPKD, including the rate of loss of kidney function, the development of end‐stage renal disease, and mortality. 26
As a result, total kidney volume was qualified as a prognostic enrichment biomarker with both the FDA and the EMA in 2015. 27 , 28 In 2018, the evidence provided by the model also led to the FDA designating total kidney volume as a measure to track disease progression and measure efficacy of novel drugs in ADPKD clinical trials (a “reasonably likely surrogate”). Total kidney volume has been used in the approval of the first ADPKD treatment, the vasopressin V2‐receptor antagonist tolvaptan, which is indicated to slow kidney function decline in adults at risk of rapidly progressing ADPKD. The identification of the total kidney volume biomarker has galvanized drug development in ADPKD, and the preclinical‐to‐phase III developmental pipeline currently includes 10 novel compounds.
Imaging biomarkers in Alzheimer's disease
The Alzheimer's Disease Neuroimaging Initiative (ADNI) is a natural history, multicenter study of AD progression, launched in 2004. It is acquiring multimodal data, including MRI, fluorodeoxyglucose, and beta‐amyloid PET, along with clinical and other biomarker data, in patients at different stages of the disease. This initiative has set a precedent of public‐private partnership by creating an open‐source data set accessible to both industry and academia. This has had a major impact in understanding preclinical changes in biomarkers (early pathological changes in the brain prior to the onset of symptoms), affirming the recognition by the research community that pathophysiological changes occur decades prior to symptoms. 29
Patient organizations played an important role in advancement of this initiative. Their contributions range from patient recruitment to data contribution, to understanding the disease from the patient's perspective. For example, the third phase of the initiative has recruited an additional 1200 volunteers to donate their data, to assess cognitive function through computer tests at home and in the clinic measuring changes in ability to handle money, and early signs of the disease. 30 , 31
Years of research, progress of establishing validation, and POC for beta amyloid PET imaging took place under the umbrella of the ADNI. 32 Recently, the FDA has rendered beta‐amyloid PET as a reasonably likely surrogate of cognitive decline, based on analysis of publicly available neuroimaging data and clinical outcomes from multiple independent trials which tested drug candidates targeting beta amyloid. 33
Hippocampal volume in Alzheimer's disease
The EMA qualified the use of MRI measurement of hippocampal volume in AD as a biomarker for enrichment in clinical trials that targeted the mild cognitive impairment stage of the disease based on the review of existing literature as well as de novo image analysis using multiple algorithms. 32 , 34 Four distinct image analysis algorithms (developed by different device manufacturers) were used to evaluate neuroimaging data that was publicly available through the ADNI. Although the absolute numeric values were not identical, the results showed nearly identical predictive accuracy for defining the rate of progression from mild cognitive impairment to AD. An important learning here is that the measurement of the underlying physiological variable – the hippocampal volume – is critical to the validation. The biomarker qualification journey required extensive technical validation performance characteristics be performed to assure confidence in the accuracy, reliability, and consistency of each of the imaging methodologies. The technical validation of individual measures is likely to evolve over time and new methodologies can be refined if they follow the evidentiary standards and criteria needed to be deemed as fit for purpose. The EMA qualification opinion 6 was successfully led by Critical Path Institute's Coalition Against Major Diseases Consortium (now Critical Path for Alzheimer's Disease). This case is a useful example, because it is a device‐agnostic approach that took place through public‐private partnerships.
Parkinson progression marker initiative
The ADNI approach was successfully replicated by the Parkinson Progression Marker Initiative (PPMI), which is an observational multi‐center study designed to identify Parkinson's disease progression biomarkers, not only to improve the understanding of the disease etiology, but also to facilitate clinical trials. PPMI has detailed the biomarker signature for an early cohort defined by clinical features and imaging biomarkers. Data sharing, enabled by PPMI, allowed researchers to develop disease progression biomarkers to support clinical trials in Parkinson’s disease. 35 Two key success factors include open sharing of all data to the broad community and, importantly, agreement on the specific standardized acquisition protocols for all imaging biomarkers (which is key to harmonizing and integrating data across different sites and manufacturers). The PPMI study was one of the key studies that supported the first imaging biomarker qualified by regulators for Parkinson's disease trials. 36 , 37
Imaging biomarker conclusions (Figure 2)
An appropriate level of validation, both technical and clinical, provides a pragmatic approach to choose and implement biomarkers for drug development. The fit‐for‐purpose principle has been instrumental in enabling go/no go decisions in early‐stage clinical development. Validating imaging tools to support surrogate endpoints and provide patient‐stratification biomarkers has enabled drug development across multiple indications.
There are multiple methods to measure pathology and disease progression; it is important to focus on measurement of indication relevant pathophysiology, rather than measurement of a signal. Standardization of imaging modality selection, data acquisition protocols, and image analysis enable various levels of precision for different methods to measure the same disease characteristic. To encourage competition to continuously improve methods, it is important to avoid definitions of the biomarker that include specific technologies.
The best‐known examples of a positive impact on the development of novel medicines come from studies done via pre‐competitive collaboration, which enables input from multiple stakeholders, creates large comprehensive data sets, and facilitates analyses performed by multiple institutions in an unrestricted way.
Data standardization by creating data annotation libraries, evaluating performance of different scanning and image analysis technologies, and understanding the data behavior as well as potential confounding factors enabled discovery and clinical validation of imaging biomarkers.
Partnership with patients and patient advocacy organizations provides important contributions to recruitment, to patient‐reported outcome data collection, and to tying biomarker‐based findings to signs and symptoms.
Where do we stand with DHT‐based measures?
DHT‐based measures encompass a variety of assessments. They include measures obtained by wearable sensors, applications on smart phones, or software on computers that could be functional tests (e.g., speed of typing or walking, or electronic patient reported outcomes; Table 2). Moreover, some DHT applications fit neatly into conventional definitions of biomarkers 8 , 10 , 38 (e.g., remote cardiac monitoring by means of echocardiogram 39 ), whereas other examples constitute a biomarker and performance outcome at the same time. 40
Development and validation of DHTs for drug development is also done according to the “fit for purpose” principle. However, given the relatively nascent field of DHTs, compared with imaging, global consensus on “fit for purpose” for DHTs is still evolving. The V3 framework, 41 developed by the scientific community, stipulates a three‐step process for determining whether a DHT is fit for purpose: verification, analytical validation, and clinical validation (Table 1). It is consistent with recent FDA guidance. 9 Despite a growing consensus on the framework for validation experiments, however, the amount of publicly available data remains limited. Many DHT validation studies are single‐institution efforts and are often not disclosed publicly. This issue is compounded by the great variability of DHTs. Unlike imaging technologies, which are developed from medical diagnostic tools with established clinical utility, DHTs include both commercial technologies (e.g., fitness trackers), as well as medical devices endorsed for an intended use by regulators (Table 3), with a highly variable amount of technical performance data. 42 The duration of data collection data structure also varies tremendously and includes point‐in‐time assessments (e.g., a 6‐Minute Walk Test [6MWT] recorded by a smartphone or a wrist‐worn accelerometer); frequent measures done multiple times a day (e.g., a pulmonary function test done by means of mobile spirometry), or multi‐day monitoring by wrist‐worn sensors (e.g., sleep assessment and physical activity measured by means of actigraphy). 43
Below, we consider the experience accumulated by the DHT field, and provide recommendations for leveraging imaging knowledge to advance drug development.
Early‐stage clinical development
The use of DHTs in early‐stage clinical development is of a somewhat smaller scope compared to imaging biomarkers. DHTs can be used in preclinical models to, for example, measure behavior such as gait and balance function in mice. 44 Although the accuracy of digitally derived measures in rodents is much higher than of a human observer, these models do not truly translate because they do not capture what patients feel or how they function. 45 Additionally, DHTs are not currently able to interrogate drug‐target interactions or tissue distribution. However, some technologies, like continuous glucose monitors 14 or sensors, analyzing drug concentration 46 or cytokines in sweat, 47 have the potential to provide data for PK/PD modeling for early mechanistic studies. Some digital biomarkers can serve as measures of distal PD or MOA (e.g., ambulatory/at‐home blood pressure monitoring for hypertension, or heart‐rate monitoring for arrhythmia). 42 Body‐worn sensors have also been evaluated for safety monitoring in phase I clinical trials. 39
Moreover, digital biomarkers have the potential to increase the confidence in POC by measuring clinically meaningful endpoints objectively. For example, daily step count is a prognostic factor for survival and a dynamic predictor of short‐term hospitalizations in patients with cancer undergoing chemoradiotherapy. 48 , 49 DHTs can be incorporated into early POC studies or even dose‐escalation studies. 50 , 51 The opportunity to explore the relationship between digital endpoints related to patient function and novel medicine's pharmacology or pathology measures is almost unique in early development. The insights gained with digital device measures in early development can be extended to later confirmatory use (Figure 1). Unfortunately, at this time, DHT‐based measures are often incorporated as exploratory end points and are not disclosed. Given that DHT use in drug development is relatively new, examples in the public domain remain limited. However, data transparency requirements are evolving. 52 One example of success is the Digital Medicine Society's crowdsourcing library, which provides examples of DHT use across diseases. 53
Late‐stage clinical development
The ability of digital tools to objectively capture functional, physiological, or cognitive performance to generate efficacy data for novel therapies is often viewed as the most promising DHT application. The remote nature of many digital assessments can improve clinical trial accessibility, while delivering data to clinical staff in real or near real time. Furthermore, remote capture of the patients’ activity or ability to perform a certain task in a natural environment, complementing standard physician assessments done during clinic visits, can reduce measurement error, leading to a smaller‐size clinical trial of a shorter duration. 54 This can be achieved by moving an existing assessment into a remote mode, or by creating novel measures. 55
However, the unique proposition of DHTs (capturing functional outcomes by objective means) also presents challenges for measure design, development, and validation. Standard disease clinical assessments often interrogate multiple disease aspects and may combine physician assessment, functional tests, and patient‐reported outcomes (e.g., the Movement Disorder Society Unified Parkinson's Disease Rating Scale). 56 Although clinical assessments have well‐known limitations (including inter‐rater variability, as well as capturing a snapshot of data that can be highly influenced by patient's motivation at a specific time when the test is taken, and the need for frequent visitation to a clinical center), 57 these instruments are well‐validated and their properties are established. 56 Physician rating scales are often used as benchmarks for cross‐validation studies, and their limitations are inherently embedded in the validation results.
An alternative approach may include collecting both traditional clinical assessments, as well as disease‐relevant DHT‐enabled measures, in a longitudinal fashion to elucidate the relationship between the two types of measures and determine which disease aspects are better captured by a particular instrument. This idea has begun to gain ground. For example, the PPMI initiative, mentioned above, collects both imaging and digital assessments, in addition to standard clinician rating scales. The PPMI digital data have provided extremely useful information for digital measure design and deployment in clinical investigations (ESI, personal communication).
The DHT field struggles with the fact that there are (as yet) not always well‐established consensus parameters for digitized performance outcomes with thresholds for validation acceptance criteria. This has been achieved for some imaging‐based endpoints. For example, the Response Evaluation Criteria in Solid Tumors (RECIST) methodology was established as a standardized framework to define meaningful thresholds of tumor size change. 58 The approach has since been adopted broadly across stages of clinical development. Importantly, the framework continues to evolve, informed by data (e.g., optimizing the number of tumors selected) and reflecting technology adoption (e.g., addition of fluorodeoxyglucose‐PET to the framework). 59 The framework has also adapted to changing treatment paradigms (e.g., immunotherapies resulting in iRECIST) 60 where different imaging responses need to be captured inflammatory response and increase in tumor volume have had to be taken into account. 61 This emphasizes the importance of generating large datasets initially to establish clinically meaningful thresholds, developing pragmatic approaches to standardization in order to promote broad adoption, continuing to evolve criteria based on data and technology.
This issue has been largely resolved in the field of imaging and laboratory biomarkers by agreeing upon a list of parameters and thresholds for defined technological modalities. 14 , 62 This has also been addressed for digital blood pressure monitors due to work by professional societies that created validation protocols and enabled unrestricted information‐sharing about validation results carried out by independent investigators, 63 in a similar fashion to imaging biomarker data collection done by ADNI and PKDOC. A relevant example in the DHT field is the concept of measuring physical activity, which requires defining specific measures and appropriate thresholds to quantify moderate to vigorous with corresponding cutoff points 64 or whether people are inactive, moderately, or highly active. 65 However, the DHT applications span across a range of diverse conditions, including systemic and nervous system disorders. 66 Additionally, there is a big unmet need of DHT enabled therapeutics in mental health disorders, although this is a rapidly emerging area. 67 Moreover, the recent review by Jones and Wright 68 highlights a potential role of DHT in addressing rising costs of health care and enabling wellness and disease prevention. Some of the DHTs are capable of generating an ever‐increasing amount of data that requires tools for retrospective or near real time data processing. 14 The gap between existing statistical methodologies and data complexity requires alternative approaches that include machine learning and artificial intelligence. Indeed, these methods were used in the FDA regulatory submissions for purposes of end point/biomarker assessment to evaluate the effectiveness of therapeutic interventions across different indications. 69
CASE STUDIES
The examples of DHTs receiving regulatory endorsement for use in clinical investigations are limited and represent efforts by both single institutions and precompetitive collaborations. Below, we consider these use cases to examine the potential path forward for digital measures.
Parkinson's disease digital motor examination and mobility outcome assessments
Many ongoing efforts of developing novel digital measures interrogate disease signs and symptoms that involve abnormal body movements, decreased mobility, and exercise, because these can be effectively assessed by body‐worn accelerometers. These measures can be used as early POC/efficacy readouts. The distinct feature of digital measures is the direct interface of patients with technology, requiring usability studies, but also implying a patient‐centric approach in line with recent FDA guidance on patient‐focused drug development. 70 The requirements for DHT validation go beyond establishing technical performance and include understanding the relationship between the DHT and conventional outcome assessments. 9 Evidence is required that digital measures capture meaningful aspects of health, if a digital measure constitutes an electronic Clinical Outcome Assessment (eCOA), demonstrating relevance to the patients’ ability to function in day‐to‐day life, so data can be interpreted as representing meaningful change in patient function. 40 , 71 Similar to imaging biomarkers, partnerships with patients and patient organizations are vital, because they can leverage patient input in multiple ways: aiding in recruitment into natural history or validation studies, understanding the impact of disease on patients’ lives, and opportunities to leverage technology innovation to create measures which are more likely to succeed in drug development. The Critical Path for Parkinson's (CPP) Digital Drug Development Tool (3DT) initiative serves as an example that is advancing regulatory maturity of DHTs by optimizing an observational natural history study of disease progression, developed with multiple stakeholders, including people living with Parkinson's disease. 72
Certain measures, such as digital mobility outcomes, can play a prominent role in capturing people's ability to move and exercise, which are relevant to many conditions. These measures serve as additional monitoring biomarkers in assessing efficacy of new treatments. The Mobilise‐D initiative, which received two letters of support from the EMA, intends to use digital measures as complementary to those already in use as biomarkers of disease status. 73 , 74
Stride velocity 95th percentile as a secondary end point in Duchenne Muscular Dystrophy
Stride velocity 95th percentile (SV 95th) is a measure that was developed as a recognition of the limitations of registration end points for Duchenne Muscular Dystrophy, which uses functional tests such as the 6MWT or the North Star Ambulation Assessment (NSAA). The episodic nature of these assessments, which are dependent on patients’ motivation and clinical condition at the time of the assessment, do not represent patient performance during daily life, and therefore drive variability in the outcome measure.
The SV 95th received a full qualification opinion by the EMA as a secondary end point on the grounds of cross‐validation of this measure against 6MWT and NSAA. The agency has indicated that more work is required, including the long‐term correlation of the SV 95th with additional functional tests, expanding normative data and further supporting the critical relevance of the proposed minimal clinically important difference. 57
This successful example of regulatory endorsement clearly demonstrates the volume and complexity of experiments that are needed to validate digital measures. This work requires an input from multiple stakeholders and are best carried out in the form of precompetitive public‐private partnerships. 75 , 76
OTHER CONSIDERATIONS
It is important to recognize the challenges the DHT community is facing as well as the initial work that is being done to address them. The work has begun under Critical Path Initiative leadership to develop DHT data standards, starting with standards for metadata. 77 Moreover, a number of precompetitive collaborations – typically observational clinical trials – are underway in neurological diseases, such as AD, Parkinson's disease, and other movement disorders. 7 , 78 , 79 , 80 They include efforts to design new studies, collect data, understand data behavior (both standalone and in conjunction with conventional clinical assessments), and characterize early disease stages. These studies have not yet yielded results reported in peer‐reviewed publications; however, they may provide a solid foundation for addressing challenges that single institutions often encounter when submitting data packages to regulators. 7 Moreover, the precompetitive efforts done via public‐private partnerships provide an opportunity to engage with regulators early and proactively address challenges in qualifying these measures. 79 Lessons learned from imaging technologies can guide development and qualification of DHTs by focusing efforts on specific areas of research, known to be impactful in advancing scientific knowledge and clinical‐trial experience. Moreover, as indicated in recent FDA guidance, 9 the data privacy, rights, and access should be explicitly stipulated in the informed consent, including the data reuse in future investigations. The utility of this approach is demonstrated by Zhu et al., 33 analysis of aggregate data across multiple studies to answer important questions pertaining to establishing biomarkers as surrogate measures in AD.
We believe that publishing the methodologies and results of validation studies done according to scientific frameworks 41 and emerging regulatory guidance, 9 , 81 as demonstrated by Ellis et al., 82 helps establishing validation acceptance criteria along with real life examples of validated tool properties. These validated tools are likely to have the biggest impact in rare diseases as they may solve multiple problems, such as the need for frequent site visits, resilience to changes in environment, and attrition during the pandemic, at the same time offering convenience, inclusion, and data quality. 42 , 83 The deployment of DHTs in rare diseases is likely to become more important as our understanding of disease subgroups continues evolving, requiring studies in subpopulations with a robust signal in a small number of participants. 54 , 84
In summary, lessons from imaging biomarkers can be leveraged to advance DHT use in clinical investigations in the following ways:
Technical validation parameters and technology performance acceptance thresholds need to be established and accepted in the scientific community. A recent positive opinion from the EMA on digital mobile outcomes 40 and the WATCH‐PD study by Critical Path Initiative 7 are important milestones in this direction.
Approaches that are agnostic to hardware and data‐processing algorithms need to be developed by sharing DHT data and cross‐validating different algorithms, demonstrating comparability of data generated by different methods. Developing measures fully agnostic to hardware and software may be difficult due to specific requirements (e.g., access to sample‐level data not available for every digital sensor). However, it is feasible to develop such approaches across multiple sensors and algorithms that meet prespecified requirements.
Data standardization and creation of publicly shared databases will be key factors for DHT acceptance in drug development. This can be achieved via precompetitive data sharing and developing data dictionaries, structure, and parameters of data reporting which must be harmonized with technical validation parameters.
Precompetitive consortia via public‐private partnerships and professional societies can provide the biggest impact and advance DHT use in drug development. To be successful, these initiatives should focus on data sharing, to enable DHT measure development in a technology‐agnostic way.
Collaboration with patient organizations and patients themselves is a must, because many DHTs capture data depicting function or activities of daily living, where patient input is critical for success, and is required by regulators. 71
Innovating clinical trials provides a significant opportunity to expedite the successful delivery of impactful medicines to patients. The measurement technology base continues to expand, providing further opportunity and more challenge. After decades of experience adapting diagnostic imaging methods into drug‐development techniques, there have been many successes, but progress has typically been slow, and gaps remain. DHTs are arguably more complex, given the rapidly expanding and heterogenous nature of technologies. To progress this field, it is critical to learn from successes and challenges across all technology domains. Early collaborative engagement is a key theme that enables sufficient data generation to develop and qualify methods that will have a timely impact on successful drug development.
FUNDING INFORMATION
No funding was received for this work.
CONFLICT OF INTEREST
E.S.I. is an employee of Koneksa Health and may own company stock. T.J.M. is an employee of Pfizer, Inc. R.P.M. is an employee of UCB. P.M. is an employee of Janssen. D.T.S. and M.L.T.M. have no conflicts to disclose.
ACKNOWLEDGMENTS
The authors thank Sarah Morgan for her help with editing the manuscript and Marc Fairstein for figure graphic design assistance.
Izmailova ES, Maguire RP, McCarthy TJ, Müller MLTM, Murphy P, Stephenson D. Empowering drug development: Leveraging insights from imaging technologies to enable the advancement of digital health technologies. Clin Transl Sci. 2023;16:383‐397. doi: 10.1111/cts.13461
[Correction added on 22 December 2022, after first online publication: the word ‘health’ in the article title was mis‐spelled and it has been corrected in this version.]
REFERENCES
- 1. Paul SM, Mytelka DS, Dunwiddie CT, et al. How to improve R&D productivity: the pharmaceutical industry's grand challenge. Nat Rev Drug Discov. 2010;9(3):203‐214. [DOI] [PubMed] [Google Scholar]
- 2. Critical Path Initiative. Accessed November 30, 2022. https://www.fda.gov/science‐research/science‐and‐research‐special‐topics/critical‐path‐initiative
- 3. Gromova M, Vaggelas A, Dallmann G, Seimetz D. Biomarkers: opportunities and challenges for drug development in the current regulatory landscape. Biomark Insights. 2020;15:1177271920974652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schwarz AJ. The use, standardization, and interpretation of brain imaging data in clinical trials of neurodegenerative disorders. Neurotherapeutics. 2021;18(2):686‐708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morgan P, Van Der Graaf PH, Arrowsmith J, et al. Can the flow of medicines be improved? Fundamental pharmacokinetic and pharmacological principles toward improving phase II survival. Drug Discov Today. 2012;17(9–10):419‐424. [DOI] [PubMed] [Google Scholar]
- 6. European Medicines Agency . Qualification opinion of low hippocampal volume (atrophy) by mri for use in clinical trials for regulatory purpose ‐ in pre‐dementia stage of Alzheimer's disease. Accessed March 31, 2022. https://www.ema.europa.eu/en/documents/regulatory‐procedural‐guideline/qualification‐opinion‐low‐hippocampal‐volume‐atrophy‐magnetic‐resonance‐imaging‐use‐clinical‐trials_en.pdf
- 7. Stephenson D, Alexander R, Aggarwal V, et al. Precompetitive consensus building to facilitate the use of digital health technologies to support Parkinson disease drug development through regulatory science. Digit Biomark. 2020;4(Suppl 1):28‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vasudevan S, Saha A, Tarver ME, Patel B. Digital biomarkers: convergence of digital health technologies and biomarkers. NPJ Digit Med. 2022;5(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Digital health technologies for remote data acquisition in clinical investigations. Draft Guidance for Industry, Investigators, and Other Stakeholders. Accessed March 30, 2022. https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/digital‐health‐technologies‐remote‐data‐acquisition‐clinical‐investigations
- 10. European Medicines Agency . Questions and answers: Qualification of digital technology‐based methodologies to support approval of medicinal products. Accessed November 30, 2022. https://www.ema.europa.eu/en/documents/other/questions‐answers‐qualification‐digital‐technology‐based‐methodologies‐support‐approval‐medicinal_en.pdf
- 11. Stringer MS, Lee H, Huuskonen MT, et al. A review of translational magnetic resonance imaging in human and rodent experimental models of small vessel disease. Transl Stroke Res. 2021;12(1):15‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vicini P, van der Graaf PH. Systems pharmacology for drug discovery and development: paradigm shift or flash in the pan? Clin Pharmacol Ther. 2013;93(5):379‐381. [DOI] [PubMed] [Google Scholar]
- 13. Izmailova ES, McLean IL, Hather G, et al. Continuous monitoring using a wearable device detects activity‐induced heart rate changes after Administration of Amphetamine. Clin Transl Sci. 2019;12(6):677‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Godfrey A, Vandendriessche B, Bakker JP, et al. Fit‐for‐purpose biometric monitoring technologies: leveraging the laboratory biomarker experience. Clin Transl Sci. 2021;14(1):62‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clinical trial imaging endpoint process standards guidance for industry. Accessed March 30, 2022. https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/clinical‐trial‐imaging‐endpoint‐process‐standards‐guidance‐industry
- 16. FDA . About biomarkers and qualification. Accessed September 9, 2022. https://www.fda.gov/drugs/biomarker‐qualification‐program/about‐biomarkers‐and‐qualification#How_can_qualified_biomarkers_improve_the_drug_development_process
- 17. FDA . List of qualified biomarkers. Accessed September 26, 2022. https://www.fda.gov/drugs/biomarker‐qualification‐program/list‐qualified‐biomarkers
- 18. EMA . Opinions and letters of support on the qualification of novel methodologies for medicine development. Accessed September 26, 2022. https://www.ema.europa.eu/en/human‐regulatory/research‐development/scientific‐advice‐protocol‐assistance/novel‐methodologies‐biomarkers/opinions‐letters‐support‐qualification‐novel‐methodologies‐medicine‐development
- 19. Woodcock J, Woosley R. The FDA critical path initiative and its influence on new drug development. Annu Rev Med. 2008;59:1‐12. [DOI] [PubMed] [Google Scholar]
- 20. deSouza NM, Achten E, Alberich‐Bayarri A, et al. Validated imaging biomarkers as decision‐making tools in clinical trials and routine practice: current status and recommendations from the EIBALL* subcommittee of the European Society of Radiology (ESR). Insights Imaging. 2019;10(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Østergaard M, Peterfy CG, Bird P, et al. The OMERACT rheumatoid arthritis magnetic resonance imaging (MRI) scoring system: updated recommendations by the OMERACT MRI in arthritis working group. J Rheumatol. 2017;44(11):1706‐1712. [DOI] [PubMed] [Google Scholar]
- 22. Conaghan PG, Østergaard M, Troum O, et al. Very early MRI responses to therapy as a predictor of later radiographic progression in early rheumatoid arthritis. Arthritis Res Ther. 2019;21(1):214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. European Medicines Agency . Guideline on clinical investigation of medicinal products for the treatment of rheumatoid arthritis. Accessed June 3, 2022. https://www.ema.europa.eu/en/documents/scientific‐guideline/guideline‐clinical‐investigation‐medicinal‐products‐treatment‐rheumatoid‐arthritis_en.pdf
- 24. Sormani MP, Bruzzi P. MRI lesions as a surrogate for relapses in multiple sclerosis: a meta‐analysis of randomised trials. Lancet Neurol. 2013;12(7):669‐676. [DOI] [PubMed] [Google Scholar]
- 25. Lavu S, Vaughan LE, Senum SR, et al. The value of genotypic and imaging information to predict functional and structural outcomes in ADPKD. JCI Insight. 2020;5(15):e138724. doi: 10.1172/jci.insight.138724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perrone RD, Mouksassi MS, Romero K, et al. Total kidney volume is a prognostic biomarker of renal function decline and progression to end‐stage renal disease in patients with autosomal dominant polycystic kidney disease. Kidney Int Rep. 2017;2(3):442‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qualification of biomarker total kidney volume in studies for treatment of autosomal dominant polycystic kidney disease draft guidance for industry. Accessed March 30, 2022. https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/qualification‐biomarker‐total‐kidney‐volume‐studies‐treatment‐autosomal‐dominant‐polycystic‐kidney
- 28. European Medicines Agency . Qualification opinion Total Kidney Volume (TKV) as a prognostic biomarker for use in clinical trials evaluating patients with Autosomal Dominant Polycystic Kidney Disease (ADPKD). Accessed March 30, 2022. https://www.ema.europa.eu/en/documents/regulatory‐procedural‐guideline/qualification‐opinion‐total‐kidney‐volume‐tkv‐prognostic‐biomarker‐use‐clinical‐trials‐evaluating_en.pdf
- 29. Jones‐Davis DM, Buckholtz N. The impact of the Alzheimer's disease neuroimaging initiative 2: what role do public‐private partnerships have in pushing the boundaries of clinical and basic science research on Alzheimer's disease? Alzheimers Dement. 2015;11(7):860‐864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weber CJ, Carrillo MC, Jagust W, et al. The worldwide Alzheimer's disease neuroimaging initiative: ADNI‐3 updates and global perspectives. Alzheimers Dement (N Y). 2021;7(1):e12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weiner MW, Aisen PS, Beckett LA, et al. How will aducanumab approval impact AD research? J Prev Alzheimers Dis. 2021;8(4):391‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jagust WJ, Landau SM, Koeppe RA, et al. The Alzheimer's disease neuroimaging initiative 2 PET Core: 2015. Alzheimers Dement. 2015;11(7):757‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhu H, Mehta M, Huang S‐M, Wang Y. Toward bridging unmet medical need in early Alzheimer's disease: an evaluation of Beta‐amyloid (Aβ) plaque burden as a potential drug development tool. Clin Pharmacol Therap. 2022;111(4):728‐731. [DOI] [PubMed] [Google Scholar]
- 34. Hill DLG, Schwarz AJ, Isaac M, et al. Coalition against major diseases/European medicines agency biomarker qualification of hippocampal volume for enrichment of clinical trials in predementia stages of Alzheimer's disease. Alzheimers Dement. 2014;10(4):421‐429.e423. [DOI] [PubMed] [Google Scholar]
- 35. Marek K, Chowdhury S, Siderowf A, et al. The Parkinson's progression markers initiative (PPMI) ‐ establishing a PD biomarker cohort. Ann Clin Transl Neurol. 2018;5(12):1460‐1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Romero K, Conrado D, Burton J, et al. Molecular neuroimaging of the dopamine transporter as a patient enrichment biomarker for clinical trials for early Parkinson's disease. Clin Transl Sci. 2019;12(3):240‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. European Medicines Agency . Qualification opinion on dopamine transporter imaging as an enrichment biomarker for Parkinson's disease clinical trials in patients with early Parkinsoniansymptoms. Accessed March 31, 2022. https://www.ema.europa.eu/en/documents/regulatory‐procedural‐guideline/qualification‐opinion‐dopamine‐transporter‐imaging‐enrichment‐biomarker‐parkinsons‐disease‐clinical_en.pdf
- 38. BEST (Biomarkers, EndpointS, and other Tools) Resource. Accessed March 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK338448/
- 39. Izmailova ES, Wood WA, Liu Q, et al. Remote cardiac safety monitoring through the lens of the FDA biomarker qualification evidentiary criteria framework: a case study analysis. Digit Biomark. 2021;5(1):103‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Viceconti M, Hernandez Penna S, Dartee W, et al. Toward a regulatory qualification of real‐world mobility performance biomarkers in Parkinson's patients using digital mobility outcomes. Sensors (Basel). 2020;20(20):5920. doi: 10.3390/s20205920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Goldsack JC, Coravos A, Bakker JP, et al. Verification, analytical validation, and clinical validation (V3): the foundation of determining fit‐for‐purpose for biometric monitoring technologies (BioMeTs). NPJ Digit Med. 2020;3:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Manta C, Jain SS, Coravos A, Mendelsohn D, Izmailova ES. An evaluation of biometric monitoring Technologies for Vital Signs in the era of COVID‐19. Clin Transl Sci. 2020;13(6):1034‐1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Izmailova ES, Wood WA. Biometric monitoring Technologies in Cancer: the past, present, and future. JCO Clin Cancer Inform. 2021;5:728‐733. [DOI] [PubMed] [Google Scholar]
- 44. Mendes CS, Bartos I, Márka Z, Akay T, Márka S, Mann RS. Quantification of gait parameters in freely walking rodents. BMC Biol. 2015;13:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Baran SW, Bratcher N, Dennis J, et al. Emerging role of translational digital biomarkers within home cage monitoring Technologies in Preclinical Drug Discovery and Development. Front Behav Neurosci. 2021;15:758274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brasier N, Widmer A, Osthoff M, et al. Non‐invasive drug monitoring of β‐lactam antibiotics using sweat analysis‐a pilot study. Front Med (Lausanne). 2020;7:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jagannath B, Lin KC, Pali M, Sankhala D, Muthukumar S, Prasad S. Temporal profiling of cytokines in passively expressed sweat for detection of infection using wearable device. Bioeng Transl Med. 2021;6(3):e10220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ohri N, Halmos B, Bodner WR, et al. Daily step counts: a new prognostic Factor in locally advanced non‐small cell lung cancer? Int J Radiat Oncol Biol Phys. 2019;105(4):745‐751. [DOI] [PubMed] [Google Scholar]
- 49. Izmailova E, Huang C, Cantor M, Ellis R, Ohri N. Daily step counts to predict hospitalizations during concurrent chemoradiotherapy for solid tumors. J Clin Oncol. 2019;37(27_suppl):293. [Google Scholar]
- 50. Dockendorf MF, Hansen BJ, Bateman KP, Moyer M, Shah JK, Shipley LA. Digitally enabled, patient‐centric clinical trials: shifting the drug development paradigm. Clin Transl Sci. 2021;14(2):445‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li Y, Guan Y, Wang J, Calvin A, Kyle J, Miller B. Use Digital Sensor and Deep Learning to Evaluate Motor Performance in the D1PAM (LY3154207) Phase 1B Parkinson's Disease Clinical Trial MDS International Congress; 2019.
- 52. Kozlov M. NIH issues a seismic mandate: share data publicly. Nature. 2022;602(7898):558‐559. [DOI] [PubMed] [Google Scholar]
- 53. DiMe's library of digital endpoints. Accessed March 31, 2022. https://www.dimesociety.org/communication‐education/library‐of‐digital‐endpoints/
- 54. Huang C, Izmailova ES, Jackson N, et al. Remote FEV1 monitoring in asthma patients: a pilot study. Clin Transl Sci. 2021;14(2):529‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Izmailova ES, Ellis R, Benko C. Remote monitoring in clinical trials during the COVID‐19 pandemic. Clin Transl Sci. 2020;13(5):838‐841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society‐sponsored revision of the unified Parkinson's disease rating scale (MDS‐UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129‐2170. [DOI] [PubMed] [Google Scholar]
- 57. European Medicines Agency . Qualification opinion on stride velocity 95th centile as a secondary endpoint in Duchenne Muscular Dystrophy measured by a valid and suitable wearable device. Accessed March 30, 2022. https://www.ema.europa.eu/en/documents/scientific‐guideline/qualification‐opinion‐stride‐velocity‐95th‐centile‐secondary‐endpoint‐duchenne‐muscular‐dystrophy_en.pdf
- 58. Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. JNCI: Journal of the National Cancer Institute. 2000;92(3):205‐216. [DOI] [PubMed] [Google Scholar]
- 59. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228‐247. [DOI] [PubMed] [Google Scholar]
- 60. Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143‐e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schwartz LH, Litière S, de Vries E, et al. RECIST 1.1‐update and clarification: from the RECIST committee. Eur J Cancer. 2016;62:132‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sullivan DC, Obuchowski NA, Kessler LG, et al. Metrology standards for quantitative imaging biomarkers. Radiology. 2015;277(3):813‐825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Muntner P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73(5):e35‐e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kim Y, Beets MW, Welk GJ. Everything you wanted to know about selecting the "right" Actigraph accelerometer cut‐points for youth, but: a systematic review. J Sci Med Sport. 2012;15(4):311‐321. [DOI] [PubMed] [Google Scholar]
- 65. Ewald B, Duke J, Thakkinstian A, Attia J, Smith W. Physical activity of older Australians measured by pedometry. Australas J Ageing. 2009;28(3):127‐133. [DOI] [PubMed] [Google Scholar]
- 66. Izmailova ES, Wagner JA, Perakslis ED. Wearable devices in clinical trials: hype and hypothesis. Clin Pharmacol Ther. 2018;104(1):42‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sverdlov O, van Dam J, Hannesdottir K, Thornton‐Wells T. Digital therapeutics: an integral component of digital innovation in drug development. Clin Pharmacol Therap. 2018;104(1):72‐80. [DOI] [PubMed] [Google Scholar]
- 68. Jones GB, Wright JM. The economic imperatives for technology enabled wellness centered healthcare. J Public Health Policy. 2022;43:456‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Liu Q, Huang R, Hsieh J, et al. Landscape analysis of the application of artificial intelligence and machine learning in regulatory submissions for drug development from 2016 to 2021. Clin Pharmacol Ther. 2022. Epub ahead of print. doi: 10.1002/cpt.2668 [DOI] [PubMed] [Google Scholar]
- 70. FDA Patient‐Focused Drug Development Guidance Series for Enhancing the Incorporation of the Patient's Voice in Medical Product Development and Regulatory Decision Making. Accessed March 30, 2022. https://www.fda.gov/drugs/development‐approval‐process‐drugs/fda‐patient‐focused‐drug‐development‐guidance‐series‐enhancing‐incorporation‐patients‐voice‐medical
- 71. Letter Of Intent Determination DDT COA #000142. Accessed March 30, 2022. https://www.fda.gov/media/149517/download
- 72. Adams J, Dorsey E, Ruiz Herrero T, et al. WATCH‐PD: wearable assessments in the clinic and home in parkinson's disease: baseline analyses. MDS Virtual Congress 2021, abstract number 364.
- 73. European Medicines Agency . Letter of support for Mobilise‐D digital mobility outcomes as monitoring biomarkers. 20 May 2021 EMA/CHMP/SAWP/277641/2021. Accessed June 3, 2022. https://www.ema.europa.eu/en/documents/other/letter‐support‐mobilise‐d‐digital‐mobility‐outcomes‐monitoring‐biomarkers‐follow_en.pdf
- 74. European Medicines Agency . Letter of support for Mobilise‐D digital mobility outcomes as monitoring biomarkers. 29 April 2020 EMA/234828/2020. https://www.ema.europa.eu/en/documents/other/letter‐support‐mobilise‐d‐digital‐mobility‐outcomes‐monitoring‐biomarkers_en.pdf
- 75. Sacks L, Kunkoski E. Digital health technology to measure drug efficacy in clinical trials for Parkinson's disease: a regulatory perspective. J Parkinsons Dis. 2021;11(s1):S111‐S115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mantua V, Arango C, Balabanov P, Butlen‐Ducuing F. Digital health technologies in clinical trials for central nervous system drugs: an EU regulatory perspective. Nat Rev Drug Discov. 2021;20(2):83‐84. [DOI] [PubMed] [Google Scholar]
- 77. Hill DL, Stephenson D, Brayanov J, et al. Metadata framework to support deployment of digital health Technologies in Clinical Trials in Parkinson’s disease. Sensors. 2022;22(6):2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Everything ALS. Accessed March 31, 2022. https://www.everythingals.org/
- 79. Stephenson D, Badawy R, Mathur S, Tome M, Rochester L. Digital progression biomarkers as novel endpoints in clinical trials: a multistakeholder perspective. J Parkinsons Dis. 2021;11(s1):S103‐s109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sood M, Aborageh M, Domingo‐Fernández D, et al. Evaluating digital device technology in Alzheimer's disease via artificial intelligence. medRxiv. 2021; 2021.2011.2007.21265705. https://www.medrxiv.org/content/10.1101/2021.11.07.21265705v2.full [Google Scholar]
- 81. European Medicines Agency . Questions and answers: Qualification of digital technology‐based methodologies to support approval of medicinal products. Accessed April 27, 2022. https://www.ema.europa.eu/en/documents/other/questions‐answers‐qualification‐digital‐technology‐based‐methodologies‐support‐approval‐medicinal_en.pdf
- 82. Ellis R, Kelly P, Huang C, Pearlmutter A, Izmailova ES. Sensor verification and analytical validation of algorithms to measure gait and balance and pronation/supination in healthy volunteers. Sensors (Basel). 2022;22(16):6275. doi: 10.3390/s22166275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Moore J, Goodson N, Wicks P, Reites J. What role can decentralized trial designs play to improve rare disease studies? Orphanet J Rare Dis. 2022;17(1):240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mori H, Wiklund SJ, Zhang JY. Quantifying the benefits of digital biomarkers and technology‐based study endpoints in clinical trials: project Moneyball. Digit Biomark. 2022;6(2):36‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Thompson A, Parekh A. Value of data sharing to advance drug development: a regulatory perspective. Ther Innov Regul Sci. 2021;55(4):850‐852. [DOI] [PubMed] [Google Scholar]
- 86. US Food and Drug Administration (FDA) Letter of Support to the Critical Path Institute's Coalition Against Major Diseases (CAMD). Accessed June 7, 2022. https://www.fda.gov/media/112623/download
- 87. Conrado DJ, Nicholas T, Tsai K, et al. Dopamine transporter neuroimaging as an enrichment biomarker in early Parkinson's disease clinical trials: a disease progression modeling analysis. Clin Transl Sci. 2018;11(1):63‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. US Food and Drug Administration (FDA) . How to determine if your product is a medical device. Accessed March 30, 2022. https://www.fda.gov/medical‐devices/classify‐your‐medical‐device/how‐determine‐if‐your‐product‐medical‐device


