Abstract
Stroke is closely associated with carotid plaques. The assessment of carotid plaque is still the key issue of stroke prevention in clinical practice. This prospective cross‐sectional study included patients with carotid plaque evaluated by ultrasonography (US). The intima‐media thickness (IMT), lumen stenosis severity, thickness, and length of carotid plaque were measured by the routine US, and the amplitudes of subharmonics in the upstream shoulder, top, and downstream shoulder of all plaques and corresponding lumens were observed by Subharmonic Aided Pressure Estimation (SHAPE) US examination from the US contrast agent perflubutane microbubbles (Sonazoid), which analyzed the clinical parameters of patients, the subharmonic amplitude characteristics of all plaques and lumens, and the parameter differences between the ischemic stroke (IS) group and control group. From May 2021 to February 2022, 46 carotid plaques of 23 patients were included. For plaques, the subharmonic amplitude in the plaque (−60.52 ± 4.46) was lower than that in the opposing level lumen (−56.82 ± 5.68 dB), the subharmonic gradient across the plaque cap was negatively correlated with plaque thickness (r = −0.51, p < 0.001), and with the lumen stenosis severity (r = −0.42, p = 0.003). The median IMT of the IS group was thicker than the control group. The subharmonic gradient of the intraplaque of the IS group was larger than the control group (p = 0.004). In this analysis, we use the receiver operating characteristic (ROC) curve to establish the cutoff value of the difference to predict a new monitoring method for plaque without invasion to predict IS. It still needs a large‐scale study with long‐term follow‐up to validate these findings.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Ischemic stroke (IS) is closely associated with carotid plaques. Thus, the quantitative analysis and monitoring of plaque is very important for the prevention of IS. Ultrasound (US) contrast agent microbubbles will vibrate nonlinearly under the excitation of sound pressure, resulting in subharmonics which will change with the ambient pressure. The local hemodynamic conditions around a plaque can be reflected by the Subharmonic Aided Pressure Estimation (SHAPE) results.
WHAT QUESTION DID THIS STUDY ADDRESS?
The quantitative analysis and monitoring of plaque is very important for the prevention of IS. SHAPE US is developed to monitoring plaques as a quantitative measurement tools.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
The subharmonic amplitude distribution in plaque and lumen was different. The subharmonic gradient across the plaque cap was negatively correlated with plaque thickness, and the lumen stenosis severity. With the cutoff value 3.95 dB, the positive and negative predictive values for identifying IS of patients were 68% and 76% and the accuracy was 70%.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
The SHAPE US can indirectly reflect the pressure changes of carotid plaques, which may provide a new monitoring method for evaluating plaque vulnerability to prevent the occurrence of IS in the future.
INTRODUCTION
Globally, stroke is a major cause of mortality and permanent disability, 1 and ischemic stroke (IS) is closely associated with carotid plaques. 2 Previous research focusing on plaque morphology points out that plaque vulnerability is characterized by a large necrotic core, high macrophage content, a concomitant reduction in collagen, and a thin fibrous cap (<65 μm). 3 , 4 , 5 , 6 , 7 Nevertheless, studies have shown that plaque morphology alone was not enough to predict plaque rupture. 8 , 9 The focus of vulnerable plaque research has shifted from the morphology of vulnerable plaques to exogenous stress that may affect the development of plaques. Biomechanical factors are essential to reveal plaque rupture, which is a predictor of identifying vulnerable plaques. 10 , 11 , 12 , 13 Therefore, the assessment of carotid plaque has extraordinary significance in preventing the incidence of occurrence and reoccurrence of ischemic cerebrovascular events. The pressure gradient between the blood flow in the plaque and the lumen can reflect the local biomechanical conditions affecting the plaque, when the internal stress of the plaque exceeds the bearing strength of the plaque fibrous cap, the plaque may rupture. 14 , 15 Some researchers emphasized that despite the continuous progress of diagnostic techniques, the criteria for assessing plaque vulnerability in a broader sense have not yet been established 16 and individualized risk assessment and guiding treatment effects of the vulnerable plaque‐oriented clinical diagnosis methods still need to be improved. In addition, studies have shown that plaque may change alternately between vulnerable and non‐vulnerable. 17 Therefore, a quantitative analysis without invasion that can dynamically reflect internal and external conditions is essential for more accurate detection of plaque rupture.
Contrast‐enhanced ultrasound (CEUS) displays the neovascularization, size, and shape of plaques. 18 In addition, ultrasound (US) contrast agent microbubbles will vibrate nonlinearly under the excitation of sound pressure, resulting in subharmonics (f0/2 of receiving frequency), which will change with the ambient pressure. An inverse relationship has been demonstrated between the subharmonic amplitude and the ambient pressure, 19 , 20 , 21 and the sensitivity of this inverse relationship changes with increasing sound pressure. Therefore, the ambient pressure can be estimated after adjusting the emission acoustic settings to maximize the sensitivity of the inverse relationship. This technique is called Subharmonic Aided Pressure Estimation (SHAPE). It has been successfully applied to evaluate portal hypertension and monitor neoadjuvant chemotherapy response. 21 , 22
In this study, our group has proposed to use SHAPE US examination for noninvasive quantitative estimation of the pressure change inside the plaque and carotid artery lumen. The stress distribution in the plaque was evaluated regarding various plaque formations and the correlation with IS were analyzed to provide a new method for clinical monitoring plaque to prevent IS.
METHODS
Study patients
This prospective cross‐sectional study consecutively included patients with carotid plaque diagnosed by routine US which was performed by two physicians (both with more than 10 years of experience) with US specialization at Beijing Tiantan Hospital of Capital Medical University from May 2021 to February 2022. Patients who met the following inclusion criteria were included in the stroke group: (1) over 18 years old; (2) diagnosed with IS confirmed by the associated symptoms and imaging results from magnetic resonance imaging (MRI) or non‐enhanced computed tomography (CT) within 30 days before study inclusion; (3) TOAST classification of IS was large‐artery atherosclerosis. Patients who met the following inclusion criteria were included in the control group: (1) carotid arteriosclerosis disease diagnosed by carotid US; (2) no IS confirmed by MRI or CT; (3) no ischemic symptoms, including transient global amnesia, acute confusion, syncope, bilateral weakness, paraesthesia, etc. The exclusion criteria were as follows: (1) patients with a transient ischemic attack and lacunar ischemic stroke; (2) patients suspected of cardioembolic stroke, evidence of cardioembolism (recent myocardial infarction <3 weeks, atrial fibrillation, etc.), unexplained stroke; (3) non‐atherosclerotic arterial diseases, such as arterial dissection and vasculitis; (4) other pathogenesis such as hematological diseases and tumor; (5) patients with coronary artery stents, angioplasty, or coronary artery bypass grafts; (6) patients who were allergic to the US contrast agent; and (7) no clear sonogram of the plaque SHAPE US could be obtained. All patients with US‐confirmed carotid atherosclerosis were asked to undergo SHAPE US examination. Finally, 23 patients were included in this study (Figure 1). All patients or the appropriate family members had signed their informed consents before CEUS, and the Ethics Committee approved the protocol of this study at the Beijing Tian tan Hospital Affiliated to Capital Medical University (KY 2019‐113‐01). The clinical information and ultrasonic parameters of patients were collected and sorted out by Excel 2016 systematically.
FIGURE 1.
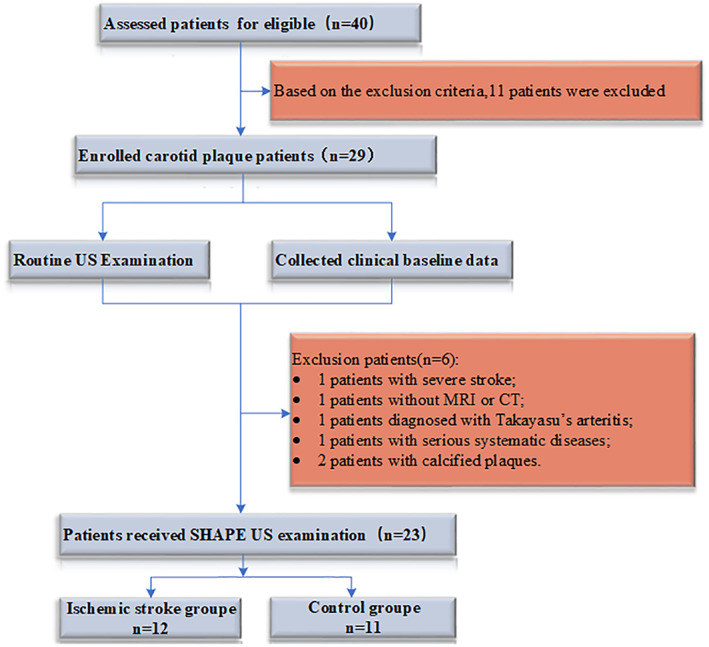
Flowchart of enrollment procedures in this study. Values are numbers of patients.
US contrast agent
Sonazoid (GE Healthcare, Oslo, Norway), composed of lipid‐coated microspheres and filled with perfluorobutane gas whose diameter typically ranges between 1 and 5 μm, 23 was used as a contrast agent in this study because it was the most sensitive to pressure changes and SHAPE measurements. 22 According to the manufacturer's recommendations, the 2 ml of sterile water for injection and perflubutane microbubbles were well reconstituted.
Routine US examination
After measuring blood pressure, all patients received bilateral carotid artery routine US scanning by LOGIQ E20 US scanner with L2‐9VN transducer (GE LOGIQ E20; GE Healthcare). The patients were positioned supine with their heads tilted back to expose the neck fully. The longitudinal and transverse sections were selected, and the measurement was performed at the thickest part of the common carotid artery intima‐media. The diagnostic criteria for carotid plaque formation were intima‐media thickness (IMT) greater than or equal to 0.15 cm and calcified plaque (class V) was excluded according to Gray‐Weale's classification. 24 The stenosis severity was measured by the method of the European Carotid Surgery Trial.
SHAPE US examination
After the routine US examination, The SHAPE mode was set to transmit at 2.5 MHz and to receive subharmonic signals at 1.25 MHz with a convex probe (GE LOGIQ E20; GE Healthcare). The grayscale gain was adjusted to 35–48 dB, and the depth was adjusted to 4 cm. Then, the subharmonic mode was selected. The patients received co‐infusion of the reconstituted US contrast agent and 0.9% sodium chloride injection intravenously at 0.024 ml/kg per minute 22 through a 20‐gauge intravenous cannula in the antecubital vein. Three minutes after the start of the infusion, and region of interest (ROI; 3 mm sampling frame) within the common carotid artery was selected. The adjusted mechanical index (MI) to 0.001 and the time‐intensity curve analysis software was initiated to determine the MI for maximum SHAPE sensitivity. Briefly, the MI gradually increased from 0.001 to 1. The subharmonic amplitude in the ROI echo signal was calculated when the microbubbles were excited by each MI, and the MI‐subharmonic amplitude curve was obtained. The first derivative of the abovementioned curve was calculated, yielding the transmission power‐subharmonic amplitude slope curve, and the midpoint of the maximum section of the slope curve (in the growth phase) was selected as the optimum MI. 22 It shows an example of this process (Figure 2c), which was performed once for each participant to account for individual variations in depth and signal attenuation levels. After the MI (range, 0.16–0.26) was adjusted to the best, the carotid arteries with plaques were observed and acquired as 9–11 s segments in the subharmonic mode. Then, ROIs with a 1 mm sampling frame were selected in the upstream shoulder, downstream shoulder, the top section of atherosclerotic plaques, and the corresponding midpoint of the remaining lumen perpendicular to the plaque is selected as the ROIs in the lumen to obtain the subharmonic amplitude of each part. The time‐intensity curve analysis software of LOGIQ E20 was used to analyze the subharmonic amplitude of ROIs (Figure 2d). The subharmonic gradient across the plaque cap was expressed by subtracting the subharmonic amplitude in the same level lumen from the subharmonic amplitude in the plaque. The subharmonic gradient of intraplaque was expressed by subtracting the minimum subharmonic amplitude in the plaque from the maximum subharmonic amplitude.
FIGURE 2.
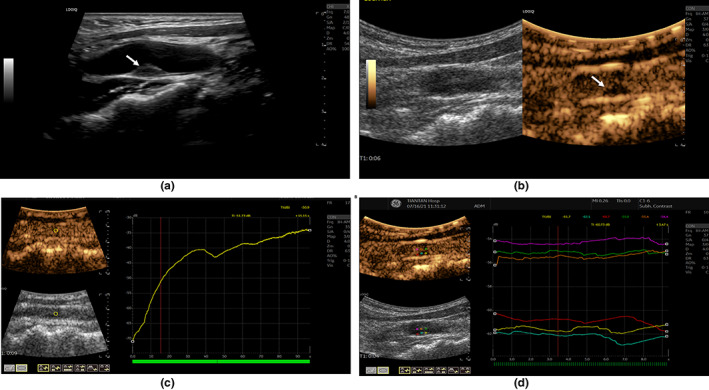
Routine US and SHAPE US Examination of AS Plaque. (a) Routine ultrasonography of carotid artery with AS plaque, the white arrow indicates an isoechoic plaque; (b) CEUS of carotid artery with AS plaque, the white arrow indicates that the plaque can be clearly displayed in subharmonic mode. (c) The point (the intersection of red and yellow lines in the growth phase) was selected as the optimum MI; (d) ROIs with 1 mm sampling frame were selected in the upstream shoulder, downstream shoulder, top section of atherosclerotic plaques, and the above three corresponding horizontal lumens of the plaque. AS, atherosclerosis; CEUS, contrast‐enhanced ultrasound; MI, mechanical index; ROI, region of interest; US, ultrasound.
Statistical analysis
All data are presented with descriptive statistics. Descriptive statistics are shown using number (percentage), mean ± SD (), and median (interquartile range) as applicable. The Kruskal‐Wallis test was used for the comparison of SHAPE data in three regions of plaques. Comparison of clinical and US parameters between the IS group and the control group were by the Mann–Whitney U test, the independent sample t‐test, or Fisher's Exact test as appropriate. Pearson correlation coefficient was used to analyze the correlation among plaque thickness, stenosis severity, and the subharmonic gradient across the plaque cap in the thickest section of atherosclerotic plaques. Reported p values were for two‐sided tests, with p less than 0.05 considered significant and statistical analysis was done using IBM SPSS Statistics (version 28; IBM). Calculation of sensitivities and specificities for diagnosing IS with SHAPE data was conducted with receiver operating characteristic (ROC) analysis. Calculation of sensitivities and specificities for diagnosing IS with SHAPE data was conducted with ROC analysis. The optimal cutoff values for ROC curves were established using the Youden index, 25 which was performed with MedCalc Statistical Software (version 20.022; MedCalc Software Ltd.).
RESULTS
Twenty‐nine patients were included in the carotid plaque screening process. Two (6.9%) patients with extensive calcification of carotid plaque, three (10.3%) patients with other diseases, and one (3.4%) patient with incomplete CT or MRI data were excluded from the study. Finally, 23 (79.3%) patients met the inclusion criteria. A total of 46 carotid plaques were collected from 23 patients, including 17 (73.91%) men and six (26.09%) women. There were 12 patients (mean ± SD age, 61.25 ± 9.35 years) in the IS group, including eight (66.67%) men and four (33.33%) women, and 11 patients (mean age ± S, 57.0 ± 3.71 years) in the control group which included nine (81.82%) men and two (18.18%) women.
For plaques, increased plaque thickness had a higher degree of stenosis (r = 0.904, p < 0.001). ROIs were taken from three parts of the carotid plaque, and the subharmonic amplitude was measured. The subharmonic amplitude in the plaque was unevenly distributed (p = 0.018), and the subharmonic amplitude in the thickest part of the plaque was the lowest (median, −62.35 dB). The average subharmonic amplitude at the plaque (−60.52 ± 4.46 dB) was lower than that in the same level lumen (−56.82 ± 5.68 dB, p < 0.001; Figure 3a).
FIGURE 3.
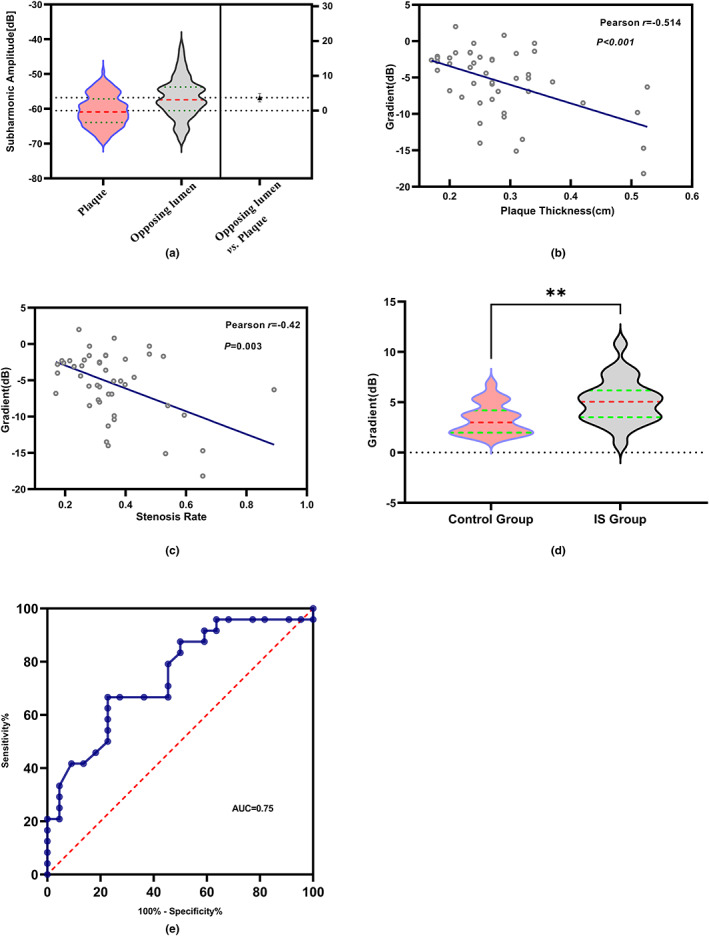
Statistical analysis of SHAPE US parameters. (a) Comparison of subharmonic amplitude of plaque and opposing lumen by independent sample t‐tests; p value less than 0.001. (b) Correlation between the subharmonic gradient across the plaque cap and thickness in the narrowest lumen. (c) Correlation between lumen stenosis severity and the subharmonic gradient across the plaque cap in the narrowest lumen. (d) The subharmonic gradient of intraplaque of the IS group and control group; **, p value = 0.0038. (e) Receiver operating characteristic curves demonstrate ability to use subharmonic‐aided pressure estimation (SHAPE) to identify patients with IS. AUC, area under receiver operating characteristic curve = 0.75; IS, ischemic stroke; SHAPE, Subharmonic Aided Pressure Estimation; US, ultrasound.
The subharmonic gradient across the plaque cap was negatively correlated with the plaque thickness (r = −0.51, p < 0.001; Figure 3b) in the narrowest area, and it was also negatively correlated with the stenosis severity in the narrowest area (r = −0.42, p = 0.003; Figure 3c).
In group analysis, there was no significant difference in age, body mass index, sex, blood pressure, plaque thickness, MI, intraplaque subharmonic amplitude, and corresponding lumen subharmonic amplitude, and subharmonic gradient across the plaque cap between the two groups (Table 1). We observed significant difference in IMT between the two groups (p = 0.04). The median IMT of the IS group (median, 1.25 mm) was thicker than the control group (median, 1.1 mm). The subharmonic gradient of intraplaque of the IS group (median 5.05 dB) was significantly larger than that in the control group (median 3.00 dB, p = 0.004; Figure 3d). The areas under the curve for the subharmonic gradient of intraplaque were 0.75 (95% confidence interval [CI]: 0.60–0.89) for patients, with IS, as shown in Figure 3e. From this curve, the optimal operating point was 3.95 dB for separating patients for cutoff. In the identification of IS, SHAPE achieved a sensitivity of 66.67% (95% CI: 46.71–82.03) and a specificity of 77.27% (95% CI: 56.56–89.88). The positive and negative predictive values for identifying IS of patients were 68% and 76% and the accuracy was 70%.
TABLE 1.
Comparison of clinical and US parameters between the IS group and the control group
| Characteristics | Total | Group | p value | |
|---|---|---|---|---|
| Ischemic stroke | Control | |||
| Age (years) a | 59.31 ± 10.46 | 61.25 ± 9.35 | 57.0 ± 3.71 | 0.35 |
| Blood pressure | ||||
| Systolic blood pressure (mmHg) a | 128.1 ± 4.95 | 130.0 ± 4.37 | 126.1 ± 4.93 | 0.06 |
| Diastolic blood pressure (mmHg) a | 76.7.8 ± 6.79 | 77.58 ± 6.73 | 75.8 ± 7.007 | 0.55 |
| BMI b | 23.15 (21.67, 26.49) | 24.05 (22.08, 27.83) | 22.61 (21.27, 24.65) | 0.18 |
| Sex, n (%) c | 23 (100) | 12 (54.55) | 11 (45.45) | 0.27 |
| Male, n (%) | 17 (73.91) | 8 (66.67) | 9 (81.82) | |
| Female, n (%) | 6 (26.09) | 4 (33.33) | 2 (18.18) | |
| Plaque, n (%) | 46 (100) | 24 (52.17) | 22 (47.83) | / |
| IMT (mm) b | 1.2 (1.1, 1.3) | 1.25 (1.13, 1.30) | 1.1 (1.1, 1.2) | 0.04 |
| Stenosis severity (%) a | 33.42 (27.52, 39.85) | 33.56 (30.76, 46.63) | 31.39 (24.98, 36.25) | 0.23 |
| Plaque | ||||
| Thickness (cm) b | 0.27 (0.23, 0.32) | 0.27 (0.23, 0.33) | 0.25 (0.21, 0.29) | 0.409 |
| Length (cm) b | 1.25 (1.00, 1.88) | 1.20 (0.99, 2.08) | 1.38 (1.05, 1.71) | 0.904 |
| Mechanical index b | 0.18 (0.16, 0.20) | 0.17 (0.16, 0.22) | 0.20 (0.16, 0.20) | 0.695 |
| Subharmonic amplitude of plaque (dB) b | ||||
| Upstream shoulder | −59.6 (−62.53, −55.57) | −58.50 (−61.53, −55.75) | −61.0 (−62.73, −55.13) | 0.301 |
| Top | −62.35 (−65.10, −58.54) | −62.05 (−65.05, −60.10) | −63.2 (−65.10, −57.90) | 0.767 |
| Downstream shoulder | −59.90 (−64.18, −56.58) | −59.35 (−62.27, −56.28) | −63.55 (−65.33, −56.83) | 0.088 |
| Subharmonic amplitude of opposing lumen of plaque (dB) b | ||||
| Upstream shoulder | −58.20 (−60.57, −53.05) | −58.05 (−59.55, −54.07) | −59.6 (−62.20, −51.42) | 0.644 |
| Top | −57.00 (−58.65, −53.27) | −57.40 (−58.30, −53.97) | −54.70 (−60.80, −53.13) | 0.455 |
| Downstream shoulder | −56.4 (−61.50, −54.15) | −55.55 (−59.60, −53.37) | −57.45 (−63.95, −54.27) | 0.333 |
| Subharmonic gradient of intraplaque (dB) b | 3.80 (2.55, 5.60) | 5.05 (3.50, 6.18) | 3.00 (1.98, 4.20) | 0.004 |
| Subharmonic gradient across the plaque cap | −4.85 (−8.13, −2.28) | −5.35 (−6.88, −2.15) | −3.75 (−10.03, −2.28) | 0.843 |
Abbreviations: BMI, body mass index; IMT, intima‐media thickness; IS, ischemic stroke; US, ultrasound.
Variable reported as mean ± SD (). The t‐test was used to analyze the comparison between two groups, the significance level is 0.05.
Variable reported as the median (interquartile range [IQR]), Mann–Whitney U test for comparison between the two groups, the significance level is 0.05.
Variable reported as frequency and percentage, Chi‐Square Test was used to compare the differences between the two groups.
DISCUSSION
Carotid vulnerable plaque is a critical cause of many cardiovascular diseases, 26 it may be abruptly complicated by rupture or erosion of an atherosclerotic plaque with an overlying thrombosis precipitating acute IS and sudden coronary death. 27 , 28 Therefore, the quantitative analysis and monitoring of plaque is very important for the prevention of IS.
However, the quantitative measurement tools for monitoring plaques were lack, especially noninvasive quantitative methods. An earlier pilot study indicated the local hemodynamic conditions around a plaque can be reflected in the SHAPE results on a rabbit model. 29 In this study, we applied SHAPE technology to carotid plaque. In the SHAPE US examination, few patients had their own conditions (deep and tortuous vessels in elderly patients, frequent jitters, etc.) or thick calcification in the plaque was always hyperechoic. These factors made it impossible for us to obtain clear plaque sonograms, making subharmonic acquisition and analysis impossible. Therefore, we also excluded these patients and calcified plaques (class V) classified by Gray‐Weale's classification. 24 , 30 We found that the subharmonic amplitude distribution in plaque and lumen was different. This reflects the previous research, that was, the components of the plaque were easy to deform under pressure, resulting in inconsistent pressure distribution in the plaque. 31 The larger the plaque, the more obvious the deformation would be, which leads to the change of pressure in the plaque and the lumen, that was, the variation of subharmonic amplitude. Then, we used the subharmonic gradient to reflect the pressure gradient across the plaque cap. The subharmonic gradient across the plaque cap was negatively correlated with plaque thickness, and the degree of lumen stenosis. When the plaque became thicker, the relative surface area of the plaque expanded, the fibrous cap on the surface of the plaque got uneven and thinner, the grade of neovascularization and lumen stenosis degree increased. 32 Consequently, the stress of the plaque increased significantly. 33
Therefore, in further analysis, we found that IMT in the IS group was thicker than that in the control group as in previous studies, 34 and our results also indicate that, through the quantitative analysis of SHAPE US, the subharmonic gradient intra the plaque of the IS group was higher than that in the control group with the quantitative analysis of SHAPE US, but there was no difference between the groups in the stenosis rate, the subharmonic gradient across the cap, etc. From the perspective of hemodynamic, after the formation of plaque, the blood flow through the lumen was unchanged in unit time. It became thinner through the cross‐sectional area of the lumen, resulting in the acceleration of blood flow velocity: the faster the blood flow velocity, the greater the change of plaque surface pressure, and the low‐pressure area was prone to collapse. 35 The greater the pressure difference, the easier the relatively weak area of the plaque was to rupture. 36 Meanwhile, the kinetic energy obtained by the blood will further aggravate the instability of the fibrous cap on the plaque's surface, which will eventually lead to rupture. Therefore, the high‐pressure difference on the plaque surface may induce the plaque to break easily. Related studies have illustrated that neovascularization and hemorrhage in plaque increase the pressure in plaque. 33 This also confirmed the conclusion of previous research of which the limitation of carotid stenosis in evaluating the occurrence of stroke and the importance of assessing the internal composition and morphological changes of large artery stroke. 37 , 38 In the current study, we observed that the subharmonic gradient of intraplaque was relatively larger in the IS group, and the threshold value of 3.95 dB was obtained, with specificity of about 77.27% and sensitivity of about 66.67%, which provides a new way for us to quantitatively monitor the pressure in plaque. Therefore, the change of subharmonic amplitude can be used to reflect the pressure change in plaque and lumen, and the subharmonic gradient of intraplaque is expected to be used to monitor the development trend of plaque clinically.
Despite the subharmonic amplitudes of carotid plaques being analyzed, satisfactory results were obtained. However, there were still limitations. First of all, the current SHAPE mode used the subharmonic frequency, which did not provide high enough resolution to evaluate small plaques, and the experimental accuracy needed to be further improved. Second, the size of experimental samples was small, and the differences between individuals were not considered, which would require more extensive sample statistics. Third, the blood pressure and heart rate were not monitored in real‐time during the extraction and processing of experimental data. Forthcoming period, the application of SHAPE technology to the risk prediction of IS may require a large sample size and long‐term follow‐up.
CONCLUSION
Our group applied SHAPE technology to assess carotid plaque and analyzed the relationship between subharmonic amplitude of carotid plaque and lumen and IS. Although the number of experimental studies was limited, the technique of SHAPE can indirectly reflect the pressure changes of carotid plaques, which may provide a new monitoring method for evaluating plaque vulnerability to prevent the occurrence of IS in the future.
AUTHOR CONTRIBUTIONS
R.L. wrote the manuscript. W.Z. and W.H. designed the research. R.L. and Y.K.Z. performed the research. S.Z. and Y.F.Z analyzed the data. LG.C. and Z.G.C. contributed analytical tools.
FUNDING INFORMATION
This study was funded by the State Key Program of National Natural Science of China (No. 8173000716).
CONFLICT OF INTEREST
The authors declared no competing interests for this work.
Supporting information
Table S1.
Li R, Zhang Y, Zheng S, et al. Noninvasive assessment of carotid plaque with subharmonic aided pressure estimation from a US contrast agent: A preliminary study. Clin Transl Sci. 2023;16:502‐511. doi: 10.1111/cts.13465
Contributor Information
Wen He, Email: hewen@bjtth.org.
Wei Zhang, Email: ultrazhangwei@126.com.
REFERENCES
- 1. Global, regional, and national burden of stroke, 1990‐2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;5(18):439‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nies K, Smits L, Kassem M, Nederkoorn PJ, van Oostenbrugge RJ, Kooi ME. Emerging role of carotid MRI for personalized ischemic stroke risk prediction in patients with carotid artery stenosis. Front Neurol. 2021;12:718438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Falk E, Nakano M, Bentzon JF, Finn AV, Virmani R. Update on acute coronary syndromes: the pathologists' view. Eur Heart J. 2013;10(34):719‐728. [DOI] [PubMed] [Google Scholar]
- 4. Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;21(368):2004‐2013. [DOI] [PubMed] [Google Scholar]
- 5. Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;5(20):1262‐1275. [DOI] [PubMed] [Google Scholar]
- 6. Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the committee on vascular lesions of the council on arteriosclerosis, American Heart Association. Circulation. 1995;5(92):1355‐1374. [DOI] [PubMed] [Google Scholar]
- 7. Virmani R, Burke A, Farb A. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. Eur Heart J. 1998;5(19):678‐680. [PubMed] [Google Scholar]
- 8. Stone GW, Maehara A, Lansky AJ, et al. A prospective natural‐history study of coronary atherosclerosis. N Engl J Med. 2011;3(364):226‐235. [DOI] [PubMed] [Google Scholar]
- 9. Tanaka A, Imanishi T, Kitabata H, et al. Morphology of exertion‐triggered plaque rupture in patients with acute coronary syndrome: an optical coherence tomography study. Circulation. 2008;23(118):2368‐2373. [DOI] [PubMed] [Google Scholar]
- 10. Tang D, Teng Z, Canton G, et al. Local critical stress correlates better than global maximum stress with plaque morphological features linked to atherosclerotic plaque vulnerability: an in vivo multi‐patient study. Biomed Eng Online. 2009;8:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tang D, Teng Z, Canton G, et al. Sites of rupture in human atherosclerotic carotid plaques are associated with high structural stresses: an in vivo MRI‐based 3D fluid‐structure interaction study. Stroke. 2009;10(40):3258‐3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tang D, Yang C, Zheng J, et al. Local maximal stress hypothesis and computational plaque vulnerability index for atherosclerotic plaque assessment. Ann Biomed Eng. 2005;12(33):1789‐1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Akyildiz AC, Speelman L, van Brummelen H, et al. Effects of intima stiffness and plaque morphology on peak cap stress. Biomed Eng Online. 2011;1(10):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stone PH, Fotiadis DI. Enhanced multifactorial biomechanical stress metrics to predict plaque rupture: rapid assessment of morphology and plaque structural stress. JACC Cardiovasc Imaging. 2020;3(13):817‐819. [DOI] [PubMed] [Google Scholar]
- 15. Brown AJ, Teng Z, Evans PC, Gillard JH, Samady H, Bennett MR. Role of biomechanical forces in the natural history of coronary atherosclerosis. Nat Rev Cardiol. 2016;4(13):210‐220. [DOI] [PubMed] [Google Scholar]
- 16. Stefanadis C, Antoniou CK, Tsiachris D, Pietri P. Coronary atherosclerotic vulnerable plaque: current perspectives. J Am Heart Assoc. 2017;6(3):e005543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kubo T, Maehara A, Mintz GS, et al. The dynamic nature of coronary artery lesion morphology assessed by serial virtual histology intravascular ultrasound tissue characterization. J Am Coll Cardiol. 2010;15(55):1590‐1597. [DOI] [PubMed] [Google Scholar]
- 18. Feinstein SB. Contrast ultrasound imaging of the carotid artery vasa vasorum and atherosclerotic plaque neovascularization. J Am Coll Cardiol. 2006;2(48):236‐243. [DOI] [PubMed] [Google Scholar]
- 19. Shi W, Forsberg F, Raichlen J, Needleman L, Goldberg B. Pressure dependence of subharmonic signals from contrast microbubbles. Ultrasound Med Biol. 1999;2(25):275‐283. [DOI] [PubMed] [Google Scholar]
- 20. Eisenbrey JR, Dave JK, Halldorsdottir VG, et al. Chronic liver disease: noninvasive subharmonic aided pressure estimation of hepatic venous pressure gradient. Radiology. 2013;2(268):581‐588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nam K, Eisenbrey JR, Stanczak M, et al. Monitoring neoadjuvant chemotherapy for breast cancer by using three‐dimensional subharmonic aided pressure estimation and imaging with US contrast agents: preliminary experience. Radiology. 2017;1(285):53‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gupta I, Eisenbrey JR, Machado P, et al. Diagnosing portal hypertension with noninvasive subharmonic pressure estimates from a US contrast agent. Radiology. 2021;1(298):104‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sontum PC. Physicochemical characteristics of Sonazoid, a new contrast agent for ultrasound imaging. Ultrasound Med Biol. 2008;5(34):824‐833. [DOI] [PubMed] [Google Scholar]
- 24. Toutouzas K, Drakopoulou M, Aggeli C, et al. In vivo measurement of plaque neovascularisation and thermal heterogeneity in intermediate lesions of human carotid arteries. Heart. 2012;23(98):1716‐1721. [DOI] [PubMed] [Google Scholar]
- 25. Gijsen FJ, Wentzel JJ, Thury A, et al. Strain distribution over plaques in human coronary arteries relates to shear stress. Am J Physiol Heart Circ Physiol. 2008;4(295):H1608‐H1614. [DOI] [PubMed] [Google Scholar]
- 26. Schinkel A, Bosch JG, Staub D, Adam D, Feinstein SB. Contrast‐enhanced ultrasound to assess carotid intraplaque neovascularization. Ultrasound Med Biol. 2020;3(46):466‐478. [DOI] [PubMed] [Google Scholar]
- 27. Spacek M, Zemanek D, Hutyra M, Sluka M, Taborsky M. Vulnerable atherosclerotic plaque – a review of current concepts and advanced imaging. Biomed Pap Med Fac Univ Palacký, Olomouc, Czechoslovakia. 2018;1(162):10‐17. [DOI] [PubMed] [Google Scholar]
- 28. Arbustini E, Dal Bello B, Morbini P, et al. Plaque erosion is a major substrate for coronary thrombosis in acute myocardial infarction. Heart. 1999;3(82):269‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nam K, Liu JB, Eisenbrey JR, et al. Three‐dimensional subharmonic aided pressure estimation for assessing arterial plaques in a rabbit model. J Ultrasound Med. 2019;7(38):1865‐1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Clevert DA, Sommer WH, Helck A, Saam T, Reiser M. Improved carotid atherosclerotic plaques imaging with contrast‐enhanced ultrasound (CEUS). Clin Hemorheol Microcirc. 2011;1(48):141‐148. [DOI] [PubMed] [Google Scholar]
- 31. Schulte‐Altedorneburg G, Droste DW, Haas N, et al. Preoperative B‐mode ultrasound plaque appearance compared with carotid endarterectomy specimen histology. Acta Neurol Scand. 2000;3(101):188‐194. [DOI] [PubMed] [Google Scholar]
- 32. Staub D, Partovi S, Schinkel AF, et al. Correlation of carotid artery atherosclerotic lesion echogenicity and severity at standard US with intraplaque neovascularization detected at contrast‐enhanced US. Radiology. 2011;2(258):618‐626. [DOI] [PubMed] [Google Scholar]
- 33. Li Z, Wang Y, Wu X, et al. Studying the factors of human carotid atherosclerotic plaque rupture, by calculating stress/strain in the plaque, based on CEUS images: a numerical study. Front Neuroinform. 2020;14:596340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tsivgoulis G, Vemmos K, Papamichael C, et al. Common carotid artery intima‐media thickness and the risk of stroke recurrence. Stroke. 2006;7(37):1913‐1916. [DOI] [PubMed] [Google Scholar]
- 35. Steinman DA. Image‐based computational fluid dynamics modeling in realistic arterial geometries. Ann Biomed Eng. 2002;4(30):483‐497. [DOI] [PubMed] [Google Scholar]
- 36. Li ZY, Taviani V, Tang T, et al. The mechanical triggers of plaque rupture: shear stress vs pressure gradient. Br J Radiol. 2009;82 Spec No 1:S39‐S45. [DOI] [PubMed] [Google Scholar]
- 37. Kramer CM, Treiman GS. Vulnerable plaque in carotid arteries without “significant” stenosis. J Am Coll Cardiol. 2020;19(76):2223‐2225. [DOI] [PubMed] [Google Scholar]
- 38. Kopczak A, Schindler A, Bayer‐Karpinska A, et al. Complicated carotid artery plaques as a cause of cryptogenic stroke. J Am Coll Cardiol. 2020;19(76):2212‐2222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.


