Abstract
Background
Both secondary tricuspid regurgitation (STR) and heart failure with preserved ejection fraction (HFpEF) are relevant public health problems in the elderly population, presenting with potential overlaps and sharing similar risk factors. However, the impact of severe STR on hemodynamics and cardiorespiratory adaptation to exercise in HFpEF remains to be clarified.
Aim
To explore the impact of STR on exercise hemodynamics and cardiorespiratory adaptation in HFpEF.
Methods
We analyzed invasive hemodynamics and gas-exchange data obtained at rest and during exercise from HFpEF patients with severe STR (HFpEF-STR), compared with 1:1 age-, sex-, and body mass index (BMI)- matched HFpEF patients with mild or no STR (HFpEF-controls).
Results
Twelve HFpEF with atrial-STR (mean age 72 years, 92% females, BMI 28 Kg/m2) and 12 HFpEF-controls patients were analyzed. HFpEF-STR had higher (p < 0.01) right atrial pressure than HFpEF-controls both at rest (10 ± 1 vs. 5 ± 1 mmHg) and during exercise (23 ± 2 vs. 14 ± 2 mmHg). Despite higher pulmonary artery wedge pressure (PAWP) at rest in HFpEF-STR than in HFpEF-controls (17 ± 2 vs. 11 ± 2, p = 0.04), PAWP at peak exercise was no more different (28 ± 2 vs. 29 ± 2). Left ventricular transmural pressure and cardiac output (CO) increased less in HFpEF-STR than in HFpEF-controls (interaction p-value < 0.05). This latter was due to lower stroke volume (SV) values both at rest (48 ± 9 vs. 77 ± 9 mL, p < 0.05) and at peak exercise (54 ± 10 vs. 93 ± 10 mL, p < 0.05). Despite these differences, the two groups of patients laid on the same oxygen consumption isophlets because of the increased peripheral oxygen extraction in HFpEF-STR (p < 0.01). We found an inverse relationship between pulmonary vascular resistance and SV, both at rest and at peak exercise (R2 = 0.12 and 0.19, respectively).
Conclusions
Severe STR complicating HFpEF impairs SV and CO reserve, leading to pulmonary vascular de-recruitment and relative left heart underfilling, undermining the typical HFpEF pathophysiology.
Keywords: right heart catheterization, tricuspid regurgitation, heart failure with preserved ejection faction, exercise, oxygen consumption, hemodynamics
Background
The development of transcatheter interventions might enlarge the number of candidates to tricuspid regurgitation (TR) repair, including elderly patients with several comorbities and secondary TR (STR) (1–4). Among patients with STR, a high prevalence of patients with heart failure with preserved ejection fraction (HFpEF) might be expected: (i) aging represents a strong risk factor both HFpEF (5) and STR (2, 6); (ii) atrial fibrillation is inextricably linked to HFpEF (7) and to atrial-STR (6, 8–13); (iii) long-standing HFpEF may induce pulmonary hypertension (14), which might predispose to ventricular-STR (12, 13, 15). Thus, the clinical manifestations of HFpEF and severe STR may overlap and “confound” each other. Additionally, the net hemodynamic effect of developing STR in patients with an underlying hemodynamic abnormality, such as HFpEF, has not been clarified. Nonetheless, such information might be useful to figure out symptoms' pathophysiology of HFpEF-STR more clearly. In turn, this may allow to better tailor specific interventions in this population that it is expected to grow exponentially in the future, due to the progressive aging of the general population. Thus, the aim of this study was to explore the hemodynamic alterations related to STR in patients with hemodynamically-proven HFpEF by combining right heart catheterization with cardiopulmonary exercise test.
Methods
This study was approved by the Ethics Committee of the Istituto Auxologico Italiano (protocol n 2020_04_21_03 approved on April 21st, 2020). All patients signed a written informed consent to allow the use of their clinical data for research purposes.
We analyzed the cohort of patients who underwent an elective, clinically indicated cardiac catheterization at rest and during exercise at Istituto Auxologico Italiano between January 2018 and January 2022.
Cases were the patients with HFpEF and severe STR (HFpEF-STR). HFpEF was defined based on the presence of signs and/or symptoms of heart failure, a left ventricular (LV) ejection fraction (EF) >50%, associated with invasive hemodynamic demonstration of HFpEF. Invasive diagnosis of HFpEF was established in patients with an end-expiratory pulmonary artery wedge pressure (PAWP) at peak exercise equal or higher than 25 mmHg, and/or PAWP/ cardiac output (CO) slope higher than 2 mmHg/L/min) (16–18). From 74 patients with HFpEF without severe TR, we selected an equal number of controls for an individual matching to cases (ratio 1:1) considering sex-, age- (±5 years), and body mass index- (±2 Kg/m2).
We excluded patients with LV EF < 50%, secondary forms of HFpEF (cardiomyopathy, infiltrative diseases, pericardial constriction), more than mild left-sided valvular heart disease, congenital heart disease, pulmonary vascular diseases (pulmonary arterial hypertension, chronic thromboembolic pulmonary hypertension), pulmonary hypertension due to lung disease and/or hypoxia, severe comorbidities. Additionally, we excluded patients with congenital or acquired primary TR, cardiac implanted electronic device-related TR, and previous tricuspid surgery. Among the cases, we distinguished between atrial- and ventricular-STR, based on the detection of invasive pulmonary artery systolic pressure lower or higher than 50 mmHg (15).
Echocardiography
Echocardiography was performed according to current recommendations of the European Association of Cardiovascular Imaging and the American Society of Echocardiography by experienced cardiologists on the same day of the right heart catheterization (19), using a Vivid E9/E95 scanners (GE Vingmed, Horten, Norway). LV and left atrial (LA) volumes were measured using biplane disk summation algorithm on dedicated 4-chamber and 2-chamber apical views, taking care to avoid chamber foreshortening. Right atrial (RA) volume was measured in a right ventricle (RV) -focused 4-chamber view. Echocardiographic evaluation of STR severity was based on an integrative approach considering multiple qualitative and quantitative parameters (6, 20, 21). Due to the retrospective nature of the study, RV dimensions were qualitatively estimated in all patients.
Right heart catheterization and cardiopulmonary exercise test
Patients were studied on optimized medical therapy and in euvolemic state, in non-fasting state, without sedation, and in supine position. They wore a non-rebreathing Hans-Rudolph mask connected to the V-MAX metabolic cart (Vmax SensorMedics 2200, Yorba Linda, CA, USA) to directly measure gas-exchange data and ventilation (17). A 7-F fluid-filled Swan-Ganz catheter was placed in the pulmonary artery through the right internal jugular vein under fluoroscopic guidance. Proper pulmonary artery wedge positioning was confirmed by the appearance of a typical PAWP trace as well as by an oxygen saturation >94% sampled at the tip of the catheter. The right radial artery was cannulated with the Seldinger technique. The transducer was zeroed at the midthoracic line, halfway between the anterior sternum and the bed surface. Hemodynamic measurements were performed at rest, after 1 min of passive leg raise (feet on the pedals), and during the last minute of each step of a symptom-limited, maximal exercise test (18). Two milliliters of blood were sampled at the same time from the tip of the Swan-Ganz catheter and from the radial artery for blood gases analysis. The increment in workload was personalized in order to obtain at least three steps of exercise before exhaustion. Subjects were encouraged to exercise up to their maximal volitional effort. Pulmonary artery pressure, PAWP and RA pressure (RAP) were reported as an average of several respiratory cycles (18). Hemodynamic data reflect the agreement of two readers who visually reviewed all pressure traces. CO was calculated by direct Fick method, solving the oxygen consumption (VO2) equation as follows: CO = VO2/C(a-v)O2, where C(a-v)O2 is the oxygen arteriovenous difference. Furthermore, to evaluate the relative contribution of the elements of the Fick equation to exercise capacity, we plotted CO as a function of C(a-v)O2 on which we represented VO2-isophlets (22).
LV trans-mural pressure (LVTMP), as a measure of LV preload, independent of right heart filling and pericardial restraint, was calculated as PAWP–RAP (23). We plotted the relationship between pulmonary vascular resistance (PVR) and stroke volume (SV) to explore whether low anterograde SV may be linked to pulmonary vascular derecruitment and higher than normal PVR (24).
Key ergospirometric measurements included standard breath-by-breath cardiorespiratory and breathing pattern parameters. Peak VO2 was measured as the highest 30-s value obtained at the end of the effort. The slope of the relationship between minute ventilation and carbon dioxide production (VE/VCO2 slope) was calculated over the linear component of VE vs. VCO2 (25).
Statistics
Continuous variables are reported as mean and standard deviation (SD) or median and interquartile range if the data did not follow a normal distribution. The categorical variables are shown as absolute frequencies and proportions. For data at rest, unpaired T-test (or Wilcoxon signed rank sum test in case of non-normal distribution) was applied to compare the continuous variables between HFpEF-STR and HFpEF-controls, while Chi-square test (or Fisher test) was used to compare the categorical variables. For each hemodynamic variable measured during exercise, an ANOVA model for repeated measures was fitted, considering an unstructured variance-covariance matrix to take into account the correlation among measurements on the same subjects. The included covariates in each model were group (HFpEF-STR or HFpEF-controls), time (Rest, Leg Raise and Peak) and their interaction. The statistical significance of interaction term suggested a different trend of the hemodynamic variables between groups. Moreover, we tested the least square means (LS-means) differences among groups at each time by means of unpaired t-test applying the False Discovery Rate (FDR) approach to control the inflation of the type I error.
The relationship between continuous hemodynamic variables at specific time-point was investigated by means quadratic B-spline with 1 knot. The goodness of fit of the model was estimated by means of R2. This value, ranging from 0 to 1, represents the proportion of total variance of an independent variable explained by a dependent variable.
All analyses were performed using SAS version 9.4 software (SAS Institute, Cary, NC, USA). Statistical significance was set at the 0.05 level. All P-values were two-sided.
Results
Clinical characteristics
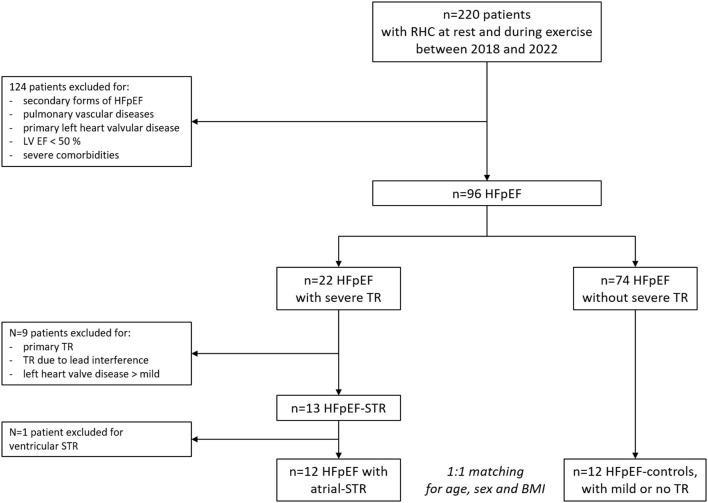
Patients' selection flowchart is depicted in Figure 1. HFpEF-STR represented 14% of our HFpEF population who had undergone exercise right heart catheterization during the study period. All HFpEF-STR patients but one (who had a typical HFpEF profile, but presented with a systolic PAP >50 mmHg) fulfilled the criteria for atrial-STR. In order to provide results on a more homogeneous patients' phenotype, we excluded from the analysis the patient that presented with a ventricular-STR. Indeed, the analyses focused on HFpEF with atrial-STR as compared with HFpEF-controls.
Figure 1.
Patients' selection flow-chart. BMI, body mass index; HFpEF, heart failure with preserved ejection fraction; LV EF, left ventricular ejection fraction; RHC, right heart catheterization; STR, secondary tricuspid regurgitation; TR, tricuspid regurgitation.
The clinical characteristics of our study groups are summarized in Table 1. They represented a quite typical elderly, overweight and predominantly female HFpEF population. Of note, 83% of patients with HFpEF-STR had permanent atrial fibrillation, as compared with only 8% of permanent atrial fibrillation in HFpEF-controls (p < 0.01). The rhythm at the time of right heart catheterization was atrial fibrillation in 83% of HFpEF-STR and 8% of HFpEF-controls. Among the other cardiovascular comorbidities, arterial hypertension was the most common affecting 75% of HFpEF-controls and 83% of HFpEF-STR patients (p = 1.000).
Table 1.
Clinical characteristics of the study cohort.
| Whole cohort (n = 24) | HFpEF-controls (n = 12) | HFpEF-STR (n = 12) | P-value | |
|---|---|---|---|---|
| Anthropometrics and demographics | ||||
| Age, years | 72 ± 5 | 72 ± 5 | 72 ± 5 | 0.867† |
| Female sex, n (%) | 22 (92%) | 11 (92%) | 11 (92%) | 1.000¥ |
| BMI, Kg/m2 | 28 ± 5 | 27 ± 4 | 28 ± 5 | 0.768† |
| Comorbidities | ||||
| Arterial hypertension, n (%) | 19 (79%) | 9 (75%) | 10 (83%) | 1.000¥ |
| Diabetes mellitus, n (%) | 1 (4%) | 0 (0%) | 1 (8%) | 1.000¥ |
| Coronary artery disease, n (%) | 3 (13%) | 2 (17%) | 1 (8%) | 1.000¥ |
| Paroxysmal/permanent AFib, n (%) | 12 (50%) | 2 (17%) | 10 (83%) | 0.001|| |
| Pace-maker, n (%) | 2 (8) | 1 (8) | 1 (8) | 1.000¥ |
| Previous cardiac surgery, n (%) | 2 (8) | 1 (8) | 1 (8) | 1.000¥ |
| Treatment | ||||
| Furosemide, n (%) | 16 (68%) | 7 (58%) | 9 (75%) | 0.667¥ |
| Spironolactone, n (%) | 8 (33%) | 4 (33%) | 4 (33%) | 1.000¥ |
| Hydrochlorothiazide, n (%) | 3 (13%) | 2 (17%) | 1 (8%) | 1.000¥ |
| ACE-I/ARB, n (%) | 14 (58%) | 7 (58%) | 7 (58%) | 1.000|| |
| Beta-blocker, n (%) | 17 (71%) | 6 (50%) | 11 (92%) | 0.069¥ |
| Oral anticoagulant, n (%) | 12 (50%) | 2 (17%) | 10 (83%) | 0.001|| |
| Symptoms | ||||
| NYHA class III-IV, n (%) | 15 (63%) | 9 (75%) | 6 (50%) | 0.400|| |
| Peripheral edema, n (%) | 10 (42%) | 4 (33%) | 6 (50%) | 0.408|| |
| JVD, n (%) | 3 (13%) | 0 (0%) | 3 (25%) | 0.217¥ |
| Blood tests | ||||
| Hemoglobin, g /dL | 12.7 ± 1.4 | 12.7 ± 1.4 | 12.7 ± 1.5 | 0.933† |
| BNP | 251 ± 185 | 220 ± 202 | 289 ± 168 | 0.451† |
| eGFR, mL/min/1.73 m2 | 57 ± 20 | 61 ± 23 | 54 ± 17 | 0.457† |
| AST, mg/dL | 23 [21–30] | 22.5 [19-25.5] | 24 [21–30] | 0.204‡ |
| ALT, mg/dL | 17.5 [14–26] | 17 [13–19] | 24.5 [18–40] | 0.013‡ |
| Echocardiography | ||||
| LVEF, % | 63 ± 5 | 63 ± 5 | 63 ± 5 | 0.980† |
| LVEDVI, mL | 47 ± 9 | 50 ± 8 | 44 ± 9 | 0.166† |
| RV dilation, n (%) | 8 (38%) | 1 (11%) | 7 (58%) | 0.067¥ |
| LAVI, mL/m2 | 44 ± 13 | 36 ± 10 | 50 ± 12 | 0.007† |
| RAVI, mL/m2 | 37 ± 18 | 25 ± 10 | 46 ± 18 | 0.003† |
| Cardiopulmonary exercise test | ||||
| VO2, % of predicted | 74 ± 18 | 77 ± 21 | 71 ± 15 | 0.446† |
| VO2, mL/Kg/min | 13 ± 4 | 13 ± 3 | 12 ± 4 | 0.690† |
| CO/VO2 slope | 4.7 ± 1.8 | 5.5 ± 1.7 | 3.9 ± 1.5 | 0.024† |
| VE/VCO2 slope | 33 ± 5 | 35 ± 6 | 32 ± 5 | 0.327† |
Continuous data are shown as mean ± standard deviation or median [interquartile range]. Categorical data are shown as number (percentage).
AFib, atrial fibrillation; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BNP, brain natriuretic peptide; CO, cardiac output; eGFR, estimated glomerular filtration rate; JVD, jugular vein distension; LAVI, left atrial volume index; LVEDVI, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; RAVI, right atrial volume index; RV, right ventricle; VCO2, carbon dioxide production; VE, minute ventilation; VO2, oxygen consumption.
†T-test;‡Wilcoxon test; ||Chi-square test; ¥Fisher test.
Representation of HF signs and symptoms did not significantly differ between HFpEF-STR and HFpEF-controls (Table 1). Brain natriuretic peptide values were slightly elevated but similar between the groups. Most of the blood test data reflecting secondary organ damage (renal, hepatic) showed similar values in HFpEF-STR as compared with HFpEF-controls. Only alanine aminotransferease values resulted significantly higher in HFpEF-STR patients than in HFpEF-controls. Estimated glomerular filtration rate was lower than expected, especially in HFpEF-STR, but did not significantly differ between the two groups (Table 1).
LV geometry and function were similar between the two groups, while RV dilation was non-significantly more frequent in HFpEF-STR than in HFpEF-controls (58 vs. 11%, p = 0.067). As expected, because of the incidence of atrial fibrillation, patients with HFpEF-STR presented larger LA and RA volumes that were, respectively, 43 and 84% larger than in HFpEF-controls (respectively, p < 0.01). No patient with HFpEF-STR presented with leaflet tenting.
Right heart catheterization and exercise capacity
Complete hemodynamic data are shown in Table 2 and Supplementary Table 1, while relative changes in hemodynamics from rest to feet on the pedals and between rest and peak are shown in Table 3.
Table 2.
Hemodynamics at rest, with feet on the pedals and at peak exercise in the study cohort.
| Rest | Leg raise | Peak | ||||
|---|---|---|---|---|---|---|
| HFpEF-controls | HFpEF- STR | HFpEF-controls | HFpEF- STR | HFpEF-controls | HFpEF- STR | |
| HR, bpm | 66 ± 4 | 81 ± 4* | 68 ± 4 | 81 ± 4* | 99 ± 6 | 120 ± 6* |
| SVI, mL/min/m2 | 43 ± 4 | 28 ± 4* | 45 ± 4 | 32 ± 4* | 52 ± 2 | 32 ± 5* |
| CI, mL/min/m2 | 2.8 ± 0.2 | 2.2 ± 0.2 | 2.9 ± 0.2 | 2.5 ± 0.2 | 4.9 ± 0.3 | 3.7 ± 0.3* |
| C(a-v)O2, mL/dL | 4.2 ± 0.3 | 5.3 ± 0.3** | 4.3 ± 0.3 | 6.0 ± 0.3** | 11.2 ± 0.7 | 13.3 ± 0.7* |
| PVR, WU | 1.5 ± 0.3 | 2.1 ± 0.3 | 1.3 ± 0.3 | 1.6 ± 0.3 | 1.1 ± 0.3 | 1.7 ± 0.3 |
| mPAP, mmHg | 18 ± 1 | 24 ± 1* | 23 ± 2 | 27 ± 2 | 36 ± 2 | 39 ± 2 |
| PAWP, mmHg | 11 ± 2 | 17 ± 2* | 16 ± 2 | 21 ± 2 | 28 ± 2 | 29 ± 2 |
| LVMTP, mmHg | 6 ± 1 | 7 ± 1 | 8 ± 1 | 8 ± 2 | 13 ± 2 | 7 ± 2* |
| RAP, mmHg | 5 ± 1 | 10 ± 1** | 8 ± 1 | 14 ± 1** | 14 ± 2 | 23 ± 2** |
| RAP V wave, mmHg | 6 ± 1 | 13 ± 1*** | 9 ± 2 | 18 ± 2*** | 17 ± 2 | 28 ± 2*** |
| RAP/PAWP | 0.45 ± 0.08 | 0.64 ± 0.08 | 0.52 ± 0.05 | 0.67 ± 0.05* | 0.50 ± 0.06 | 0.79 ± 0.06** |
CI, cardiac index; HFpEF, heart failure with preserved ejection fraction; HR, heart rate; LVMTP, left ventricular transmural pressure; mPAP, mean pulmonary artery pressure; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; STR, secondary tricuspid regurgitation; SVI, stroke volume index. *p < 0.05; **p < 0.01; ***p < 0.001.
Table 3.
Changes in hemodynamics between rest and feet on the pedals, and between rest and at peak exercise in the study cohort.
| Delta feet on pedals—Rest | Delta peak exercise—Rest | |||||
|---|---|---|---|---|---|---|
| HFpEF-controls | HFpEF-STR | P -value | HFpEF-controls | HFpEF-STR | P -value | |
| HR, bpm | 2 ± 4 | −0.3 ± 4 | 0.713 | 33 ± 4 | 39 ± 5 | 0.296 |
| SVI, mL/min/m2 | 2 ± 2 | 4 ± 2 | 0.519 | 9 ± 2 | 4 ± 2 | 0.174 |
| CI, mL/min/m2 | 0.2 ± 0.2 | 0.3 ± 0.2 | 0.669 | 2.2 ± 0.2 | 1.5 ± 0.2 | 0.036 |
| C(a-v)O2, mL/dL | 0.26 ± 0.47 | 0.72 ± 0.47 | 0.492 | 6.84 ± 0.47 | 7.69 ± 0.47 | 0.099 |
| PVR, WU | −0.2 ± 0.2 | −0.6 ± 0.2 | 0.286 | −0.5 ± 0.2 | −0.4 ± 0.2 | 0.924 |
| mPAP, mmHg | 5 ± 1 | 4 ± 1 | 0.562 | 18 ± 1 | 15 ± 1 | 0.167 |
| PAWP, mmHg | 5 ± 1 | 5 ± 1 | 0.731 | 17 ± 1 | 13 ± 1 | 0.017 |
| LVMTP, mmHg | 2 ± 1 | 1 ± 1 | 0.548 | 8 ± 1 | 0 ± 1 | 0.0001 |
| RAP, mmHg | 4 ± 1 | 4 ± 1 | 0.724 | 9 ± 1 | 13 ± 1 | 0.019 |
| RAP V wave, mmHg | 3 ± 1 | 5 ± 1 | 0.294 | 11 ± 1 | 15 ± 1 | 0.019 |
CI, cardiac index; HFpEF, heart failure with preserved ejection fraction; HR, heart rate; LVMTP, left ventricular transmural pressure; mPAP, mean pulmonary artery pressure; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; STR, secondary tricuspid regurgitation; SVI, stroke volume index.
At rest, patients with HFpEF-STR had higher mean pulmonary artery pressure (24 ± 1 mmHg vs. 18 ± 1 mmHg, p = 0.006) and PAWP (17 ± 2 mmHg vs. 11 ± 2 mmHg, p = 0.006) than HFpEF-controls, consistent with mild post-capillary pulmonary hypertension (PH). PH, defined by a mean pulmonary artery pressure >20 mmHg, was present in 83% of HFpEF-STR and in 25% of HFpEF-controls. SV was lower in HFpEF-STR than in HFpEF-controls (28 ± 4 mL vs. 43 ± 4 mL/m2, p = 0.03), which was compensated by higher heart rate (81 ± 4 bpm vs. 66 ± 4 bpm, p = 0.022) to maintain cardiac index, which resulted on average at the lower limit of normal in HFpEF-STR (2.2 ± 0.2 L/min/m2 vs. 2.8 ± 0.2 L/min/m2, p = 0.147). PVR was slightly increased in HFpEF-STR patients but not significantly higher to that of HFpEF-controls (2.1 ± 0.3 WU vs. 1.5 ± 0.3 WU, p = 0.196). RAP was twice as high in HFpEF-STR than in HFpEF-controls (10 ± 1 mmHg vs. 5 ± 1 mmHg, p = 0.007), with tall V waves (13 ± 1 mmHg vs. 6 ± 1 mmHg, p < 0.001) and a high RAP/PAWP ratio (0.64 ± 0.08 vs. 0.45 ± 0.08, p = 0.079).
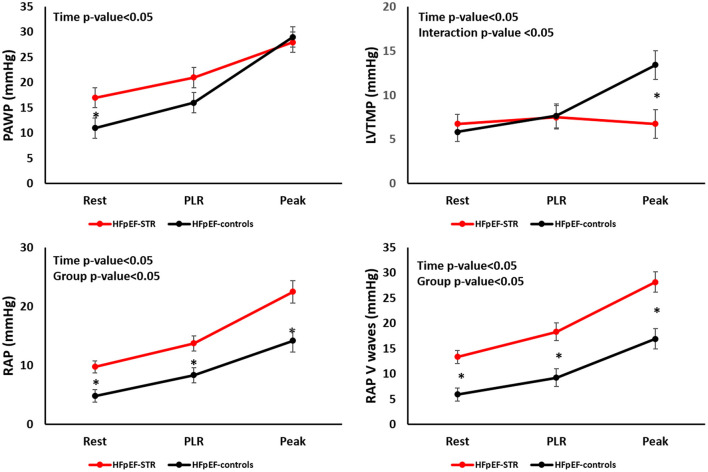
During exercise (Figure 2, Table 3, and Supplementary Table 1), PAWP increased less in HFpEF-STR patients than in HFpEF-controls. Indeed, despite higher PAWP in HFpEF-STR than in HFpEF-controls at rest, at peak exercise PAWP was not different in the two groups (28 ± 2 vs. 29 ± 2, p = 0.584). RAP V waves displayed an opposite behavior, increasing more in HFpEF-STR patients than in HFpEF-controls (interaction p-value = 0.06), with persistently higher RAP and RAP/PAWP ratio in HFpEF-STR patients than in HFpEF-controls (group p-value < 0.01). Accordingly, LVTMP increased only in HFpEF-controls during exercise (interaction p-value = 0.006), coherent with LV underfilling in HFpEF-STR patients.
Figure 2.
Evolution of left and right heart hemodynamics during exercise in our patients' population. HFpEF, heart failure with preserved ejection fraction; LVTMP, left ventricular transmural pressure; PAWP, pulmonary artery wedge pressure; PLR, passive leg raising; RAP, right atrial pressure; STR, secondary tricuspid regurgitation. *P < 0.05.
Exercise capacity, as evaluated through peak VO2, was not different at peak exercise between HFpEF-STR and HFpEF-controls, who reached the 71 and 77% of predicted values, respectively (p = 0.446), as shown in Table 1. However, the determinants of VO2 (Table 2, Supplementary Table 1, and Figure 3) behaved differently in the two groups, with a lower increase in CO and a higher C(a-v)O2 in HFpEF-STR patients as compared with HFpEF-controls (group p-value < 0.05 for both variables). In particular, HFpEF-STR and HFpEF-controls roughly lay roughly on the same VO2 isophlets at each step of exercise, with HFpEF-STR rightward and downward shifted (Figure 3). Accordingly, the CO/VO2 slope, as a measure of CO reserve, was lower in HFpEF-STR patients than in HFpEF-controls (3.9 vs. 5.5, p = 0.024). Exercise hyperventilation (VE/VCO2 slope) did not differ between HFpEF-STR and HFpEF-controls.
Figure 3.
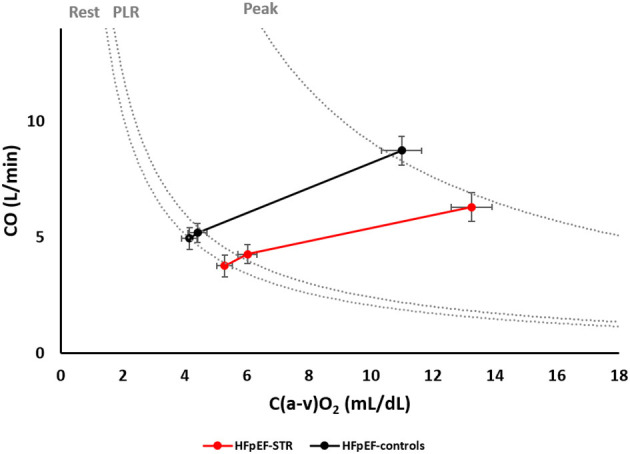
Relative weight of cardiac output and arteriovenous oxygen difference in determining oxygen consumption at rest, during passive leg raising and at peak exercise. Dotted lines represent oxygen consumption isophlets, i.e., cardiac output and arteriovenous oxygen difference coordinates whose product is a given oxygen consumption, at rest, during passive leg raising, and at peak exercise. C(a-v)O2, arteriovenous oxygen difference; CO, cardiac output; HFpEF, heart failure with preserved ejection fraction; PLR, passive leg raising; STR, secondary tricuspid regurgitation.
The SV was lower in HFpEF-STR than in HFpEF-controls (group p-value = 0.018). In particular, at peak exercise, it resulted 40 mL lower in HFpEF-STR (p = 0.03). The rate of increase of SV in the two groups however did not significantly differ (Table 3). Similarly, PVR similarly decreased by 0.4–0.5 WU in both groups during exercise. SV and PVR were inversely correlated both at rest and peak, with higher PVR at lower SV (Supplementary Figure 1). However, the low R2 values (0.12 and 0.19 for rest and peak timepoint, respectively) suggested high heterogenity in the relationship.
Discussion
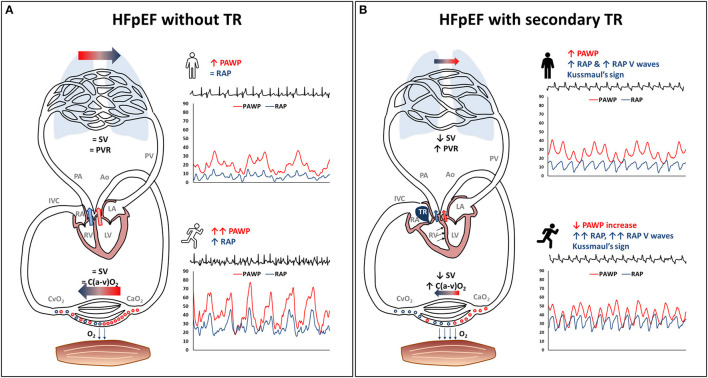
Our study highlights the hemodynamic impact of developing STR in a cohort of patients with HFpEF. Indeed, in patients with HFpEF, STR was associated with (i) marked RA dilation and RA hypertension, both at rest and during exercise; (ii) reduced SV and CO reserve, with patients relying on heart rate and on peripheral compensation (O2 extraction) to maintain exercise performance; (iii) pulmonary vascular derecruitment and LV underfilling, leading to lower than expected increase in left heart filling pressure during exercise (Figure 4).
Figure 4.
Exercise pathophysiology of secondary tricuspid regurgitation (STR) in heart failure with preserved ejection fraction (HFpEF). The typical HFpEF patient without STR (panel on the left) may present with (A) high pulmonary artery wedge pressure, either at rest or during exercise, with a steep pulmonary artery wedge pressure rise, and mildly increased right atrial pressure. (B) substantially normal stroke volume, pulmonary vascular resistance and peripheral oxygen extraction. This HFpEF pathophysiology is altered in the presence of STR (panel on the right), being characterized by: (i) high right atrial pressure that lacks inspiratory decrease or may present with overt Kussmaul's sign, tall V waves in the right atrium, right atrial enlargement and venous congestion; (ii) reduced forward stroke volume with pulmonary vascular derecruitment (increasing pulmonary vascular resistance), left ventricular underfilling, flatter pulmonary artery wedge pressure rise during exercise, and high ratio between right atrial pressure and pulmonary artery wedge pressure; (iii) higher reliance upon peripheral oxygen extraction to exercise despite low stroke volumeAo, aorta; C(a-v)O2, arteriovenous oxygen difference; CaO2, arterial oxygen content; CvO2, venous oxygen content; IVC, inferior vena cava; LA, left atrium; LV, left ventricle; O2, oxygen; PA, pulmonary artery; PAWP, pulmonary artery wedge pressure; PV, pulmonary vein; PVR, pulmonary vascular resistance; RAP, right atrial pressure; SV, stroke volume; TR, tricuspid regurgitation.
In our relatively small cohort of highly-selected and hemodynamically-proven HFpEF patients who underwent a clinically-indicated right heart catheterization at rest and during exercise, STR prevalence was not irrelevant (14%). The etiology of severe STR was atrial-functional in the great majority of these patients, on whom we decided to focus our analyses. Of note, 83% of patients with atrial-STR had a hemodynamic diagnosis of PH at rest according to the new definition (14), even though the degree of mean pulmonary artery pressure elevation was only mild, and no patient presented with leaflet tenting. Indeed, the definition of atrial-STR (previously known as isolated TR) (6, 8–15) relies on exclusion of other more commonly known secondary causes of TR and on an imprecise echocardiographic surrogate to exclude severe PH, i.e., a systolic pulmonary artery pressure < 50 mmHg (15). This distinction between ventricular-STR and atrial-STR may be better refined in the future, taking advantage of 3D reconstruction of the RV, the RA and of the tricuspid annulus, and considering the relative contribution of each of these elements in the pathogenesis of TR, as well as more precise measures of RV afterload.
Coherent with the non-negligible prevalence of atrial-STR in our hemodynamically-proven HFpEF population, both atrial-STR and HFpEF share common denominators, such as aging and atrial fibrillation. First, the prevalence of STR increases with age (26). Similarly, HFpEF is a disease of cardiovascular aging (27), which promotes diastolic stiffening of the LV and LA myopathy (28). Second, atrial fibrillation is associated with RA enlargement, negative remodeling of the tricuspid annular-valvular complex, and development of atrial-functional STR (8, 9, 11–13, 29–32). On the other hand, it has been proven that HFpEF diagnosis is a risk factor for the development or the progression of atrial fibrillation (28) and that atrial fibrillation is a strong clue for HFpEF diagnosis (33). Additionally, atrial fibrillation plays a role in HFpEF progression: its persistence may be associated over time with adverse bi-atrial remodeling, volume expansion, development of STR and right ventricular dysfunction (5, 10, 34, 35).
RAP, although often within normal limits in early-stage HFpEF, is generally 2-fold higher in this population than in healthy controls (16). STR causes an additional impact on the right heart of HFpEF patients, elevating RAP by another factor 2 in our HFpEF-STR. Despite this marked RAP elevation both at rest and during exercise, symptoms and peak VO2 did not relevantly differ between HFpEF-STR patients and HFpEF controls. Nonetheless, HFpEF-STR systematically presented with lower SV, and had to rely more on heart rate and peripheral O2 extraction [despite this latter being slightly lower than normal (36), pointing to the additional component of peripheral limitation in HFpEF] to maintain an exercise performance similar to HFpEF-controls. This highlights the importance of avoid strict rate-control strategies in HFpEF patients with atrial-STR (37). Additionally, our results suggest that VO2 at peak exercise might not be an optimal surrogate for STR severity, since HFpEF patients showed an ability to compensate for low CO by increasing peripheral extraction and, consequently, that interventions aimed at reducing STR severity might not necessarily improve peak VO2 despite improving symptoms, nonetheless reducing muscular fatigue. Furthermore, low SV was associated with pulmonary vascular de-recruitment, that can lead to spuriously increased PVR (24), not necessarily reflecting pulmonary vascular remodeling. Indeed, the rate of decrease in PVR during exercise was similar in the two groups, with HFpEF-STR set at higher PVR values because of low SV. Thus, slightly increased PVR at rest should not necessarily contraindicate transcatheter interventions for the correction of STR (38), albeit potentially indicating a worse patients' profile (39, 40). Finally, low SV together with the marked increase in RAP probably contributed to LV underfilling in HFpEF-STR patients by reducing LVTMP (23). This apparently counterintuitive finding, that volume-expanded HFpEF-STR patients had actually lower than expected rise in PAWP during exercise, might have clinical implications. Indeed, one might guess that STR may “protect” HFpEF-STR patients from pulmonary congestion, at the expense of reduced forward flow, even though pulmonary congestion might be favored by impaired lymphatic drainage associated with RA hypertension (41). After STR correction, unimpeded LV filling as a result of increased SV, may be associated with a sharp rise in left heart filling pressure with more overt pulmonary congestion, potentially fading the net clinical benefit of tricuspid valve repair.
Study limitations
This is a small, single-center retrospective study, conducted on a highly selected patients' population. To investigate the effect of STR on HFpEF pathophysiology, we sought to limit potential confounders by applying a 1:1 matching for age, sex, and body mass index, as well as rigorously identifying patients with HFpEF using exercise invasive hemodynamics. The careful and precise hemodynamic evaluation we performed, which could provide significant and physiologically-meaningful results, might at least partially compensate for the small sample size, albeit we cannot exclude the possibility of a type II error. However, we could not control for atrial fibrillation, which was expectedly more frequent in HFpEF-STR than in HFpEF-controls, and that might have contributed to some of the above-mentioned hemodynamic differences between these two population, which may eventually represent two extremes of the HFpEF progression (41). Additionally, the limited sample size of our well-characterized population might have hindered to highlight significant differences in some clinical variables between HFpEF-STR and HFpEF-controls. Due to the retrospective nature of the study, patients had only qualitative assessment of RV geometry and function, while three-dimensional evaluation would have been desirable for a better understanding of this complex chamber. Moreover, the use of exercise-stress echocardiography would have improved our understanding of hemodynamic behaviors, especially in terms of ventricular interdependence (42).
Conclusion
Occurrence of atrial-STR in patients with HFpEF is associated with atrial fibrillation. The hemodynamic characteristics at rest and during exercise of HFpEF-STR patients are consistent with afterload-independent right heart failure. Due to low SV, HFpEF-STR have to rely more on heart rate and peripheral O2 extraction to maintain exercise capacity. Pulmonary vascular resistance in HFpEF-STR may be slightly increased due to pulmonary vascular derecruitment. LV underfilling may lead to lower than expected PAWP. Understanding this peculiar pathophysiology may be relevant in the perspective of transcatheter correction of STR in HFpEF patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was approved by the Ethics Committee of the Istituto Auxologico Italiano (protocol n 2020_04_21_03 approved on April 21st, 2020). All patients signed a written informed consent to allow the use of their clinical data for research purposes.
Author contributions
CB contributed for conceptualization, data curation, investigation, methodology, visualization, and writing the original draft. SC contributed for conceptualization, data curation, investigation, methodology, supervision, and visualization and writing the original draft. GC contributed to data curation, investigation, methodology, and writing—review and editing. DS contributed for data analysis and writing—review and editing. AZ contributed for data analysis and writing—review and editing. CD contributed for supervision and writing—review and editing. MG, FH, MT, NR, and FP contributed to writing—review and editing. GPe contributed to supervision and writing—review and editing. J-LV contributed to conceptualization, supervision, and writing—review and editing. GPa contributed to supervision and writing—review and editing. LB and DM contributed to conceptualization, supervision, and writing—review and editing. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors are indebted with the nurses of the cath lab for their invaluable support in data acquisition. The authors are grateful to Laboratori Guidotti S.p.A., for providing free-of-charge software for echocardiographic analysis.
Funding Statement
This study was partially supported by the Italian Ministry of Health.
Abbreviations
C(a-v)O2, arteriovenous oxygen difference; CO, cardiac output; EF, ejection fraction; HFpEF, heart failure with preserved ejection fraction; LA, left atrium; LV, left ventricle; LVTMP, left ventricular transmural pressure; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; RA, right atrium; RAP, right atrial pressure; RV, right ventricle; STR, secondary tricuspid regurgitation; TR, tricuspid regurgitation; VCO2, carbon dioxide production; VE, minute ventilation; VO2, oxygen consumption.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer GI declared a shared parent affiliation with the authors CB, SC, GC, DS, AZ, MG, FH, MT, NR, FP, GPe, GPa, LB, and DM to the handling editor at the time of the review.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1061118/full#supplementary-material
References
- 1.Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. ESC/EACTS scientific document group. ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. (2022) 43:561–632. 10.1093/ejcts/ezac209 [DOI] [PubMed] [Google Scholar]
- 2.Praz F, Muraru D, Kreidel F, Lurz P, Hahn RT, Delgado V, et al. Transcatheter treatment for tricuspid valve disease. EuroIntervention. (2021) 17:791–808. 10.4244/EIJ-D-21-00695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russo G, Taramasso M, Pedicino D, Gennari M, Gavazzoni M, Pozzoli A, et al. Challenges and future perspectives of transcatheter tricuspid valve interventions: adopt old strategies or adapt to new opportunities? Eur J Heart Fail. (2022) 24:442–54. 10.1002/ejhf.2398 [DOI] [PubMed] [Google Scholar]
- 4.Ricci F, Bufano G, Galusko V, Sekar B, Benedetto U, Awad WI, et al. Tricuspid regurgitation management: a systematic review of clinical practice guidelines and recommendations. Eur Heart J Qual Care Clin Outcomes. (2022) 8:238–48. 10.1093/ehjqcco/qcab081 [DOI] [PubMed] [Google Scholar]
- 5.Borlaug BA. Evaluation and management of heart failure with preserved ejection fraction. Nat Rev Cardiol. (2020) 17:559–73. 10.1038/s41569-020-0363-2 [DOI] [PubMed] [Google Scholar]
- 6.Caravita S, Figliozzi S, Florescu DR, Volpato V, Oliverio G, Tomaselli M, et al. Recent advances in multimodality imaging of the tricuspid valve. Expert Rev Med Devices. (2021) 18:1069–81. 10.1080/17434440.2021.1990753 [DOI] [PubMed] [Google Scholar]
- 7.Obokata M, Reddy YNV, Melenovsky V, Pislaru S, Borlaug BA. Deterioration in right ventricular structure and function over time in patients with heart failure and preserved ejection fraction. Eur Heart J. (2019) 40:689–97. 10.1093/eurheartj/ehy809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Florescu DR, Muraru D, Volpato V, Gavazzoni M, Caravita S, Tomaselli M, et al. Atrial functional tricuspid regurgitation as a distinct pathophysiological and clinical entity: no idiopathic tricuspid regurgitation anymore. J Clin Med. (2022) 11:382. 10.3390/jcm11020382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guta AC, Badano LP, Tomaselli M, Mihalcea D, Bartos D, Parati G, Muraru D. The pathophysiological link between right atrial remodeling and functional tricuspid regurgitation in patients with atrial fibrillation: a three-dimensional echocardiography study. J Am Soc Echocardiogr. (2021) 34:585–94. 10.1016/j.echo.2021.01.004 [DOI] [PubMed] [Google Scholar]
- 10.Muraru D, Caravita S, Guta AC, Mihalcea D, Branzi G, Parati G, et al. Functional tricuspid regurgitation and atrial fibrillation: which comes first, the chicken or the egg? CASE. (2020) 4:458–63. 10.1016/j.case.2020.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muraru D, Guta AC, Ochoa-Jimenez RC, Bartos D, Aruta P, Mihaila S, et al. Functional regurgitation of atrioventricular valves and atrial fibrillation: an elusive pathophysiological link deserving further attention. J Am Soc Echocardiogr. (2020) 33:42–53. 10.1016/j.echo.2019.08.016 [DOI] [PubMed] [Google Scholar]
- 12.Hahn RT, Badano LP, Bartko PE, Muraru D, Maisano F, Zamorano JL, et al. Tricuspid regurgitation: recent advances in understanding pathophysiology, severity grading and outcome. Eur Heart J Cardiovasc Imaging. (2022) 23:913–29. 10.1093/ehjci/jeac009 [DOI] [PubMed] [Google Scholar]
- 13.Florescu DR, Muraru D, Florescu C, Volpato V, Caravita S, Perger E, et al. Right heart chambers geometry and function in patients with the atrial and the ventricular phenotypes of functional tricuspid regurgitation. Eur Heart J Cardiovasc Imaging. (2022) 23:930–40. 10.1093/ehjci/jeab211 [DOI] [PubMed] [Google Scholar]
- 14.Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. ESC/ERS scientific document group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. (2022) 5:ehac237. 10.1183/13993003.00879-2022 [DOI] [PubMed] [Google Scholar]
- 15.Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, 3rd, Gentile F, et al. 2020 ACC/AHA Guideline for the management of patients with valvular heart disease: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. (2021) 143:e72–227. 10.1161/CIR.0000000000000923 [DOI] [PubMed] [Google Scholar]
- 16.Baratto C, Caravita S, Soranna D, Dewachter C, Bondue A, Zambon A, et al. Exercise haemodynamics in heart failure with preserved ejection fraction: a systematic review and meta-analysis. ESC Heart Fail. (2022). 10.1093/eurheartj/ehac544.810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baratto C, Caravita S, Soranna D, Faini A, Dewachter C, Zambon A, et al. Current limitations of invasive exercise hemodynamics for the diagnosis of heart failure with preserved ejection fraction. Circ Heart Fail. (2021) 14:e007555. 10.1161/CIRCHEARTFAILURE.120.007555 [DOI] [PubMed] [Google Scholar]
- 18.Kovacs G, Herve P, Barbera JA, Chaouat A, Chemla D, Condliffe R, et al. An official European respiratory society statement: pulmonary haemodynamics during exercise. Eur Respir J. (2017) 50:1700578. 10.1183/13993003.00578-2017 [DOI] [PubMed] [Google Scholar]
- 19.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. (2015) 16:233–70. 10.1093/ehjci/jev014 [DOI] [PubMed] [Google Scholar]
- 20.Lancellotti P, Pibarot P, Chambers J, La Canna G, Pepi M, Dulgheru R, et al. Scientific document committee of the European association of cardiovascular imaging. Multi-modality imaging assessment of native valvular regurgitation: an EACVI and ESC council of valvular heart disease position paper. Eur Heart J Cardiovasc Imaging. (2022) 23:e171–232. 10.1093/ehjci/jeab253 [DOI] [PubMed] [Google Scholar]
- 21.Zaidi A, Oxborough D, Augustine DX, Bedair R, Harkness A, Rana B, et al. Echocardiographic assessment of the tricuspid and pulmonary valves: a practical guideline from the British Society of Echocardiography. Echo Res Pract. (2020) 7:G95–G122. 10.1530/ERP-20-0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baratto C, Caravita S, Faini A, Perego GB, Senni M, Badano LP, Parati G. Impact of COVID-19 on exercise pathophysiology: a combined cardiopulmonary and echocardiographic exercise study. J Appl Physiol. (1985). 130:1470–8. 10.1152/japplphysiol.00710.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersen MJ, Nishimura RA, Borlaug BA. The hemodynamic basis of exercise intolerance in tricuspid regurgitation. Circ Heart Fail. (2014 N) 7:911–7. 10.1161/CIRCHEARTFAILURE.114.001575 [DOI] [PubMed] [Google Scholar]
- 24.Naeije R, Gerges M, Vachiery JL, Caravita S, Gerges C, Lang IM. Hemodynamic phenotyping of pulmonary hypertension in left heart failure. Circ Heart Fail. (2017) 10:e004082. 10.1161/CIRCHEARTFAILURE.117.004082 [DOI] [PubMed] [Google Scholar]
- 25.Caravita S, Faini A, Deboeck G, Bondue A, Naeije R, Parati G, et al. Pulmonary hypertension and ventilation during exercise: role of the pre-capillary component. J Heart Lung Transplant. (2017) 36:754–62. 10.1016/j.healun.2016.12.011 [DOI] [PubMed] [Google Scholar]
- 26.Topilsky Y, Maltais S, Medina Inojosa J, Oguz D, Michelena H, Maalouf J, et al. Burden of tricuspid regurgitation in patients diagnosed in the community setting. JACC Cardiovasc Imaging. (2019) 12:433–42. 10.1016/j.jcmg.2018.06.014 [DOI] [PubMed] [Google Scholar]
- 27.Caravita S, Iacovoni A, Senni M. The right side of the circulation in not secondary heart failure with preserved ejection fraction: an elephant in the room? Eur J Heart Fail. (2021) 23:1659–61. 10.1002/ejhf.2294 [DOI] [PubMed] [Google Scholar]
- 28.Reddy YNV, Obokata M, Verbrugge FH, Lin G, Borlaug BA. Atrial dysfunction in patients with heart failure with preserved ejection fraction and atrial fibrillation. J Am Coll Cardiol. (2020) 76:1051–64. 10.1016/j.jacc.2020.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volpato V, Badano LP, Figliozzi S, Florescu DR, Parati G, Muraru D. Multimodality cardiac imaging and new display options to broaden our understanding of the tricuspid valve. Curr Opin Cardiol. (2021) 36:513–24. 10.1097/HCO.0000000000000890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muraru D, Addetia K, Guta AC, Ochoa-Jimenez RC, Genovese D, Veronesi F, et al. Right atrial volume is a major determinant of tricuspid annulus area in functional tricuspid regurgitation: a three-dimensional echocardiographic study. Eur Heart J Cardiovasc Imaging. (2021) 22:660–9. 10.1093/ehjci/jeaa286 [DOI] [PubMed] [Google Scholar]
- 31.Badano LP, Caravita S, Rella V, Guida V, Parati G, Muraru D. The added value of 3-dimensional echocardiography to understand the pathophysiology of functional tricuspid regurgitation. JACC Cardiovasc Imaging. (2021) 14:683–9. 10.1016/j.jcmg.2020.04.029 [DOI] [PubMed] [Google Scholar]
- 32.Muraru D, Parati G, Badano LP. The tale of functional tricuspid regurgitation: when atrial fibrillation is the villain. Eur Heart J Cardiovasc Imaging. (2020) 21:1079–81. 10.1093/ehjci/jeaa223 [DOI] [PubMed] [Google Scholar]
- 33.Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. (2018) 138:861–70. 10.1161/CIRCULATIONAHA.118.034646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Utsunomiya H, Itabashi Y, Mihara H, Berdejo J, Kobayashi S, Siegel RJ, et al. Functional tricuspid regurgitation caused by chronic atrial fibrillation: a real-time 3-dimensional transesophageal echocardiography study. Circ Cardiovasc Imaging. (2017) 10:e004897. 10.1161/CIRCIMAGING.116.004897 [DOI] [PubMed] [Google Scholar]
- 35.Ortiz-Leon XA, Posada-Martinez EL, Trejo-Paredes MC, Ivey-Miranda JB, Pereira J, Crandall I, et al. Understanding tricuspid valve remodeling in atrial fibrillation using three-dimensional echocardiography. Eur Heart J Cardiovasc Imaging. (2020) 21:747–55. 10.1093/ehjci/jeaa058 [DOI] [PubMed] [Google Scholar]
- 36.Pugliese NR, Mazzola M, Fabiani I, Gargani L, De Biase N, Pedrinelli R, et al. Haemodynamic and metabolic phenotyping of hypertensive patients with and without heart failure by combining cardiopulmonary and echocardiographic stress test. Eur J Heart Fail. (2020) 22:458–68. 10.1002/ejhf.1739 [DOI] [PubMed] [Google Scholar]
- 37.Palau P, Seller J, Domínguez E, Sastre C, Ramón JM, de La Espriella R, et al. Effect of β-blocker withdrawal on functional capacity in heart failure and preserved ejection fraction. J Am Coll Cardiol. (2021) 78:2042–56. 10.1016/j.jacc.2021.08.073 [DOI] [PubMed] [Google Scholar]
- 38.Lurz P, Orban M, Besler C, Braun D, Schlotter F, Noack T, et al. Clinical characteristics, diagnosis, and risk stratification of pulmonary hypertension in severe tricuspid regurgitation and implications for transcatheter tricuspid valve repair. Eur Heart J. (2020) 41:2785–95. 10.1093/eurheartj/ehaa138 [DOI] [PubMed] [Google Scholar]
- 39.Caravita S, Dewachter C, Soranna D, D'Araujo SC, Khaldi A, Zambon A, et al. Haemodynamics to predict outcome in pulmonary hypertension due to left heart disease: a meta-analysis. Eur Respir J. (2018) 51:1702427. 10.1183/13993003.02427-2017 [DOI] [PubMed] [Google Scholar]
- 40.Caravita S, Faini A, Carolino D'Araujo S, Dewachter C, Chomette L, Bondue A, et al. Clinical phenotypes and outcomes of pulmonary hypertension due to left heart disease: Role of the pre-capillary component. PLoS ONE. (2018) 13:e0199164. 10.1371/journal.pone.0199164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Omote K, Sorimachi H, Obokata M, Reddy YNV, Verbrugge FH, Omar M, et al. Pulmonary vascular disease in pulmonary hypertension due to left heart disease: pathophysiologic implications. Eur Heart J. (2022) 3:ehac184. 10.1093/eurheartj/ehac184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. (2017) 136:6–19. 10.1161/CIRCULATIONAHA.116.026807 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.